Abstract
BACKGROUND:
Patients with a failing Fontan continue to have decreased survival after heart transplant (HT), particularly those with preserved ventricular function (PVF) compared with impaired ventricular function (IVF). In this study we evaluated the effect of institutional changes on post-HT outcomes.
METHODS:
Data were retrospectively collected for all Fontan patients who underwent HT. Mode of failure was defined by the last echocardiogram before HT, with mild or no dysfunction considered PVF and moderate or severe considered IVF. Outcomes were compared between early era (EE, 1995 to 2008) and current era (CE, 2009 to 2014). Management changes in the CE included volume load reduction with aortopulmonary collateral (APC) embolization, advanced cardiothoracic imaging, higher goal donor/recipient weight ratio and aggressive monitoring for post-HT vasoplegia.
RESULTS:
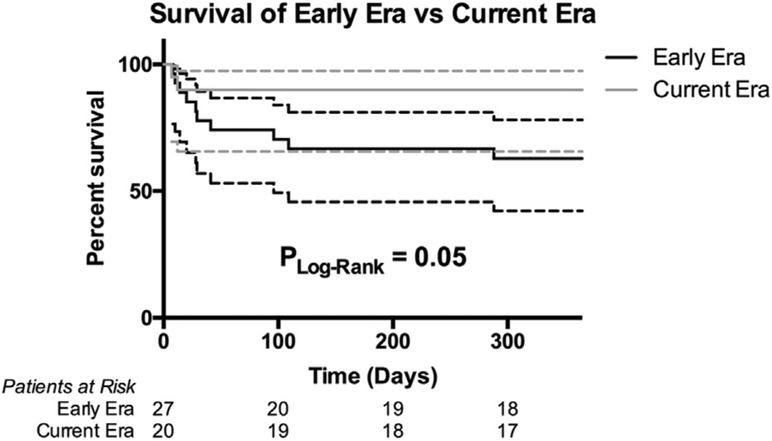
A total of 47 patients were included: 27 in the EE (13 PVF, 14 IVF) and 20 in the CE (12 PVF, 8 IVF). Groups were similar pre-HT, except for more PLE in PVF patients. More patients underwent APC embolization in the CE (80% vs 28%, p < 0.01). There was no difference in donor/recipient weight ratio between eras. There was a trend toward higher primary graft failure for PVF in the EE (77% vs 36%, p = 0.05) but not the CE (42% vs 75%, p = 0.20). Overall, 1-year survival improved in the CE (90%) from the EE (63%) (p = 0.05), mainly due to increased survival for PVF (82 vs 38%, p = 0.04).
CONCLUSIONS:
Post-HT survival for failing Fontan patients has improved, particularly for PVF. In the CE, our Fontan patients had a 1-year post-HT survival similar to other indications.
Keywords: aortopulmonary collateral, failing Fontan physiology, Fontan, heart transplant, preserved ventricular function, single ventricle
The Fontan procedure surgically palliates many congenital cardiac defects. Unfortunately, many of these patients eventually develop heart failure,1,2 resulting in an increased number of single ventricle patients being considered for heart transplant (HT).3 Although this is a heterogeneous patient population, Fontan patients traditionally present with either impaired ventricular function (IVF) or preserved ventricular function (PVF).4,5 For either mode of failure, once medical therapies have been exhausted, HT is the only remaining option. Currently, post-HT survival for Fontan patients is 65% to 80%, significantly less than that for patients with cardiomyopathy or biventricular congenital heart disease.3,4,6-13 However, in a recent multi-institutional pediatric transplant registry review, history of Fontan procedure was associated with worse outcomes in the early era, but was not predictive of decreased survival when applied within a risk assessment model in the later era.6
Our institution has previously published data illustrating reduced short-term post-HT survival for patients with PVF compared with IVF,5 corroborating what was seen in other studies.4,12 Furthermore, we noted that PVF patients had a significantly greater aortopulmonary collateral (APC) burden and early graft failure. Since 2009, our team has implemented several changes to our pre-HT management of Fontan patients. These changes included aggressive catheter-based APC embolization to reduce volume load, evaluation of thoracic anatomy with cross-sectional imaging, higher goal donor/recipient weight ratio and proactive monitoring and therapy for post-operative vasoplegia. We hypothesized that these systematic changes would lead to improved 1-year post-HT survival in our Fontan patients, particularly in those with PVF.
Methods
An institutional database was queried for all patients who underwent HT at St. Louis Children’s Hospital from January of 1995 through December of 2014. All patients transplanted with a previous Fontan, but who had not undergone Fontan takedown, were included. Only single-organ transplant patients were included. This study protocol was approved by the internal review board of the Washington University School of Medicine, with need for patient consent waived.
