Abstract
There is a need for effective wound treatments that retain the bioactivity of a cellular treatment, but without the high costs and complexities associated with manufacturing, storing, and applying living biological products. Previously, we developed an amnion membrane‐derived hydrogel and evaluated its wound healing properties using a mouse wound model. In this study, we used a full thickness porcine skin wound model to evaluate the wound‐healing efficacy of the amnion hydrogel and a less‐processed amnion product comprising a lyophilized amnion membrane powder. These products were compared with commercially available amnion and nonamnion wound healing products. We found that the amnion hydrogel and amnion powder treatments demonstrated significant and rapid wound healing, driven primarily by new epithelialization versus closure by contraction. Histological analysis demonstrated that these treatments promote the formation of a mature epidermis and dermis with similar composition to healthy skin. The positive skin regenerative outcomes using amnion hydrogel and amnion powder treatments in a large animal model further demonstrate their potential translational value for human wound treatments.
Keywords: amnion membrane, amnion powder, extracellular matrix, hydrogel, skin, wound
1.
Significance statement.
This study demonstrates the efficacy of amnion hydrogel and amnion powder wound healing products in a large animal model. This further demonstrates their potential translational value for human wound treatments.
2. INTRODUCTION
Despite current standard of care treatments, extensive burns and full thickness skin wounds are devastating to patients. The current clinical gold standard treatment utilizes an autologous split‐thickness skin graft and involves removing an area of skin from the patient, often increasing the skin surface area through meshing, and reapplying the graft on the wound or burn.1, 2, 3, 4 Although this treatment yields a reasonable clinical outcome, the availability of patient graft samples is significantly limited for extensive wounds or burns.5 The requirement of immunosuppressive drugs to avert immune rejection of allographs eliminates this approach as a viable long‐term treatment option for many patients.6, 7 These limitations led to the development of noncellular dermal substitutes, which are often comprised of scaffolds of synthetic or natural materials, such as silicone and collagen (eg, Integra and Biobrane). This category also includes natural extracellular matrix (ECM)‐based tissue scaffolds derived from tissues such as human or porcine skin, small intestinal submucosa, urinary bladder matrix, and decellularized liver matrix. One such example is Graftjacket Regenerative Tissue Matrix, which is comprised of a decellularized human dermis matrix, freeze‐dried to remove moisture while preserving biologic components and structure of the dermal matrix.8 Although dermal substitutes often result in improved wound healing over controls,8, 9, 10 they can be costly to produce and result in relatively poor cosmetic outcomes. Tissue engineering approaches have developed more complex biological skin equivalents, predicted to yield more suitable wound treatment options for patients (eg, Dermagraft, Apligraf, and TransCyte). These products are generally comprised of a polymer scaffold patch seeded with human skin cells such as fibroblasts and/or keratinocytes. Although some of these products have been shown to promote wound closure and more rapid healing of chronic diabetic foot ulcers when compared with standard therapy,11 they are also expensive to produce, require complex storage, thawing, and application methodologies, and pose risks for immune rejection. Therefore, there remains a significant need for a pro‐regenerative wound‐healing product with: (a) proven clinical efficacy, (b) nonliving, room temperature stable components, and (c) retained bioactivity.
Amnion membranes have been successfully used for wound and reconstructive purposes since the early 20th century.12 In 1910, Davis was the first to report the use of amnion membranes as surgical material in skin transplantation.13 Since these early findings that identified the amnion membrane as a potent wound healing tool, the amnion membrane has become relatively widespread as an application for wound covering, ocular surface disorders, diabetic neurovascular ulcers, venous stasis ulcers, and various types of postsurgical and post‐traumatic wound dehiscence.14, 15, 16, 17, 18, 19 The use of amnion membranes for skin burn management and treatment has been reported in over 200 clinical trials. In a prospective clinical trial, amnion membrane was used as a wound covering for burn wounds treated with split thickness skin grafts.20 That study included patients with symmetric chronic burn wounds in upper or lower limbs. After debridement of granulation tissue and meshed split thickness skin grafting, the graft surfaces of the right limb wounds were covered with amnion membrane dressing, while conventional skin grafting was performed on the left limb wounds. That study concluded that human amnion membrane dressing significantly increased graft take in these wounds. Branski et al. compared amnion membrane application for children with partial‐thickness facial burns to standard topical treatment.21 Participants were divided into two groups and received either amnion membrane or topical antimicrobials. The results indicated that amnion membrane is safe and advantageous for wound coverage compared with the standard topical ointments. In a study by Ravishanker et al., glycerol‐preserved amnion membranes were used for treating superficial and superficial thickness burns.22 The study reported complete healing of superficial partial burns within 7‐10 days with the same successful result in mid‐dermal burns within 20 days. Multiple clinical trials describe similar results with various amnion preparation and application methodologies23, 24, 25 and there are multiple amnion‐based commercial products, such as AmnioGraft—a cryopreserved amniotic membrane sheet, Epifix—dehydrated human amnion/chorion membrane sheet, and NuCel—a cryopreserved, liquid suspension derived from human amnion and amniotic fluid cells, among others.
Although the use of amnion membrane in these clinical settings is clearly advantageous, the material has been difficult to incorporate into routine clinical use. Limitations include difficulty of handling the thin amnion membrane sheets without folding or tearing and the requirement for sutures or adhesives to hold the membrane in place over the wound. Additionally, similar to current tissue‐engineered skin substitutes, the transportation and storage of the living, cellular tissue introduces unwanted complexity and costs for routine clinical applications. Thus, our goal has been to develop an amnion‐based wound‐healing product, which does not require a living cellular component, but retains the bioactivity and clinical efficiency of a living cellular product. We have previously described the development and wound healing efficacy of an amnion hydrogel, comprising a hyaluronic acid‐based hydrogel combined with solubilized amnion membrane (HA‐SAM).26 In the current study, we have evaluated the wound‐healing efficacy of the amnion hydrogel was compared with a lyophilized amnion membrane powder in a full thickness skin wound model. The performance of these amnion‐based products was also compared with two commercially available products, AmnioGraft (a cryopreserved amniotic membrane sheet) and Graftjacket (a decellularized human dermis graft). These commercial products were chosen to allow for the evaluation of potential therapeutic differences resulting from the matrix source, processing technique and form factor.
3. MATERIALS AND METHODS
3.1. Experimental animals and regulations
This study was reviewed and approved by the Wake Forest University Institutional Animal Care and Use Committee (A13‐015—Amnion Products for Wound Healing in Pigs). Six specific pathogen free Yorkshire pigs were purchased and allowed to acclimatize for the required 2‐week period. At the start of the study, the pigs weighed approximately 40‐50 kg. Prior to surgery, the animals were trained to tolerate the plastic saddle with animal crackers providing a distraction and incentive for human contact. First, the animals were acclimated to the odor of the plastic. Once acclimated, the saddle was placed on the animal under supervision for a period of time, gradually increasing the time until the animal was comfortable wearing the saddle for the duration of the study.
3.2. Full thickness wound creation
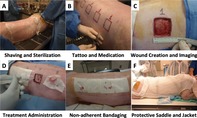
The animals were sedated and anesthetized with a combination of ketamine, xylazine, and acepromazine, and maintained under anesthesia using inhaled isoflurane via an endotracheal tube. The anesthetized pigs had their backs depilated by shaving. The animals were immobilized and placed in a dorsal position. The animals were tattooed with eight 4 cm × 4 cm squares on the dorsum to denote the area of the excisional wound. The dorsal skin was cleaned with water and soap, and sterilized with β‐iodine and 70% alcohol. For the creation of the defect, eight areas of skin wound were created by removing 4 cm × 4 cm of full thickness skin in the central back along the thoracic and lumbar area. Incisions were made along the wound edges with a surgical blade to the panniculus carnosus layer and the overlying skin was excised (Figure 1A‐C).
