Summary
Several reports have highlighted the abnormal increments of serum immunoglobulin free light chains (FLCs) in the course of systemic autoimmune rheumatic diseases (SARD), but a comparative analysis among different conditions is still lacking. A strong association between elevated FLC and hepatitis C virus (HCV)‐related mixed cryoglobulinaemia (HCVMC) has been well established. Here, we aimed to analyse serum FLC levels in patients with four different SARD in comparison with HCVMC. Using a turbidimetric assay, free κ and λ chains were quantified in sera from 198 SARD patients (37 rheumatoid arthritis, RA; 47 systemic lupus erythematosus, SLE; 52 anti‐phospholipid syndrome, APS; 62 primary Sjogren's syndrome, pSS), 62 HCVMC and 50 healthy blood donors (HD). All patient groups showed increased κ levels when compared to HD: 33·5 ± 2·6 mg/l in HCVMC, 26·7 ± 2·3 mg/l in RA, 29·7 ± 1·9 mg/l in SLE, 23·8 ± 1·1 mg/l in APS, 24·2 ± 1·1 mg/l in pSS; 10·1 ± 0·6 mg/l in HD. Free λ levels displayed a significant increase only for HCVMC (20·4 ± 1·4 mg/l) and SLE (18·4 ± 1·0 mg/l) compared to HD (13·6 ± 0·9 mg/l). The increase of κ compared to λ takes into account a κ /λ ratio of 1·6 for all groups. Our results substantially analyse and strengthen the association between FLC and SARD focusing the questions regarding their role in the pathogenesis and diagnosis of human diseases. Unfortunately, the biochemical differences distinguishing normal from pathological FLC have not been identified. Production of different isotypes is probably connected to still‐unknown pathways.
Keywords: B lymphocytes, FLC, mixed cryoglobulinaemia, mini autoantibodies, SARD
Our study confirms the occurrence of abnormal increases of immunoglobulin free light chains (FLC) in systemic autoimmune rheumatic diseases (SARD), with a comparative analysis of serum levels in four different disorders. Our results strongly suggest that the addition of FLC analysis in routine panels can improve the quality of laboratory diagnosis, offering physicians a direct viewpoint on B‐cell activation. Based on our data and well‐demonstrated biological properties, we hypothesize the pathogenic potential of FLCs that could act as ‘mini autoantibodies'.
Introduction
Activation of autoreactive B lymphocytes, leading to their differentiation into autoantibody‐producing plasma cells, represents the most important pathogenetic mechanism in several autoimmune diseases. Immunoglobulin (Ig) free light chains (FLCs) are produced in excess of heavy chains during synthesis of Igs by plasma cells. Thanks to their short half‐life and in subjects with normal kidney function, their serum levels can be considered as a direct marker of B cell activation, as has been reported in several autoimmune diseases. An abnormal FLC ratio has been shown to be a risk factor for progression of monoclonal gammopathy of undetermined significance, smouldering (asymptomatic) myeloma and solitary plasmacytoma of bone, and is prognostic in multiple myeloma 1, 2, 3. Even if the functional relevance is still poorly investigated, FLCs could be considered bioactive molecules rather than a secondary product of the synthesis of Igs. Each clone of FLC displays distinct physicochemical properties and different bioactivities that may lead to differential injury in damaged tissues. Their contribution to inflammation has been reported in experimental disease models 4. FLCs modulate inhibition of neutrophil apoptosis and viral replication and the induction of mast cell degranulation 5; the increased levels of FLCs, detected in the course of autoimmune disorders, correlate with disease activity 6. All these data suggest that they could act as ‘mini‐autoantibodies'.
FLCs belong to a heterogeneous family of molecules produced by different B cell clones that share only the molecular weight and constant region. Light chains' complementarity determining region (CDR) consists of a part of the antigen‐binding site of self‐ and non‐self‐antibodies despite Ig light chains, when separated from antigen‐specific polyclonal antibodies, displaying little or no antigen‐binding activity 7. The light chain replacement appears to be the dominant form of ‘receptor editing' that can switch the unfavourable antibody reactivity and convert dangerous self‐reactive into innocuous antibodies 8, 9. A peripheral B cell tolerance checkpoint further eliminates autoreactive new emigrant B cells before they enter the mature naive B cell compartment. In systemic autoimmune rheumatic diseases (SARD), both the central and peripheral checkpoints are defective 10.
SARD consist of a heterogeneous group of diseases that affect connective tissues mainly resulting from uncontrolled autoimmune responses, complement activation and associated inflammation. B cells carry out a central role in the pathogenesis of autoimmune disease. In addition to the production of different specific autoantibody patterns, B cells may contribute to disease development by presenting autoantigens to autoreactive T cells and by secreting proinflammatory cytokines and chemokines 11, 12. In nearly all SARD, autoantibody production and immune dysregulation precede the clinical onset. The detection of autoantibodies is currently widely used for diagnosis. However, the ongoing research is focused on improving biomarker utilization for diagnosis, prognosis, treatment selection and optimized therapy. Since 2001, when an assay for determining serum FLCs levels was developed, increased levels of FLC have been investigated and described in patients with generalized B cell stimulation 6, 13, 14, 15.
