Abstract
In humans and songbirds, neuronal activation for language and song shifts from bilateral- or diffuse-activation to left-hemispheric dominance while proficiency increases. Further parallels exist at the behavioural level: unstructured juvenile vocalizations become highly stereotyped adult vocalizations through a process of trial and error learning. Greater left-hemispheric dominance in the songbird caudomedial Nidopallium (NCM), a Wernicke-like region, is related to better imitation of the tutor’s song learned early in development, indicating a role for left NCM in forming auditory memories. Here, we hypothesize that inhibition of the left NCM during interaction with a song tutor would impair imitation of the tutor’s song more than inhibition of the right NCM. We infused a transient sodium channel blocker (TTX) immediately prior to tutoring sessions in either left or right auditory lobule of previously isolated juvenile male zebra finches (Taeniopygia guttata). Upon maturation, both right-infused and left-infused birds tutor-song imitation was significantly impaired. Left-infused birds also showed less consistency in the rhythmic stability of their song as well as increased pitch, suggesting a subtle division of function between the left and right auditory lobules.
Keywords: birdsong, songbird, language, lateralization, memory
INTRODUCTION
For humans, the first step in learning to speak is successful acquisition of auditory memories. Neural activity underlying language perception and production is predominantly left-lateralized, which can already be observed in neonates listening to spoken language as well as in babbling infants [1–3]. Humans share this pattern of left-hemispheric dominance with songbirds, which learn their songs as juveniles, and have analogous anatomical circuits underlying vocal production and perception to those found in humans [4]. Among other parallels, similarities exist at the behavioural level: both species form auditory memories during a sensitive period for learning by listening to adult conspecifics (or ‘tutors’). A comparable vocal developmental trajectory follows; from ‘cooing’ to ‘babbling’ in infants and from ‘subsong’ to ‘plastic song’ in birds.
The caudomedial Nidopallium (NCM), which is functionally analogous to regions in the human superior temporal lobe including Wernicke’s area, exhibits predominant activation in zebra finches’ (Taeniopygia guttata) left hemisphere in response to re-exposure to the tutor song [4–7]. The left NCM has an innate bias towards song processing and left-hemispheric dominance develops with song maturation [7, 8]. As in human learners, the emergence of left-hemispheric dominance during skill acquisition seems to be a key component of successful vocal imitation.
Bilateral inhibition of molecular signalling through the MAP kinase pathway, as well as through the mechanistic target of the rapamycin (mTOR) signalling cascade in the auditory lobule (which includes the NCM, the caudomedial Mesopallium [CMM] and Field L) during song tutoring impairs learning, and bilateral permanent lesions to the NCM in adults impair their ability to recognize the tutor’s song [5, 9, 10]. Thus, the NCM has been proposed to contain (part of-) the neural substrate for the representation of tutor song memory [5, 11–16]. As there is left-hemispheric dominance in the NCM in response to tutor song re-exposure, we hypothesized that disrupting neuronal activity in the left NCM during sessions with a song tutor would impair song acquisition more than disrupting the right NCM. We tested this hypothesis by infusing the transient sodium channel blocker tetrodotoxin (TTX) unilaterally into the auditory lobule of acoustically isolated juvenile males immediately prior to tutor song exposure, then analysing their adult songs for imitation of species-specific spectro-temporal features.
MATERIALS AND METHODS
Subjects
24 male juvenile zebra finches were reared in the animal facility of Wellesley College (left NCM injection N = 6, right NCM injection N = 7, control birds N = 8; 3 birds died during surgery). Birds were maintained on a 16:8 light:dark cycle, lights on at 10:00 AM. All birds were kept in breeding cages with their parents and siblings until 6−11 days post-hatching (dph). At 7.6 dph (± 1.3 SD; range 6–11 dph), the juveniles and their mother were separated from the father and transferred to a different room equipped with acoustically isolated cages each holding a single clutch. At 32.3 dph (± 0.6 SD; range 32–34 dph), juvenile males were transferred into individual, acoustically isolated holding cages in order to control auditory and social exposure to adult song tutors or to song produced by their brothers. Experimental procedures were in accordance with US law and approved by the Institutional Animal Care and Use Committee of Wellesley College (IACUC #1405).
