Summary
Host−microbiota interaction plays fundamental roles in the homeostasis of mucosal immunity. Dysbiosis of intestinal microbiota has been demonstrated to participate in various immune responses and many multifactorial diseases. Study of intestinal microbiota has moved beyond the consequences of dysbiosis to the causal microbiota associated with diseases. However, studies of pulmonary microbiota and its dysbiosis are still in their infancy. Improvement of culture‐dependent and ‐independent techniques has facilitated our understanding of lung microbiota that not only exists in healthy lung tissue but also exerts great impact on immune responses under both physiological and pathological conditions. In this review, we summarize recent progresses of lung microbiota dysbiosis and its impact on the local immune system that determines the balance of tolerance and inflammation. We discuss the causal roles of pulmonary dysbiosis under disease settings, and propose that the interaction between lung microbiota and host is critical for establishing the immune homeostasis in lung.
Keywords: dysbiosis, inflammation, lung, microbiota, pulmonary disease
The gradual emergence of lung commensals is associated with the establishment of pulmonary mucosal homeostasis. Various factors can lead to lung microbiota dysbiosis and the dysregulated lung microbiota, especially the expansion of specific microbes contributes to chronic inflammation that promotes the progression of pulmonary diseases including fibrosis and tumour.
Abbreviations
- BPT
background predominant taxa
- cDCs
conventional dendritic cells
- CF
cystic fibrosis
- COPD
chronic obstructive pulmonary disease
- DCs
dendritic cells
- ICOS‐L
inducible co‐stimulatory molecule ligand
- iNKT
invariant natural killer T‐cells
- IPF
idiopathic pulmonary fibrosis
- LPS
lipopolysaccharides
- OMVs
outer membrane vesicles
- pDCs
plasmacytoid dendritic cells
- SCFAs
short‐chain fatty acids
- SPF
specific pathogen‐free
- SPT
supraglottic predominant taxa
- Treg
regulatory T‐cells
Introduction
Microbiota coexists with host as symbiotic commensals to form a mutualistic relationship. Microbiota has co‐evolved with its host and colonizes the host’s mucosal tissues, including oral cavity, gastrointestinal tract, skin, vaginal tract and respiratory tract.1 The National Institutes of Health launched the Human Microbiome Project in 2007 and, since then, microbiome at five major body sites of health subjects and microbiome from three cohorts of microbiota‐associated conditions have been characterized.2, 3 However, owing to the traditional notion that lung is sterile and technical challenges of sampling, the lung microbiota was not included into this project.4 Over the past several years, the field of lung microbiota has been revolutionized by the advance of culture‐dependent and ‐independent techniques.4
Several pioneering studies using culture‐independent techniques have confirmed that there are diverse communities, although much less than that in the gastrointestinal tract, of microbes even in lung under health conditions,5, 6, 7 and the microbial composition of healthy lung is distinct from that of individuals with pulmonary diseases.8 With appropriate protocols, bacteria can be isolated and cultured from lung lobes, indicating that there are live microbes in lung.9 The history and evolution of this field has been well reviewed by Dickson et al. 4 Healthy lung usually harbours a small community of bacteria, comprising mainly of four phyla: Firmicutes, Proteobacteria, Bacteroidetes and Actinobacteria, which are similar in human and mice. While being a small amount but important, like the roles that gut microbiota plays in shaping immune system development in the gastrointestinal tract, pulmonary microbiota has been gradually realized to play important roles in maintaining the homeostasis in lung.
As the main function of the respiratory system is gas exchange and the air is not always innocuous, maturation of lung immune homeostasis occurs in a nearly open environment full of microorganisms and inhaled particles. The pulmonary immune system comprises of a network of participants: the lung epithelial cells stand for the first barrier that protects against external harms, while various lung‐resident lymphocytes and alveolar macrophages can continually monitor the potential threats. Exposure to external microorganisms after birth helps the host to establish the immune system of the lung. However, the homeostasis state of the pulmonary immune system is not so stable as its interaction with microbiota is dynamic, and it changes with age, genetics and environmental exposure. Dysbiosis of lung microbiota will lead to disturbance of pulmonary homeostasis and predispose the host to the occurrence or progression of lung diseases. Up to now, it is still a challenge to reveal the mechanisms of the generation and alteration of lung microbiota dysbiosis and to figure out its contributions to the development of lung diseases. In this review, we summarize recent studies of both external and internal pressures that lead to lung microbiota dysbiosis. We highlight the effects that dysbiosis of lung microbiota exerts on reshaping the pulmonary immune system and predisposition to lung diseases. Previous studies usually attribute the dysbiosis of lung diseases to the associated consequences; however, there are much more than just associations. We discuss the potential causal microbes out of the chaotic microbiota dysbiosis that contribute to pulmonary immune responses and diseases. Exploration of the causal roles of lung microbiota dysbiosis will help us better understand how the pulmonary immune homeostasis is established and provide potential precise strategies to target dysbiosis for lung disease prevention and therapy.
