Abstract
Sjogren Larsson syndrome (SLS) is a rare autosomal recessive inborn error of lipid metabolism due to mutations in the ALDH3A2 that result in a deficiency of fatty aldehyde dehydrogenase (FALDH). The syndrome has a high prevalence in Sweden where it was first described, but now known to occur worldwide. The classical triad of ichthyosis, mental retardation and spasticity characterizes clinical features. Preterm birth is common. “Glistening white dots” in the retina is a pathognomic clinical feature. Magnetic resonance imaging of the brain demonstrates leukoencephalopathy predominant in the periventricular region. Cerebral MR spectroscopy reveals a characteristic abnormal lipid peak at 1.3ppm and a small peak at 0.9ppm. The primary role of FALDH is oxidation of medium and long-chain aliphatic aldehydes derived from fatty alcohol, phytanic acid, ether glycerolipids and sphingolipids. The diagnosis is based on the typical phenotype, demonstration of the enzyme deficiency and presence of biallelic mutations in the ALDH3A2. The management of SLS largely remains symptomatic currently. However, several potential therapeutic options are being developed, keeping in view of the fundamental metabolic defects or correcting the genetic defect. This review aims to summarize the clinical, genetic and biochemical findings, pathogenetic mechanisms and the current therapeutic options, in SLS.
Keywords: Sjogren Larsson syndrome, spastic paraplegia, icthyosis, glistening white dots, leukoencephalopathy, FALDH, ALDH3A2, fatty aldehydes, leukotriene B4
Introduction
Sjogren-Larsson syndrome (Online Mendelian Inheritance in Man [OMIM]-270200) is an autosomal recessive neurocutaneous disorder caused by mutations in the ALDH3A2 gene that encodes fatty aldehyde dehydrogenase (FALH). The FALDH enzyme deficiency results in the accumulation of long-chain aliphatic aldehydes and alcohols. The disease was first described fifty years ago by Sjogren from Northern Sweden.1 The autosomal recessive inheritance of the disease was established in the monograph written by Sjogren and Larsson.2 The estimated prevalence in Sweden where the largest number of patients have been identified is about one in 250,000.3 However, it has been described worldwide.4–7 Diagnosis requires measurement of FALDH activity in cultured fibroblasts or mutation analysis of the FALDH gene. Deficiency of fatty acid aldehyde dehydrogenase (FALDH) causes an accumulation of fatty alcohols and fatty aldehydes, leading to altered cell-membrane integrity primarily affecting skin, eyes, and the central nervous system. The disorder is defined by the classical triad of symptoms, ichthyosis, mental retardation and spastic paraparesis. Additional findings include preterm birth, pruritis and macular dystrophy, leukoencephalopathy and seizures. The biochemical pathways and genetic abnormalities underlying the pathogenesis of this disorder has been unraveled by focused and continuing research paving the way for novel treatment strategies.8 This review aims to summarize the clinical, genetic and biochemical findings, pathogenetic mechanisms and the current therapeutic options, in SLS.
Clinical Features
Sjogren Larsson syndrome is characterized by the triad of ichthyosis, mental retardation and spastic diplegia. The onset is during infancy or even could be prenatal. The main clinical features are described below.
Preterm Birth
Preterm birth is common in patients with SLS.6,9,10 In the Dutch cohort consisting of 33 patients, preterm birth occurred in 73% of the patients and the median gestational age was 36 weeks.9 This is presumably caused by abnormal lipid metabolism. One of the metabolic pathways involved in SLS is the breakdown of the leukotriene B4 (LTB4), a pro-inflammatory mediator synthesized from arachidonic acid via the lipooxygenase pathway.11,12 Products of the lipoxygenase pathway of arachidonic acid metabolism is considered to have a role in causing uterine contractions.13 It is hypothesized that preterm birth in SLS is directly caused by increased fetal urinary excretion of LTB4 into the amniotic fluid.9
Cutaneous Manifestations
Ichthyosis is usually apparent at birth or develops in infancy.6,14 At birth the skin has an erythematous and hyperkeratotic appearance. Collodion membrane is not seen. This transform over days to dry scaly appearance of ichthyosis. The pathologic skin involvement has been described as early as 23 weeks of gestation.15 Often the entire body is affected, though a relative sparing of the central face is seen. The icthyosis is prominent in flexural areas of the neck and lower abdomen and has a disturbing pruritic character. This symptom differentiates ichthyosis in SLS from other cornification disorder.6 Other ectodermal structures are not affected, and teeth and hair are healthy.
Neurological Features
Spastic Paraplegia
Neurologic symptoms have onset during either first year or second year of life.16 Delay in motor milestones is typical and manifest as delay in sitting, crawling, and walking. This is due to spasticity, which manifests with hypertonia, brisk deep tendon reflexes, extensor plantar response and ankle clonus. Motor disability with spasticity is prominent in legs more than arms. Contractures are common. Independent ambulation is difficult. Even though some of them eventually walk with the use of braces or canes, the majority becomes wheelchair dependent. Upper limb involvement and tetraplegia are uncommon.
Cognitive Deficits
A spectrum of severity of cognitive deficits is seen and mostly ranges from mild to moderate.16 Majority has moderate cognitive impairment. The developmental age is mostly 5–6 years, which limit their ability for social interaction but can reach a certain level of independence.6 Notably there is no cognitive deterioration at least during the first three to four decades of life.16
Delayed Speech and Dysarthria
Speech difficulties are common in patients with SLS. Usually, the dysarthria is pseudo bulbar type and mild to moderate in severity.6,17 Orofacial motor functioning is impaired. There is no correlation between motor functioning and dysarthria or cognitive development; however, cognitive functioning and language development seem to correlate. In order to optimize the speech-language development, early intervention is recommended.17
Seizures
Isolated or infrequent seizures occur in 35–40% of the patients.3,7 Seizures are usually generalized tonic-clonic in type. They are not a prominent feature and usually controlled with anti-epileptic agents. Interictal EEGs are reported to be normal.16 No specific anticonvulsant agents are particularly effective or ineffective in SLS.
Leukoencephalopathy
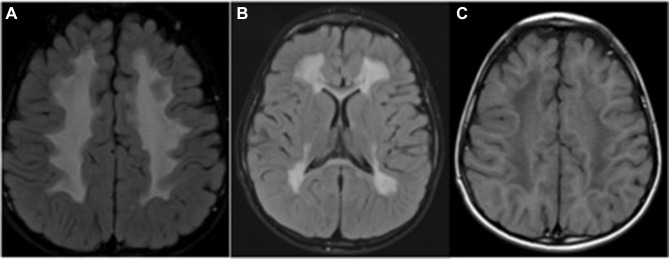
The abnormal neurologic findings are associated with white matter signal changes on MRI (Figure 1A and B).18–22 The zone of increased white matter signal intensity is seen in the periventricular white matter on T2 weighted &FLAIR (fluid-attenuated inversion recovery) images.22 The corresponding T1-weighted images show normal or mildly decreased signal intensity (Figure 1C). The frontal or parieto-occipital zone is preferentially involved. The cerebellar white matter is usually spared. Subcortical association fibers in all white matter regions show unmyelinated areas.16,22
Figure 1.
Magnetic resonance imaging of the brain in a 4year old child with Sjogren Larsson syndrome demonstrating hyperintense signal changes in the periventricular and deep white matter on FLAIR axial view (A and B) and hypointense signal changes on T1weighted axial image (C).