Transient Inactivations
At 46 dph (SD = 1.2; range 45–49 dph), birds were lightly anesthetized with 1.5% isoflurane/oxygen gas mixture and small holes (approximately 300 μm in diameter) were drilled through the skull bilaterally above NCM using stereotaxic coordinates (head-angle 65°, 0.6 mm anterior, 0.75 mm lateral, 1.5 mm depth). A 2 mm long double guide cannula (1.5 mm between cannulas, 26GA, Plastics One, Roanoke, VA) was implanted at a depth of 1.5 mm and fixed to the skull with dental cement with a 33GA dummy cannula inserted to prevent tissue clog. Immediately before injection the dummy cannula was removed and replaced with a 33GA internal cannula, projecting 0.2 mm outside of the guide cannula to a depth of 1.7 mm, which was connected via PE20 tubing to a 1 μL Hamilton microsyringe driven by a microinfusion pump (RWD Life Science Co., Ltd Model RWD202). At 10:30am on experimental days, birds were lightly anesthetized with 1.5% isoflurane, and 0.75 μL TTX (50 μM, # T5651, Sigma, St. Louis, Missouri, United States, in saline with Lucifer Yellow) was injected in either the left (N = 6) or right (N = 7) NCM (dose based on [17] and [18]) while 0.75 μL saline was injected in the NCM of the opposite hemisphere at the same time. In all birds in the Experimental Group, implantation of bilateral guide cannulas and injection of saline in the other hemisphere provided an intra-subject control for the effects of surgery and injection. Birds in the Control Group (N = 8) were exposed to 2 minutes (the duration of a 0.75 μL injection) of 1.5% isoflurane to mimic the effects of injection sessions and handling immediately prior to song tutoring. All birds were between 50 and 60 dph for inactivation and tutoring sessions, which is in their sensorimotor phase of song learning, and as effective for song learning as the 40–50 dph window used in other studies [9, 18]. As injection/tutoring days were alternated with rest days and the birds had ~30dph left in their sensorimotor period to practice songs, it is likely that effects from the experimental manipulations are due to interference with the tutor song memorization process and not the sensorimotor learning process.
Tutoring Sessions
Birds underwent 5 days of unilateral TTX injections between 50–60 dph, each of which was immediately followed by a 90-minute tutoring session (Suppl. Fig. S1). Tutors were novel to the experimental subjects (not the experimental birds’ fathers). When possible, tutoring sessions consisted of two juveniles with a single tutor in order to mimic a more natural song-learning environment (N = 14 out of 21 subjects had paired sessions; pairs consisted of one left-inactivated and one right-inactivated bird, or two control birds). At 10:30 am, one experimental subject received a unilateral TTX injection (see Transient inactivations) and was then transferred to a cage containing the tutor. Immediately following, a second experimental subject received a TTX injection and was subsequently introduced to the same cage. Each bird received 90 minutes of tutoring beginning at the time of their individual introduction to the tutor.
Song Analysis
The bird’s vocalizations were continuously recorded (see [19] or details) from the point of individual isolation 32.3 dph (SD = 0.6; range 32–34 dph) until date of perfusion (150.7 dph, SD = 2.0; range 149–156 dph).
Twenty songs were randomly selected for each experimental subject at 120 dph, and for each tutor (exact tutor age at recording unknown but >120 dph) for further analysis from after 2pm, which are less variable as compared to morning songs [20]. Sound Analysis Pro [21] was used to assess the fidelity of tutor song imitation of the experimental subjects by calculating the “percentage similarity” to their tutor and to unfamiliar (novel) birds with the default settings for zebra finches (see Suppl. Methods for more detail). A song imitation score was derived by subtracting the NOV-song-similarity score from the TUT-song-similarity score, and it reflects how much more similar a bird’s song is to its tutor as compared to unfamiliar zebra finches.
In order to perform an in-depth analysis of song structure, we used the SAP feature-extraction tool to obtain feature values for spectral features on a syllable-to-syllable basis. Motifs were manually parsed into individual syllables by an observer blind to treatment, by setting segmentation values based on amplitude and Weiner entropy, which is unique to each individual. Feature values for Wiener entropy, pitch, goodness of pitch, amplitude and frequency modulation were then extracted for individual syllables for all the birds.
