Introduction
Immunoglobulin light chain amyloidosis (AL) is a plasma cell (PC) dyscrasia characterized by a neoplastic malignant PC population producing highly toxic light chains. Despite overlapping features with multiple myeloma (MM), therapeutic options are limited as a result of disease-specific treatment toxicities. Given delays in diagnosis, patients often present with advanced disease, making them poor candidates for autologous stem-cell transplantation.
Current therapeutic agents include proteasome inhibitors, cyclophosphamide, dexamethasone, and daratumumab; however, the use of thalidomide and lenalidomide are limited as a result of toxicity with worsening heart failure.1
Although t(11;14) is relatively common in MM, it is even more prevalent in systemic light chain amyloidosis, with 60% of patients harboring this translocation. This results in a higher BCL-2:MCL-1 ratio and lower response rates to standard therapies,2 so inhibiting BCL-2 is a logical step. Recently venetoclax, an oral small-molecule BCL-2 inhibitor approved by the US Food and Drug Administration for use in both chronic lymphocytic leukemia and acute myeloid leukemia, was studied in relapsed/refractory MM patients harboring t(11;14). Although venetoclax monotherapy showed modest response rates,3 combining venetoclax with bortezomib and dexamethasone in t(11;14) agnostic MM showed better results, with a 67% overall response rate and 43% of patients experiencing very good partial response or better.4 Given that AL has a high prevalence of t(11;14) with similar disease biology, venetoclax warrants examination in this population.
Case Report
Case 1 was that of a 73-year-old man who was referred for treatment of AL. Initial assessment showed kappa light chains of 0.55 mg/dL, lambda light chains (LLC) of 50.8 mg/dL (ratio, 0.11; difference between involved and uninvolved free light chains [dFLC], 50.25 mg/dL), hypogammaglobulinemia, and 14 g of proteinuria in 24 hours. Bone marrow biopsy was performed; results revealed 10% PCs, negative for amyloid; however, kidney biopsy results indicated Congo Red positivity, with immunohistochemistry confirming AL. The patient began therapy with bortezomib, melphalan, and prednisone and achieved very good partial response, but eventually experienced disease progression. Over the next 7 years, he experienced transient responses to bendamustine, ixazomib, CAEL-101 (chimeric fibril-reactive monoclonal antibody 11-1F4), carfilzomib, lenalidomide, pomalidomide, and daratumumab, but he would ultimately experience disease progression.
Repeat bone marrow biopsy was performed in August 2018 to look for potential actionable mutations, which revealed t(11;14) in 28% of cells. He initiated therapy with venetoclax 400 mg daily, bortezomib 1.3 mg/m2 weekly, and dexamethasone 10 mg weekly.
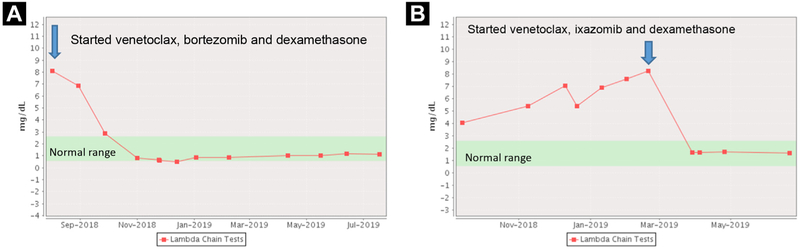
He tolerated treatment without toxicity, and after just 2 therapy cycles, he experienced complete remission (CR). His kappa light chains decreased to 0.89 mg/dL, and his LLC decreased to 0.79 mg/dL with dFLC of −0.20 (Figure 1A). His N-terminal–pro hormone B-type natriuretic peptide level decreased from 5597 pg/mL to 3452 pg/mL, and his 24-hour urinary protein improved from 8.19 g to 2.31 g.
Figure 1.
Lambda Free Light Chains for patient 1 (A) and patient 2 (B)
After cycle 2 of therapy, however, he developed pneumonia with hypogammaglobulinemia (immunoglobulin G, 133 mg/dL) requiring hospitalization and treatment cessation. After discharge, he received intravenous immunoglobulin (IVIg), which led to an improvement in immunoglobulins. Given the severity of his pneumonia, we decided to restart treatment only if he was no longer in CR. As of July 2019, 11 months after initiating only 2 cycles of treatment, he remains in CR.
Case 2 was that of a 72-year-old man who was referred after polypectomy, which stained positively for Congo Red, with immunohistochemistry confirming AL. He was noted to have LLC of 27.32 mg/dL and kappa light chains of 0.76 mg/dL (ratio, 0.03; dFLC, 26.56 mg/dL). Further assessment revealed skin and cardiac involvement. He received multiple therapies, including melphalan, ixazomib, cyclophosphamide, lenalidomide, CAEL-101, daratumumab, and pomalidomide, however his disease eventually progressed. Despite the lack of t(11;14) on repeat bone marrow biopsy, on March 4, 2019, the patient initiated therapy with venetoclax 400 mg daily with ixazomib 2.3 mg and dexamethasone 8 mg on days 1, 8, and 15 of a 28-day cycle. He tolerated treatment well; however, after the release of the interim results of the BELLINI trial (), treatment was discontinued on March 21, 2019. After just 17 days of treatment, the patient experienced a CR (LLC decreased from 8.28 mg/dL to 1.68 mg/dL and dFLC decreased from 7.82 mg/dL to 1.30 mg/dL). His immunoglobulin G remained unchanged at 424 mg/dL, and he was administered prophylactic IVIg. He had minor improvement in cardiac and renal markers of disease. As of June 2019, he remained in CR (Figure 1B).