Different immunological dysfunctions have been frequently reported in hepatitis C virus (HCV)‐positive patients, including mixed cryoglobulinaemia (MC) 16, 17. HCV chronic infection triggers aberrant immunoglobulin production by non‐specific autoreactive B cell clones and may contribute to the pathogenesis of cryoglobulinaemic vasculitis 18, 19, 20. FLC patterns have been associated with MC 21, 22, 23.
Chronic diseases such as SARD and HCVMC represent a burden to humans because of lifelong debilitating illness, increased mortality and morbidity due to the involvement of multiple organ systems and to the high costs of therapy and care 24. B lymphocyte activation associated with the increased FLC production prompted us to investigate serum free κ and λ levels in these conditions. Here, we report our results on FLCs analysis in four patient groups with different SARD [rheumatoid arthritis (RA); systemic lupus erythematosus (SLE); anti‐phospholipid syndrome (APS); primary Sjogren's syndrome (pSS)] in comparison with HCVMC as a prototype of a B cell‐sustained chronic inflammation induced by a well‐known aetiological agent.
Patients and methods
Patients
Sera from 260 patients (62 HCVMC, 37 RA, 47 SLE, 52 APS, 62 pSS) enrolled at the Dipartimento di Medicina Interna e Gastroenterologia, Fondazione Policlinico Universitario ‘A. Gemelli', IRCCS (Rome, Italy) were collected between January 2010 and December 2015 for the determination of FLC levels. Sera from 50 sex‐ and age‐matched healthy blood donors (HD) were tested as control.
Exclusion criteria comprised subjects with diagnoses of plasma cell dyscrasia disorders, cancer and renal failure (estimated glomerular filtration rate was < 60 ml/min/1·73 m2).
Sixty‐two HCVMC patients [40 females (F); 22 males (M)] were defined by the presence of serum type II CGs. Alanine aminotransferase was available for all patients (mean value = 108·4, 9 ± 10 U/l) and the METAVIR score, assessed by transient elastography Fibroscan, ranged from F2 to F3, according to the classification criteria for MC as proposed by the Italian Group for the Study of Cryoglobulinemias in 1989 and later revised in 2002 16. A positive pattern for anti‐nuclear autoantibody (ANA) was assessed. All HCVMC patients displayed a type II cryoglobulinaemia.
The study included 37 RA patients (25F, 12M) with a diagnosis based on the 1987 American College of Rheumatology (ACR) criteria and/or 2010 ACR/European League Against Rheumatism (EULAR) classification criteria for RA 25, 26; 47 SLE patients (35F, 12M), with at least four ACR criteria for the classification of SLE 27, 28; 52 APS patients (40F and 12M) fulfilled the Sydney criteria for APS 29; 62 pSS patients (50F, 12M) who fulfilled 2016 ACR–EULAR criteria for pSS were also included 30.
The study was approved by the ethical committee of Università Cattolica of Rome. The protocol was carried out in accordance with the Declaration of Helsinki as revised in Seoul 2008. Upon assessment of the importance of the study and that the information resources were appropriately expressed, patients and HD gave their informed consent.
Laboratory investigations
Sera were collected and stored at –80° C, except samples for cryoglobulin (CG) determination that was performed on the same day. CGs were characterized by immunofixation electrophoresis, with a G26 fully automated system (Interlab, Rome, Italy) 31, 32.
Rheumatoid factor (RF) measurements were performed according to the manufacturer's protocol on the BNII automated analyzer from Siemens Healthcare (Erlangen, Germany). An RF value of > 20 IU/ml was considered positive.
ANA levels were determined by an indirect immunofluorescence assay (IIFA) on a HEp‐2 cell line. Baseline ANA levels greater than 1 : 80 were regarded as positive 33, 34. ANA detection by IIFA was followed by a double‐strength DNA enzyme‐linked immunosorbent assay (ELISA) that allows the determination of antigen specificity; subsequently an immunofluorescence test for antibodies to native DNA, using the kinetoplast of Crithidia luciliae as substrate, has been assessed and appeared to have great specificity as a diagnostic test for SLE.
The quantitative HCV‐RNA detection was measured by a routine method and virus genotype was determined for each sample (Siemens Healthcare).
Laboratory criteria included persistent presence of anti‐phospholipid antibodies, a heterogeneous group of autoantibodies, lupus anti‐coagulant, anti‐cardiolipin and anti‐beta 2‐glycoprotein I antibodies of IgG or IgM isotypes at medium/high titres. Chemiluminescent immunoassays employing antigen‐specific paramagnetic beads were used in accordance with the manufacturer's instructions.
A specific single test diagnostic for pSS is lacking. Currently, the diagnosis requires either the presence of extractable nuclear antigen antibodies, anti‐SSA (Ro) or anti‐SSB (La) in ELISA and ANA in IIFA‐positive.