Figure 1.
Porcine animal model wound creation and treatment strategy. Visual description of the steps involved in the preparation, wound creation, treatment administration, and protective bandaging of animals in this study. A, The anesthetized pigs had their backs depilated by shaving. B, The animals were tattooed with eight 4 cm × 4 cm squares on the dorsum to denote the area of the excisional wound. C, For the creation of the wounds, eight areas of 4 cm × 4 cm full thickness skin was removed in the central back along the thoracic and lumbar area. D, Wounds were immediately treated either with commercially available products according to the manufacturers' instructions, with hydrogel only, or with amnion‐derived products; an additional group underwent no treatment. E, The wound areas were covered by dressing materials to provide a protective barrier. F, The materials included a topical antibiotic cream, Tegaderm, cast padding, cohesive bandaging, stockinette, a protective plastic saddle, and finally a specially designed jacket to hold the bandage in place
3.3. Product description and application
Wounds were immediately treated either with commercially available products according to the manufacturer's instructions, with hydrogel only, or with amnion‐derived products, an additional group underwent no treatment (Figure 1D). The six experimental groups were: (a) untreated other than standard of care bandaging, (b) amnion powder, (c) amnion hydrogel (HA‐SAM), (d) AmnioGraft, (e) Graftjacket, or (e) HA hydrogel only.
3.3.1. Amnion powder
Placentae were collected from consented donors by hospital personnel and placed into a sterile collection bag. Placental tissue was washed with saline to remove blood clots from the membrane. The amniotic membrane was then removed by manual dissection and/or cutting with scissors. The amnion was cut into pieces approximating 5 cm × 5 cm then placed into a series of five saline washes and a final brief wash with water. The tissue was placed into sterile conical tubes and frozen at −80°C. Frozen tissue was transferred into glass lyophilizer flasks and lyophilized, before the undergoing cryomilling. The amnion tissue was then aliquoted into sterile type I borosilicate clear serum glass vials and sterilized by γ irradiation using a dose of 10‐12 kGy. Following irradiation, the product was be stored at −80°C until use.
3.3.2. Amnion hydrogel (HA‐SAM)
HA‐SAM was prepared as previously described.26 Briefly, 220 mg of sterile amnion powder and 22 mg of pepsin was added into a 15 mL tube with 10 mL of sterilized 0.01 N HCl. The materials within the tube were then mixed, allowing digestion for 48 hours at 37°C. The digest was centrifuged at 4000g (4500 rpm) for 10 minutes. The supernatant was removed and placed in another 15 mL tube. The solution (SAM) was neutralized with NaOH to a pH of 7. Next, Heprasil, Gelin‐S, and extralink were then mixed in a 2:2:1 ratio by volume, and SAM solution was incorporated into the hydrogel solution at a 1:1 ratio prior UV crosslinking. For crosslinking, HA‐SAM hydrogels were irradiated with UV light (365 nm, 18 W/cm2) at a distance of 3 cm to initiate a thiol‐ene stepwise crosslinking reaction.
3.3.3. AmnioGraft
AmnioGraft is a commercially available human amniotic membrane that is designated by the Food and Drug Administration (FDA) as a human cell/tissue product for use as a homologous graft for ocular wound repair and healing. Current indications for AmnioGraft include a variety of corneal and conjunctival diseases and conditions, such as pterygium, Stevens–Johnson syndrome, and chemical burns. AmnioGraft is processed in a Dulbecco's modified Eagle's medium/glycerol (1:1) storage medium containing ciprofloxacin and amphotericin B. The manufacturers claim that the processing method, in which AmnioGraft is cryopreserved at −80°C is performed to kill the cells present and eliminate the possibility of graft rejection. Although not currently indicated for skin wound healing, AmnioGraft was chosen as a comparator due to it being a minimally manipulated amnion sheet, providing a useful amnion‐based comparator to the experimental amnion products developed by our group. Other than the application of this product for skin wounds rather than ocular wounds, the product was prepared and applied according to manufacturer's instructions. This involved removing the white nitrocellulose paper backing from the “sticky” stromal side of the membrane, and placing the stromal side of the AmnioGraft down on to the wound surface.
3.3.4. Graftjacket
Graftjacket regenerative tissue matrix is donated allograft human dermis, aseptically processed to remove cells and freeze‐dried to remove moisture while preserving biologic components and structure of the dermal matrix. The manufacturers claim that this processing method creates a collagen‐rich, noncytotoxic matrix, which retains many of the ECM and growth factor components. Graftjacket is regulated by the US FDA as human tissue for transplantation and is indicated for augmentation for soft tissue repairs. Graftjacket was selected for this study due to reports of its successful use in the management of a wide range of wounds, from diabetic foot ulcers to rotator cuff repairs.8, 27, 28 Graftjacket was prepared and applied according to manufacturer's instructions, which included and application to the wound bed with the dermal side placed against the wound bed with the basement membrane side facing up.
3.3.5. Hydrogel only
Hydrogel only treatments comprised of the heprasil, Gelin‐S, and extralink prepared as above for the amnion hydrogel (HA‐SAM), but with SAM solution replaced with sterile saline, incorporated into the HA hydrogel solution at a 1:1 ratio prior UV crosslinking. Hydrogel only treatments were applied identically to the amnion hydrogel (HA‐SAM) product application.
All treatments were applied as a single application at the time of wound creation and received standard of care bandaging, which is described below. The six experimental options were distributed over the eight skin defects to control for differences in wound locations, resulting in a total of eight applications of each product. Each experimental round ran for 28 days, during which the wounds were inspected two times each week for: (a) documentation of wound size, re‐epithelialization, and closure, and (b) cleaning and administration of antibiotics, and (c) rebandaging.
3.4. Bandaging
After treatment application and for scheduled bandage changes, the wound area was covered by dressing materials to provide a protective barrier. The materials included a topical antibiotic cream, Tegaderm, cast padding, cohesive bandaging, stockinette, a protective plastic saddle, and finally a specially designed jacket to hold the bandage in place. Edges of the saddle were covered with porous surgical tape along the front of the saddle to protect the pig's neck. Four Velcro straps were used to position a stretchable strap on the saddle as a means to secure it to the pig (Figure 1E,F).
3.5. Euthanasia
At 28 days post‐treatment, the study was terminated and the wound areas harvested. Animals were sedated with ketamine, xylazine, and acepromazine. The animal was then brought to the necropsy room. A lethal overdose of pentobarbital euthanasia solution was given by intravenous injection. Wounds were divided for histology and immunohistochemistry analysis.
3.6. Image analysis
Digital photos were taken for each of the time points (days 0, 4, 7, 11, 14, 18, 21, 24, and 28) and compiled for each of the wounds. Standardized, quantitative digital image analysis was performed, based on Molnar et al.29 Wound closure and epithelialization was measured by using ImageJ to determine the area of open wound and epithelialization, which can be identified by color and texture of the healing wound (Supporting Information Figure S1). Generally, open wounds were dark red and shiny, and epithelium ranged from light red to pink/white and was generally opaque/matte. Contraction is a relative value measuring the area inside the tattoo square against the original tattoo size at each time point using ImageJ. A wound contraction ratio was used as an additional means to describe contracted wounds. In addition to the wound contraction measurement, the contraction ratio describes the extent of which the wound has changed shape from the initial square wound. Wounds healing by re‐epithelialization normally maintain their initial square shape while contracting slightly and symmetrically. Highly contractile wounds, however, usually contract from the edges of the wound, resulting in a 4‐pointed star‐shaped wound.30 These components were expressed as individual measurements relative to the original wound size, after which they were combined to demonstrate the contribution of all components to the wound healing over time.