Patient cohort
The patients were compared by era of transplantation, including the early era (EE), from January 1995 through December 2008, and the current era (CE), from January 2009 through December 2014. Patients were further divided based on modality of Fontan failure. Systemic ventricular systolic function was qualitatively defined by 2-dimensional transthoracic echocardiogram using the most recent echocardiogram before HT. Systolic ventricular function considered moderately to severely dysfunctional comprised the IVF group, whereas mild or no dysfunction comprised the PVF group, as reported elsewhere.4,5
Fontan patients were referred by their primary cardiologist, with the final decision to list for HT made by a multidisciplinary transplant team. Typically, patients were listed for HT with progressive dysfunction with Class III or IV symptoms, refractory arrhythmias, cyanosis or severe protein-losing enteropathy (PLE) or plastic bronchitis (PB). These criteria without further interventional, surgical or medical options indicated the need for HT. Diagnosis of PLE and/or PB was made by the referring cardiologist. Typical findings included albumin <2 g/dl, with intermittent diarrhea, peripheral edema or ascites or a fecal α1-antitrypsin level >50 mg/dl for PLE and the presence of bronchial casts either expectorated or visualized with bronchoscopy for PB.
Management changes across eras
APC embolization.
Patients were considered to have APCs if visualized on cardiac catheterization. APC embolization was performed in the EE at the treating cardiologist’s discretion. In the CE, all Fontan patients listed for HT underwent evaluation for APCs, and embolization was performed once it was identified. If the APC burden was too high to be addressed during one procedure due to concerns for contrast toxicity, serial embolizations were performed. Thereafter, cardiac catheterization with APC embolization was performed in patients with prolonged waitlist times and/or any deterioration in clinical status.
Evaluation of intrathoracic anatomy.
In the EE, thoracic imaging was not standard and performed at the discretion of the managing physician. In the CE, all patients underwent either computerized tomography or magnetic resonance imaging, with 3-dimensional (3D) reconstruction when available.
Donor/recipient weight range.
In the EE, the typical goal donor/recipient weight ratio range was 80% to 200%. In the CE, the goal donor/recipient weight ratio range was increased to 100% to 200%.
Vasoplegia.
In the CE, our team increasingly recognized that the extensive dissection and the systemic inflammatory response of cardiopulmonary bypass associated with these re-do operations resulted in post-transplant vasoplegia and its associated complications. Post-transplant management changed in the CE to include aggressive monitoring of vasoplegia. Intra-operative placement of monitoring devices was continued in the post-operative period. This included either a pulmonary artery catheter or a pulse contour cardiac output catheter, which allowed for the differentiation between graft dysfunction and vasoplegia. As defined, vasoplegia is associated with low systemic vascular resistance with normal to high cardiac output. This was aggressively managed with vasopressor titration, particularly the use of vasopressin, and delaying thymoglobulin initiation or adjusting induction therapy as needed.
Survival and post-HT complications
Survival was compared at 1-year post-HT between eras and among PVF and IVF patients. The 1-year survival cut-off was chosen due to the previously reported high early mortality and the normalization in survival similar to other HT indications after the acute post-operative period.3,7,11,13 A patient was considered to have a hemorrhagic complication if they required a re-operation for mediastinal bleeding or tamponade or for any transfusion requirement of >20 ml/kg in the first 24 hours post-HT. Any patient who required 2 inotropes (including epinephrine) or mechanical circulatory support (MCS) in the first 24 hours post-HT was considered to have early graft dysfunction. Any seizures, prolonged focal neurologic deficit or new radiographic ischemic or hemorrhagic lesion during the early post-HT period was considered a central nervous system (CNS) event. A positive culture from any source during the post-HT admission was considered an infectious complication.
Statistical analysis
Data were compared between the EE and the CE and further analyzed based on PVF and IVF. Laboratory data and cardiac catheterization data are from the most recent evaluation before HT. Continuous variables are reported as mean ± standard deviation and were compared using an unpaired Student’s t-test. Categorical variables were compared using a Fisher’s exact or a chi-square test. For comparing groups of ordinal variables, a Mann-Whitney U-test was utilized. A Kaplan-Meier survival curve was constructed and survival results were compared by log-rank statistics. For all comparisons, p < 0.05 was considered statistically significant.
Results
Patient characteristics by era
During the study period, 337 HTs were performed at St. Louis Children’s Hospital: 217 in the EE and 120 in the CE. Of these, 47 Fontan patients underwent heart transplant, including 27 in the EE and 20 in the CE. Among the EE patients, 13 had PVF and 14 had IVF, as compared with the CE with 12 PVF and 8 IVF cases.