To compare effects on song temporal structure between treatment groups we generated rhythm spectra for birds in each condition (tutor, left TTX injection, right TTX injection, and control group) [22, 23]; methods identical to those described in [24]. The rhythm spectrum analyses the stereotypy of the song’s rhythmic structure (motif duration, syllables, notes, syllable onset/offset, etc.) and its repeating units across renditions. Using this analysis, we were able to assess and compare the temporal consistency of the songs from birds in each experimental group.
Histological Analysis
At 150 dph, birds were exposed to a set of song playbacks from the tutor, immediately followed by collection of tissue, and immunocytochemical staining for Zenk (egr-1; Santa Cruz Biotechnology, Cat. No. sc-189) to confirm normal song-induced neuronal activation in NCM in adulthood following juvenile cannula implantations (see [8] for details on stimulus preparation, playback procedures, and the immunocytochemistry protocol, and [19] for tissue collection).
To establish the neuroanatomical position of injection sites within NCM, egr-1 stained sections were examined under a compound light microscope with a 4x objective to locate cannula tracks (see Suppl. Fig. 2B). Cannula tracks were identified on parasagittal sections with an average distance of 556 μm from the midline (range: 250–800, +/−201 SD). Tracks could not be located for 5 birds (2 left-TTX-injected, 3 right-TTX-injected) due to technical problems during sectioning. Alternate sections were examined for Lucifer yellow at 150 dph to determine the spread of the TTX injection throughout the auditory lobule. However, given that this was >90 days post injection, no clear traces of the dye were observed most likely as a result of dye longevity issues. Therefore, the results could technically be due to inactivation of the entire auditory lobule in the experimental hemisphere, comparable to drug spread reported by London & Clayton [9].
Statistical Analysis
A one-way ANOVA was conducted with song imitation scores and treatment groups as factors, followed by Bonferroni corrected post-hoc tests. For the rhythm analysis, we conducted a one-way ANOVA with mean RMS residuals of fitted rhythm spectra and treatment groups as factors, followed by Fisher’s Least Significant Difference post-hoc tests. We performed one-way permutation-based analysis of variance (PERMANOVA) with PAST software (version 3.23, [28]) on the five features (Frequency and amplitude modulation, pitch, Wiener entropy and goodness of pitch) that were extracted on the single syllable level, followed by pair-wise Bonferroni-corrected comparisons between groups and one-way ANOVAs to delineate which specific song features changed upon treatment with TTX.
RESULTS
Juvenile zebra finches were raised in isolation (no exposure to adult song) then injected for 5 days between 50 – 60 dph with TTX (Tetrodotoxin, a general inhibitor of neuronal activity) in either the left or right NCM, immediately prior to tutoring sessions (Suppl. Fig. 1; see Supplementary Results and Suppl. Fig. 2 for histology).
Song imitation
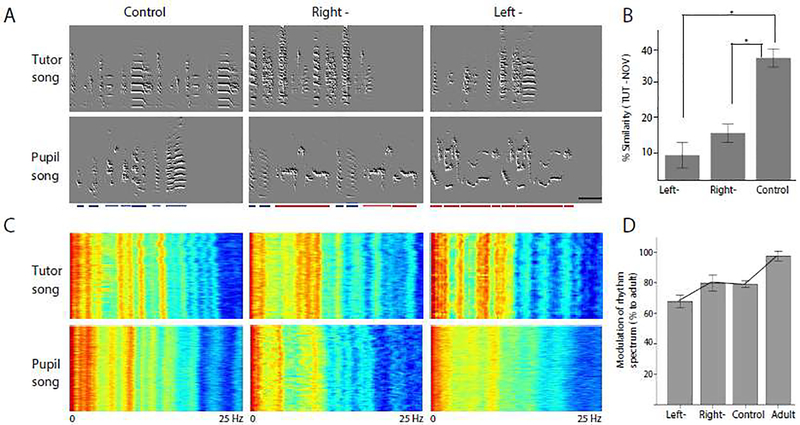
When the birds reached adulthood (120 dph), we assessed the similarity between their song and that of their tutor in order to determine their success in forming a representation of the tutor song. There was a significant effect of treatment on song imitation scores (F (2, 18) = 26.69, p < 0.001 Fig. 1B). Left-TTX injected (M = 7.75, SD = 9.1) and right-TTX injected birds (M = 14.25, SD = 7.2) had lower song imitation scores than birds in the control group (M = 37.17, SD = 7.8). Post-hoc comparisons showed that birds with either the left or right hemisphere inactivated during tutor song exposure incorporated less of the tutor song in to their motifs (p < 0.001 for both comparisons, Fig. 1B).