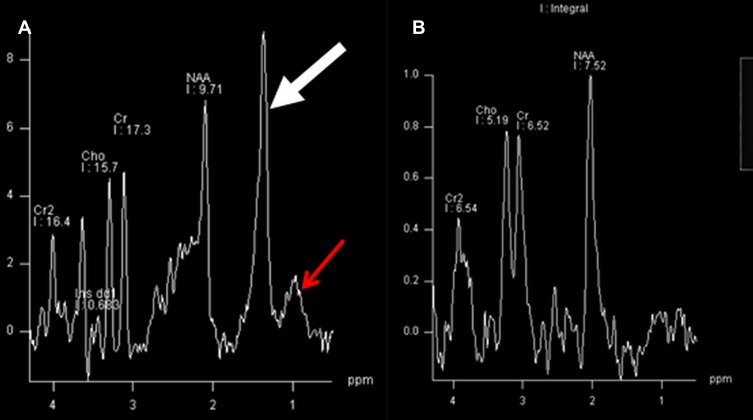
Proton MR spectroscopy of white matter characteristically reveals a prominent peak at 1.3 ppm and also at 0.8–0.9 ppm (Figure 2A) compared to age matched control (Figure 2B).22 This may represent accumulated fatty alcohols or their metabolites nature of which remains unclear.
Figure 2.
Magnetic resonance spectroscopy of white matter reveals a prominent peak at 1.3 ppm (thick arrow) and also at 0.9 ppm (thin arrow) Figure 2A) in a child with Sjogren Larsson syndrome compared to age matched control (Figure 2B).
Ophthalmologic Findings
SLS patients demonstrate distinctive ophthalmic features, characterized by retinal crystalline inclusions appearing as white glistening dots.23 This sign may not be present in all the patients, but when present, represent the pathognomonic feature of the disease. This may appear later in life and absent in infancy.23 This distinctive clinical sign helps to distinguish SLS from other neurocutaneous syndromes. Optical coherence tomography demonstrates macular crystals and pseudocysts.24
Genetics
SLS is caused by mutations in the ALDH3A2 gene that codes for FALDH and is located on chromosome 17p 11.2.25–27 The gene is 31kb long and consists of 11 exons that are numbered 1–10 with an additional exon (exon 9#x2019).28 This produces two transcripts, which encode protein isoforms that differ at their carboxy terminals. The most abundant transcript is derived from splicing of exons 1–10 and produces 485 amino acids protein. There is another minor transcript that accounts for less than total FALDH mRNA and is produced by splicing of exon 9#x2019, between exons 9&10. This transcript encodes a variant protein isoform FALDHv of 508 amino acids that contains a variant carboxy-terminal sequence.28 The carboxy-terminal of the abundant transcript is required to anchor the enzyme to the microsomal membrane.29 Transcripts also arise from the utilization of distinct polyadenylation sites in the 3#x2019, which are 2.0, 3.8, 4kb length.28 ALDH3A2 gene is widely expressed in tissues. The longer transcripts are abundant in brain, heart, skeletal muscle and pancreas, whereas liver has an excess of shorter transcripts.30
Mutations have been identified throughout the ALDH3A2 gene. Recently, the phenotypic data of the SLS patients was merged into a concise genotype based open- access database (www.LOVD.nl/ALDH3A2).7 There were 178 patients identified in the database with 90 unique SLS causing variants. The mutations include amino acid substitutions/deletions/insertions, splicing defects and complex rearrangements. Most are private mutations. However, there are common mutations, which reflect founder effects, consanguinity or recurrent mutational events. This has been reported from Europe, the Middle East, and Brazil. The c.943C>T (p. Pro315Ser) mutation is common in Swedish patients and one, which has been most commonly reported, in the recently compiled database.7,31 The other commonly reported mutation is c.1297_1298delGA (p. Glu433Argfs*3) allele in European patients.32 Both these mutations are each associated with a single haplotype and originate from recurrent mutational events.
Only a few mutations have been studied for their functional significance. Rizzo et al studied 63 kindreds with SLS and identified 49 different mutations, which included ten deletions, two insertions, 22 amino acid substitutions, three nonsense mutations, nine splice site defects and three complex mutations.33 Majority of the missense variants resulted in a severe reduction in the FALDH enzyme catalytic activity when expressed in mammalian cells. One of the mutations had an effect on mRNA stability. The mutations were both shared and private. The common mutations were associated with different haplotypes suggesting that they have originated independently on more than one occasion or are ancient SLS genes, which have undergone intragenic recombination. The mutation c.798G>C (p. Lys266Asp) caused only mild reduction in FALDH activity. Even though the mutations appear to be randomly distributed, four of them involved nucleotides at or around nt 798. They resulted in disrupted normal splicing, and the missense mutations result in unstable mRNA.
Missense mutations account for about one-third of the pathogenic variants reported and are scattered throughout the gene.7 Functional studies in Chinese hamster cells have shown that these mutations result in proteins with little or no activity. A few mutant enzymes possess residual catalytic activity and appear to have altered kinetic properties and protein stability. The splice site mutations cause exon skipping or lead to utilization of cryptic splice sites.
Deletions/insertions of various sizes have been found in the ALDH3A2 gene. The frequency of large gene deletions in SLS is not currently precise. It is thought to affect approximately 5% of the mutant alleles. The reported intragenic deletions in SLS ranged from 1 or two nucleotides to as large as 6kb,34 but complete gene deletions have been reported by Engelstad et al.35 The deletions were defined using long-distance inverse PCR and microarray-based comparative genomic hybridization. The first one was 352 kb deletion involving ALDH3A2 and four contiguous genes including ALDH3A1. The second one was a 1.44-Mb contiguous gene deletion along with a missense mutation. It was concluded that large gene deletions might account for up to 5% of the mutant alleles in SLS. This study highlights the importance of parental testing to confirm carrier status.
In some patients with SLS, only one mutant allele has been identified. Sequencing strategies have not examined the promoter region of the gene or most of the 3#x2019. No mutations have been found in exon 9ʹ that code for FALDHv isoform.
Biochemical Features
Patients with SLS have deficient activity of fatty aldehyde dehydrogenase (FALDH), a microsomal nicotinamide-adenine-dinucleotide-dependent enzyme. FALDH, otherwise known as ALDH3A2, is a member of large ALDH family encoded by at least 19 genes.36 The ALDH superfamily members metabolize both physiologically and pathophysiologically important aldehydes, which prevents the accumulation of toxic aldehydes derived from both endogenous and exogenous sources. Traditionally aldehydes proteins have been grouped into three classes. FALDH is a class 3 enzyme and is known as microsomal ALDH, the class 1 isozymes being located in the cytosol and class 2 in mitochondria.
The FALDH enzyme, expressed in various human tissues, is metabolically active as a dimer and comprised of two similar 54kd subunits.28,37 There are four isoforms of FALDH, which are separated by isoelectric focusing of the native enzymes. SLS patients are deficient in all isoforms. Experiments have localized human FALDH in endoplasmic reticula.38 The major isoform has a carboxy-terminal 35 aa residue that constitutes the hydrophobic domain and anchors the protein to the ER membrane.