Pre-transplant characteristics were similar between eras, including age at transplant, body surface area (BSA), gender, systemic ventricle morphology and presence of Fontan fenestration (Table 1). There was a shift in type of Fontan from 70% non-extracardiac type in the EE to 90% extracardiac in the CE. The median duration from Fontan to HT (Fontan duration) was similar between eras. PVF was the indication for HT in 48% and 60% in the EE and CE, respectively. As expected, PLE and PB were mainly observed in patients with PVF, with all of the PLE/PB patients having PVF in the EE and 10 of 11 in the CE. The number of patients receiving inotropic support before HT trended higher in the CE (56% vs 85%, p = 0.06) and the number of patients receiving phosphodiesterase type 5 (PDE5) inhibitors was higher in the CE (7% vs 50%, p < 0.01). Median number of days on the waitlist was similar between eras. Severe atrioventricular valve regurgitation was evenly distributed between eras, consisting of 5 (11%) total patients: 1 in the CE with PVF and the remaining 4 with IVF.
Table 1.
Pre-transplant Patient Characteristics
| Patient characteristics | Early era: 1995 to 2008 (n = 27) |
Current era: 2009 to 2014 (n = 20) |
p-value |
|---|---|---|---|
| Age (years) [mean ± SD] | 13.3 ± 6.4 | 12.8 ± 6.1 | 0.80 |
| BSA (m2) [mean ± SD] | 1.2 ± 0.4 | 1.2 ± 0.5 | 1 |
| Gender (male) [n (%)] | 15 (56%) | 8 (40%) | 0.38 |
| Systemic ventricle | |||
| Left | 14 (52%) | 11 (55%) | 1 |
| Right | 13 (48%) | 9 (45%) | 1 |
| Type of Fontan at HT | |||
| RA to PA | 4 (15) | 0 | 0.13 |
| Bjork modification | 1 (4) | 0 | 1 |
| Lateral tunnel | 14 (52) | 2 (10) | <0.01 |
| Extracardiac | 8 (30) | 18 (90) | <0.01 |
| Fenestrated at time of HT | 15 (56) | 14 (70) | 0.37 |
| Fontan durationa (m) | 70 (14–137) | 85 (48–196) | 0.229 |
| [median (IQR)] | |||
| Listing status at transplant | |||
| 1A | 15 (56%) | 16 (80%) | 1 |
| 1B | 8 (30%) | 4 (20%) | 0.75 |
| 2 | 4 (15%) | 0 | 1 |
| Waitlist duration (days) | 73 (23–194) | 129 (17–236) | 0.34 |
| [median (IQR)] | |||
| Inotropes at listing [n (%)] | 15 (56) | 17 (85) | 0.06 |
| PDE5 inhibitor at listing [n (%)] | 2 (7) | 10 (50) | <0.01 |
| PLE [n (%)] | 7 (26) | 9 (45) | 0.22 |
| PB [n (%)] | 0 | 2 (10) | 0.18 |
| AV valve regurgitation | |||
| Mild/moderate | 15 (56) | 17 (85) | 0.06 |
| Severe | 3 (11) | 2 (10) | 1 |
AV, atrioventricular; BSA, body surface area; HT, heart transplant; IQR, interquartile range; PDE5, phosphodiesterase type 5; PLE, protein-losing enteropathy; PB, plastic bronchitis; RA to PA, right atrium to pulmonary artery.
Duration from time of Fontan operation to transplant.
Pre-transplant organ function and hemodynamics
Creatinine, total bilirubin and albumin levels were similar between eras for both PVF and IVF patients (Table 2). PVF patients had a lower albumin concentration than IVF patients in both eras, correlating with the higher incidence of PLE (60 vs 5%, p < 0.01). Mean indexed pulmonary vascular resistance (PVRi) was not significantly different between eras or between IVF and PVF patients in each era. The cardiac index (CI) was higher in PVF patients compared with IVF in the EE (3.4 ± 1.2 vs 2.1 ± 0.6 liters/min/m2, p < 0.01), but not in the CE. Mean Fontan pressures were also similar between eras and between PVF and IVF groups.
Table 2.