3.7. Histology
Immediately after euthanasia, wound areas were surgically removed by excising a full‐thickness area at least 1 cm outside the tattoo boundary. The tissue was then divided into quarters and labeled for histology to identify the outer edge healthy tissue side (outside tattoo area) and the central regenerated wound side of the skin tissue. Wound sections were fixed for approximately 48 hours in 4% paraformaldehyde, after which the samples were transferred to 70% ethanol prior to paraffin processing. A microtome (Leica) was used to generate 4‐6 μm sections comprised of the center and edge portions of the regenerating wounds. Sections were stained with either hematoxylin and eosin (H&E), pentachrome, picrosirius red, or iron colloid stains for histology, and slides were imaged by light microscopy. For picrosirius red stained sections, an additional polarized filter was used. Examination criteria included degree of epidermal formation and extent of epithelialization, dermal organization, evaluation of ECM composition and organization, in comparison with normal undamaged skin sections.
3.8. Pathology
Representative lesions from each of the groups were examined by a board‐certified veterinary pathologist (N.D.K.), in a blinded fashion with respect to treatment group. All lesions were identified by major process, distribution, and if possible time course, and graded as within normal limits, minimal, mild, moderate, or marked.
3.9. Statistical analysis
Data were expressed for each experimental group as mean ± SD and statistical significance determined using statistical analysis software (GraphPad Prism, Graphpad Software, Inc.). Mixed models analysis of variance techniques were used to compare outcomes. Within‐animal variability was modeled using a compound symmetry structure. Models included the factors TREATMENT, DAY, and a TREATMENT × DAY interaction. A confidence interval of 95% assumes significance.
4. RESULTS
4.1. Wound treatment and product application
Full thickness wounds were created with a uniform size and depth. Representative images of wounds and treatments for each of the time points (days 0, 7, 14, 21, and 28) are shown in Figure 2. Generally, wound treatment products were easy to administer, although AmnioGrafts thin and fragile nature required that this product to be handled by two surgeons to remove the protective backing material and prevent inopportune folding. Graftjacket was easier to administer; however, the size variation of this product paired with the products thickness and positioning on the wound made suturing difficult. The amnion hydrogel was easy to apply by one person and did not require any contact with the wounds. Amnion hydrogel precursor liquid was applied with a hand held syringe whereas the other hand held a UV light approximately 5 cm above the wound surface. As expected, the liquid rapidly filled the wound bed and gelatinized within several seconds. Identical methods were used to apply the hydrogel without the amnion component. Amnion powder was the easiest overall product to administer. A controlled measured dose of powder was distributed evenly by tapping the vial gently over the wound before applying 1 mL of sterile saline to wet the product.
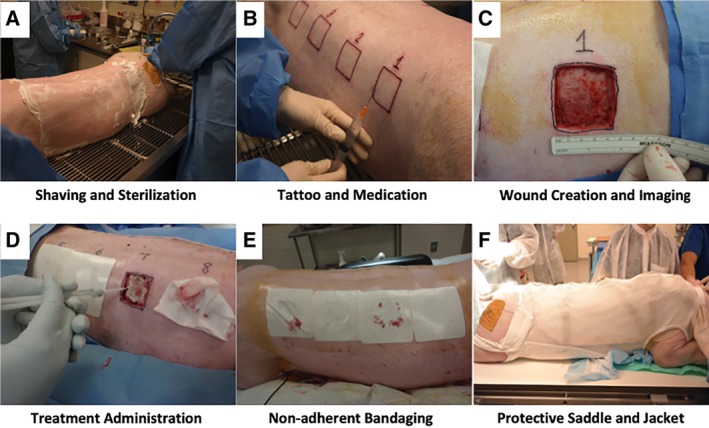
Figure 2.
Images of each wound treatment over 28 days. Digital photos were taken for each of the time points (days 0, 7, 14, 21, and 28) and compiled for each of the wounds. Amnion powder and amnion hydrogel treatments were easy to administer to full thickness wounds and resulted in the most rapid wound closure rates driven primarily by new epithelialization
4.2. Wound area
For each time point, the percentage of wound area present relative to the original wound area was measured to describe wound closure. These measurements are shown combined and separately in Figures 3A and 4, respectively. At the day 4 time point following wound treatment, there was an initial average increase of the wound area for the untreated (108.2% of original), Graftjacket (111.7% of original), and AmnioGraft‐treated (112.3% of original) wounds due to additional wound stretching and drooping, suggesting that application of these treatments do not immediately stabilize the wound area at days 4 and 7. In contrast, amnion hydrogel (99.3% of original) and amnion powder (95.1% of original) better stabilized the wound areas.
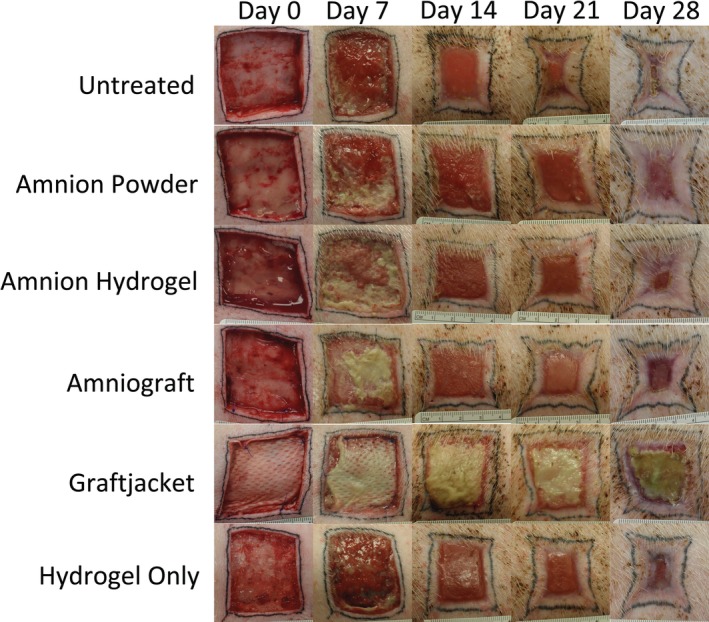
Figure 3.
Individual parameter wound image analysis. Individual plots for (A) wound area, (B) total epithelialization, (C) contraction, and (D) contraction ratio over 28 days. Based on individual characteristics, amnion powder and amnion hydrogel were the best performing treatments, with an average of 0.8% and 0.9% wound area, 98.6% and 98.7% wound epithelialization, 34.5% and 41.9% wound contraction, and contraction ratios of 0.14 and 0.12, respectively, at day 28
Figure 4.