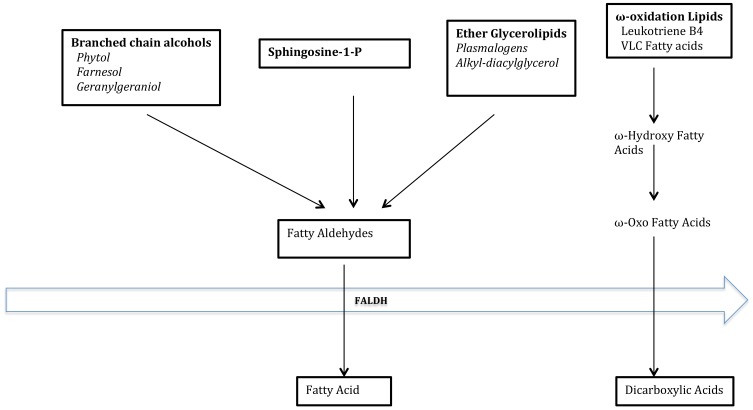
The most crucial function of FALDH is the NAD-dependent oxidation of long-chain aliphatic aldehydes to fatty acids.38 The FALDH enzyme does not require any additional cofactors or proteins for aldehyde oxidase activity except for NAD+.38 FALDH is also a component of the fatty alcohol: NAD+ oxidoreductase (FAO) complex, which catalyzes the oxidation of fatty alcohol. Patients with SLS are deficient in both FALDH and FAO, and therefore, measurement of both enzymes serves as a diagnostic test in patients with SLS. The rate-limiting step in the oxidation of fatty alcohol to fatty acids is the fatty alcohol dehydrogenase activity. FALDH has a high affinity for saturated and unsaturated aliphatic aldehydes 6–24 carbon long, but the preference is for substrates with 16–20 carbon atoms. These fatty aldehydes are derived from the metabolism of fatty alcohols,39 phytanic acids,40 leukotriene B4,11,12 and ether glycerolipids.41 Various studies in SLS patients have defined the biological role of FALDH. The role of FALDH in lipid metabolism is depicted in Figure 3.42
Figure 3.
Substrates and products of FALDH.
Notes: Adapted from Rizzo WB. The role of fatty aldehyde dehydrogenase in epidermal structure and function. Dermato-Endocrinology. 2011;3(2):91–99.42
Fatty Alcohol Metabolism
FALDH is a component of FAO and is therefore necessary for the oxidation of long-chain aliphatic fatty aldehydes produced by fatty alcohol metabolism.39,43 There are elevated levels of fatty alcohols in plasma, urine and cultured cells, accumulating to 25 fold more fatty alcohols in SLS keratinocytes in patients with SLS.44 Most affected patients accumulated long chain fatty alcohols in plasma, with a higher relative accumulation of octadecanol than hexadecanol.
Phytol Metabolism
It has been shown that oxidation of phytol to phytanic acid is deficient in SLS fibroblasts.40,45 Besides pristanal, derived from α-oxidation of phytanic acid is a substrate for FALDH.40 This implicates FALDH in steps in phytol/phytanic acid metabolism.42 However, SLS patients do not accumulate phytol or phytanic acid in plasma, implicating other ALDs in bypassing the FALDH dependent steps.
Isoprenoid Alcohol metabolism
In addition to the phytol/phytanic acid metabolism, studies have indicated that FALDH is involved in the oxidation of other branched chain alcohols. Isoprenoid alcohol, one of the products of the mevalonate pathway, is oxidized by FAO. Mevalonic acid is involved in the generation of farnesyl-pp and geranylgeranyl- pp, which are oxidized to their respective fatty acids through a series of steps.46 SLS patients are noted to have impaired oxidative reaction of the isopropanol pathway.42
Fatty Aldehyde Metabolism
Apart from its role in fatty alcohol metabolism, FALDH is involved in the oxidation of several other fatty aldehydes, most important ones being derived from glycerolipid and eicosanoid metabolism
Glycerolipid Metabolism
Ether glycerolipids are commonly known as plasmalogens and are present in most mammalian tissues including brain, kidney, heart, skeletal muscle and skin. Ether glycolipids, a subgroup of the phospholipids, mainly phosphatidylcholine and phosphatidylethanolamine- represent a high proportion of the total lipids in tissues such as heart, brain and muscle. FALDH has a role in the cleavage of the alkyl group from 1-O-alkylglycerol lipids- such as plasmalogens and 1-0 alkyldiacyl glycerol, which generate long chain-aldehydes that are subsequently oxidized to fatty acids.41 Even though the cleavage step is normal in SLS patients the oxidation of the fatty aldehyde to fatty acids is impaired.41
Eicosanoid Metabolism
FALDH has been implicated in ω-oxidation of eicosanoid, LTB4.11 This is a lipid inflammatory mediator, synthesized from the 20-carbon fatty acid arachidonic acid via the lipooxygenase pathway. Leukotriene B4 is a potent proinflammatory mediator that plays a role in a variety of disease process. LTB4- Epsilon hydroxyl-LTB4- Epsilon aldehyde LTB4 and epsilon –carboxy-LTB4- carboxy LTB4 is further degraded by peroxisomal beta-oxidation. Cytochrome P450 catalyzes the microsomal oxidation of epsilon aldehyde LTB4 to carboxy LTB4. Another aldehyde dehydrogenase is preferentially involved. LTB4 undergoes rapid degradation after intravenous administration. Experiments have proved that the degradation of LTB4 is impaired in patients with SLS.11,12 This indicates the crucial involvement of FALDH in the conversion of epsilon-aldehyde-LTB4 to epsilon-carboxy-LTB4. SLS is the only condition described with profound urinary excretion of LTB4. Analysis of these metabolites in urine offers a non-invasive diagnostic approach for patients with SLS.
Others
FALDH is also implicated in oxidation of fatty aldehydes generated through ceramide metabolism, oxidative stress and lipid peroxidation.
Pathogenesis
Unraveling the pathophysiological mechanisms in SLS patients has remained challenging, given the involvement of the FALDH, in many lipid pathways. Two possible mechanisms are contributing to biochemical pathogenesis in SLS patients, accumulation of lipid substrates of FALDH/FAO and deficiency of critical fatty acid products.
Cutaneous and neurologic symptoms in SLS patients result from abnormal lipid accumulation in the membranes of skin and brain. Epidermal differentiation and function depend on normal fatty aldehyde and alcohol metabolism.47 FALDH deficiency leads to accumulation of fatty aldehydes, fatty alcohols, related lipids-ether glycerolipids and wax esters. in cultured keratinocytes.48 This, in turn, results in abnormal lamellar bodies in the stratum granulosum and impaired delivery of their precursor membranes to the stratum corneum (SC).42 The defective extracellular stratum corneum membranes are responsible for a leaky epidermal water barrier and results in ichthyosis. Light microscopy in SLS patients shows pronounced hyperkeratosis, papillomatosis and acanthosis.49 Ultra structurally, skin exhibits global disruption of lamellar body formation and secretion.50 The lamellar bodies are misshapen and possess granular contents rather than cargo membranes or are empty.50
The specific biochemical pathway responsible for the pathogenesis remains speculative. Fatty aldehydes are potentially harmful products to the cells when they exceed the physiological levels. Various mechanisms have been postulated for this cytotoxicity. This includes cytotoxicity to keratinocytes originating from their propensity to form covalent adducts with other molecules.51–53 Aldehydes are not readily detected in tissues or cultured cells from SLS patients because they are chemically reactive groups that readily form covalent adducts with other molecules. Indirect evidence of fatty aldehyde accumulation is the increased accumulation of N-alkyl-Phosphatydil Ethanolamine. This is the reduction product of an aldehyde dependent Schiff base with phosphatidyl Ethanolamine. Fatty aldehydes are toxic to cultured CHO cells, and FALDH deficient cells are particularly susceptible to the lipids compared to wild type cells. This paper concluded that ether glycerolipids are a source of fatty aldehyde that accumulates in SLS.52
There are also recent reports showing their role in the JNK signaling pathway.54 It has been shown that the hexadecanol product of SIP degradation can induce cell apoptosis.55 FALDH also protects skin from the damaging effects of oxidative stress and ER stress.56–58
There is only limited information on the toxic effects of fatty alcohols. It is unclear if the pathologic changes are due to fatty alcohols or its metabolic products. It was postulated that fatty alcohol accumulation in the skin might lead to ichthyosis by altering the epidermal lipid composition, critical for water-barrier function. SLS patients have had clinical improvement with a low-fat diet supplemented with medium chain fatty acids. The diet restriction removes the source of long chain fatty acids, which is used as a substrate for fatty alcohol synthesis. The dietary regimen with a diet supplemented by medium chain triglycerides also has resulted in clinical improvement. This was associated with a significant reduction in palmitate and stearate composition. Hexadecanol and octadecanol are derived from palmitate and stearate, respectively.