FLCs were assessed by means of turbidimetric assay (Freelite Human Kappa and Lambda Free Kits, The Binding Site, Birmingham, UK) and performed with the Optilite® instrument (The Binding Site). The immunoassay consisted of two separate measurements, for free κ (normal range = 3·3–19·4 mg/l) and free λ (normal range = 5·7–26·3 mg/l). A ratio of κ/λ < 0·26 or > 1·65 is abnormal, according to the manufacturer's recommendations. Calibrators and controls were provided by the manufacturer and consisted of stabilized human sera containing polyclonal λ‐ and κ‐FLC; calibrators and controls were diluted to the appropriate concentrations for serum determinations, following the manufacturer's instructions.
Total IgG, IgM and IgA, C3 and C4 were part of the routine clinical care of each patient and were assessed on the same day of blood sample collection.
The analysis was performed by an operator without knowledge of the clinical information of the handled sample.
Statistical analysis
Statistical analyses were performed by using the software package r (3.5.2 release). Four candidate biomarkers were considered: serum κ‐levels (mg/l), serum λ levels (mg/l), κ/λ ratio (unit‐less quantity) and κ + λ (mg/l). Continuous variables were reported as mean ± standard error of the mean (SEM). Biomarkers were analysed by the modified Levene equal variance test (F‐test) to determine whether the variances in the groups are equal, followed by Welch's analysis of variance (anova) test for unequal variances. Biomarkers were also tested for normality by a visual inspection of the QQ‐plot, followed by a Shapiro–Wilk test. Although it was found that selected data sets showed some degree of deviation from normality, Welch's anova was preferred over Kruskal–Wallis because many authors have reported that the former is robust to deviation from normality, while the latter is adversely affected by heteroscedastic data, as determined by the Levene test.
Post‐hoc analysis was carried out by a Games–Howell test for multiple means comparisons. The results were displayed by plotting the difference in means together with the corresponding 95% confidence interval (CI). Correlations between variables were evaluated by a linear regression analysis and by calculating the Spearman's correlation coefficients. Strength of correlation was judged using correlation coefficients of > 0·70 as strong, 0·3–0·7 as moderate and <0·3 as weak.
Results
Determinations of FLC levels in sera from 62 HCVMC (mean age = 61·4 ± 12), 37 RA (mean age = 57·6 ± 13), 47 SLE (mean age = 60·0 ± 14), 52 APS (mean age = 63·2 ± 13) and 62 pSS patients (mean age = 61·8 ± 11) are reported in Table 1; sera from 50 HD (mean age = 51 ± 10·2) were tested as negative control.
Table 1.
Age and concentration of free light chains (FLC) in the study population expressed as mean ± standard deviation
| n | Age (years) | κ (mg/l) | λ (mg/l) | κ /λ | κ + λ (mg/l) | |
|---|---|---|---|---|---|---|
| HCVMC | 62 | 61·4 ± 12 | 33·5 ± 2·6 (P < 0·0001) | 20·4 ± 1·4 (P = n.s.) | 1·60 ± 0·08 (P < 0·0001) | 56·2 ± 4·0 (P < 0·0001) |
| RA | 37 | 57·6 ± 13 | 26·7 ± 2·3 (P < 0·0001) | 14·4 ± 1·0 (P = n.s.) | 1·70 ± 0·07 (P < 0·0001) | 43·0 ± 3·6 (P < 0·0001) |
| SLE | 47 | 60·0 ± 14 | 29·7 ± 1·9 (P < 0·0001) | 18·4 ± 1·0 (P = n.s.) | 1·59 ± 0·05 (P < 0·0001) | 49·1 ± 2·8 (P < 0·0001) |
| APS | 52 | 63·2 ± 13 | 23·8 ± 1·1 (P < 0·0001) | 14·7 ± 0·8 (P = n.s.) | 1·56 ± 0·05 (P < 0·0001) | 38·1 ± 1·6 (P < 0·0001) |
| pSS | 62 | 61·8 ± 11 | 24·2 ± 1·1 (P < 0·0001) | 14·4 ± 0·6 (P = n.s.) | 1·50 ± 0·05 (P < 0·0001) | 37·8 ± 1·3 (P < 0·0001) |
| HD | 50 | 51 ± 10·2 | 10·1 ± 0·6 | 13·6 ± 0·9 | 0·78 ± 0·03 | 23·7 ± 1·5 |
Normal range for FLCs: 3·30–19·40 mg/l for κ; 5·71–26·30 mg/l for λ. A ratio of κ/λ < 0·26 or > 1·65 is abnormal.
P was calculated between each patients group and healthy donors (HD).
HCVMC = hepatitis C virus‐related mixed cryoglobulinaemia; RA = rheumatoid arthritis; SLE= systemic lupus erythematosus; APS = anti‐phospholipid syndrome; pSS = primary Sjogren's syndrome; n.s.= not significant.