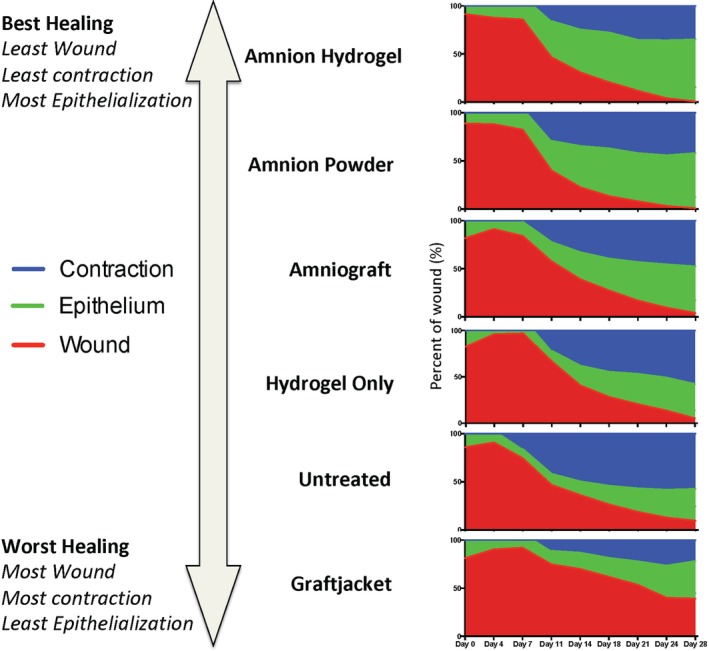
Graphs showing a combination of each individual component of the wound area during healing. Combined wound healing analysis represents the total wound area at each time point divided into the percentage of wound area (red), percentage contraction (blue), and percentage epithelialization (green). With these analyses describing the healing wound over time, we can observe wound closure via competing parameters of contraction and epithelialization for each treatment. The best performing treatments were categorized has having the following: (a) small wound area, (b) least contraction, and (c) most epithelialization; the worst performing treatments had the opposing characteristics. Amnion powder and amnion hydrogel were the best performing products using the above criteria. These wound treatment products demonstrated the most rapid and complete closure of the wounds driven by increased epithelialization and minimal contraction
The amnion hydrogel‐treated group showed a lowest percentage wound area during the study time course, with significant improvement compared with hydrogel‐only treated wound on day 11 (44.7% vs 80.9%), day 14 (25.3% vs 49.5%), and day 18 (15.0% vs 34.5%; P < .05). Amnion powder‐treated groups showed the next lowest percentage wound area during the study time course, with significantly lower wound area compared with untreated wounds at day 18 (22.5% vs 32.4%) and day 24 (3.6% vs 14.1%; P < .05). By day 28, wounds treated with amnion hydrogel demonstrated an average of 0.9% percentage wound area and amnion powder treated wounds had an average of 0.8% percentage wound area. AmnioGraft‐treated wounds showed a percentage wound area comparable to the untreated and hydrogel only‐treated wounds, showing no significant differences between these treatments while performing worse than amnion hydrogel‐treated groups at day 11, 14 (P < .001), 18, and 21 (P < .05), and worse than amnion powder‐treated groups at day 4, 14, 18, and 21 (P < .05). Hydrogel only‐treated groups appeared to show a slight improvement over untreated wounds for days 7 and 11 (P < .05); however, this effect was not sustained. Graftjacket‐treated wounds demonstrated the slowest rate of wound closure, showing significantly greater percentage wound area remaining at all time points after day 14, compared with all other groups (P < .0001). The applied Graftjacket product formed a dry, hard scab‐like structure, potentially due to the mismatch between product integration and underlying wound healing and/or the need for hydrogel wound hydration for external applications. This scab‐like structure paired with slow product degradation resulted in poor closure of with a percentage wound area average of only 47.9% by day 28.
4.3. Wound epithelialization
Epithelialization was measured at all time points with epithelium defined visually by the pink/white color and presence of a matte appearing epithelial coating distinct from the wound area. The area within the tattoo borders of epithelialization was measured, and the area of wound subtracted. These measurements are shown combined and separately in Figures 3B and 4, respectively. All tissues initially had some level of epithelialization due to the incisional wound being created inside the tattoo line that was consistent over all treatments and pigs. Amnion hydrogel and amnion powder‐treated wounds were the best performing for total wound epithelialization. At days 11, 14, 18, 21, 24, and 28, amnion hydrogel and amnion powder treatment groups showed significantly greater levels of epithelialization than untreated (P < .001), hydrogel only (P < .001), Graftjacket (P < .001), and AmnioGraft‐treated wounds (P < .05). By day 28, amnion hydrogel and amnion powder treated wounds demonstrated a final total epithelialization of an average 98.6% and 98.7%, respectively. AmnioGraft‐treated wounds showed a moderate level of early wound epithelialization (average of 42.1% at day 14) and an average epithelialization of 92.2% at the end of the study. Untreated, hydrogel‐only, and Graftjacket‐treated wounds all showed the least wound epithelialization, with a very slow onset of epithelialization (average of 28.1%, 34.6%, and 20.1%, respectively, at day 14) and a low total epithelialization 79.9%, 86.9%, and 51.5%, respectively, by day 28.
4.4. Wound contraction
For each time point, the area within the tattoo border relative to the original tattoo area was expressed as a percent to measure the wound contracture. These measurements are shown combined and separately in Figures 3C and 4, respectively. As with the wound closure data, we observed an initial increase in the wound/tattoo area over the first 7 days resulting in an initial negative contraction percentage of an average of −14%. Graftjacket's stiff, scab‐like structure prohibited wound closure and thus, resulted in the least amount of wound contracture, with an average of only 21.8% contraction by day 28. Amnion powder‐treated wounds demonstrated an average of only 19.3% contraction by day 14 and 36.3% contraction by day 28. Wounds treated with amnion powder contracted an average of 24.1% less than untreated wounds by day 28 (P < .001) and an average of 21.5% less than hydrogel only‐treated wounds by day 28 (P < .05). Amnion hydrogel and AmnioGraft‐treated wounds showed a delayed increase in contraction and reduced contraction by day 14 (average contraction of 34.5% and 32.8%, respectively); at the final time point these products showed 41.9% and 47.5% contraction, respectively. By day 28, wounds treated with the amnion hydrogel contracted 18.5% less than untreated and 15.9% less than hydrogel only‐treated wounds (P < .05). Hydrogel only‐treated wounds showed slightly less average contraction of 37.9% at day 14%, and 57.8% by the end of the study. Untreated wounds showed the most contraction, with rapid 50.6% contraction of the original tattoo area occurring by day 14. At the final time point, these wounds showed an average of 60.4% contraction and were consistent with our observations throughout the study.
4.5. Wound contraction ratio
To provide an additional measure of contraction we performed several measurements of the wounds over all time points (Figure 3D). The distance of the left edge of the wound to the center point between the two left wound corners was measured and expressed as a ratio of the total distance between these corners. This ratio provides an approximate description of the amount of wound shape change. Noncontracted square wounds have a ratio close to 0, whereas highly contracted “star” shaped wounds have a ratio close to 0.2. Confirming the observations of the minimal contraction of Graftjacket‐treated wounds, these wounds only had a minimal increase of contraction ratio to an average of 0.02. Amnion powder, amnion hydrogel, and hydrogel only‐treated wounds all increased slightly, with contraction ratios of 0.14, 0.12, and 0.11, respectively. Confirming the contraction data, the contraction ratio of untreated wounds and AmnioGraft‐treated wounds was 0.18 and 0.17, respectively, at day 28, which accurately represents the contracted “star” shape of the wounds.
4.6. Combined analysis of wound area, contraction, and epithelialization
Vital information is gained from analyzing individual components of wound healing parameters such as wound closure, contraction, and epithelialization. However, a combined analysis of the wound during healing can better illustrate the overall wound healing quality and the relative contributions of each parameter. In this study, we represented this combined wound healing analysis by representing the total wound area at each time point divided into the percentage of wound area (red), percentage contraction (blue), and percentage epithelialization (green; Figure 4). With these analyses describing the healing wound over time, we can observe wound closure via competing parameters of contraction and epithelialization for each treatment. These data highlight the importance of the type and quality of wound healing. The best performing treatments were categorized has having the following: (a) small wound area, (b) least contraction, and (c) most epithelialization; the worst performing treatments had the opposing characteristics. Graftjacket‐treated wounds demonstrated minimal contraction and very little epithelialization resulting in a persistent open wound. Therefore, Graftjacket ranked last of the products tested. Hydrogel only and untreated wounds grouped together as the next best performing products. Untreated wounds had a similar rate and extent of wound closure compared with hydrogel only treated wounds, hydrogel only‐treated wounds had less contraction. AmnioGraft‐treated wounds, demonstrated a faster rate of wound closure driven by approximately 50% contraction and 50% epithelialization. Amnion powder and amnion hydrogel were the best performing products using the above criteria. These wound treatment products demonstrated the most rapid and complete closure of the wounds driven by increased epithelialization and minimal contraction.