Discussion
Although conclusions from case reports are limited, here we show venetoclax administered in conjunction with a proteasome inhibitor and steroid can produce deep and extraordinarily rapid light chain reductions in AL, with responses of some durability—over 11 months in one patient. Recently the US Food and Drug Administration has placed a hold on venetoclax in MM, given the preliminary results from BELLINI (bortezomib, venetoclax, and dexamethasone vs. bortezomib, dexamethasone, and placebo in relapsed/refractory MM) showing an increased risk of death (21.1% vs. 11.3%) in the venetoclax arm. Progression-free survival, however, was improved with venetoclax therapy (22.4 vs. 11.5 months). Analysis revealed that 13 of 41 deaths in the venetoclax arm were treatment emergent (compared to 1 of 11 in the placebo arm), and 8 of 13 were from severe infection.5 Aside from disease progression, the most common causes of death while receiving venetoclax therapy were sepsis, pneumonia, and cardiac arrest.
Conclusion
Although venetoclax targets normal PCs, resulting in severe hypogammaglobulinemia (as seen in case 1) and increased risk of infection, it is highly potent against malignant PCs, and careful consideration should be given before abandoning its use. We should identify ways to reduce the putative increased mortality rate. The patients in the BELLINI trial were exposed to 1 to 3 prior therapies, resulting in impaired cellular immunity. Furthermore, venetoclax, being a potent suppressor of PCs, can depress humoral immunity. While we await the final safety evaluation of BELLINI, we expect that the increased mortality rate was due to infection and insufficient use of prophylactic antibiotics and IVIg. Protocol amendments mandating prophylactic antibiotic and IVIg use (once immunoglobulin G falls below 400 mg/dL) could ameliorate the increased risk of infection and death.
Seventy-five percent of AL patients are ineligible for transplant at diagnosis. Those whose disease has limited or no response to standard treatment have few, if any, therapeutic options. For our patients’ sake, we hope that this is not the end of venetoclax in PC dyscrasias and that the detailed safety data forthcoming will allow us to carefully design trials with aggressive infection prophylaxis in order to determine whether venetoclax has a place in the treatment armamentarium of AL amyloidosis.
Clinical Practice Points.
Immunoglobulin light chain amyloidosis (AL) is a plasma-cell disease marked by production and deposition of toxic free light chains in organs including the heart, kidney, and nervous system.
While treatment is generally borrowed from multiple myeloma (MM), patients typically have difficulty tolerating many of these drugs, namely the immunomodulatory drugs, as a result of high cardiac and hematologic toxicities.
Recently daratumumab has been shown to be active in AL; however, for patients whose disease becomes refractory to daratumumab, options remain bleak.
Given the relative prevalence of t(11;14) in patients with AL and subsequent overexpression of BCL-2, we report 2 cases of deep and durable responses to treatment with venetoclax in combination with a proteasome inhibitor.
Recently the US Food and Drug Administration has put a hold on studying venetoclax in MM, given the results of the BELLINI trial () examining the efficacy of venetoclax in MM.
Preliminary results indicate an increased rate of death in the venetoclax arm compared to placebo, largely the result of infection.
With proper infectious prophylaxis, we can abrogate the perceived increased rate of death and the hold on venetoclax can be lifted.
Given the high quality and durable responses seen in these cases, venetoclax warrants further study in AL.
Acknowledgments
Disclosure
R.C. serves as an advisor to Takeda Millennium, Unum, Prothena, and Janssen, and has received clinical research support from Takeda Millennium, Janssen, Prothena, and AbbVie, the latter in support of a phase 1 trial in relapsed AL, currently on hold (). S.L. is a shareholder of Caelum Biosciences and has received consultant fees from Caelum Biosciences, Bayer, AbbVie, Janssen, Proclara, and Takeda. S.L. receives research funding from Karyopharm and Sanofi. V.P. has no conflict of interest to disclose.
References
- 1.Varga C, Titus SE, Toskic D, Comenzo RL. Use of novel therapies in the treatment of light chain amyloidosis. Blood Rev 2019. [DOI] [PubMed] [Google Scholar]
- 2.Gertz MA, Lacy MQ, Dispenzieri A, et al. Clinical implications of t(11;14)(q13;q32), t(4;14)(p16.3;q32), and –17p13 in myeloma patients treated with high-dose therapy. Blood 2005; 106:2837–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S, Kaufman JL, Gasparetto C, et al. Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood 2017; 130: 2401–9. [DOI] [PubMed] [Google Scholar]
- 4.Moreau P, Chanan-Khan A, Roberts AW, et al. Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood 2017; 130:2392–400. [DOI] [PubMed] [Google Scholar]
- 5.Food US and Administration Drug . Drug safety and availability—FDA warns about the risks associated with the investigational use of venclexta in multiple myeloma. March 21, 2019. Available at: https://www.fda.gov/Drugs/DrugSafety/ucm634120.htm. Accessed: July 19, 2019.