Figure 1:
NCM inactivation during tutor song exposure disrupts song imitation and rhythmicity. (A) Representative sonograms of subjects in each experimental group at 120 dph (left TTX injected (N = 6), right TTX injected (N = 7), and surgical controls (N = 8; birds receiving anesthesia and handling exposure) and their respective tutors. In the pupils’ songs, normal syllables are indicated with blue bars, and isolate-like syllables with red bars. Note the abnormal duration of inter-syllable gaps between isolate-like syllables. Scale bar represents 200 ms. (B) Mean scores (± SEM) of song imitation (TUT-NOV) for each experimental group. There was a significant difference in mean song imitation scores between the treatment groups (F(2,19) = 26.67, p < 0.001). Post-hoc comparisons with Bonferroni correction revealed a significant difference between left-TTX inactivated and control group (t(19) = 6.718, p < 0.001) as well as right-TTX inactivated and control group (t(19) = 5.419, p < 0.001). (C) Rhythm spectra (frequency on x-axis) for a full day of singing (on y-axis) for subjects in each experimental group at 120 dph and their respective tutors. The rhythm spectra shown represent the songs of the same birds as presented in the sonograms from (A). Clearly delineated high-power (red) bands are indicative of crystallized adult song with highly stereotyped motif durations (the fundamental frequency of the rhythm spectrum) and consistent intervals between syllable onsets (bands at higher frequencies). Although pupils do not necessarily copy the rhythm from their tutors, rhythm spectra of birds singing isolate song are less modulated (appearing more washed-out) than those from birds singing normal, crystallized song (characterized by clearly delineated bands at higher frequencies). (D) Modulation of rhythm spectra for all experimental groups. Mean RMS residuals of fitted rhythm spectra are expressed as a percentage of the normal adult songs from their tutors (± SEM; left-: N = 6, right-: N = 7, control: N = 8, adult: N = 4).
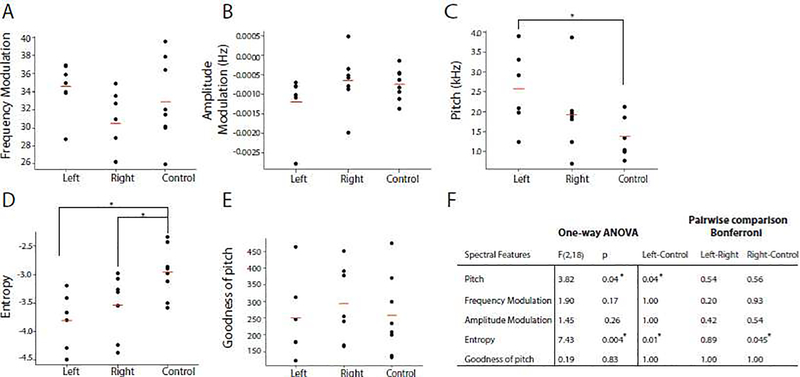
One-way PERMANOVA (9999 permutations) showed there was a significant difference in the song structure between songs of the left and right- TTX injected birds and control birds (PseudoF2,18 = 3.54, p = 0.047). A pairwise comparison with Bonferroni correction showed that left-TTX injected birds differed in their song structure as compared to the control group birds (p = 0.03). To further delineate the contribution of individual features, one-way analysis of variance showed that TTX injection in the left hemisphere resulted in higher wiener entropy and pitch as compared to the control birds (Fig. 2F, p values are indicated in the table). Even though most features from the right-TTX injected birds did not reach statistical significance, wiener entropy was significantly higher than in control birds, just like in the left-TTX injected birds (Fig. 2 D, F).
Figure 2:
NCM inactivation during song tutoring results in changes in acoustic properties of the song. Songs were segmented into syllables based on their amplitude and Wiener entropy. To investigate the acoustic properties of individual syllables, the mean values of frequency (A), amplitude modulation (B), pitch (C), Wiener entropy (D) and goodness of pitch (E) were calculated (red bar indicates the mean for each group, each data point represents the average of all syllables for one bird). F) Table of results from one-way ANOVAs for each spectral feature. P-values are adjusted with Bonferroni correction across treatment groups for each feature. Asterisk represents significance at alpha = 0.05.