Generation of lung microbiota
It is generally believed that the fetus in utero is sterile,10 and the lung is filled with bacteria‐free amniotic fluid.11 Immediately after birth, the newborn’s mucosal tissues are colonized by bacteria, which are relatively homogeneous across all body sites, but very quickly differentiate into organ‐specific communities with body sites serving as the primary determinant of the bacterial composition and its functional capacity.12 Gollwitzer et al.13 quantified the bacterial load in mouse lungs at multiple time points after birth, and found the bacterial load increases and the composition changes with age. Interestingly, they found the neonatal airways mainly harbour Firmicutes and Gammaproteobacteria, which have been proved to be associated with asthmatic phenotypes in human6 and mice14 and, with age, Bacteroidetes gradually expands.
To better understand the microbiology of the lung, one proposed model is the ‘adapted island model’,15 where the total microbial burden and relative composition are determined by a balance between immigration of microbes from the ‘mainland’ and their local distinction.4 While aspiration of oral cavity microbes from the upper respiratory tract to the lower tract is a major factor that affects the lung microbiota,16, 17 airborne microbes serve as another important contributor. Therefore, the lung microbiota displays great spatial variation between individuals and continual renewal within individuals. Although the compositions of lung microbiota of healthy subjects are relatively stable,18 various patterns of dysbiosis of lung microbiota are associated with many lung diseases, like idiopathic pulmonary fibrosis (IPF),19, 20, 21 cystic fibrosis (CF),22, 23 chronic obstructive pulmonary disease (COPD),24 asthma,25 and cancer.26, 27
Internal and external factors that lead to lung microbiota dysbiosis
The human lung, mainly comprising of a single epithelial cell layer coated with thin mucus, has approximately 70 m2 of surface area, which is in nearly direct contact with the exterior environment.28 It is not exaggerating to say that the lung and its associated microbiota are just one step away from disaster. A set of internal and external factors can perturb the relatively fragile microbial ecosystem of the lung to an extent that exceeds its resistance capability and finally leads to dysbiosis (Fig. 1).
Figure 1.
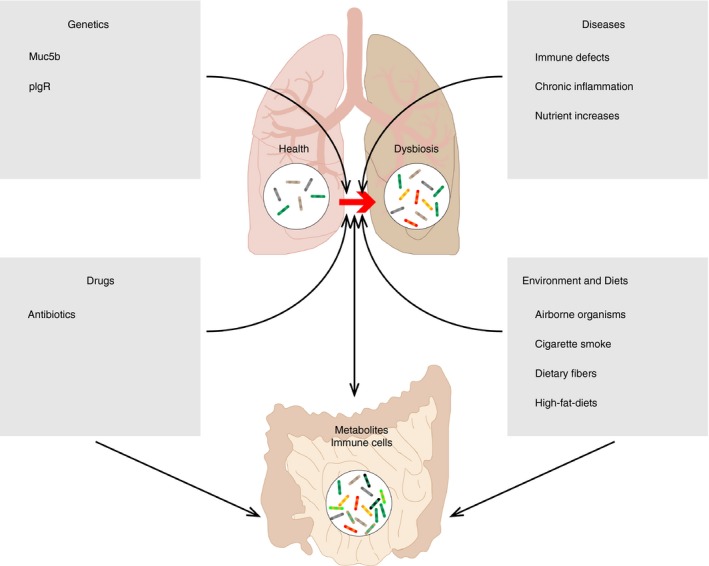
Internal and external factors that lead to lung microbiota dysbiosis. Genetic deficiency of Muc5b or pIgR causes direct barrier disruption in lung; drugs including antibiotics can either target local immune cells to inhibit their activity or target bacteria to prevent their survival; progression of many pulmonary diseases including viral infections and fibrosis is usually accompanied with immune defects, chronic inflammation and nutrient increases; airborne organisms and cigarette smoking may directly or indirectly shift pulmonary bacterial composition while dietary changes are associated with changes of metabolites that have systemic effects on the immune system; the lung may closely interact with the gut to form the lung−gut axis, which is mainly mediated by metabolites changes and status changes of immune cells.