There are only limited neuropathologic reports of the brain available in patients with SLS.59,60 The reported findings include degeneration of the neurons in the cortex and basal ganglia.60 Demyelination was noted in the white matter of the cerebral cortex, corticospinal and vestibulospinal tracts. Purkinje cell loss and small foci of atrophy are noted in the cerebellum. Subsequently, Brain MRI studies have suggested that white matter disease is the most prominent findings.22 The changes were thought to be due to dysmyelination rather than demyelination. Plasmalogens are particularly abundant lipids in myelin membrane, comprising 40–50% of the total Phoshatidylethanolamine (PE). PE in myelin is metabolically active with a half-life of several days. It was postulated that the metabolic turnover of ether sphingolipids in the skin and myelin of SLS patients generates a lot of fatty aldehydes that may be responsible for the pathogenesis of SLS.41
The pathogenesis of the ophthalmological features in SLS patients also remains speculative. The intraretinal deposition of lipid metabolites likely lead to Muller cell degeneration with subsequent formation of cystoid spaces or atrophic changes within the fovea.24
Therapeutic Options
Currently, there is no effective or curative therapy for SLS. Treatment is mainly symptomatic and involves treatment of the neurologic and cutaneous symptoms as they arise. Management requires a multidisciplinary team consisting of neurologists, dermatologists, ophthalmologists and rehabilitation specialists.
Symptomatic Treatment
Cutaneous Symptoms
Current treatment for the cutaneous symptoms in SLS is mostly non-specific. This mainly consists of measures to restore the epidermal water barrier, which includes moisturizing lotions, removing excess scales with keratolytic agents or using retinoids. Topical creams containing 2–10% urea are commonly used. The frequency of application included 1–2 times per day.
Spasticity
The current treatment of spasticity in patients with SLS is symptomatic which include muscle relaxants, benzodiazepines and anticholinergic drugs as well as oral baclofen. The systemic side effects of these drugs often limit their usefulness. Intrathecal baclofen therapy has been tried in patients with SLS with a favorable therapeutic response.61 They are also benefitted by early physiotherapy and surgical procedures such as tendon lengthening, adductor release and dorsal rhizotomy.62
Prospective Therapeutic Options
These are targeted to specific metabolic defects associated with FALDH deficiency or to correct the genetic defect by gene transfer. The most promising pharmacologic approach involves blocking the formation of potentially harmful fatty aldehydes adducts using aldehyde scavenging drugs.8 Other approaches include ALDH specific activator drugs and PPAR –alpha agonists to increase mutant FALDH activity, inhibitors of the JNK phosphorylation cascade, antioxidants to decrease aldehyde load, dietary lipid modification and gene therapy.8 Given below is a summary of the potential therapeutic options in SLS as detailed by Rizzo et al.8
Fatty Aldehyde Scavengers
Since fatty aldehyde adduct formation is an essential pathogenic mechanism in SLS, it is possible theoretically that aldehyde scavengers could be used to scavenge or compete as therapeutic targets for endogenously produced aldehydes.63 The aldehyde scavengers include hydroxylamine derivatives and amino-containing small molecules.64 In SLS, the non-toxic drug NS2 (2-[3-amino-6-chloro-quinolin-2yl]-propan-2-ol) has been shown to react with hexadecimal and inhibit the formation of N-alkyl PE in mouse models.8 NS2 also has been shown to reduce N-alkyl-PE in FALDH deficient CHO cells. A phase 2 trials has been initiated.8 It remains to be seen if systemic use of aldehyde trapping agents could be useful in treating the neurological symptoms and if it needs to be administered before the neurological symptoms.
PPAR Agonists
It has been shown that bezafibrate, a PPAR-α ligand improved the ALDH3A2 gene expression and increased the residual enzyme activity in cultured fibroblasts from patients with missense mutations in ALDH3A2 and small residual enzyme activity.65 This raises the possibility of this being a potential therapeutic agent for the subgroup of SLS patients with residual enzyme activity. However, this has not been tried clinically so far.
ALDH Activators
There are small molecules, which enhance the enzyme activity of FALDH. They act either by acting as chemical chaperons by stabilizing the enzyme from denaturation or binding to enzyme active site. Alda-89, a compound has explicitly been shown to stimulate FALDH activity by three-fold invitro selectively. They have not been clinically tried.
Antioxidants
The role of FALDH in protecting cells from toxic effects of fatty aldehydes generated during oxidative stress and during ER stress has been highlighted in studies.56–58 In this regard, the use of antioxidants may be a potential therapeutic option.
Retinoids and Vitamin D Analogues
Short-acting retinoid acitretin is effective in patients with SLS. Oral retinoid treatment resulted in a marked improvement in the cutaneous symptoms in a cohort of patients with SLS.14 Besides, the treatment was well tolerated. Regular medical supervision and follow up and titration of the doses by a dermatologist are essential for patients receiving retinoic acid therapy. Topical calcipotriol has been used to improve ichthyosis in SLS patients.66
Modulation of Sphingosine Signaling Pathway
Sphingosine-1-phosphate lyase (SPL) is involved in the degradation of sphingolipids and cleavage of the bioactive molecule sphingosine-1-phosphate (SIP) by SPL results in the production of the trans-2-hexadecenal. Trans-2-hexadecenal has been shown to activate JNK signaling pathway and exert the apoptotic effects.54 Modulation of JNK activation has been proposed as a therapeutic target and inhibitors of JNK activation have been proven useful in animal models.67
Replacement of Cutaneous Lipids
Topical application of lipids have been proposed as a possible treatment option, given the fact that abnormal lipid composition contributes to the pathogenesis of cutaneous symptoms in SLS. An optimal lipid mixture has not been developed so far; however slight improvement has been reported with the use of topical cholesterol and lovastatin.68
Reduction of Fatty Alcohol Production
Given the role of fatty alcohols in the pathogenesis of SLS, pharmacologic approaches to reduce the accumulation by enhancing their breakdown or inhibiting the production is a potential therapeutic approach.8 It has been postulated if this is possible by regulation of the biosynthetic enzyme, fatty acyl-CoA reductase (FAR1). FAR1 activity is down regulated by ether glycerolipids.69 Dietary treatment of SLS with ether lipids might decrease the fatty alcohol production, but the caveat is that break down of ether lipids themselves is a source of fatty aldehydes.