Pre-transplant Patient Status
| Early era: 1995 to 2008 |
Current era: 2009 to 2014 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient characteristics | Total (n = 27) |
PVF (n = 13) |
IVF (n = 14) |
p-value: IVF vs PVF |
Total (n = 20) |
PVF (n = 12) |
IVF (n = 8) |
p-value: IVF vs PVF |
Era comparison: p-value |
| Pre-transplant evaluation | |||||||||
| Creatinine (mg/dl) | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.3 | 1 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 1 | 1 |
| Albumin (mg/dl) | 3.4 ± 0.8 | 3.2 ± 0.9 | 3.7 ± 0.4 | 0.07 | 3.3 ± 0.9 | 2.9 ± 0.8 | 3.8 ± 0.6 | 0.01 | 0.69 |
| Total bilirubin (mg/dl) | 1.0 ± 0.8 | 0.8 ± 0.4 | 1.1 ± 1.1 | 0.36 | 1.1 ± 1.0 | 0.9 ± 1.1 | 1.5 ± 0.7 | 0.19 | 0.71 |
| Hemodynamics at listing CI (liters/min/m2) [mean ± SD] | 2.8 ± 1.2 | 3.4 ± 1.2 | 2.1 ± 0.6 | < 0.01 | 3.2 ± 1.2 | 3.4 ± 1.3 | 2.8 ± 1.0 | 0.28 | 0.26 |
| PVRI (WU/m2) [mean ± SD] | 1.9 ± 1.1 | 2.0 ± 1.0 | 2.5 ± 1.2 | 0.25 | 2.3 ± 1.1 | 2.5 ± 1.3 | 2.1 ± 1.4 | 0.41 | 0.90 |
| Fontan pressure (mm Hg) [mean ± SD] | 16.7 ± 4.2 | 15.5 ± 3.8 | 18.3 ± 4.4 | 0.09 | 16.0 ± 3.6 | 17.0 ± 3.7 | 14.8 ± 3.4 | 0.20 | 0.55 |
| Presence of APCs [n (%)] | 14 (52) | 8 (62) | 6 (43) | 0.45 | 17 (85) | 10 (83) | 7 (86) | 1 | 0.03 |
| Patients with ≥1 APC embolization | 6 (22) | 3 (23) | 3 (21) | 1 | 17 (85) | 10 (83) | 7 (86) | 1 | <0.01 |
| Total embolization events per group | 7 | 4 | 3 | 0.84 | 30 | 22 | 8 | 0.31 | <0.01 |
| Events per patient (range) | 0.3 (0–2) | 0.3 (0–1) | 0.2 (0–2) | 1.5 (0–7) | 1.8 (0–7) | 1 (0–2) | |||
APC, aortopulmonary collateral; CI, cardiac index; IVF, impaired ventricular function; PVF, preserved ventricular function; PVRI, pulmonary vascular resistance, indexed; WU, Wood units.
APC embolization
Reflective of our change in evaluation of APCs pre-HT, more patients had APCs identified in the CE compared with the EE (85% vs 52%, p = 0.03; Table 2). Concurrently, the number of cardiac catheterizations with APC embolization per patient increased significantly in the CE (0.3 vs 1.5, p < 0.01). This included an increase from 0.2 to 1.0 (p < 0.01) for IVF patients and from 0.3 to 1.8 (p < 0.01) for PVF patients.
Post-transplant outcomes and major adverse events
Between eras, there was no significant difference in mean donor ischemic time, percent of patients with a positive crossmatch or median donor/recipient weight ratio (Table 3). There was no significant difference in donor ischemic times for PVF patients across eras. However, IVF patients in the CE had a significantly longer donor ischemic time compared with IVF patients in the EE (p = 0.03). Further, cardiopulmonary bypass (CPB) time increased in the CE, although significantly only for IVF patients (p < 0.01). The ischemic and CPB times in the CE are similar those of earlier studies.9 Hospital length of stay was also similar across eras and between PVF and IVF within eras.
Table 3.
Transplant and Post-transplant Characteristics
| Early era: 1995 to 2008 |
Current era: 2009 to 2014 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Transplant and post-transplant characteristics |
Total (n = 27) |
PVF (n = 13) |
IVF (n = 14) |
p-value IVF vs PVF |
Total (n = 20) |
PVF (n = 12) |
IVF (n = 8) |
p-value IVF vs PVF |
Era comparison: p-value |
| Donor ischemic time (min)[mean ± SD] | 219 ± 76 | 215 ± 73 | 223 ± 60 | 0.78 | 259 ± 63 | 241 ± 59 | 287 ± 61 | 0.73 | 0.06 |
| CPB time (min) [mean ± SD | 200 ± 51 | 208 ± 59 | 193 ± 43 | 0.45 | 248 ± 70 | 228 ± 68 | 278 ± 66 | 0.12 | 0.01 |
| Positive crossmatch [n (%)] | 6 (22) | 3 (23) | 3 (21) | 1 | 5 (25) | 3 (25) | 2 (25) | 1 | 1 |
| D/R weight ratio [mean ± SD] | 1.1 ± 0.3 | 1.0 ± 0.4 | 1.1 ± 0.3 | 0.47 | 1.1 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.4 | 4 | 0.43 |
| Post-transplant characteristics | |||||||||
| Peri-operative bleeding [n (%)] | 13 (48) | 8 (62) | 5 (36) | 0.26 | 10 (50) | 6 (50) | 4 (50) | 1 | 1 |
| Primary graft failure [n (%)] | 15 (56) | 10 (77) | 5 (36) | 0.05 | 11 (55) | 5 (42) | 6 (75) | 0.20 | 1 |
| Infection [n (%)] | 11 (41) | 7 (54) | 4 (29) | 0.25 | 4 (20) | 1 (8) | 3 (38) | 0.26 | 0.21 |
| CNS event [n (%)] | 3 (11) | 3 (23) | 0 | 0.10 | 2 (10) | 2 (17) | 0 | 0.49 | 1 |
| Hospital LOS (days) [median (IQR)] | 14 (8–35) | 20 (10–41) | 12 (8–26) | 0.20 | 20 (12–36) | 17 (9–34) | 22 (22–64) | 0.21 | 0.38 |
CNS, central nervous system; CPB, cardiopulmonary bypass; D/R, donor to recipient; IVF, impaired ventricular function; LOS, length of stay; PVF, preserved ventricular function.