In Fig. 1a we show κ levels for HCVMC patients (κ = 33·5 ± 2·6 mg/l), for RA patients (κ = 26·7 ± 2·3 mg/l), for SLE patients (κ = 29·7 ± 1·9 mg/l), for APS patients (κ = 23·8 ± 1·1 mg/l), for 62 pSS patients (κ = 24·2 ± 1·1 mg/l) and for 50 HD (κ = 10·1 ± 0·6 mg/l). No HD display increased κ levels (> 19·4 mg/l) (dashed black lines in Fig. 1a). A Welch's anova for unequal variances revealed that FLC levels differed significantly in the six groups: (F (5,125) = 60·628, P < 1e‐6 for κ levels; F (5,125) = 5·7723, P = 7·926e‐05 for λ levels). A Games–Howell post‐hoc analysis revealed that all patient groups showed increased κ levels when compared to HD (Fig. 1b). HCVMC patients showed also higher κ levels than SLE and APS patients.
Figure 1.
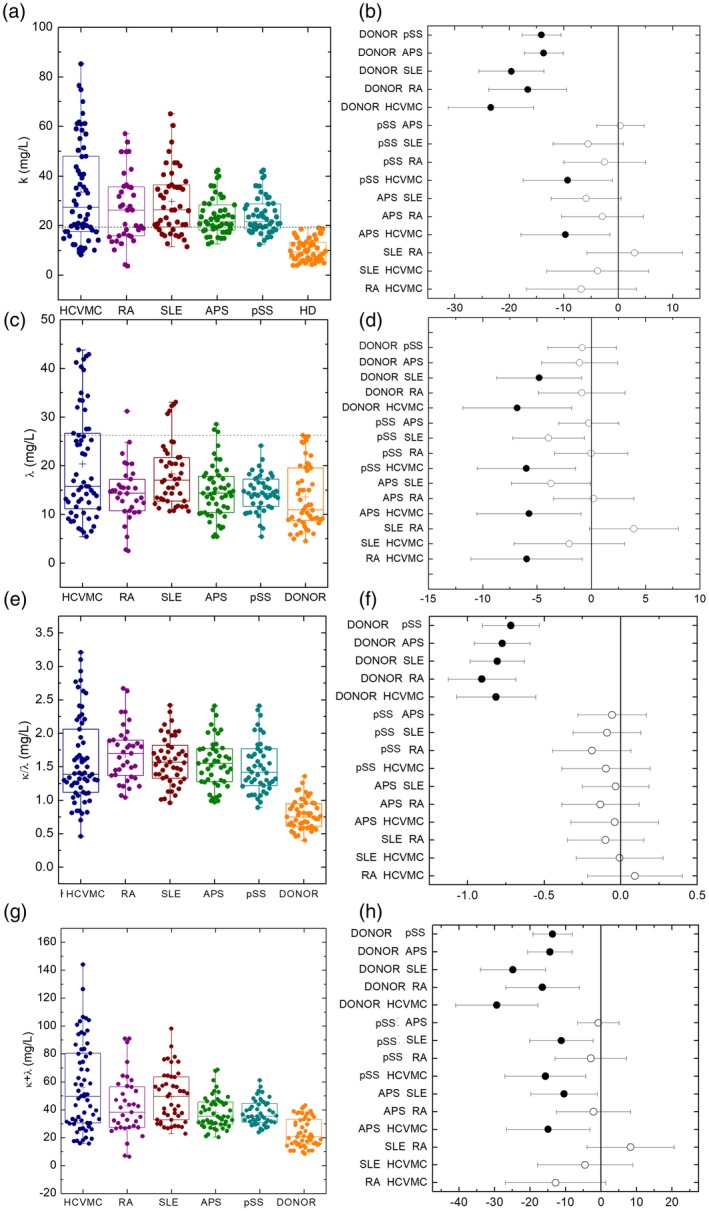
Free light chain (FLC) levels in patients and controls. (a) The κ levels in different patient subgroups and controls. (b) Comparison of κ levels among the various patient subgroups and controls. (c) The λ levels in different patient subgroups and controls. (d) Comparison of λ levels between the various patient subgroups and controls. (e) The κ/λ levels in different patient subgroups and controls. (f) Comparison of κ/λ levels among the various patient subgroups and controls. (g) The κ + λ levels in different patient subgroups and controls. (h) Comparison of κ + λ levels between the various patient subgroups and controls.
In Fig. 1c we show λ levels for HCVMC patients (λ = 20·4 ± 1·4 mg/l), for RA patients (λ = 14·4 ± 1·0 mg/l), for SLE patients (λ = 18·4 ± 1·0 mg/l), for APS patients (λ = 14·7 ± 0·8 mg/l), for 62 pSS patients (λ = 14·4 ± 0·6 = mg/l) and for 50 HD (λ = 13·6 ± 0·9 mg/l). No HD display any increase in λ levels (> 26·3) (dashed black lines in Fig. 1c). The λ levels display a different distribution, with a significant increase only for HCVMC and SLE patients compared to HD. No statistically significant differences were observed for APS, pSS and RA patients. Moreover, λ levels in HCVMC and SLE patients were statistically higher than in APS patients (Fig. 1d).