Development of species-typical song rhythm
To distinguish the role of the left and right hemisphere in acquiring the temporal properties of song and to confirm the lack of clear song motifs and the irregular rhythm that is characteristic of isolate song [29–32] (Fig. 1A, Suppl. Fig. S3 with wave files embedded), we proceeded with analysis of song rhythms, we generated rhythm spectra for a full day of singing at 120 dph (for full methods see [22, 23]; Fig 1C). The rhythm spectrum provides insight into the consistency of the song’s rhythmic features (motif duration, syllables, notes, syllable onset/offset, etc.) and its repeating units across renditions. The energy of a frequency band in the rhythm spectrum may be influenced by the periodicity of that rhythmic element or its prevalence across renditions. Therefore, clearly delineated high power (red) frequency bands in the spectra are indicative of crystallized adult song with highly stereotyped motif durations (the fundamental frequency of the rhythm spectrum), and consistent intervals between syllable onsets. We observed a notable absence of these bands in the rhythm spectra of birds in the left-TTX-injected group. We computed the root mean square (RMS) of the residuals of a linear fit to the rhythm spectra and expressed this value as a percentage to normal adult song (the average tutor RMS residual value; methods identical to [23]). Our results show that the left-, but not right-, TTX-injected birds exhibited a trend towards significant deterioration to the rhythmic, temporal quality of their song and lacked the characteristic stereotypical nature of adult song (Fig. 1D; ANOVA: F (2, 18) = 2.81, p = 0.087; left vs. right: p = 0.047, left vs. control: p = 0.055, right vs. control p = 0.88).
DISCUSSION
We found impaired tutor song imitation upon vocal maturity after reversibly inactivating the left or right auditory lobule with TTX in juvenile male zebra finches for the duration of all social interactions with an adult song tutor. We noted small differences in the temporal properties of the song upon inhibition, while the spectral properties were more sensitive to manipulations during sensitive-period learning. Thus, bilateral activity in the auditory lobule is necessary for the formation of tutor song memory. While both left- and right- inactivated birds learned less from their song tutor than control birds (measured by spectral similarity), inhibition of the auditory lobule in the left hemisphere had distinct effects on the spectral features of song, specifically Wiener entropy (a measure of tonality) and pitch. Both left- and right-inactivated birds also had whistle-like syllables in their songs, causing larger negative values for entropy due to noisy (less structured) syllables as compared to birds in the control group. The results are consistent with the idea that blocking access to auditory lobule during tutoring results in pronounced effects on song learning, with subtle differences between the left and right hemisphere.
Our findings contribute to a large body of evidence, supporting the hypothesis that numerous brain regions are involved in tutor song learning, and different song features could be encoded and/or processed asymmetrically in the zebra finch brain [9, 18, 33]. Nuclei in the song system such as HVC (proper name) and the lateral part of the magnocellular nucleus of anterior nidopallium (lMAN), which are homologous to the laryngeal motor cortex and Broca’s area in humans [4], are part of a complex circuit necessary for both song acquisition and production. Targeted optogenetic manipulation of neuronal activity in HVC during song acquisition can disrupt imitative learning of a single syllable, while leaving imitation of other syllables unaffected [18]. Morphological and physiological changes following the very first day of exposure to a tutor song have been observed in both HVC and RA, indicating that both of these nuclei represent aspects of song memory [34, 35]. In HVC, but also in lMAN, NMDA-receptor blocking during song-tutoring sessions results in impaired song imitation [18, 33]. Although all these studies demonstrate the involvement of auditory regions or song-system nuclei in song learning, our current results are the first to reveal asymmetric contributions of the left and right hemisphere to the song learning process.