Genetics
Host genetics are critical internal factors that influence the composition of lung microbiota and local immunity. Airways are lined with a thin mucus layer,29 which inhales external toxins and transports them out of the lung through ciliary beating and cough. MUC5AC and MUC5B are mucin‐encoding genes highly expressed in the airways, with the former mainly expressed in proximal airways by surface goblet cells and the latter in surface secretory cells throughout the airways.29 Roy et al.30 found that Muc5b but not Muc5ac is required for mucociliary clearance, controlling spontaneous infections in the airways and maintaining immune homeostasis in mouse lungs. Muc5b‐deficient mice harbour more culturable bacteria in the lungs over time and further more in the spontaneous moribund mice, and 16S rRNA analysis shows significantly increased Streptococci and Staphylococci, especially one important pneumonia‐causing pathogen Staphylococcus aureus. Importantly, antibiotics treatment ameliorates the spontaneous infection‐associated mortality of Muc5b‐deficient mice.30 Paradoxically, Hancock et al. showed in mice that Muc5b overexpression is also related to impaired mucociliary clearance and predisposes the host to Bleomycin‐induced fibrosis.31, 32 Mucin overproduction not only disrupts normal ciliary beat frequency and mucociliary transport rate, but may also provide nutrients for the opportunistic pathogens, as mucin is one of the important nutritional sources for pathogens like Pseudomonas aeruginosa.33, 34, 35
In addition to mucin, the airway epithelial cells support an antigen‐specific secretory IgA (SIgA) barrier that covers and protects the airway surface.36 In patients with COPD, widespread structural abnormalities of the airway epithelium are common, and are correlated with decreased expression of polymeric immunoglobulin receptor (pIgR) and disruption of the SIgA barrier in airways.37 pIgR‐deficient mice spontaneously develop COPD‐like phenotypes as they age, which is correlated with about twofold increase of bacterial taxa in the lung.38 Further analysis identified 10 taxes like Prevotella, Veillonella and Bacillus, that can discriminate the genotypes of pIgR‐deficient mice.38 Importantly, germ‐free pIgR‐deficient mice are completely protected from airway chronic inflammation, remodelling and emphysema compared with mice maintained in standard conditions,38 and reconstituting the microbiome of germ‐free pIgR‐deficient mice can recover those COPD‐like phenotypes. These data suggest that specific immunodeficiency in the lung will cause dysbiosis of lung microbiota with opportunistic pathogen infection and lead to disruption of local immune homeostasis.
Environment and diet
People are daily exposed to various sources of airborne microorganisms from both indoor and outdoor environments,39, 40 which may have beneficial or detrimental effects on human health. Previous studies have shown that high microbial diversity in the environment is associated with lower asthma risk, especially in children exposed to a farming environment.41, 42 Birzele et al.43 found that farm exposure is positively correlated with bacterial diversity in mattress dust samples, especially the bacterial genera Clostridium, Facklamia, and some genus within the family of Ruminococcaceae. Interestingly, they found stronger negative association of asthma risk with bacterial diversity in mattress dust compared with that in nasal specimens, indicating that microbial involvement contributes more than just colonization of the upper airway. Another study showed that the protective low asthma risk microbiota has a low abundance of Streptococcaceae, which serves as predictor of asthma risk and potential modifiable target for asthma prevention.44 Despite the fact that airborne microorganisms contribute to the ameliorated asthma, their effects on other pulmonary diseases and the corresponding lung microbiota composition need to be further determined.
As an important trigger of many inflammatory lung diseases, cigarette smoking also exerts a significant effect on the bacterial composition in the lung. Recently, one study showed that exposing mice to smoking for 90 days will cause denser inflammation and congestion in their lungs compared with the non‐smoking mice.45 Importantly, lung microbiota composition is different between the two groups, suggesting that lung microbiota dysbiosis caused by smoking may serve as the aetiology of the associated inflammatory pulmonary diseases. Another study explored the mechanisms underlying the association between cigarette smoking and the increased risk of acute respiratory distress syndrome after severe blunt trauma.46 They found that smoking is significantly associated with compositional changes of patient lung microbiota both at the time of ICU admission and 48 hr post ICU admission, with some opportunistic pathogens including Streptococcus, Fusobacterium, Prevotella, Haemophilus and Treponema displaying the most significant enrichment. However, through 16S rDNA sequencing of the microbiome of the upper and lower respiratory tract in healthy nonsmokers and smokers, Morris et al.47 found that the lung microbiome is not significantly altered by smoking.