4.7. Histological analysis
H&E stained sections were used to evaluate and compare the general structure and composition of each of the wounds. Representative images are shown in the first row of Figure 5. Healthy pig skin was used as a control for this analysis. Analysis of wound histology showed that most wounds had some degree of epidermal covering with the exception of Graftjacket. Graftjacket‐treated wounds generally had an exposed dermis or were covered with a variably deep scab‐like tissue. Untreated, hydrogel only, and AmnioGraft‐treated wounds appeared to have inconsistent coverage, a thinner epidermis, and lacked noticeable epithelial rete peg protrusions into the dermal area. The epidermis from amnion hydrogel and amnion powder‐treated wounds appeared similar to healthy skin with regard to the coverage, thickness, and presence of rete pegs. This analysis confirmed that amnion hydrogel and amnion powder‐treated wounds had a very mature appearing epidermis.31
Figure 5.
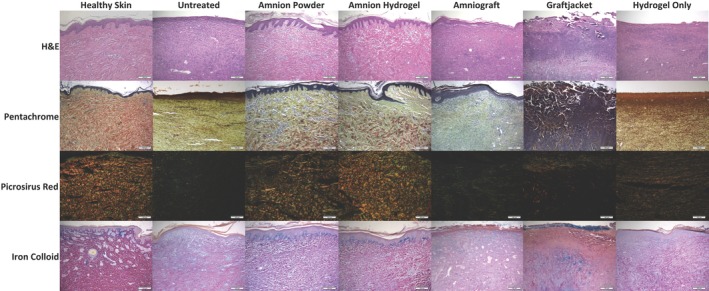
Representative histological images for hematoxylin and eosin (H&E), pentachrome, picrosirius red, and iron colloid staining. H&E staining (row 1) demonstrated that amnion hydrogel and amnion powder had an epidermis very similar to that observed in healthy pig skin. Amnion hydrogel and amnion powder also had a very similar dermis organization compared with healthy skin. Untreated, hydrogel only, and AmnioGraft‐treated inconsistent and thinner epidermis, lacking noticeable epithelial rete peg protrusions. Hydrogel only‐treated wounds showed increased presence of darker‐stained larger fibers within the epidermis, but with minimal organization. Graftjacket‐treated wounds generally had an exposed dermis or were covered with a variably deep scab‐like tissue. Pentachrome staining (row 2) demonstrated that amnion hydrogel and amnion powder showed similar organized staining for collagen (yellow) and mature fibers (red) compared with healthy skin. Amnion hydrogel, amnion powder, AmnioGraft also showed mucins/GAGs staining (green). Untreated, hydrogel only, and Graftjacket‐treated tissues show primarily densely‐packed nuclei, yellow collagen staining, with some green staining for mucins and glycosaminoglycans. Graftjacket‐treated wounds were very cellular and showed little observable red, green, or yellow staining. AmnioGraft‐treated wounds demonstrated minimal red stained fibroid/muscle staining further indicating a lack of normal mature dermis structure. Picrorsirius red staining viewed with polarized light (row 3) confirmed observations from the pentachrome staining, demonstrating that amnion hydrogel and amnion powder‐treated wounds showed similar organized staining for mature collagen (red) and immature collagen (green) compared with healthy skin. Untreated and Graftjacket‐treated wounds did not stain positive for organized collagen structures. Hydrogel only‐treated wounds showed a moderate amount of positive staining for green and orange‐stained collagen, but significantly less than observed in healthy skin. Iron colloid staining (row 4) was performed to visualize Mucins/GAGs. Amnion hydrogel and amnion powder‐treated wounds showed intense iron colloid staining directly under the epidermis similar to healthy skin, whereas Graftjacket showed some intense staining under the inflamed scab area, as well as at the surface of the scab. Untreated, AmnioGraft, and hydrogel only‐treated tissues showed absent or minimal diffuse blue staining
The dermis of healthy skin consisted of large organized fibers that were light pink in color with only minimal disorganized, thin, purple fibers. The dermis of Graftjacket‐treated wounds appeared to contain many neutrophils and fibroblasts by morphological appearance. This transitioned into a thick fibrous tissue at the surface of the wound. Untreated wounds did not show organization of the dermal fibers and consisted completely of small, purple unorganized fibers. AmnioGraft‐treated wounds had some unorganized large pink fibers present in the dermis but this was inconsistent between wounds and within individual wounds; some areas were dominated by the small, purple unorganized fibers. Hydrogel only‐treated wounds showed increased presence of darker‐stained larger fibers within the epidermis, but with minimal organization. Amnion hydrogel and amnion powder‐treated wounds both appeared to have a dermis very similar to that of healthy skin, with highly organized fibers that were light pink in color, and with minimal unorganized small fibers. Overall, it appears that histologically, amnion hydrogel and amnion powder‐treated wounds looked most similar to healthy skin. Untreated and AmnioGraft wounds all appeared to be progressing toward normal healing but had an immature epidermis and unorganized dermis. Grafjacket‐treated wounds appeared inflammatory and did not show signs of a developing epidermis or mature‐appearing dermis.
Pentachrome straining was performed to give an overview of the ECM composition of the wounds. Pentachrome staining allows the identification of multiple ECM components simultaneously. Pentachrome staining stains collagen yellow, mature fibers red, mucins and glycosaminoglycans (GAGs) blue/green, and nuclei and elastic fibers black. The second row of Figure 5 shows the representative images of the treatment groups and healthy skin. In this study, the overlapping/close proximity of collagen and mucins/GAGs resulted in a green coloration of the tissues. Healthy skin had a dermis that consisted of thick mature collagen fibers (red) and positive yellow and green staining for collagen and mucins/GAGs. Very little black elastin or purple inflammatory or fibrotic cell infiltration was observed. Untreated and hydrogel only and Graftjacket‐treated tissues show primarily densely packed nuclei. Untreated and hydrogel only‐treated wounds showed yellow collagen staining, with some green staining for mucins and glycosaminoglycans. Graftjacket‐treated wounds were very cellular and showed little observable red, green, or yellow staining. This suggests that these areas may consist of granular tissue composed of infiltrating inflammatory cells and fibroblasts. AmnioGraft‐treated wound tissues show some collagen and mucins/GAGs staining but little red stained fibroid/muscle staining further indicating a lack of normal mature dermis structure. Amnion hydrogel and amnion powder‐treated wounds appeared most similar to healthy skin with an intermediate amount of thick mature collagen fibers (red) on a yellow/green background of collagen, mucins, and GAGs. This suggests that these tissues are of similar composition to healthy skin even with slightly less mature collagen fibers and more mucins/GAGs.
Picrosirius red staining differentiates between immature and mature collagen when viewed under polarized light. Immature unorganized collagen stains green and mature bundled and organized collagen fibers stain yellow/orange. Row three of Figure 5 shows the representative images of the treatment groups and healthy skin. Healthy skin shows a strong staining of both green and orange, indicating an organized network of larger mature collagen fibers with smaller immature collagen fibers intermixed. Graftjacket‐treated wounds showed very little green staining, except within the scab region. Conversely, there is positive staining for orange‐stained mature collagen. However, staining was localized to small areas sporadically arranged throughout the tissue. Untreated and AmnioGraft‐treated groups, appeared mostly green, and displayed little to no orange stain, further indicating slower ECM regeneration. Hydrogel only‐treated wounds showed a moderate amount of positive staining for green and orange‐stained collagen, but significantly less than observed in healthy skin. The amnion hydrogel treated‐wounds appear similar to the healthy skin, showing a similar distribution of orange and green. The amnion powder treated‐wounds appear to have slightly less orange‐stained organized mature collagen, but did show a notable staining intensity and distribution similar to healthy skin.