For successful formation of a representation of song memory to occur, interactions between nuclei in the song system and regions in the auditory lobule may be required. As an alternative explanation of our findings, it could be possible that bilateral cannulas in the auditory lobule result in damage to the developing brain that impairs song learning. This explanation is unlikely, as identical double cannulas were targeted to a similar location in the NCM by London and Clayton [9], who found no significant effects on song imitation with a comparable song learning paradigm (1.5 hrs/day exposure to a song tutor between 40–50 dph, and control drug infusions 30 min prior to tutoring). In addition, there could be minor differences in the placement of cannulas between the left- and right-inactivated groups in the rostral-caudal plane (see Suppl. Fig. 2 C), but due to the large volumes of drug injected into the auditory lobule this does not change our interpretation of the subtle differences between left and right hemisphere reported here. Another possibility is that our pharmacological manipulations could have silenced the entire network in a single hemisphere, with effects not solely due to inactivation of the auditory lobule, but also due to alterations in homeostatic plasticity in downstream brain regions in the same hemisphere [36]. Surprisingly, Canopoli and colleagues found that permanent bilateral excitotoxic lesions to (parts of) the NCM before or immediately after tutoring did not affect tutor song learning, indicating that neurons that express NMDA receptors in the NCM are not exclusively necessary for acquisition of song memory [37, 38]. However, in the intact system as we have shown here, temporarily blocking activity in all neurons with a sodium channel blocker could easily disrupt the homeostatic balance critical for normal plasticity to act upon. This may be due to effects within the entire auditory lobule, or due to interactions between NCM and its afferents and efferents. Relay of tutor-song specific information between auditory and motor systems during the song acquisition process is possibly dependent on the sensorimotor nucleus Nif [18, 24, 39]. Thus, our data suggest that activity within the auditory lobule or an interaction between regions in the auditory lobule and the song system is critical for learning to occur.
A recent fMRI study in normally-reared adult zebra finches also reported lateralized processing biases for spectral and temporal features of song and corroborates these findings in juveniles [40]. Specifically, the NCM/Field L complex in the right hemisphere was more sensitive to spectral elements of song, whereas the left seemed to be specialized for temporal domain processing [40]. Thus, there seems to be a shift in auditory processing due to experience: the left hemisphere is involved in temporal and spectral processing early on (this study, and [8, 40]), whereas the right hemisphere may acquire its sensitivity for spectral elements with repeated exposure to song, and contributes fine spectral features to song acquisition ([this study, and 40]). At the level of neuronal plasticity within the NCM in adult zebra finches, both neurogenesis and epigenetic mechanisms also show left-hemispheric dominance in relation to song memory formation [41, 42]. Interestingly, the epigenetic manipulations by Phan et al. [41] may have induced a period of experience-dependent plasticity similar to that seen in juveniles and resulting in hemispheric asymmetry, but whether these processes are critical for juvenile song acquisition remains to be determined.
In summary, our results show that there is a moderate level of innate lateralization of the ascending auditory pathway that could be modified by experience with the tutor’s song. Such hemispheric asymmetry may be a convergent evolutionary adaptation in distant species of vocal learners such as humans and songbirds, which benefits the efficiency of information processing allowing for successful vocal imitation.
Supplementary Material
Highlights.
Auditory regions in both hemispheres are essential for successful song acquisition
Spectral and temporal aspects of song are represented in different hemispheres
Specific aspects of birdsong learning are lateralized, like human language
ACKNOWLEGMENTS
We thank Pat Carey and Valerie LePage for animal care, Katie Barnes ’21 for creating the Suppl. Fig..3, Sanne Moorman for comments on a previous version, Cassandra Pattanayak for statistical advice, and Richard Hahnloser for providing custom software to compute rhythm spectra. This work was supported by the Susan Todd Horton Class of 1910 Trust, Patterson Neuroscience Summer Research Fellowship, Arnold and Mabel Beckman Foundation, Early Career funds from Wellesley College, Brachman-Hoffman fellowship, and by award R15HD085143 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health.
Footnotes
ADDITIONAL INFORMATION
Competing interests: The authors declare no competing interests.