Figure 1e shows the κ/λ ratio for the five groups of patients compared to the HD. A Welch's anova test revealed statistically significant differences among the six groups (F (5,126) = 74·19, P‐value < 2·2e‐16). Interestingly, the post‐hoc pairwise comparisons with the Games–Howell test showed that the κ/λ ratio were statistically higher in the five groups of patients than in the control group; no statistically significant differences were observed by comparing different groups of patients (Fig. 1f). The same Welch's anova test followed by a Games–Howell post‐hoc test was carried out for κ + λ values (Fig. 1g,h), and the behaviour was similar to κ levels (Fig. 1a,b).
The correlation among κ and λ levels and age were investigated by computing the Spearman's correlation coefficient. No statistically significant correlation was observed between κ levels and age and λ levels and age. A strong correlation between κ and λ levels was found for RA, SLE patients and HD (Fig. 2a), and a moderate correlation for HCVMC, pSS and APS patients (Fig. 2a).
Figure 2.
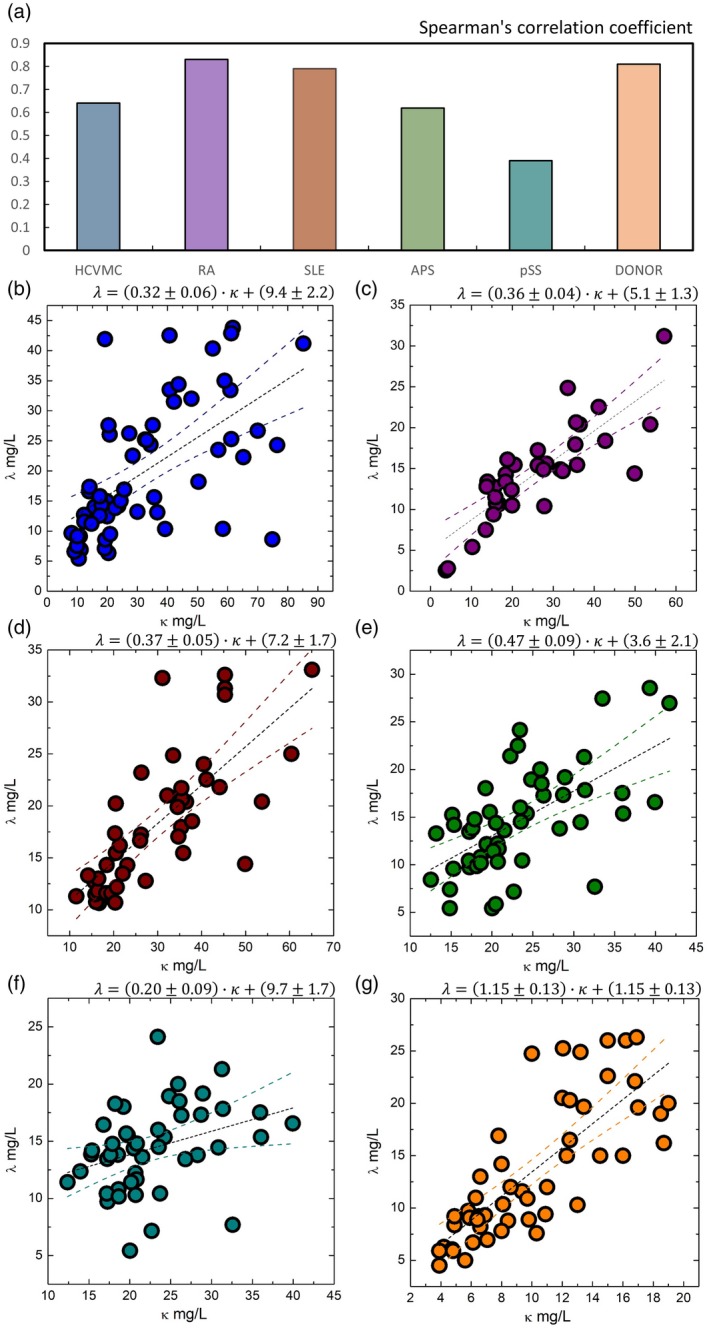
Free light chain distribution between different patient subgroups and controls. (a) Correlation between κ and λ levels in patient subgroups. (b) The λ levels as a function of κ levels for patients with hepatitis C virus‐related mixed cryoglobulinaemia (HCVMC). (c) The λ levels as a function of κ levels for rheumatoid arthritis (RA) patients. (d) The λ levels as a function of κ levels for systemic lupus erythematosus (SLE) patients. (e) The λ levels as a function of κ levels for anti‐phospholipid syndrome (APS) patients. (f) The λ levels as a function of κ levels for primary Sjogren's syndrome (pSS) patients. (g) The λ levels as a function of κ levels for healthy blood donors.