Dietary Therapy
Dietary lipid modification in SLS has been tried in a few instances. The regimen consisted of reduction of dietary fat to 30% of the total intake of calories. The diet was also supplemented with n-3 and n-6 fatty acids to obtain a linoleic/linolenic acid ratio of 6 with low erucic acid rapeseed oil, plus high unsaturated fatty acids.70 The studies included two SLS patients and with proven fatty alcohol/NAD+ oxidoreductase deficiency. Clinical benefit was noted in the patient receiving early intervention.71 In some instances, the diets included medium chain triglycerides supplemented with essential fatty acids.72 No convincing effects were reported in the neurologic symptoms.
Other potential dietary management includes restriction of phytanic acid and its alcohol precursor phytol, which are metabolic precursors to the fatty aldehyde pristine. However, neither the phytanic acid nor phytol is elevated in patients with SLS and is not implicated in the causation of neurologic disease.
Inhibition of LTB4 Synthesis
Leukotriene B4, a proinflammatory mediator, is synthesized from arachidonic acid through the lipooxygenase pathway.73 It is mainly synthesized in polymorphonuclear leukocytes. LTB4 is metabolized to microsomal ω oxidation to the less active ω-Carboxy LTB4. Subsequently, ω-Carboxy LTB4 undergoes microsomal degradation to ω-aldehyde-LTB4 and ω-Carboxy-LTB4. Ω-Carboxy-LTB4 further undergoes peroxisomal β oxidation. Aldehyde dehydrogenase likely has a role in microsomal oxidation of ω-aldehyde-LTB4 to ω-carboxy-LTB4. It has been shown that the urinary excretion of leukotriene B4 and ω-hydroxy-LTB4 were highly elevated while ω-carboxy-LTB4 was absent.11 Further studies provided unambiguous evidence for defective LTB4 degradation in SLS patients.12 LTB4 and 20-OH-LTB4 have been implicated in the pathophysiological processes leading to pruritis and pain.74,75 This has lead to the treatment of SLS patients with zileuton, an agent that blocks the synthesis of LTB4 and cysteinyl leukotrienes. Treatment of five SLS patients with zileuton for three months resulted in favorable effects on pruritis score, general well being and background activity of electroencephalographic studies.76 However, these findings could not be replicated in a double-blind cross over study involving 10 SLS patients.77 The consistent beneficial therapeutic effect was demonstrated in only one patient. However, the study recommended that it is worthwhile to give a therapeutic trial of zileuton for 4–6 weeks in patients with severe disabling pruritis.
Gene Therapy
It has been demonstrated taht gene transfer of functional FALDH using adeno-associated virus-2 vectors(rAAV-2) can increase FALDH activity in Chinese hamster Ovary (CHO) cell line model resembling the gene defect of SLS.78 Subsequently, transduction studies were performed on human keratinocytes derived from SLS patients.79 SLS keratinocytes were transduced with rAAV-2/C-FALDH vectors, and FALDH activity was measured after four weeks. The transduced keratinocytes had an average increase of 15 times FALDH activity up to a level of 60–70% of normal keratinocytes. The fact that this level of enzyme deficiency is seen in some of the heterozygote carriers without clinical manifestations, this range of activity is deemed to produce clinical benefit in homozygous patients with SLS. Besides this study also studied the effects of long-chain aldehydes in transduced cells. Long chain aldehydes such as octadecane (C-18) are highly toxic for in-vitro cultured FALDH defective cells.52 Cytotoxic assays have revealed that only 17% of survivors of SLS keratinocytes in comparison to more than 91% of healthy keratinocytes. It was demonstrated that FALDH gene transfer resulted in 84% of surviving cells, which regained resistance to long-chain aldehydes. This confirmed the gene transfer of FALDH stabilized, the resistance of SLS keratinocytes to long chan aldehydes. The study also demonstrated the re-expression of FALDH in a skin equivalent model. The disadvantage was that the model did not allow evaluation of the morphologic appearance of epidermal changes. It is also not clear, Though the rAAV-2 vectors can successfully target keratinocyte stem cells, it is not clear if the correction of skin cells can influence the neurological symptoms by lowering the systemic load of accumulated fatty aldehydes. This might be possible since a genetically engineered skin as a “metabolic sink” for systemic detoxification has been shown in several diseases such as PKU and gyrate atrophy.80
In order to consolidate the results, a transgenic mouse model is necessary which would allow assessment of neurological as well as cutaneous changes. However the development of a such a mouse model has not been successful in other ichthyotic syndromes and was hampered by neonatal death of transgenic mice.81,82 Recently haematopoietic stem cell gene therapy has also been tried in mouse models using lentiviral containing human ALDH3A2 cDNA.83
Even though there has been considerable advancement in SLS research in the last few decades, which largely unravelled the biochemical pathogenesis and genetic basis, there remain grey areas. The most important one being lack of definition of the single pathogenic mechanism, explaining all the symptoms of the disease. Much remains unknown regarding the pathogenesis of central nervous system findings. The different lipid pathways and pathogenic mechanisms in skin, brain and eyes remain the most significant challenge and necessitate, therapeutic approaches tailored to each pathway and tissues. It is envisaged that, as our understanding of the pathogenetic mechanisms improve, new better therapeutic options might emerge for this rare disorder.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.Sjogren T. Oligophrenia combined with congenital ichthyosiform erythrodermia, spastic syndrome and macularretinal degeneration; a clinical and genetic study. Acta Genet Stat Med. 1956;6(1Part 2):80–91. [PubMed] [Google Scholar]
- 2.Sjogren T, Larsson T. Oligophrenia in combination with congenital ichthyosis and spastic disorders; a clinical and genetic study. Acta Psychiatrica Neurologica Scandinavica Supplementum. 1957;113:1–112. [PubMed] [Google Scholar]
- 3.Rizzo WB. Sjögren–larsson syndrome: fatty aldehyde dehydrogenase deficiencyIn:Scriver CR,Beckman K,Small GM et al,editors. The Metabolic & Molecular Bases of Inherited Disease. New York, NY: McGraw-Hill; 2001:2239–2258. [Google Scholar]