The intestinal microbiota is remarkably influenced by diet,48, 49 which may also indirectly affect lung microbiota. Dietary fibres have displayed their beneficial roles in ameliorating gastrointestinal disorders, and recently they have also been proved to play a protective role in the lung against allergic airway inflammation.14 Interestingly, dietary fermentable fibre content can modulate not only intestinal microbiota but also lung microbiota, especially by altering the ratios of Firmicutes and Bacteroidetes.14 However, whether the changes of the lung microbiota under different dietary styles can ameliorate allergic airway inflammation needs further determination.
Drugs
Inappropriate antibiotics usage can produce long‐lasting deleterious effects for human health, which has been well revealed in many gastrointestinal researches, as it may also clear beneficial bacteria and provide niches for outgrowth of opportunistic pathogens.50, 51, 52 Early life or perinatal antibiotics exposure can cause shifts in the intestinal microbiota and predispose the host to Th2‐ or Th1/Th17‐driven allergic airway inflammatory diseases,53, 54 suggesting that antibiotics may also cause lung microbiota dysbiosis. Using different antibiotics to treat mouse lungs has displayed different effects on the progression of Bleomycin‐induced fibrosis,19 and another study also found that vancomycin plus neomycin‐treated mice have decreased bacterial load associated with reduced regulatory T‐cells and enhanced T‐cell and natural killer (NK) cell activation that paralleled a significant reduction of melanoma B16 lung metastasis in their lungs.55 These data indicate that antibiotics can cause lung microbiota dysbiosis similar to the situations in the gastrointestine56 and affect pulmonary disease development.
Co‐trimoxazole and Azithromycin have been used in clinical trials to treat IPF and achieved beneficial effects,57, 58 whose effects are generally thought owing to their anti‐inflammatory activity. However, these drugs are also known as broad‐spectrum antibiotics, suggesting that they may exert their beneficial effects by bactericidal functions, as more than one‐third of IPF patients are colonized with pathogenic bacteria or Pneumocystis jirovecii, most of which are susceptible to co‐trimoxazole.58 These clinic trials provide important insights into future clinical therapy of dysbiosis‐associated pulmonary diseases by targeting opportunistic pathogenic bacteria with selective antibiotics.
Diseases
Lung microbiota dysbiosis occurs in nearly all kinds of lung diseases, yet the driving mechanism is largely unknown. Several investigators have now revealed that human immunodeficiency virus (HIV) infection changes the composition of lung microbiota and identified the enrichment of Prevotella, Veilonella and Streptococcus in the lower airways, which positively correlate with advanced HIV infection and HIV‐associated pulmonary diseases.59 HIV infection is accompanied with immune defects and chronic inflammation, which is likely to cause dysbiosis in the lung. Lozupone et al. found that the bronchoalveolar fluid of HIV‐positive individuals was frequently enriched with Tropheryma whipplei, the aetiological agent of Whipple’s disease, which was reduced by effective antiretroviral therapy.60
Among many factors affecting microbiota composition, nutrients are most vulnerable to be disrupted. The nutrient supply of the airways, normally scarce in health, but is likely increased by the presence of mucus and vascular permeability during lung injury. Dysbiosis of lung microbiota in both IPF patients and Bleomycin‐induced fibrosis models has been widely reported,19, 20, 21 yet the reason remains to be explored. Dysbiosis of lung microbiota after Bleomycin exposure precedes the onset of fibrosis, showing increased bacterial load and alpha diversity.19 Further analyses show that the Gram‐negative Bacteroidetes is the most upregulated bacteria phylum, among which Bacteroides and Prevotella are the most obviously increased genera. Intestinal Bacteroides abundance is strongly correlated with richness of many host amino acids and fatty acid metabolites, whereas Prevotella abundance is associated with carbohydrate metabolism in the host. RNA‐seq assay shows that the corresponding genes associated with those metabolites are indeed increased in Bleomycin‐treated mouse lungs; therefore, like in the gut, those increased metabolites under the situation of lung injury may endow Bacteroides and Prevotella growth advantage. Another acute lung injury model induced by lipopolysaccharides (LPSs) intratracheal administration also shows increased bacterial loads and remarkably changed composition.61 Further analyses show that bacteria from families Xanthomonadaceae and Brucellaceae increase their abundance in the injured mouse lungs, and metabolic profiling of BAL from these mice detects the presence of bacterial substrates suitable for both isolates, suggesting metabolic changes in the pulmonary niches after lung injury may provide these bacteria with a growth advantage.