We carried out a linear regression analysis to analyse more clearly a relationship between κ and λ levels. In Fig. 2, we show λ levels as a function of κ levels for HCVMC (Fig. 2b), RA (Fig. 2c), SLE (Fig. 2d), APS (Fig. 2e), pSS patients (Fig. 2e) and HD (Fig. 2f). Data were fitted to a linear trend (dashed black line). Prediction bands are also reported. On one hand, linear regression confirmed a positive correlation between κ and λ levels in each of the six groups of subjects (Fig. 2b–g). In all the subject groups, we found the intercept of the linear regression systematically higher than zero. This result suggests that, at low levels, λ mean levels were higher than κ levels. Of note, in the five patient groups slopes were systematically lower than λ (see insets of Fig. 2b–f), and were thus between 0 and 1. On the other hand, in the control group we measured a slope of 1·15 ± 0·13, which is consistent with λ within one standard deviation. The results of the linear regression analysis also accounted for the behaviour of the κ/λ ratio, as shown in Fig. 1e. In the control group, κ and λ levels increased at the same rate, but λ was higher than κ at low FLC levels, thus giving a κ/λ ratio lower than λ (0·78 ± 0·03; data are expressed as mean ± s.e.m.). Conversely, the increase of κ compared to λ takes into account a κ/λ ratio of approximately 1·6 for all the patient groups (Fig. 1e).
Discussion
Due to the multiple mechanisms that can lead to a breakdown of tolerance and to the multitude of lesions associated with autoimmune diseases, specific patterns of biomarkers are still widely needed to identify patients at risk of relapse and for the management of patients under drug treatments in order to limit organ damage 23, 35, 36. Significant increments of polyclonal FLCs are reported in a wide spectrum of inflammatory and autoimmune conditions in correlation with disease activity, suggesting their role as therapeutic targets and biomarkers of B cell activity 2, 6, 37. Here we evaluated FLCs levels in four patient groups with different systemic autoimmune rheumatic disorders (RA, SLE, APS, pSS) in comparison with patients with HCVMC, an inflammatory immune condition sustained by a well‐defined stimulus.
From our data analysis, only an increase of κ levels occurred in each rheumatic condition that we analysed, while κ and λ levels rose at the same rate in HD. Moreover, the κ/λ ratio was statistically higher in the five groups of patients (SARD + MC) than in HD.
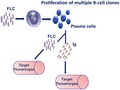
Each SARD is characterized by specific patterns of autoantibodies (towards unknown antigens) that resemble the different B cell clones' origin, and is paralleled by an increase in FLC production, as we report here. Different papers on the biological functions of FLCs have demonstrated their immunological properties; in some cases, FLCs may activate mast cells through the binding on specific surface receptors 5, 38, 39. It is conceivable that not only mast cells but also B lymphocytes could display specific surface receptors for FLCs. In this way, through their CDRs, FLCs might influence the individual antigen‐binding capacity, the pathogenic potential and the ability to interact with immune cells, acting as ‘mini‐autoantibodies'. In this hypothetical scenario, antigen‐bound FLCs may activate B cells, through an autocrine activation loop, into plasma cells producing FLC and autoreactive Igs (Fig. 3). From this point, the outcome of immune reaction should depend upon (auto)antigenic stimulus persistence. Production of different FLC isotypes by B lymphocytes is probably connected to any still‐unknown pathways. In these conditions, FLCs could sustain inflammation and disease progression 40, 41.
Figure 3.
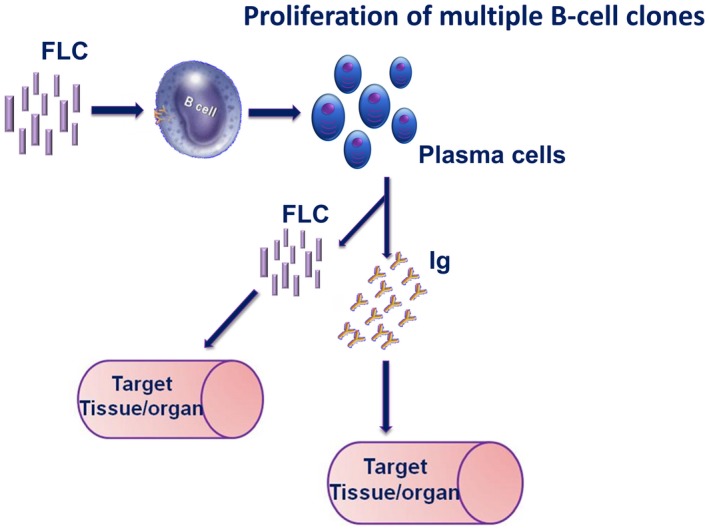
Free light chains as activators of B cells and/or putative mini‐autoantibodies.
MC is characterized by circulating cryoglobulins resulting from an abnormal interaction between a host predisposition and a well‐defined environmental trigger (e.g. HCV infection) that leads to an aberrant B cell dysfunction. Chronic inflammation selects a multi‐step progression from a simple serological alteration towards more complex disorders and ultimately to overt malignant B lymphoproliferation. The increment of FLCs that we observed in MC represents an index of plasma cell activity; an altered κ/λ ratio seems to be positively correlated with the worsening progression of the HCV‐related lymphoproliferative disorder 2. Unfortunately, specific biochemical differences distinguishing normal from pathological FLCs have not been identified, and currently no experimental evidence supports our hypothesis.