The number of patients with any infection was similar between eras overall. However, there was a decreased infection rate among the PVF in the CE compared with EE (54% vs 8%, p = 0.03). CNS events were similar between eras, but were only seen in PVF patients. In the EE, 2 patients experienced intracranial hemorrhage and 1 patient had both a subdural hematoma and acute infarcts, whereas in the CE 1 patient had an embolic stroke and another had a subdural hematoma.
Patients in both eras had similar rates of significant post-operative bleeding, observed in approximately 50% of patients in each era. Although there was no difference in primary graft failure between eras, there was a trend toward higher graft failure in the PVF patients compared with IVF patients during the EE (77% vs 36%, p = 0.05) that was not seen in the CE (42% vs 75%, p = 0.20). Extracorporeal membrane oxygenation (ECMO) remained the main mode of MCS for graft dysfunction across eras, with only 2 of 8 and 1 of 5 patients being placed on VAD support in the EE and CE, respectively.
Post-transplant survival
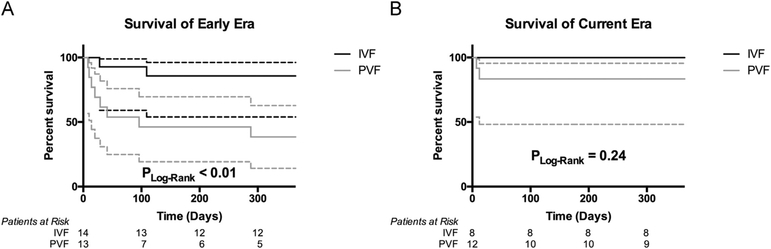
Overall 1-year post-transplant survival improved with 63% survival in the EE and 90% in the CE (log rank, p = 0.05; Figure 1). IVF had a better 1-year survival than PVF in the EE (86% vs 39%; log rank, p < 0.01; Figure 2A), but there was no longer any significant difference in the CE (100% vs 83%; log rank, p = 0.24; Figure 2B). IVF patients had a non-significant improvement in 1-year survival across eras from the EE to the CE (86% vs 100%; log rank, p = 0.28), although it should be noted 1 patient had only reached 10 months of follow-up since HT in the CE and was censored in the survival analysis. Conversely, PVF patients had a significant improvement in 1-year survival from 38% in the EE to 83% in the CE (log rank, p = 0.05).
Figure 1.
Overall 1-year post-transplant survival by era.
Figure 2.
Survival at 1 year post-transplant by era for each mode of failure. (A) Survival at 1 year post-transplant in the early era for PVF vs IVF patients. (B) Survival at 1 year post-transplant in the current era for PVF vs IVF patients.
Although not statistically significant, there were notable trends in the cause of death between eras (Table 4). In the EE, rejection and primary graft dysfunction with multiorgan failure were the primary causes of death compared with the CE, where mortality was due to rejection only.
Table 4.
Survival and Causes of Death
| Early era: 1995 to 2008 |
Current era: 2009 to 2014 |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Post transplant outcomes | Total (n = 27) |
PVF (n = 13) |
IVF (n = 14) |
p-value IVF vs PVF |
Total (n = 20) |
PVF (n = 12) |
IVF (n = 8) |
p-value: IVF vs PVF |
Era comparison: p-value |
| 1-year mortality [n (%)] | 10 (37) | 8 (62) | 2 (14) | 0.02 | 2 (10) | 2 (17) | 0 | 0.49 | 0.05 |
| Cause of death | |||||||||
| [n (% of total deaths)] | |||||||||
| MOF with graft dysfunction | 4 (40) | 4 (50) | 0 | 0.04 | 0 | 0 | 0 | 1 | 0.52 |
| Rejection | 3 (30) | 2 (25) | 1 (50) | 0.60 | 2 (100) | 2 (100) | 0 | 0.49 | 0.15 |
| Hemorrhage | 2 (20) | 1 (13) | 1 (50) | 1 | 0 | 0 | 0 | 1 | 1 |
| Infection | 1 (10) | 1 (13) | 0 | 0.48 | 0 | 0 | 0 | 1 | 1 |
IVF, impaired ventricular function; MOF, multiorgan failure; PVF, preserved ventricular function.