Iron colloid staining shows carboxylated and sulfated mucins and GAGs in blue and cytoplasm in pink/red. Additionally, dark red staining of the organized dermis tissue was apparent in the healthy skin. Row four of Figure 5 shows the representative images of the treatment groups and healthy skin. Graftjacket‐treated wounds showed blue staining in the scab but lacked organization below the scab. Untreated, AmnioGraft, and hydrogel only‐treated tissues showed absent or minimal diffuse blue staining. The amnion hydrogel and amnion powder‐treated wounds showed similar localization and intensity of blue staining compared with the healthy skin—specifically, blue staining directly under the epidermis. Similar dark red staining, but to a lesser degree than in healthy skin, was also observed in the amnion hydrogel and amnion powder groups. Less of this red staining was observed in the hydrogel only‐treated group with little to none of this red staining observed in the untreated, AmnioGraft, and Graftjacket‐treated groups.
4.8. Pathology findings
4.8.1. Untreated
Approximately half of a section of haired skin has regions of ulceration, with superficial dermal infiltration by mixed‐type inflammatory cells, mostly mononuclear cells and fewer neutrophils. This blends into a mature collagenous scar, which extends to the subcutis and effaces the adnexal units.
Diagnosis
Dermatitis, diffuse, chronic, moderate with extensive dermal fibrosis.
Interpretation
The wound appears to be healing but residual inflammation is superficially in the dermis.
4.8.2. Amnion powder
The epidermis over a section of haired skin is intact and of uniform, normal thickness throughout. Dermal adnexal units are within normal limits and evenly spaced. Inflammation is not present.
Diagnosis
Essentially normal skin.
Interpretation
Skin within normal limits.
4.8.3. Amnion hydrogel
The epidermis of a section of haired skin is intact and has evenly spaced normal appearing adnexal units in the dermis except for about a quarter of the section in which the dermis is replaced by mature collagenous connective tissue, which extends into the subcutis. Adnexal units and inflammation are absent in this region.
Diagnosis
Fibrosis, focal, moderate, dermis, haired skin.
Interpretation
The wound appears to have completely healed.
4.8.4. AmnioGraft
Approximately half of a section of haired skin has an ulcerated surface, with superficial infiltration by mixed‐type inflammatory cells, mostly mononuclear cells and fewer neutrophils. This blends into a mature collagenous scar, which extends into the subcutis, and effaces the adnexal units. Four 2‐3 mm diameter regions of pyogranulomatous inflammation (many neutrophils and few multinucleated giant cells) are present within the scar, one of which is associated with linear foreign material. The adjacent intact epidermis is mildly hyperplastic.
Diagnosis
Dermatitis, focal, extensive, chronic, moderate, ulcerative with dermal pyogranulomas with intralesional foreign material.
Interpretation
Wound in the process of attempted healing but not complete yet. The inflammation is not severe but the ulceration is extensive. The granuloma formation in the dermis appears to be associated with foreign material, the nature of which is uncertain.
4.8.5. Graftjacket
Approximately three‐quarters of a section of haired skin is absent (either ulcerated or missed in sectioning) and the dermis, nearly to the level of the subcutis, is infiltrated by variably sized round accumulations of mixed type‐inflammatory cells, mostly mononuclear cells separated by collagenous connective tissue, all of which efface adnexal units. The epidermis adjacent is slightly hyperplastic.
Diagnosis
Dermatitis, multifocal, chronic, severe.
Interpretation
The epidermis over the wound is absent, either ulcerated or missed in sectioning. The dermal inflammation is mostly mononuclear and appears nodular.
4.8.6. Hydrogel only
Approximately half of a section of haired skin has an ulcerated surface, with superficial infiltration by mixed‐type inflammatory cells, mostly mononuclear cells with occasional neutrophils. This blends into a mature collagenous scar in which collagen fibers are separated by pale material (edema). This extends to the level of the subcutis and effaces adnexal units.
Diagnosis
Dermatitis, focal, extensive, mild, ulcerative.
Interpretation
Wound in the process of attempted healing but not complete yet. The inflammation is not mild, but the ulceration is extensive.
5. DISCUSSION
This study demonstrated that amnion hydrogel and amnion powder were easy to administer to full thickness wounds, and that application of these products resulted in the most rapid wound closure rates driven primarily by new epithelialization. These observations were supported by histological analysis, which demonstrated that these treatments promote the rapid healing of these full‐thickness wounds, resulting in the formation of a mature epidermis and dermis with similar composition to healthy skin.
Amnion hydrogel and amnion powder were superior to other test products in accelerating wound closure and epithelialization, and were among the best performers in preventing contraction. This suggests that wounds treated using these products will not only heal faster, but heal with mature and healthy epidermal coverage with less contraction. We observed that the amnion hydrogel and amnion powder‐treated wounds showed increased epidermal coverage that was confirmed by histological analysis of the tissues. The presence of a thick and mature epidermis supports the findings of accelerated epithelialization in the amnion hydrogel and amnion powder‐treated groups. Although other treatments did have an epidermal covering, it appeared thinner and lacked dermal protrusions. This suggests that the epidermis in these tissues was less mature, perhaps developing at a slower rate than amnion hydrogel and amnion powder‐treated wounds.
The quality of the wound ECM is of key importance to the long‐term success of the healing wound.32 A healthy ECM facilitates normal cellular and mechanical properties, whereas an abnormal ECM composition can result in scarring, contraction, and loss of mechanical properties such as strength and elasticity.33, 34, 35 Histological staining confirmed our initial observations, showing that amnion hydrogel and amnion powder‐treated wounds had a dermal ECM composition consisting of thick mature collagen fibers intertwined with less immature collagen and mucins/GAGs with localizations and staining intensities consistent with mature skin.36 Picrosirius red staining demonstrated that the amnion hydrogel and amnion powder‐treated groups contained red‐stained, collagen fibers arranged in a basket‐like weave pattern, indicative of normal skin. In contrast, other groups showed finer, unorganized green‐colored fibers, which is a characteristic of hypertrophic scars.37 Additionally, we observed increased iron colloid staining of mucins/GAGs in the amnion hydrogel and amnion powder‐treated groups compared with other groups. This further suggests that deposition of other ECM components such as HA, heparan sulfate, chondroitin sulfate, and other GAGs/proteoglycans, is important for healthy matrix and tissue. Moreover, the correct balance between collagen deposition and these other ECM components likely contributes to healthier regenerated skin.