DATA AVAILABILITY
The dataset generated during the current study is available from the corresponding author on reasonable request.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Holland SK, Plante E, Byars AW, Strawsburg RH, Schmithorst VJ, Ball WS, Normal fMRI brain activation patterns in children performing a verb generation task, Neuroimage 14 (2001) 837–843. [DOI] [PubMed] [Google Scholar]
- [2].Pena M, Maki A, Kovacic D, Dehaene-Lambertz G, Koizumi H, Bouquet F, Mehler J, Sounds and silence: An optical topography study of language recognition at birth, Proc. Natl. Acad. Sci. U. S. A 100 (2003) 11702–11705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L, Functional neuroimaging of speech perception in infants, Science 298 (2002) 2013–2015. [DOI] [PubMed] [Google Scholar]
- [4].Jarvis ED, Neural systems for vocal learning in birds and humans: a synopsis, J. Ornithol 148 (2007) S35–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gobes SMH, Bolhuis JJ, Birdsong memory: a neural dissociation between song recognition and production, Curr. Biol 17 (2007) 789–793. [DOI] [PubMed] [Google Scholar]
- [6].Gobes SMH, Zandbergen MA, Bolhuis JJ, Memory in the making: localized brain activation related to song learning in young songbirds, Proc. R. Soc. B-Biol. Sci 277 (2010) 3343–3351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moorman S, Gobes SMH, Kuijpers M, Kerkhofs A, Zandbergen MA, Bolhuis JJ, Human-like brain hemispheric dominance in birdsong learning, Proc. Natl. Acad. Sci. U. S. A 109 (2012) 12782–12787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chirathivat N, Raja SC, Gobes SMH, Hemispheric dominance underlying the neural substrate for learned vocalizations develops with experience. Scientific Reports, Vol. 5, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].London SE, Clayton DF, Functional identification of sensory mechanisms required for developmental song learning, Nat. Neurosci 11 (2008) 579–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ahmadiantehrani S, London SE, Bidirectional manipulation of mTOR signaling disrupts socially mediated vocal learning in juvenile songbirds, Proc. Natl. Acad. Sci. U. S. A 114 (2017) 9463–9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bolhuis JJ, Gahr M, Neural mechanisms of birdsong memory, Nat. Rev. Neurosci 7 (2006) 347–357. [DOI] [PubMed] [Google Scholar]
- [12].Bolhuis JJ, Okanoya K, Scharff C, Twitter evolution: converging mechanisms in birdsong and human speech, Nat Rev Neurosci 11 (2010) 747–759. [DOI] [PubMed] [Google Scholar]
- [13].Gobes SMH, Fritz JB, Bolhuis JJ, Brain mechanisms of auditory learning and memory in songbirds and mammals In: Bolhuis JJ, Everaert M (Eds.), Birdsong, Speech, and Language: Exploring the Evolution of Mind and Brain, The MIT Press, Cambridge, Massachusetts, 2013, pp. 295–315. [Google Scholar]
- [14].Woolley SMN, Early experience shapes vocal neural coding and perception in songbirds, Developmental Psychobiology 54 (2012) 612–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bolhuis JJ, Moorman S, Birdsong memory and the brain: In search of the template, Neuroscience and Biobehavioral Reviews 50 (2015) 41–55. [DOI] [PubMed] [Google Scholar]
- [16].Hahnloser RHR, Kotowicz A, Auditory representations and memory in birdsong learning, Curr. Opin. Neurobiol 20 (2010) 332–339. [DOI] [PubMed] [Google Scholar]
- [17].Olveczky BP, Andalman AS, Fee MS, Vocal experimentation in the juvenile songbird requires a basal ganglia circuit, PLoS. Biol 3 (2005) 902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Roberts TF, Gobes SMH, Murugan M, Olveczky BP, Mooney R, Motor circuits are required to encode a sensory model for imitative learning, Nat. Neurosci 15 (2012) 1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Olson EM, Maeda RK, Gobes SMH, Mirrored patterns of lateralized neuronal activation reflect old and new memories in the avian auditory cortex, Neuroscience 330 (2016) 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Deregnaucourt S, Mitra PP, Feher O, Pytte C, Tchernichovski O, How sleep affects the developmental learning of bird song, Nature 433 (2005) 710–716. [DOI] [PubMed] [Google Scholar]
- [21].Tchernichovski O, Nottebohm F, Ho CE, Pesaran B, Mitra PP, A procedure for an automated measurement of song similarity, Anim. Behav 59 (2000) 1167–1176. [DOI] [PubMed] [Google Scholar]