Discussion
Previous reports have shown that failing Fontan patients have a decreased post-HT survival compared with other indications, particularly for patients with PVF.4,8,14 Earlier experience from our institution also suggested a trend toward decreased post-HT survival in the PVF Fontan patient population.5 In line with these reports, our EE overall Fontan 1-year survival was a discouraging 63%, with the majority of mortality concentrated in the PVF population. However, the CE survival analysis is encouraging with an improvement in overall survival to 90%—again, mainly due to improvements in PVF survival. Our results show that for Fontan patients in the CE, including those with PVF, post-transplant survival at 1 year is on par with other indications.15
Multiple strategies have been proposed to improve post-HT survival in the PVF Fontan population.12,16 After noting a high incidence of primary graft failure and peri-operative bleeding, aggressive pre-HT APC embolization became routine at our institution.5 Single ventricle patients are known to have a propensity for forming APCs after the Glenn and Fontan,17 reportedly accounting for approximately 10% to 50% of total systemic cardiac output.18,19 This leads to recirculation, resulting in an increased volume load on the systemic ventricle19,20 or, in the case of transplant, on the new graft. Short-term effects of APC embolization revealed significant increases in systemic flow.21 Currently, there are limited data on the clinical impact of APC embolization in children with single ventricle physiology who undergo HT. Krishnan et al reported a series of children with cyanotic heart disease who underwent HT, yet they were unable to wean from inotropic and ventilator support despite good graft function in the setting of significant APC flow.22 The majority of those patients were able to wean from support and clinically improved after APC embolization. In our study, the percent of primary graft failure and peri-operative bleeding was not significantly different in the CE; however, severe primary graft failure with multiorgan failure, which accounted for 40% of the deaths in the EE, was eliminated in the CE.
Our pre-transplant evaluation also changed to include cross-sectional imaging to define intrathoracic anatomy and 3D relationships to avoid significant intra-operative hemorrhage noted in the EE. In addition, the decision was made to avoid undersized donors with a goal donor/recipient weight ratio of at least 100%. Despite this, our actual donor/recipient weight ratio did not change significantly over time. Although some studies showed decreased survival with lower weight ratios in children,23 other reports did not find significant differences,24-26 except in infant recipients who had higher mortality at the lowest ratio range.25 In clinical practice, the previous emphasis of targeting larger donor/recipient weight ratios at transplant may not have as much impact on survival as hypothesized, if at all.
In the CE we increased the routine use of hemodynamic monitoring during the immediate post-operative period and aggressively treated vasoplegia in all HT patients. Postoperative vasoplegia is multifactorial and is a result of prolonged CPB and extensive adhesiolysis with blood loss from repeated operations. It has been associated with decreased survival in adult transplant recipients.27,28 Fontan patients, by their very nature, have several risk factors for significant vasoplegia post-HT, including previous operations, long bypass times and pre-HT aspirin or angiotensin-converting enzyme inhibitor use.27,29
Our overall 1-year post-HT survival of 90% in the CE reflects a significant improvement from previously published studies.7,30 PVF had the greatest increase in survival, at least in part from modifications of risk factors specific to this population, as demonstrated by the trend in causes of death over time. However, the overall reason for improvement is likely multifactorial and cannot be attributed entirely to our specific management changes alone. Other advancements in surgical technique, post-operative care and institutional experience with this challenging patient population have undoubtedly contributed to improved outcomes over time. Regardless, the difference in post-HT survival between Fontan patients and other indications for HT has narrowed significantly, emphasizing that Fontan patients may not carry an increased risk for mortality in the CE. Clinicians need to recognize the challenges and risks unique to Fontan patients undergoing HT and develop strategies to manage their care pre- and post-HT to optimize patient outcomes.