- 4.Nagappa M, Bindu PS, Chiplunkar S, et al. Child neurology: Sjögren- Larsson syndrome. Neurology. 2017;88(1):e1–e4. doi: 10.1212/WNL.0000000000003456 [DOI] [PubMed] [Google Scholar]
- 5.Didona B, Codispoti A, Bertini E, et al. Novel and recurrent ALDH3A2 mutations in Italian patients with Sjögren–Larsson syndrome. J Hum Genet. 2007;52(10):865–870. doi: 10.1007/s10038-007-0180-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fuijkschot J, Theelen T, Seyger MM, et al. Sjogren-Larsson syndrome in clinical practice. J Inherit Metab Dis. 2012;35(6):955–962. doi: 10.1007/s10545-012-9518-6 [DOI] [PubMed] [Google Scholar]
- 7.Weustenfeld M, Eidelpes R, Schmuth M, Rizzo WB, Zschocke J, Keller MA. Genotype and phenotype variability in Sjogren-Larsson syndrome. Hum Mutat. 2019;40(2):177–186. doi: 10.1002/humu.23679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rizzo WB. Genetics and prospective therapeutic targets for Sjogren-Larsson Syndrome. Expert Opinion Orphan Drugs. 2016;4(4):395–406. doi: 10.1517/21678707.2016.1154453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staps P, Hogeveen M, Fuijkschot J, van Drongelen J, Willemsen M. Understanding fetal factors that contribute to preterm birth: Sjogren-Larsson syndrome as a model. J Perinat Med. 2018;46(5):523–529. doi: 10.1515/jpm-2017-0187 [DOI] [PubMed] [Google Scholar]
- 10.Willemsen MA, Rotteveel JJ, van Domburg PH, Gabreels FJ, Mayatepek E, Sengers RC. Preterm birth in Sjogren-Larsson syndrome. Neuropediatrics. 1999;30(6):325–327. doi: 10.1055/s-2007-973513 [DOI] [PubMed] [Google Scholar]
- 11.Willemsen MA, de Jong JG, van Domburg PH, Rotteveel JJ, Wanders RJ, Mayatepek E. Defective inactivation of leukotriene B4 in patients with Sjogren-Larsson syndrome. J Pediatr. 2000;136(2):258–260. doi: 10.1016/S0022-3476(00)70113-2 [DOI] [PubMed] [Google Scholar]
- 12.Willemsen MA, Rotteveel JJ, de Jong JG, et al. Defective metabolism of leukotriene B4 in the Sjogren-Larsson syndrome. J Neurol Sci. 2001;183(1):61–67. doi: 10.1016/S0022-510X(00)00474-3 [DOI] [PubMed] [Google Scholar]
- 13.Romero R, Emamian M, Wan M, Grzyboski C, Hobbins JC, Mitchell MD. Increased concentrations of arachidonic acid lipoxygenase metabolites in amniotic fluid during parturition. Obstet Gynecol. 1987;70(6):849–851. [PubMed] [Google Scholar]
- 14.Ganemo A, Jagell S, Vahlquist A. Sjogren-larsson syndrome: a study of clinical symptoms and dermatological treatment in 34 Swedish patients. Acta Derm Venereol. 2009;89(1):68–73. doi: 10.2340/00015555-0561 [DOI] [PubMed] [Google Scholar]
- 15.Kousseff BG, Matsuoka LY, Stenn KS, Hobbins JC, Mahoney MJ, Hashimoto K. Prenatal diagnosis of Sjogren-Larsson syndrome. J Pediatr. 1982;101(6):998–1001. doi: 10.1016/S0022-3476(82)80030-9 [DOI] [PubMed] [Google Scholar]
- 16.Willemsen MA, IJlst L, Steijlen PM, et al. Clinical, biochemical and molecular genetic characteristics of 19 patients with the Sjogren-Larsson syndrome. Brain. 2001;124(Pt 7):1426–1437. doi: 10.1093/brain/124.7.1426 [DOI] [PubMed] [Google Scholar]
- 17.Fuijkschot J, Maassen B, Gorter JW, Gerven M, Willemsen M. Speech-language performance in Sjogren-Larsson syndrome. Dev Neurorehabil. 2009;12(2):106–112. doi: 10.1080/17518420902800944 [DOI] [PubMed] [Google Scholar]
- 18.Mano T, Ono J, Kaminaga T, et al. Proton MR spectroscopy of Sjogren-Larsson’s syndrome. AJNR Am j Neuroradiol. 1999;20(9):1671–1673. [PMC free article] [PubMed] [Google Scholar]
- 19.Miyanomae Y, Ochi M, Yoshioka H, et al. Cerebral MRI and spectroscopy in Sjogren-Larsson syndrome: case report. Neuroradiology. 1995;37(3):225–228. doi: 10.1007/BF01578262 [DOI] [PubMed] [Google Scholar]
- 20.Nakayama M, Tavora DG, Alvim TC, Araujo AC, Gama RL. MRI and 1H-MRS findings of three patients with Sjogren-Larsson syndrome. Arq Neuropsiquiatr. 2006;64(2b):398–401. doi: 10.1590/S0004-282X2006000300009 [DOI] [PubMed] [Google Scholar]
- 21.Sijens PE, Westerlaan HE, de Groot JC, et al. MR spectroscopy and diffusion tensor imaging of the brain in Sjogren-Larsson syndrome. Mol Genet Metab. 2009;98(4):367–371. doi: 10.1016/j.ymgme.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 22.Willemsen MA, Van Der Graaf M, Van Der Knaap MS, et al. MR imaging and proton MR spectroscopic studies in Sjogren-Larsson syndrome: characterization of the leukoencephalopathy. AJNR Am j Neuroradiol. 2004;25(4):649–657. [PMC free article] [PubMed] [Google Scholar]
- 23.Willemsen MA, Cruysberg JR, Rotteveel JJ, Aandekerk AL, Van Domburg PH, Deutman AF. Juvenile macular dystrophy associated with deficient activity of fatty aldehyde dehydrogenase in Sjogren-Larsson syndrome. Am J Ophthalmol. 2000;130(6):782–789. doi: 10.1016/S0002-9394(00)00576-6 [DOI] [PubMed] [Google Scholar]
- 24.Fuijkschot J, Cruysberg JR, Willemsen MA, Keunen JE, Theelen T. Subclinical changes in the juvenile crystalline macular dystrophy in Sjogren-Larsson syndrome detected by optical coherence tomography. Ophthalmology. 2008;115(5):870–875. doi: 10.1016/j.ophtha.2007.05.063 [DOI] [PubMed] [Google Scholar]
- 25.Pigg M, Jagell S, Sillen A, Weissenbach J, Gustavson KH, Wadelius C. The Sjogren-Larsson syndrome gene is close to D17S805 as determined by linkage analysis and allelic association. Nat Genet. 1994;8(4):361–364. doi: 10.1038/ng1294-361 [DOI] [PubMed] [Google Scholar]
- 26.Rogers GR, Rizzo WB, Zlotogorski A, et al. Genetic homogeneity in Sjögren-Larsson syndrome: linkage to chromosome 17p in families of different non-Swedish ethnic origins. Am J Hum Genet. 1995;57(5):1123–1129. [PMC free article] [PubMed] [Google Scholar]
- 27.De Laurenzi V, Rogers GR, Hamrock DJ, et al. Sjogren-Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat Genet. 1996;12(1):52–57. doi: 10.1038/ng0196-52 [DOI] [PubMed] [Google Scholar]
- 28.Rogers GR, Markova NG, De Laurenzi V, Rizzo WB, Compton JG. Genomic organization and expression of the human fatty aldehyde dehydrogenase gene (FALDH). Genomics. 1997;39(2):127–135. doi: 10.1006/geno.1996.4501 [DOI] [PubMed] [Google Scholar]