Interestingly, while the amnion hydrogel and amnion powder were the best performing treatments, AmnioGraft was the third best performing product, supporting the notion that the wound‐healing efficacy of these treatments is inherent to the amnion membrane tissue. Although AmnioGraft is currently not indicated for skin wounds, these data suggest this product may be efficacious for this application. Additionally, it confirms that the processing steps taken to produce the amnion hydrogel and powder did not degrade this property but instead facilitated the availability/release of therapeutic factors, as we have demonstrated in our previous work with the amnion hydrogel.26 It is also interesting to note the processing methods of AmnioGraft when comparing to the amnion hydrogel and amnion powder. According to the manufacturer, AmnioGraft is prepared using a patented CryoTek method (US patent number US6326019B1), which involves the mounting the amniotic membrane onto a flat substrate and the “killing” of the cells by freezing in a liquid comprising a culture medium and a hyperosmotic reagent, presumably Dulbecco's modified Eagle's medium/glycerol (1:1) as described in the product information. Therefore, the processing methodology for all three amnion products removes the cellular viability of the cells, but maintains the presence of the nonviable cellular components such as growth factors. We postulate that the observed differences in the effects of the three products may be related to the cytokine release profile of the hydrogel and powder in the wound, releasing or delivering amnion‐derived factors to the wound at a rate that is more beneficial to wound healing compared with the natural degradation profile of the native amnion membrane sheet. It is interesting that we observed similar wound healing efficacy between the amnion hydrogel and the amnion powder, given the different processing methodology and final product form. This finding suggests: (a) that the processing of the amnion into the solubilized version does not result in a loss of critical therapeutic components, and (b) that the release or delivery of therapeutic components from the amnion hydrogel and amnion powder is at a similar rate. Although further studies are needed to test this hypothesis and improve our understanding of the exact therapeutic components and their release into the wounds, it appears that the processing and application methodology of either the amnion hydrogel and amnion powder was more beneficial in comparison to unprocessed or cryopreserved amnion membrane sheets. This is supported by several studies comparing fresh and processed amnion membranes demonstrating processing such as lyophilization maintains much of the tissues native ECM and growth factor composition.38, 39 In addition, while the hydrogel component provides a hydrating wound barrier and effectively prevented wound contraction, it may also have a key role in the controlled release of delivery of the SAM components.40
As our previous studies were performed in immune‐compromised animals,26 it was important to evaluate whether our human tissue‐derived materials induce an immune response in these immune competent animals. We observed mixed type‐inflammatory cell infiltration, mostly mononuclear within the dermis of the Graftjacket‐treated wounds perhaps suggesting the presence of stimulatory human antigens in this product. Interestingly we saw mildly increased inflammatory cell infiltration in the AmnioGraft‐treated wounds. This observation in AmnioGraft‐treated wounds may be due to the presence of intact human cells or antigens; however, this was not evaluated in this study. Importantly, we did not observe inflammatory cell infiltration in amnion hydrogel or amnion powder treated wounds at time of tissue harvest, suggesting that these products do not stimulate a detrimental immune reaction in with xenogeneic application. Further studies are needed to evaluate potential immune responses in various inflamed wound scenarios (burns/infection, etc.). However, these data, combined with current clinical evidence using amnion membrane for wound healing supports the application of this product in the allogeneic and potentially xenogeneic setting with minimal risk.41, 42, 43
It is worth noting some limitations and future directions for this study. First, this study used a single time point (day 28) for wound histological analysis. This time point was useful for evaluating wound closure and resolution, however for evaluation of early wound healing events such as the acute inflammatory response, or late stage events such as scarring, it would be interesting to evaluate wounds within the first days/week of treatment, as well as after a period of several months postwound closure. Although taking biopsies is a feasible approach, in other studies we have found that this prevents the evaluation of accurate imaging planimetry data. Now that we have established the efficacy of these amnion‐based treatments, future studies will use “sacrificial” wounds for repeated biopsies and analysis, as well as wounds maintained for longer time points for evaluation of scarring mechanisms. Additionally, while there is strong rationale for utilizing the two commercially available products as comparators (ie, AmnioGraft—a cryopreserved amniotic membrane sheet and Graftjacket—a decellularized human dermis graft), neither of these products are currently indicated for excisional wound treatment. By applying each of these products to this wound model, the efficacy of AmnioGraft for the acceleration of wound healing was clearly demonstrated. Conversely, Graftjacket did not perform well for this indication. However, this may have been due to following application instructions that were developed specifically for internal soft tissue repair. It is likely that the addition of a hydrogel‐based bandage to maintain product hydration when used externally and/or removal of nonintegrated product may result in improved outcomes for dermal wound applications.
As we have demonstrated the efficacy of both the amnion hydrogel and the lesser‐processed amnion powder, it will be interesting to evaluate the potential therapeutic components shared between these products to begin to describe potential mechanisms of action. Although extensive studies on potential mechanisms are ongoing and outside the scope of this article, it is likely that multiple components of the amnion membrane positively influence wound healing. We postulate that this includes delivery of amnion ECM components, as well as preserved growth factors known to promote keratinocyte proliferation and migration, promote neovascularization, and have anti‐inflammatory effects. Finally, future studies will test the efficacy of these products in other clinically relevant wound‐types and time frames, such as burns and nonhealing wounds, as well as explore the use of multiple treatment applications.
6. CONCLUSION
We found that the amnion hydrogel and amnion powder were the easiest products to administer to full thickness wounds in the porcine model. Application of these products resulted in the most rapid wound closure rates driven primarily by new epithelialization, less contraction, and without immune rejection. These observations were supported by histological analysis, which demonstrated that these treatments promote the rapid healing of these full‐thickness wounds, resulting in the formation of a mature epidermis and dermis with similar composition to healthy skin.
CONFLICT OF INTEREST
S.V.M., A.S. declared intellectual property inventor, patent holder, and research grant from IP licensee. The other authors indicated no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
S.V.M.: conception and design, collection and/or assembly of data, data analysis and interpretation, prepared the draft of the manuscript, final approval of manuscript; A.S.: conception and design, collection and/or assembly of data, data analysis and interpretation, designed the study, conducted experiments and analysis, final approval of manuscript; R.A.N., N.D.K.: data analysis and interpretation, final approval of manuscript; K.S.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; T.R., C.C.: collection and/or assembly of data, final approval of manuscript; J.J., S.S., A.A.: conception and design, final approval of manuscript.
7.
Supporting information
Supplementary Figure 1 Image Analysis Methodology. A) Contraction was measured by the area defined by the tattoo (purple) at each time point. Contraction ratio is the measurement of the shape change that occurs during wound contraction. B) Wound closure and epithelialization was determined by measuring the area of open wound (red), epithelium (yellow) and mature epithelium (green). C) Factors were described individually and D) as a combination.
ACKNOWLEDGMENT
This study was supported in part by the 3M Company.
Murphy SV, Skardal A, Nelson RA Jr., et al. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. STEM CELLS Transl Med. 2020;9:80–92. 10.1002/sctm.19-0101
Funding information 3M Company
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Blair VP, Brown JB. The use and uses of large split skin grafts of intermediate thickness. Plast Reconstr Surg. 1968;42:65‐75. [Google Scholar]
- 2. Mcdonald WS, Deitch E. Hypertrophic skin grafts in burned patients: a prospective analysis of variables. J Trauma. 1987;27:147‐150. [DOI] [PubMed] [Google Scholar]
- 3. Tanner J, Vandeput J, Olley J. The mesh skin graft. Plast Reconstr Surg. 1964;34:287‐292. [PubMed] [Google Scholar]
- 4. Ratner D. Skin grafting: from here to there. Dermatol Clin. 1998;16:75‐90. [DOI] [PubMed] [Google Scholar]
- 5. Alexander J, MacMillan B, Law E et al. Treatment of severe burns with widely meshed skin autograft and meshed skin allograft overlay. J Trauma. 1981;21:433‐438. [PubMed] [Google Scholar]
- 6. Yang S‐X, Gao H‐L, Xie S‐S et al. Immunosuppression of triptolide and its effect on skin allograft survival. Int J Immunopharmacol. 1992;14:963‐969. [DOI] [PubMed] [Google Scholar]
- 7. Burke J, Quinby W, Bondoc C et al. Immunosuppression and temporary skin transplantation in the treatment of massive third degree burns. Ann Surg. 1975;182:183‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brigido SA, Boc SF, Lopez RC. Effective management of major lower extremity wounds using an acellular regenerative tissue matrix: a pilot study. Orthopedics. 2004;27:S145‐S149. [DOI] [PubMed] [Google Scholar]
- 9. Lesher AP, Curry RH, Evans J et al. Effectiveness of Biobrane for treatment of partial‐thickness burns in children. J Pediatr Surg. 2011;46:1759‐1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rahmanian‐Schwarz A, Beiderwieden A, Willkomm L‐M et al. A clinical evaluation of Biobrane® and Suprathel® in acute burns and reconstructive surgery. Burns. 2011;37:1343‐1348. [DOI] [PubMed] [Google Scholar]
- 11. Ho C, Tran K, Hux M, Sibbald G, Campbell K. Artificial skin grafts in chronic wound care: a meta‐analysis of clinical efficacy and a review of cost‐effectiveness, Database of Abstracts of Reviews of Effects. 2005.