Limitations
This study has some important limitations. It was non-randomized and retrospective, so the causality of the improved survival cannot be directly attributed to any single intervention. Other factors were notably different between eras, including changes in the predominate type of Fontan, pre-HT PDE5 inhibitor utilization, and the trend toward increased pre-transplant inotropic support. Overall, there were likely multiple unquantifiable alterations in practice that occurred across eras. Also, only patients who survived to HT were included; consequently, if a survival reduction occurred while awaiting HT, this factor would be missed. Furthermore, there is a risk associated with repeated cardiac catheterizations that could potentially reduce survival while awaiting transplant. This, however, is unlikely to influence the results due to the low rate of severe adverse events associated with a cardiac catheterization.31,32 Last, our study had a small sample size, which limits any statistical or subgroup analysis.
In conclusion, as experience and surgical techniques have progressed from the classic Fontan, patient survival has continued to improve. The issue of post-HT survival is of growing importance as a significant number of patients are at risk for developing heart failure with Fontan anatomy. Post-HT survival in the CE has improved to a level comparable to the expected survival for other indications for pediatric HT, with the greatest improvement in the PVF patients with failed Fontan physiology. Patients with PVF were once considered to have worse post-HT survival, but this is no longer true in the current era.
Acknowledgments
Disclosure statement
C.E.C. reports having received travel reimbursement from Berlin Heart. This study was supported by a grant from the National Institutes of Health (T32 HL007776).
Footnotes
The remaining authors have no conflicts of interest to disclose.
These data were presented at the 35th annual meeting and scientific sessions of the International Society for Heart and Lung Transplantation, April 2015, Nice, France.
References
- 1.Khairy P, Fernandes SM, Mayer JE Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery. Circulation 2008;117:85–92. [DOI] [PubMed] [Google Scholar]
- 2.d’Udekem Y, Iyengar AJ, Galati JC, et al. Redefining expectations of long-term survival after the Fontan procedure: twenty-five years of follow-up from the entire population of Australia and New Zealand. Circulation 2014;130(suppl 1):S32–8. [DOI] [PubMed] [Google Scholar]
- 3.Voeller RK, Epstein DJ, Guthrie TJ, et al. Trends in the indications and survival in pediatric heart transplants: a 24-year single-center experience in 307 patients. Ann Thorac Surg 2012;94:807–15. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths ER, Kaza AK, Wyler von Ballmoos MC, et al. Evaluating failing Fontans for heart transplantation: predictors of death. Ann Thorac Surg 2009;88:558–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simpson KE, Cibulka N, Lee CK, et al. Failed Fontan heart transplant candidates with preserved vs impaired ventricular ejection: 2 distinct patient populations. J Heart Lung Transplant 2012;31:545–7. [DOI] [PubMed] [Google Scholar]
- 6.Schumacher KR, Almond C, Singh TP, et al. Predicting graft loss by 1 year in pediatric heart transplantation candidates: an analysis of the Pediatric Heart Transplant Study database. Circulation 2015;131:890–8. [DOI] [PubMed] [Google Scholar]
- 7.Kanter KR, Mahle WT, Vincent RN, et al. Heart transplantation in children with a Fontan procedure. Ann Thorac Surg 2011;91:823–30. [DOI] [PubMed] [Google Scholar]
- 8.Lamour JM, Kanter KR, Naftel DC, et al. The effect of age, diagnosis, and previous surgery in children and adults undergoing heart transplantation for congenital heart disease. J Am Coll Cardiol 2009;54:160–5. [DOI] [PubMed] [Google Scholar]
- 9.Davies RR, Sorabella RA, Yang J, et al. Outcomes after transplantation for “failed” Fontan: a single-institution experience. J Thorac Cardiovasc Surg 2012;143:1183–92. [DOI] [PubMed] [Google Scholar]
- 10.Backer CL, Russell HM, Pahl E, et al. Heart transplantation for the failing Fontan. Ann Thorac Surg 2013;96:1413–9. [DOI] [PubMed] [Google Scholar]
- 11.Everitt MD, Boyle GJ, Schechtman KB, et al. Early survival after heart transplant in young infants is lowest after failed single-ventricle palliation: a multi-institutional study. J Heart Lung Transplant 2012;31:509–16. [DOI] [PubMed] [Google Scholar]
- 12.Michielon G, van Melle JP, Wolff D, et al. Favourable mid-term outcome after heart transplantation for late Fontan failure. Eur J Cardiothorac Surg 2015;47:665–71. [DOI] [PubMed] [Google Scholar]