- 29.Masaki R, Yamamoto A, Tashiro Y. Microsomal aldehyde dehydrogenase is localized to the endoplasmic reticulum via its carboxyl-terminal 35 amino acids. J Cell Biol. 1994;126(6):1407–1420. doi: 10.1083/jcb.126.6.1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rizzo WB. Sjogren-Larsson syndrome: molecular genetics and biochemical pathogenesis of fatty aldehyde dehydrogenase deficiency. Mol Genet Metab. 2007;90(1):1–9. doi: 10.1016/j.ymgme.2006.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Laurenzi V, Rogers GR, Tarcsa E, et al. Sjogren-Larsson syndrome is caused by a common mutation in northern European and Swedish patients. J Invest Dermatol. 1997;109(1):79–83. doi: 10.1111/1523-1747.ep12276622 [DOI] [PubMed] [Google Scholar]
- 32.Rizzo WB, Carney G, De Laurenzi V. A common deletion mutation in European patients with Sjogren-Larsson syndrome. Biochem Mol Med. 1997;62(2):178–181. doi: 10.1006/bmme.1997.2640 [DOI] [PubMed] [Google Scholar]
- 33.Rizzo WB, Carney G, Lin Z. The molecular basis of Sjogren-Larsson syndrome: mutation analysis of the fatty aldehyde dehydrogenase gene. Am J Hum Genet. 1999;65(6):1547–1560. doi: 10.1086/302681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizzo WB, Carney G. Sjogren-Larsson syndrome: diversity of mutations and polymorphisms in the fatty aldehyde dehydrogenase gene (ALDH3A2). Hum Mutat. 2005;26(1):1–10. doi: 10.1002/humu.20181 [DOI] [PubMed] [Google Scholar]
- 35.Engelstad H, Carney G, S’Aulis D, et al. Large contiguous gene deletions in Sjogren-Larsson syndrome. Mol Genet Metab. 2011;104(3):356–361. doi: 10.1016/j.ymgme.2011.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson B, Brocker C, Thompson DC, et al. Update on the aldehyde dehydrogenase gene (ALDH) superfamily. Hum Genomics. 2011;5(4):283–303. doi: 10.1186/1479-7364-5-4-283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Marchitti SA, Brocker C, Stagos D, Vasiliou V. Non-P450 aldehyde oxidizing enzymes: the aldehyde dehydrogenase superfamily. Expert Opin Drug Metab Toxicol. 2008;4(6):697–720. doi: 10.1517/17425255.4.6.697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kelson TL, Secor McVoy JR, Rizzo WB. Human liver fatty aldehyde dehydrogenase: microsomal localization, purification, and biochemical characterization. Biochim Biophys Acta. 1997;1335(1–2):99–110. doi: 10.1016/S0304-4165(96)00126-2 [DOI] [PubMed] [Google Scholar]
- 39.Rizzo WB, Craft DA. Sjogren-Larsson syndrome. Deficient activity of the fatty aldehyde dehydrogenase component of fatty alcohol: NAD+oxidoreductase in cultured fibroblasts. J Clin Invest. 1991;88(5):1643–1648. doi: 10.1172/JCI115478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhoeven NM, Jakobs C, Carney G, Somers MP, Wanders RJ, Rizzo WB. Involvement of microsomal fatty aldehyde dehydrogenase in the alpha-oxidation of phytanic acid. FEBS Lett. 1998;429(3):225–228. doi: 10.1016/S0014-5793(98)00574-2 [DOI] [PubMed] [Google Scholar]
- 41.Rizzo WB, Heinz E, Simon M, Craft DA. Microsomal fatty aldehyde dehydrogenase catalyzes the oxidation of aliphatic aldehyde derived from ether glycerolipid catabolism: implications for Sjogren-Larsson syndrome. Biochim Biophys Acta. 2000;1535(1):1–9. doi: 10.1016/S0925-4439(00)00077-6 [DOI] [PubMed] [Google Scholar]
- 42.Rizzo WB. The role of fatty aldehyde dehydrogenase in epidermal structure and function. Dermato-Endocrinology. 2011;3(2):91–99. doi: 10.4161/derm.3.2.14619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rizzo WB, Dammann AL, Craft DA. Sjogren-Larsson syndrome. Impaired fatty alcohol oxidation in cultured fibroblasts due to deficient fatty alcohol: nicotinamideadenine dinucleotide oxidoreductase activity. J Clin Invest. 1988;81(3):738–744. doi: 10.1172/JCI113379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rizzo WB, Craft DA. Sjogren-Larsson syndrome: accumulation of free fatty alcohols in cultured fibroblasts and plasma. J Lipid Res. 2000;41(7):1077–1081. [PubMed] [Google Scholar]
- 45.van den Brink DM, van Miert JN, Dacremont G, Rontani JF, Jansen GA, Wanders RJ. Identification of fatty aldehyde dehydrogenase in the breakdown of phytol to phytanic acid. Mol Genet Metab. 2004;82(1):33–37. doi: 10.1016/j.ymgme.2004.01.019 [DOI] [PubMed] [Google Scholar]
- 46.Crick DC, Andres DA, Waechter CJ. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun. 1997;237(3):483–487. doi: 10.1006/bbrc.1997.7145 [DOI] [PubMed] [Google Scholar]
- 47.Rizzo WB. Fatty aldehyde and fatty alcohol metabolism: review and importance for epidermal structure and function. Biochim Biophys Acta. 2014;1841(3):377–389. doi: 10.1016/j.bbalip.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rizzo WB, Craft DA, Dammann AL, Phillips MW. Fatty alcohol metabolism in cultured human fibroblasts. Evidence for a fatty alcohol cycle. J Biol Chem. 1987;262(36):17412–17419. [PubMed] [Google Scholar]
- 49.Jagell S, Liden S. Ichthyosis in the Sjogren-Larsson syndrome. Clin Genet. 1982;21(4):243–252. doi: 10.1111/j.1399-0004.1982.tb00758.x [DOI] [PubMed] [Google Scholar]
- 50.Shibaki A, Akiyama M, Shimizu H. Novel ALDH3A2 heterozygous mutations are associated with defective lamellar granule formation in a Japanese family of Sjogren-Larsson syndrome. J Invest Dermatol. 2004;123(6):1197–1199. doi: 10.1111/j.0022-202X.2004.23505.x [DOI] [PubMed] [Google Scholar]
- 51.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6 [DOI] [PubMed] [Google Scholar]
- 52.James PF, Zoeller RA. Isolation of animal cell mutants defective in long-chain fatty aldehyde dehydrogenase. Sensitivity to fatty aldehydes and Schiff’s base modification of phospholipids: implications for Sj-ogren-Larsson syndrome. J Biol Chem. 1997;272(38):23532–23539. doi: 10.1074/jbc.272.38.23532 [DOI] [PubMed] [Google Scholar]
- 53.Stadelmann-Ingrand S, Pontcharraud R, Fauconneau B. Evidence for the reactivity of fatty aldehydes released from oxidized plasmalogens with phosphatidylethanolamine to form Schiff base adducts in rat brain homogenates. Chem Phys Lipids. 2004;131(1):93–105. doi: 10.1016/j.chemphyslip.2004.04.008 [DOI] [PubMed] [Google Scholar]
- 54.Kumar A, Byun HS, Bittman R, Saba JD. The sphingolipid degradation product trans-2-hexadecenal induces cytoskeletal reorganization and apoptosis in a JNK-dependent manner. Cell Signal. 2011;23(7):1144–1152. doi: 10.1016/j.cellsig.2011.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Upadhyaya P, Kumar A, Byun HS, Bittman R, Saba JD, Hecht SS. The sphingolipid degradation product trans-2-hexadecenal forms adducts with DNA. Biochem Biophys Res Commun. 2012;424(1):18–21. doi: 10.1016/j.bbrc.2012.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Demozay D, Mas JC, Rocchi S, Van Obberghen E. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Diabetes. 2008;57(5):1216–1226. doi: 10.2337/db07-0389 [DOI] [PubMed] [Google Scholar]