- 12. John T. Human amniotic membrane transplantation: past, present, and future. Ophthalmol Clin North Am. 2003;16:43‐65. [DOI] [PubMed] [Google Scholar]
- 13. Davis JS. Skin transplantation. Johns Hopkins Hosp Rep. 1910;15:307‐396. [Google Scholar]
- 14. Pigeon J. Treatment of second‐degree burns with amniotic membranes. Can Med Assoc J. 1960;83:844. [PMC free article] [PubMed] [Google Scholar]
- 15. Cornwell KG, Landsman A, James KS. Extracellular matrix biomaterials for soft tissue repair. Clin Podiatr Med Surg. 2009;26:507‐523. [DOI] [PubMed] [Google Scholar]
- 16. Bose B. Burn wound dressing with human amniotic membrane. Ann R Coll Surg Engl. 1979;61:444‐447. [PMC free article] [PubMed] [Google Scholar]
- 17. Quinby JW, Hoover HC, Scheflan M et al. Clinical trials of amniotic membranes in burn wound care. Plast Reconstr Surg. 1982;70:711‐717. [DOI] [PubMed] [Google Scholar]
- 18. Sawhney C. Amniotic membrane as a biological dressing in the management of burns. Burns. 1989;15:339‐342. [DOI] [PubMed] [Google Scholar]
- 19. Fu Y, Liu J, Tseng SC. Ocular surface deficits contributing to persistent epithelial defect after penetrating keratoplasty. Cornea. 2012;31:723‐729. [DOI] [PubMed] [Google Scholar]
- 20. Mohammadi AA, Jafari SMS, Kiasat M et al. Effect of fresh human amniotic membrane dressing on graft take in patients with chronic burn wounds compared with conventional methods. Burns. 2013;39:349‐353. [DOI] [PubMed] [Google Scholar]
- 21. Branski LK, Herndon DN, Celis MM et al. Amnion in the treatment of pediatric partial‐thickness facial burns. Burns. 2008;34:393‐399. [DOI] [PubMed] [Google Scholar]
- 22. Ravishanker R, Bath A, Roy R. “Amnion Bank”—the use of long term glycerol preserved amniotic membranes in the management of superficial and superficial partial thickness burns. Burns. 2003;29:369‐374. [DOI] [PubMed] [Google Scholar]
- 23. Hermans MH. Preservation methods of allografts and their (lack of) influence on clinical results in partial thickness burns. Burns. 2011;37:873‐881. [DOI] [PubMed] [Google Scholar]
- 24. Fairbairn N, Randolph M, Redmond R. The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg. 2014;67:662‐675. [DOI] [PubMed] [Google Scholar]
- 25. Lo K, Kohanim S, Trief D et al. Role of amniotic membrane transplantation in acute chemical injury. Int Ophthalmol Clin. 2013;53:33‐41. [DOI] [PubMed] [Google Scholar]
- 26. Murphy SV, Skardal A, Song L et al. Solubilized amnion membrane hyaluronic acid hydrogel accelerates full‐thickness wound healing. Stem Cells Transl Med. 2017;6:2020‐2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bond JL, Dopirak RM, Higgins J et al. Arthroscopic replacement of massive, irreparable rotator cuff tears using a GraftJacket allograft: technique and preliminary results. Arthroscopy. 2008;24:403.e1‐403.e8. [DOI] [PubMed] [Google Scholar]
- 28. Reyzelman A, Crews RT, Moore JC et al. Clinical effectiveness of an acellular dermal regenerative tissue matrix compared to standard wound management in healing diabetic foot ulcers: a prospective, randomised, multicentre study. Int Wound J. 2009;6:196‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Molnar JA, Lew WK, Rapp DA et al. Use of standardized, quantitative digital photography in a multicenter web‐based study. Eplasty. 2009;9. [PMC free article] [PubMed] [Google Scholar]
- 30. Chu H, Son D, Kwon S et al. Characteristics of wound contraction according to the shape and antomical regions of the wound in porcine model. J Korean Soc Plast Reconstr Surg. 2011;38:576‐584. [Google Scholar]
- 31. Urmacher C. Histology of normal skin. Am J Surg Pathol. 1990;14:671‐686. [DOI] [PubMed] [Google Scholar]
- 32. Midwood KS, Williams LV, Schwarzbauer JE. Tissue repair and the dynamics of the extracellular matrix. Int J Biochem Cell Biol. 2004;36:1031‐1037. [DOI] [PubMed] [Google Scholar]
- 33. Xue M, Jackson CJ. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv Wound Care. 2015;4:119‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Aarabi S, Bhatt KA, Shi Y et al. Mechanical load initiates hypertrophic scar formation through decreased cellular apoptosis. FASEB J. 2007;21:3250‐3261. [DOI] [PubMed] [Google Scholar]
- 35. Reilly GC, Engler AJ. Intrinsic extracellular matrix properties regulate stem cell differentiation. J Biomech. 2010;43:55‐62. [DOI] [PubMed] [Google Scholar]
- 36. Johnson WC, Helwig EB. Histochemistry of the acid mucopolysaccharides of skin in normal and in certain pathologic conditions. Am J Clin Pathol. 1963;40:123‐131. [DOI] [PubMed] [Google Scholar]
- 37. Ehrlich HP, Desmoulière A, Diegelmann RF et al. Morphological and immunochemical differences between keloid and hypertrophic scar. Am J Pathol. 1994;145:105. [PMC free article] [PubMed] [Google Scholar]
- 38. Nakamura T, Yoshitani M, Rigby H et al. Sterilized, freeze‐dried amniotic membrane: a useful substrate for ocular surface reconstruction. Invest Ophthalmol Vis Sci. 2004;45:93‐99. [DOI] [PubMed] [Google Scholar]
- 39. Singh R, Chacharkar M. Dried gamma‐irradiated amniotic membrane as dressing in burn wound care. J Tissue Viability. 2011;20:49‐54. [DOI] [PubMed] [Google Scholar]
- 40. Skardal A, Murphy SV, Crowell K et al. A tunable hydrogel system for long‐term release of cell‐secreted cytokines and bioprinted in situ wound cell delivery. J Biomed Mater Res B Appl Biomater. 2017;105:1986‐2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kubo M, Sonoda Y, Muramatsu R et al. Immunogenicity of human amniotic membrane in experimental xenotransplantation. Invest Ophthalmol Vis Sci. 2001;42:1539‐1546. [PubMed] [Google Scholar]
- 42. Kesting MR, Wolff K‐D, Hohlweg‐Majert B et al. The role of allogenic amniotic membrane in burn treatment. J Burn Care Res. 2008;29:907‐916. [DOI] [PubMed] [Google Scholar]
- 43. Rao TV, Chandrasekharam V. Use of dry human and bovine amnion as a biological dressing. Arch Surg. 1981;116:891‐896. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 Image Analysis Methodology. A) Contraction was measured by the area defined by the tattoo (purple) at each time point. Contraction ratio is the measurement of the shape change that occurs during wound contraction. B) Wound closure and epithelialization was determined by measuring the area of open wound (red), epithelium (yellow) and mature epithelium (green). C) Factors were described individually and D) as a combination.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