In conclusion, we believe that our results are relevant for a clearer understanding of mechanisms behind the dysregulated immune response that we observed in SARD. Our findings confirm that a modification of FLC profile is strongly associated with various autoimmune conditions, even if lacking disease specificity; however, the addition of FLC analysis in routine panels may improve the quality of laboratory diagnosis, providing clinicians with a direct viewpoint on B cell activation. Moreover, assessment of the pretreatment FLC profile may represent a useful prediction tool for the response to therapy, as we have already reported 2, 21, 42. A more in‐depth research investigation is necessary to demonstrate the pathogenetic role of FLC as ‘mini‐autoantibodies' that we hypothesize here. In a translational pathway, FLCs and their cell receptors should be explored as novel attractive therapeutic targets to reduce inflammation and disease activity (this concept is still unexplored). Another challenge will be to increase our knowledge of the bone marrow microenvironment allowing the self‐antigen presentation to newly generated B cells producing FLCs, and for the processes of selection. Further investigations are necessary to exploit their pathogenetic potential in disease activity.
Disclosures
None declared.
Author contributions
U. B., F. G. and C. N. designed the study; F. G, C. N. and G. L. R. collected patients' serum samples and clinical data; K. P., V. B. and A. S. performed the analysis; U. B., M. M., G. C., L. T. and M. D. S. analysed data; U. B., M. M., F. G., C. N. and M. V. drafted the article and/or revised it critically; U. B. and M. M. approved the final version of the article to be published.
Acknowledgements
This research and its publication have been funded from Università Cattolica del Sacro Cuore as a part of its programmes on promotion and dissemination of scientific research (Linea D1 to M. M., Linea Premio pubblicazioni di alta qualità to M. M.).
References
- 1. Pratt G. The evolving use of serum free light chain assays in haematology. Br J Haematol 2008; 141:413–22. [DOI] [PubMed] [Google Scholar]
- 2. Basile U, Gulli F, Gragnani L et al Free light chains: eclectic multipurpose biomarker. J Immunol Methods 2017; 451:11–9. [DOI] [PubMed] [Google Scholar]
- 3. Hutchison CA, Landgren O. Polyclonal immunoglobulin free light chains as a potential biomarker of immune stimulation and inflammation. Clin Chem 2011; 57:1387–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Burmeister A, Assi LK, Ferro CJ et al The relationship between high‐sensitivity CRP and polyclonal free light chains as markers of inflammation in chronic disease. Int J Lab Hematol 2014; 36:415–24. [DOI] [PubMed] [Google Scholar]
- 5. Kaplan B, Livneh A, Sela BA. Immunoglobulin free light chain dimers in human diseases. Sci World J 2011; 11:726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Napodano C, Pocino K, Rigante D et al Free light chains and autoimmunity. Autoimmun Rev 2019; 18:484–92. [DOI] [PubMed] [Google Scholar]
- 7. Porter RR, Weir RC. Subunits of immunoglobulins and their relationship to antibody specificity. J Cell Physiol 1966; 67:51–64. [DOI] [PubMed] [Google Scholar]
- 8. Gay D, Saunders T, Camper S, Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med 1993; 177:999–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wardemann H, Hammersen J, Nussenzweig MC. Human autoantibody silencing by immunoglobulin light chains. J Exp Med 2004; 200:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Menard L, Samuels J, Ng YS, Meffre E. Inflammation‐independent defective early B cell tolerance checkpoints in rheumatoid arthritis. Arthritis Rheum 2011; 63:1237–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Scuderi F, Convertino R, Molino N et al Effect of pro‐inflammatory/anti‐inflammatory agents on cytokine secretion by peripheral blood mononuclear cells in rheumatoid arthritis and systemic lupus erythematosus. Autoimmunity 2003; 36:71–7. [DOI] [PubMed] [Google Scholar]
- 12. Marino M, Bartoccioni E, Alboini PE, Evoli A. Rituximab in myasthenia gravis: a ‘to be or not to be' inhibitor of T cell function. Ann NY Acad Sci 2018; 1413:41–8. [DOI] [PubMed] [Google Scholar]
- 13. Bradwell AR, Carr‐Smith HD, Mead GP et al Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem 2001; 47:673–80. [PubMed] [Google Scholar]
- 14. Aggarwal R, Sequeira W, Kokebie R et al Serum free light chains as biomarkers for systemic lupus erythematosus disease activity. Arthritis Care Res (Hoboken) 2011; 63:891–8. [DOI] [PubMed] [Google Scholar]
- 15. Gottenberg JE, Aucouturier F, Goetz J et al Serum immunoglobulin free light chain assessment in rheumatoid arthritis and primary Sjögren's syndrome. Ann Rheum Dis 2007; 66:23–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ferri C, Zignego AL, Pileri SA. Cryoglobulins. J Clin Pathol 2002; 55:4–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brouet JC, Clauvel JP, Danon F, Klein M, Seligmann M. Biologic and clinical significance of cryoglobulins. A report of 86 cases. Am J Med 1974; 57:775–88. [DOI] [PubMed] [Google Scholar]