- 57.Demozay D, Rocchi S, Mas JC, et al. Fatty aldehyde dehydrogenase: potential role in oxidative stress protection and regulation of its gene expression by insulin. J Biol Chem. 2004;279(8):6261–6270. doi: 10.1074/jbc.M312062200 [DOI] [PubMed] [Google Scholar]
- 58.Ashibe B, Motojima K. Fatty aldehyde dehydrogenase is up-regulated by polyunsaturated fatty acid via peroxisome proliferator-activated receptor alpha and suppresses polyunsaturated fatty acid-induced endoplasmic reticulum stress. FEBS J. 2009;276(23):6956–6970. doi: 10.1111/j.1742-4658.2009.07404.x [DOI] [PubMed] [Google Scholar]
- 59.McLennan JE, Gilles FH, Robb RM. Neuropathological correlation in Sjogren-Larsson syndrome. Oligophrenia, ichthyosis and spasticity. Brain. 1974;97(4):693–708. doi: 10.1093/brain/97.1.693 [DOI] [PubMed] [Google Scholar]
- 60.Sylvester PE. Pathological findings in Sjogren-Larsson syndrome. J Ment Defic Res. 1969;13(4):267–275. doi: 10.1111/j.1365-2788.1969.tb01091.x [DOI] [PubMed] [Google Scholar]
- 61.Hidalgo ET, Orillac C, Hersh A, Harter DH, Rizzo WB, Weiner HL. Intrathecal Baclofen therapy for the treatment of spasticity in Sjögren-Larsson syndrome. J Child Neurol. 2017;32(1):100–103. doi: 10.1177/0883073816671440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Haddad FS, Lacour M, Harper JI, Fixsen JA. The orthopaedic presentation and management of Sjogren-Larsson syndrome. J Pediatr Orthop. 1999;19(5):617–619. doi: 10.1097/01241398-199909000-00012 [DOI] [PubMed] [Google Scholar]
- 63.Shapiro HK. Carbonyl-trapping therapeutic strategies. Am J Ther. 1998;5(5):323–353. doi: 10.1097/00045391-199809000-00008 [DOI] [PubMed] [Google Scholar]
- 64.Aldini G, Facino RM, Beretta G, Carini M. Carnosine and related dipeptides as quenchers of reactive carbonyl species: from structural studies to therapeutic perspectives. Biofactors. 2005;24(1–4):77–87. doi: 10.1002/biof.v24:1/4 [DOI] [PubMed] [Google Scholar]
- 65.Gloerich J, Ijlst L, Wanders RJ, Ferdinandusse S. Bezafibrate induces FALDH in human fibroblasts; implications for Sjogren-Larsson syndrome. Mol Genet Metab. 2006;89(1–2):111–115. doi: 10.1016/j.ymgme.2006.05.009 [DOI] [PubMed] [Google Scholar]
- 66.Lucker GP, van de Kerkhof PC, Cruysberg JR, der Kinderen DJ, Steijlen PM. Topical treatment of Sjogren-Larsson syndrome with calcipotriol. Dermatology. 1995;190(4):292–294. doi: 10.1159/000246719 [DOI] [PubMed] [Google Scholar]
- 67.Bogoyevitch MA, Arthur PG. Inhibitors of c-Jun N-terminal kinases: juNK no more? Biochim Biophys Acta. 2008;1784(1):76–93. doi: 10.1016/j.bbapap.2007.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merino De Paz N, Rodriguez-Martin M, Contreras-Ferrer P, et al. Topical treatment of CHILD nevus and Sjogren-Larsson syndrome with combined lovastatin and cholesterol. Eur j Dermatol. 2011;21(6):1026–1027. doi: 10.1684/ejd.2011.1549 [DOI] [PubMed] [Google Scholar]
- 69.Honsho M, Asaoku S, Fujiki Y. Posttranslational regulation of fatty acyl-CoA reductase 1, Far1, controls ether glycerophospholipid synthesis. J Biol Chem. 2010;285(12):8537–8542. doi: 10.1074/jbc.M109.083311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Auada MP, Taube MB, Collares EF, Tanaka AM, Cintra ML. Sjogren-Larsson syndrome: biochemical defects and follow up in three cases. Eur j Dermatol. 2002;12(3):263–266. [PubMed] [Google Scholar]
- 71.Taube B, Billeaud C, Labreze C, Entressangles B, Fontan D, Taieb A. Sjogren-Larsson syndrome: early diagnosis, dietary management and biochemical studies in two cases. Dermatology. 1999;198(4):340–345. doi: 10.1159/000018145 [DOI] [PubMed] [Google Scholar]
- 72.Maaswinkel-Mooij PD, Brouwer OF, Rizzo WB. Unsuccessful dietary treatment of Sjogren-Larsson syndrome. J Pediatr. 1994;124(5 Pt 1):748–750. doi: 10.1016/S0022-3476(05)81369-1 [DOI] [PubMed] [Google Scholar]
- 73.Lewis RA, Austen KF, Soberman RJ. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990;323(10):645–655. doi: 10.1056/NEJM199009063231006 [DOI] [PubMed] [Google Scholar]
- 74.Levine JD, Lau W, Kwiat G, Goetzl EJ. Leukotriene B4 produces hyperalgesia that is dependent on polymorphonuclear leukocytes. Science. 1984;225(4663):743–745. doi: 10.1126/science.6087456 [DOI] [PubMed] [Google Scholar]
- 75.Andoh T, Harada A, Kuraishi Y. Involvement of leukotriene B4 released from keratinocytes in itch-associated response to intradermal interleukin-31 in mice. Acta Derm Venereol. 2017;97(8):922–927. doi: 10.2340/00015555-2697 [DOI] [PubMed] [Google Scholar]
- 76.Willemsen MA, Lutt MA, Steijlen PM, et al. Clinical and biochemical effects of zileuton in patients with the Sjögren-Larsson syndrome. Eur J Pediatr. 2001;160(12):711–717. doi: 10.1007/s004310100838 [DOI] [PubMed] [Google Scholar]
- 77.Fuijkschot J, Seyger MM, Bastiaans DE, Wevers RA, Roeleveld N, Willemsen MA. Zileuton for pruritus in Sjogren-Larsson syndrome: a randomized double-blind placebo-controlled crossover trial. Acta Derm Venereol. 2016;96(2):255–256. doi: 10.2340/00015555-2195 [DOI] [PubMed] [Google Scholar]
- 78.Haug S, Braun-Falco M. Adeno-associated virus vectors are able to restore fatty aldehyde dehydrogenase-deficiency. Implications for gene therapy in Sjogren-Larsson syndrome. Arch Dermatol Res. 2005;296(12):568––72.. [DOI] [PubMed] [Google Scholar]
- 79.Haug S, Braun-Falco M. Restoration of fatty aldehyde dehydrogenase deficiency in Sjögren-Larsson syndrome. Gene Ther. 2006;13(13):1021-–1026. [DOI] [PubMed] [Google Scholar]
- 80.Christensen R, Jensen UB, Jensen TG. Skin genetically engineered as a bioreactor or a ‘metabolic sink’. Cells Tissues Organs. 2002;172(2):96–104. doi: 10.1159/000065612 [DOI] [PubMed] [Google Scholar]
- 81.Hewett DR, Simons AL, Mangan NE, et al. Lethal, neonatal ichthyosis with increased proteolytic processing of filaggrin in a mouse model of Netherton syndrome. Hum Mol Genet. 2005;14(2):335–346. doi: 10.1093/hmg/ddi030 [DOI] [PubMed] [Google Scholar]
- 82.Kuramoto N, Takizawa T, Takizawa T, et al. Development of ichthyosiform skin compensates for defective permeability barrier function in mice lacking transglutaminase 1. J Clin Invest. 2002;109(2):243–250. doi: 10.1172/JCI0213563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim Y-S, Johnson IM, S’Aulis D, Rizzo WB, Nienhuis AW. 166. Lentiviral hematopoietic stem cell gene therapy for sjögren-larsson syndrome. Mol Ther. 2016;24:S65. doi: 10.1016/S1525-0016(16)32975-6 [DOI] [Google Scholar]