Lung−gut axis
Except for the above factors, lung microbiota can also be systemically influenced by gut microbiota to form a lung−gut axis that has been discussed in many reviews.62, 63, 64 Reflux and aspiration can promote direct bacterial seeding into the lungs, while metabolism and nutrition are key indirect mediators of the gut−lung axis. Gut microbiota can metabolite dietary fibres into short‐chain fatty acids (SCFAs), which enhances seeding of lung with dendritic cells (DCs) that have high phagocytic capability but impaired ability to promote Th2 cell effector function thereby ameliorating the allergic airway inflammation.14 However, SCFAs only represent a small fraction of metabolites that alter with changes of dietary, antibiotics treatment, or inflammation in the gastrointestine. Thus, the potential roles of other metabolites still remain to be explored.
Interestingly, changes in lung microbiota may also influence the composition of gut microbiota. Influenza virus infection in the respiratory tract is often accompanied by increases of Enterobacteriaceae as well as reductions of Lactobacilli and Lactococci in the intestinal microbiota.65, 66 Another study shows that the dysbiosis in lung microbiota upon administration of LPS in mice is accompanied by disturbances in their gut microbiota due to movement of bacteria from their lung into the bloodstream.61 As the lung−gut axis has attracted more and more attention, manipulation of gut microbiota or its metabolites may serve as potential clinical strategies to treat lung diseases.
Lung microbiota dysbiosis affects local inflammation
Microbial colonization in the gastrointestinal tract after birth is a critical step for the education of the local and systematic immune compartment,67 and people gradually find that this dogma also suits the lung mucosal system. Although no viable bacteria have been detected in lung specimens from preterm and term infants, one study proves that even the microbial DNA in the fetus lung shows its effect on the developing pulmonary immune system, and ‘dysbiosis’ predisposes the fetus to many lung diseases like bronchopulmonary dysplasia,11 although no obvious differences in pulmonary structure, epithelium thickness or bronchus number are observed between germ‐free mice and bacteria‐colonized mice, and lungs of germ‐free mice have bigger but less alveolar as illustrated by histological examination.19, 68 The lack of microbiota leads to immaturation of the pulmonary immune system, rendering the host susceptible to lung disorders like allergic asthma.9, 69 In addition to the fact that dysbiosis of lung microbiota is associated with progression of nearly all kinds of pulmonary diseases,4 there are more and more direct evidences to propose that lung microbiota deficiency or dysbiosis, once established, dramatically affects both local pulmonary and systemic landscape of immune cells, thereby creating a feedback loop where local immune cells and microbiota cross‐talk with each other (Fig. 2).
Figure 2.
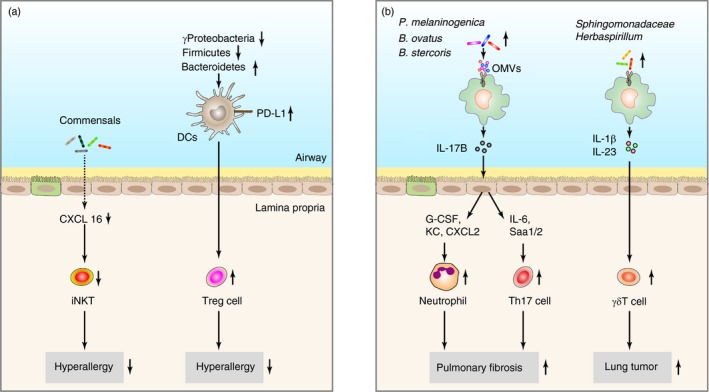
The effects of lung microbiota on the pulmonary immune system. (a) The gradual emergence of pulmonary commensals is associated with decreased allergic responses in the lung. (b) Dysregulated lung microbiota especially the expansion of specific microbes contributes to chronic inflammation that promotes the progression of pulmonary diseases including fibrosis and tumour.