- 18. Cacoub P, Renou C, Rosenthal E et al Extrahepatic manifestations associated with hepatitis C virus infection. A prospective multicenter study of 321 patients. The GERMIVIC. Medicine 2000; 79:47–56. [DOI] [PubMed] [Google Scholar]
- 19. Basile U, Gulli F, Torti E et al Anti‐nuclear antibody detection in cryoprecipitates: distinctive patterns in hepatitis C virus‐infected patients. Dig and Liv Dis 2015; 47:50–6. [DOI] [PubMed] [Google Scholar]
- 20. Roccatello D, Saadoun D, Ramos‐Casals M et al Cryoglobulinaemia. Nat Rev Dis Primers 2018; 4:11. [DOI] [PubMed] [Google Scholar]
- 21. Basile U, Gragnani L, Piluso A et al Assessment of free light chains in HCV‐positive patients with mixed cryoglobulinaemia vasculitis undergoing rituximab treatment. Liver Int 2015; 35:2100–7. [DOI] [PubMed] [Google Scholar]
- 22. Basile U, Napodano C, Pocino K et al Serological profile of asymptomatic HCV positive patients with low level of cryoglobulins. Biofactors 2018; 45:318–25. [DOI] [PubMed] [Google Scholar]
- 23. Basile U, Gulli F, Isgrò MA et al A novel biomarker score for the screening and management of patients with plasma cell proliferative disorders. Eur Rev Med Pharmacol Sci 2019; 23:4293–302. [DOI] [PubMed] [Google Scholar]
- 24. Fazal SA, Khan M, Nishi SE et al A clinical update and global economic burden of rheumatoid arthritis. Endocr Metab Immune Disord Drug Targets 2018; 18:98–109. [DOI] [PubMed] [Google Scholar]
- 25. Arnett FC, Edworthy SM, Bloch DA et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–24. [DOI] [PubMed] [Google Scholar]
- 26. Aletaha D, Neogi T, Silman AJ et al Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–81. [DOI] [PubMed] [Google Scholar]
- 27. Tan EM, Cohen AS, Fries JF et al The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1982; 25:1271–7. [DOI] [PubMed] [Google Scholar]
- 28. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997; 40:1725. [DOI] [PubMed] [Google Scholar]
- 29. Miyakis S, Lockshin MD, Atsumi T et al International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. [DOI] [PubMed] [Google Scholar]
- 30. Shiboski CH, Shiboski SC, Seror R et al 2016 American College of Rheumatology/European League Against Rheumatism classification criteria for primary Sjӧgren's syndrome: a consensus and data‐driven methodology involving three international patient cohorts. Arthritis Rheumatol 2017; 69:3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Basile U, Torti E, Dell'Abate MT et al Preanalytical phase in cryoglobulin (CG) detection: an alternative method for sample transport. Clin Chem Lab Med 2016; 54:e123–6. [DOI] [PubMed] [Google Scholar]
- 32. Gulli F, Santini SA, Napodano C et al Cryoglobulin test and Cryoglobulinemia hepatitis C‐virus related. Medit J Hematol Infect Dis 2017; 9:e2017007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Napodano C, Pocino K, Gulli F et al Comparison of fully automated and semiautomated systems for protein immunofixation electrophoresis. J Clin Lab Anal 2017; 31:e22027 10.1002/jcla.22027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meroni PL, Schur PH. ANA screening: an old test with new recommendations. Ann Rheum Dis 2010; 69:1420–22. [DOI] [PubMed] [Google Scholar]
- 35. Basile U, Gulli F, Gragnani L et al Different biochemical patterns in type II and type III mixed cryoglobulinemia in HCV positive patients. Dig Liver Dis 2018; 50:938–43. [DOI] [PubMed] [Google Scholar]
- 36. Stockley RA. Biomarkers in COPD: time for a deep breath. Thorax 2007; 62:657–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verstappen GM, Moerman RV, van Nimwegen JF et al Serum immunoglobulin free light chains are sensitive biomarkers for monitoring disease activity and treatment response in primary Sjögren's syndrome. Rheumatology 2018; 57:1812–21. [DOI] [PubMed] [Google Scholar]
- 38. Van der Heijden M, Kraneveld A, Redegeld F. Free immunoglobulin light chains as target in the treatment of chronic inflammatory diseases. Eur J Pharmacol 2006; 533:319–26. [DOI] [PubMed] [Google Scholar]
- 39. Thio M, Blokhuis BR, Nijkamp FP, Redegeld FA. Free immunoglobulin light chains: a novel target in the therapy of inflammatory diseases. Trends Pharmacol Sci 2008; 29:170–4. [DOI] [PubMed] [Google Scholar]
- 40. Bosello S, De Luca G, Tolusso B et al B cells in systemic sclerosis: a possible target for therapy. Autoimmun Rev 2011; 10:624–30. [DOI] [PubMed] [Google Scholar]
- 41. Draborg AH, Lydolph MC, Westergaard M et al Elevated concentrations of serum immunoglobulin free light chains in systemic lupus erythematosus patients in relation to disease activity, inflammatory status, B cell activity and Epstein–Barr virus antibodies. PLOS ONE 2015; 11:e0148151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Basile U, Marino M, Napodano C et al Serological immunoglobulin‐free light chain profile in myasthenia gravis patients. J Immunol Res 2018; 2018:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