- 13.Bernstein D, Naftel D, Chin C, et al. Outcome of listing for cardiac transplantation for failed Fontan: a multi-institutional study. Circulation 2006;114:273–80. [DOI] [PubMed] [Google Scholar]
- 14.Karamlou T, Diggs BS, Welke K, et al. Impact of single-ventricle physiology on death after heart transplantation in adults with congenital heart disease. Ann Thorac Surg 2012;94:1281–7. [DOI] [PubMed] [Google Scholar]
- 15.Dipchand AI, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: seventeenth official pediatric heart transplantation report—2014; Focus theme: retransplantation. J Heart Lung Transplant 2014;33:985–95. [DOI] [PubMed] [Google Scholar]
- 16.Pretre R, Haussler A, Bettex D, et al. Right-sided univentricular cardiac assistance in a failing Fontan circulation. Ann Thorac Surg 2008;86:1018–20. [DOI] [PubMed] [Google Scholar]
- 17.Triedman JK, Bridges ND, Mayer JE Jr, et al. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol 1993;22:207–15. [DOI] [PubMed] [Google Scholar]
- 18.Grosse-Wortmann L, Al-Otay A, Yoo SJ. Aortopulmonary collaterals after bidirectional cavopulmonary connection or Fontan completion: quantification with MRI. Circ Cardiovasc Imaging 2009;2:219–25. [DOI] [PubMed] [Google Scholar]
- 19.Whitehead KK, Gillespie MJ, Harris MA, et al. Noninvasive quantification of systemic-to-pulmonary collateral flow: a major source of inefficiency in patients with superior cavopulmonary connections. Circ Cardiovasc Imaging 2009;2:405–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latus H, Gummel K, Diederichs T, et al. Aortopulmonary collateral flow is related to pulmonary artery size and affects ventricular dimensions in patients after the Fontan procedure. PLoS One 2013;8:e81684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dori Y, Glatz AC, Hanna BD, et al. Acute effects of embolizing systemic-to-pulmonary arterial collaterals on blood flow in patients with superior cavopulmonary connections: a pilot study. Circ Cardiovasc Intervent 2013;6:101–6. [DOI] [PubMed] [Google Scholar]
- 22.Krishnan US, Lamour JM, Hsu DT, et al. Management of aortopulmonary collaterals in children following cardiac transplantation for complex congenital heart disease. J Heart Lung Transplant 2004;23:564–9. [DOI] [PubMed] [Google Scholar]
- 23.Kanani M, Hoskote A, Carter C, et al. Increasing donor-recipient weight mismatch in pediatric orthotopic heart transplantation does not adversely affect outcome. Eur J Cardiothorac Surg 2012;41:427–34. [DOI] [PubMed] [Google Scholar]
- 24.Kwon MH, Wong S, Kittleson M, et al. Selecting oversized donor cardiac allografts for patients with pulmonary hypertension may be unnecessary. Transplant Proc 2014;46:1497–501. [DOI] [PubMed] [Google Scholar]
- 25.Tang L, Du W, Delius RE, et al. Low donor-to-recipient weight ratio does not negatively impact survival of pediatric heart transplant patients. Pediatr Transplant 2010;14:741–5. [DOI] [PubMed] [Google Scholar]
- 26.Razzouk AJ, Johnston JK, Larsen RL, et al. Effect of oversizing cardiac allografts on survival in pediatric patients with congenital heart disease. J Heart Lung Transplant 2005;24:195–9. [DOI] [PubMed] [Google Scholar]
- 27.Patarroyo M, Simbaqueba C, Shrestha K, et al. Pre-operative risk factors and clinical outcomes associated with vasoplegia in recipients of orthotopic heart transplantation in the contemporary era. J Heart Lung Transplant 2012;31:282–7. [DOI] [PubMed] [Google Scholar]
- 28.Byrne JG, Leacche M, Paul S, et al. Risk factors and outcomes for ‘vasoplegia syndrome’ following cardiac transplantation. Eur J Cardiothorac Surg 2004;25:327–32. [DOI] [PubMed] [Google Scholar]
- 29.Lenglet S, Mach F, Montecucco F. Methylene blue: potential use of an antique molecule in vasoplegic syndrome during cardiac surgery. Exp Rev Cardiovasc Ther 2011;9:1519–25. [DOI] [PubMed] [Google Scholar]
- 30.Kovach JR, Naftel DC, Pearce FB, et al. Comparison of risk factors and outcomes for pediatric patients listed for heart transplantation after bidirectional Glenn and after Fontan: an analysis from the Pediatric Heart Transplant Study. J Heart Lung Transplant 2012;31:133–9. [DOI] [PubMed] [Google Scholar]
- 31.Bergersen L, Marshall A, Gauvreau K, et al. Adverse event rates in congenital cardiac catheterization—a multi-center experience. Cathet Cardiovasc Intervent 2010;75:389–400. [DOI] [PubMed] [Google Scholar]
- 32.Odegard KC, Bergersen L, Thiagarajan R, et al. The frequency of cardiac arrests in patients with congenital heart disease undergoing cardiac catheterization. Anesth Analgesia 2014;118:175–82. [DOI] [PubMed] [Google Scholar]