- [22].Saar S, Mitra PP, A Technique for Characterizing the Development of Rhythms in Bird Song, PLoS ONE 3 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Naie K, Hahnloser RHR, Regulation of learned vocal behavior by an auditory motor cortical nucleus in juvenile zebra finches, J. Neurophysiol 106 (2011) 291–300. [DOI] [PubMed] [Google Scholar]
- [24].Piristine HC, Choetso T, Gobes SMH, A sensorimotor area in the songbird brain is required for production of vocalizations in the song learning period of development, Developmental Neurobiology 76 (2016) 1213–1225. [DOI] [PubMed] [Google Scholar]
- [25].Bolhuis JJ, Hetebrij E, Den Boer-Visser AM, De Groot JH, Zijlstra GGO, Localized immediate early gene expression related to the strength of song learning in socially reared zebra finches, Eur. J. Neurosci 13 (2001) 2165–2170. [DOI] [PubMed] [Google Scholar]
- [26].Bolhuis JJ, Zijlstra GGO, den Boer-Visser AM, Van der Zee EA, Localized neuronal activation in the zebra finch brain is related to the strength of song learning, Proc. Natl. Acad. Sci. U. S. A 97 (2000) 2282–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Terpstra NJ, Bolhuis JJ, den Boer-Visser AM, An analysis of the neural representation of birdsong memory, J. Neurosci 24 (2004) 4971–4977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hammer O, Harper DAT, Ryan PD, PAST: Paleontological Statistics Software Package for Education and Data Analysis., Palaeontologia Electronica 4 (2001) art. 4: 9pp. [Google Scholar]
- [29].Feher O, Wang HB, Saar S, Mitra PP, Tchernichovski O, De novo establishment of wild-type song culture in the zebra finch, Nature 459 (2009) 564–U594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Gehrold A, Leitner S, Laucht S, Deregnaucourt S, Heterospecific exposure affects the development of secondary sexual traits in male zebra finches (Taeniopygia guttata), Behav. Processes 94 (2013) 67–75. [DOI] [PubMed] [Google Scholar]
- [31].Price PH, Developmental determinants of structure in zebra finch song, Journal of Comparative and Physiological Psychology 93 (1979) 260–277. [Google Scholar]
- [32].Williams H, Kilander K, Sotanski ML, Untutored song, reproductive success and song learning, Anim. Behav 45 (1993) 695–705. [Google Scholar]
- [33].Basham ME, Nordeen EJ, Nordeen KW, Blockade of NMDA receptors in the anterior forebrain impairs sensory acquisition in the zebra finch (Poephila guttata), Neurobiol. Learn. Mem 66 (1996) 295–304. [DOI] [PubMed] [Google Scholar]
- [34].Roberts TF, Tschida KA, Klein ME, Mooney R, Rapid spine stabilization and synaptic enhancement at the onset of behavioural learning, Nature 463 (2010) 948–U123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Shank SS, Margoliash D, Sleep and sensorimotor integration during early vocal learning in a songbird, Nature 458 (2009) 73–U74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Otchy TM, Wolff SBE, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SMH, Ölveczky BP, Acute off-target effects of neural circuit manipulations, Nature 528 (2015) 358–363. [DOI] [PubMed] [Google Scholar]
- [37].Canopoli A, Zai A, Hahnloser R, Bilateral neurotoxic lesions in NCM before tutoring onset do not prevent successful tutor song learning, Matters (2017). [Google Scholar]
- [38].Canopoli A, Zai A, Hahnloser R, Lesions of a higher auditory brain area during a sensorimotor period do not impair birdsong learning, Matters (2016). [Google Scholar]
- [39].Pawlisch BA, Remage-Healey L, Neuroestrogen signaling in the songbird auditory cortex propagates into a sensorimotor network via an ‘interface’ nucleus., Neuroscience 284 (2015) 522–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Van Ruijssevelt L, Washington SD, Hamaide J, Verhoye M, Keliris GA, Van der Linden A, Song Processing in the Zebra Finch Auditory Forebrain Reflects Asymmetric Sensitivity to Temporal and Spectral Structure, Frontiers in Neuroscience 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Phan ML, Gergues MM, Mahidadia S, Jimenez-Castillo J, Vicario DS, Bieszczad KM, HDAC3 Inhibitor RGFP966 Modulates Neuronal Memory for Vocal Communication Signals in a Songbird Model, Frontiers in Systems Neuroscience 11 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Tsoi SC, Aiya UV, Wasner KD, Phan ML, Pytte CL, Vicario DS, Hemispheric Asymmetry in New Neurons in Adulthood Is Associated with Vocal Learning and Auditory Memory, PLoS ONE 9 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.