Effects on innate immunity
Pulmonary epithelium is the first defense barrier that protects the lung against external toxins, which is coated with thin and mobile mucus.29 Mucus production in the intestine is a dynamic process that is affected by microbiota,70 so does it work in the lung. Histological examination reveals that, compared with bacteria‐colonized mouse lungs, lungs of germ‐free mice have reduced mucus,68 which is consistent with another study showing that Muc5ac mRNA levels are higher in the lungs of specific pathogen‐free (SPF) mice.9 Interestingly, colonizing germ‐free mouse lungs with bacteria Lactobacillus spp. restores mucus production to the level of SPF mice.68 Both intestinal and pulmonary microbiota can promote the expression of several interleukin (IL)‐17 family cytokines, like IL‐17A and IL‐17E,19, 71, 72 which have been proved to enhance airway mucus production through increasing the expression of Muc5ac,73, 74, 75 and dysbiosis of lung microbiota that occurred in many chronic lung inflammatory diseases is usually associated with either reduced or increased mucus production.30, 31 Therefore, these data suggest lung microbiota may regulate the local mucosal barrier functions through modulating mucus production.
The healthy lungs are populated with a number of different macrophages, with alveolar macrophages as the dominant subpopulation.76, 77 Alveolar macrophages are vital for maintaining lung health and function by clearing external toxins, debris, surfactant and apoptotic cells, therefore functioning as sentinels under healthy state.76 During inflammation, alveolar macrophages can mediate bacterial clearance and initiate neutrophil recruitment.78 Wang et al.79 identified that S. aureus, a common lung opportunistic pathogen, recruits peripheral CCR2+CD11B+ monocytes into alveoli and polarizes them into M2 alveolar macrophages, which significantly attenuates influenza‐induced lung injury. Microaspiration is a common phenomenon mediating seeding of oral bacteria into airways in healthy subjects. Segal et al. found that about half of the people they examined are enriched with oral taxa, therefore they classified two distinct lung microbiomes: pneumotypeSPT, characterized by high bacterial load and supraglottic predominant taxa (SPT); and pneumotypeBPT, with low bacterial burden and background predominant taxa (BPT).17 Of note, alveolar macrophages isolated from pneumotypeSPT lungs, which are enriched with bacterial taxa like Prevotella, Rothia and Veillonella, show decreased production of IL‐6 and MIP‐1α under LPS stimulation, indicating an attenuated immune response of alveolar macrophages under the presence of pneumotypeSPT lung microbiota.17 Another study shows that lung microbiota dysbiosis in lung transplantation may lead to inflammatory or remodelling profiles in macrophages.80 Importantly, Firmicutes‐ and Proteobacteria‐driven dysbiosis is associated with low macrophage and high neutrophil percentages and an inflammation gene expression profile while, in contrast, Bacteroidetes‐driven dysbiosis is linked to high macrophage and low neutrophil percentages and proremodelling profile.80
Dendritic cells bridge innate immunity and adaptive immunity in the lung mucosal system. Under homeostatic conditions, lung mucosa generally contains two functionally different DC subsets: conventional DCs (cDCs) and plasmacytoid DCs (pDCs).81 As in the gut, DCs in lungs are constantly exposed to external factors, including commensal bacteria and pathogens, and both cDCs and pDCs express a large number of pattern recognition receptors,81 and thus it is not surprising that DCs play critical roles in regulating local immune homeostasis through interacting with local microbes. Increased bacterial load in the lungs during the first 2 weeks after birth protects mice from airways hyperresponsive to allergens. Mechanistically, the changed lung microbiota, especially shifts from a predominance of Gamma proteobacteria and Firmicutes to Bacteroidetes, promotes PD‐L1 expression on CD11b+ DCs in lung, which then induce Treg cells to ameliorate the airway allergic inflammation.13 Increased numbers and altered functions of airway mucosal DCs have been found in many chronic inflammatory pulmonary diseases, like asthma82 and COPD.83 Lower expression of the inducible co‐stimulatory molecule ligand (ICOS‐L) on airway DCs is observed in individuals with severe asthma,84 while cDCs in patients with COPD show prominent expression of Langerhans’ cell markers langerin and CD1a and the co‐stimulatory molecules CD80 and CD86.83 Yet, the specific roles of lung microbiota dysbiosis in regulating airway DCs functions need to be further determined. One study shows that lung commensals and pathogenic bacteria exert different effects on human monocytes‐derived DCs in vitro,85 and that pathogenic bacteria provoke higher levels of IL‐23, IL‐12p70 and IL‐10 production than commensal like Prevotella spp. and Veilonella spp. Therefore, dysbiosis of lung microbiota, particularly outgrowth of opportunistic pathogens, may remodel DCs functions to regulate local immunity.
Invariant natural killer T (iNKT) cells are a CD1d‐restricted T‐cell population, which use an αβ T‐cell receptor heterodimer with limited diversity for the recognition of exogeneous (bacterial and nonbacterial) and endogenous lipid antigens.86 iNKT cells play an important role in the control of commensals including opportunistic pathogenic microbiota.87 In turn, the microbiota regulates iNKT cells. Olszak et al.88 illustrated that iNKT cells contribute to the enhanced airway allergic inflammation in germ‐free mice. The expression and production of the chemokine ligand CXCL16, which is responsible for iNKT recruitment, is increased in germ‐free mouse lung and intestine, and colonization of neonatal germ‐free mice with a conventional microbiota could decrease CXCL16 production and thus alleviate the hyperallergic responses.88 Interestingly, regulation of CXCL16 expression and iNKT cells accumulation by the microbiota is independent of the Toll‐like receptor adaptor protein Myd88, but depends on epigenetic modulations like CpG methylation.88 Further study showed that monocolonization with a human bacterium Bacteroides fragilis or treatment with a B. fragilis‐derived glycosphingolipid can block the increased number of iNKT cells in the adult colon but not in the lung,89 indicating that specific lung microbes are likely responsible for local pulmonary iNKT cells regulation.
Effects on adaptive immunity
Lung‐resident γδT cells are known as important effector and regulator cells maintaining pulmonary homeostasis and affecting the progress of many pulmonary diseases including lung cancers and COPD.90 Recently, Jin et al.27 provided evidence that lung microbiota is disordered in lung tumour‐bearing mice, displaying increased bacterial load and reduced bacterial diversity in the airways. Several bacterial taxa, including Herbaspirillum and Sphingomonadaceae, Aggregatibacter and Lactobacillus, are dramatically expanded in tumour‐bearing lungs. Importantly, the tumour development is significantly correlated with the dysbiosis of lung microbiota but not intestinal microbiota.27 Mechanistically, dysregulated lung microbiota provokes activation of resident γδT cells to promote lung adenocarcinoma development. Lung commensal microbes could stimulate the production of IL‐1β and IL‐23 from myeloid cells in a Myd88‐dependent manner, and then induce the proliferation and activation of specific γδT cells that produce IL‐17 to promote tumour‐associated inflammation.27 Interestingly, the differentiation and activation of γδT cells are discounted in germ‐free mice and antibiotics‐treated mice, which are significantly protected from lung cancer development induced by Kras mutation and p53 loss.27 Lung microbiota dysbiosis is associated with progressive respiratory diseases like COPD. Yadava et al.91 explored whether there is a causal relationship of the dysbiosis and COPD progression by using a LPS/elastase intranasally treated mouse model. They found both microbial abundance and diversity were decreased in the COPD‐like mouse lungs with an outgrowth of the genera Pseudomonas, Lactobacillus and a reduction of Prevotella. Intranasal transfer of the enriched microbiota from LPS/elastase‐treated mice into antibiotics‐treated mice could induce IL‐17‐producing γδT cells development, and recapitulate the IL‐17 inflammatory responses.91 Together, these data suggest an important role of γδT cells in mediating the effect of lung microbiota dysbiosis on the progression of chronic inflammatory lung diseases.
Th17 cells, the major cell sources of IL‐17, play critical roles in regulating pulmonary homeostasis and diseases.92 Our recent work shows that the dysregulated lung microbiota in mice with Bleomycin‐induced fibrosis can remarkably induce Th17 cells and the following IL‐17 production, and then promote fibrosis progression.19 Th17 cells are diminished in the lungs of either antibiotics‐treated mice or germ‐free mice, suggesting a critical role of the disordered microbiota in regulating Th17 cells development. Importantly, several bacterial species out of the genera Bacteroides are identified to dramatically induce Th17 cells when intranasally transferred into antibiotics‐treated or germ‐free mice. Mechanistically, these bacteria can act on alveolar macrophages in a Myd88‐TLR2/4‐dependent manner to secret IL‐17B, another IL‐17 family member, which in turn stimulate lung epithelial cells to produce Th17‐promoting cytokines like IL‐6 and Saa1/2. Other investigations have also found correlations of the lung microbiota dysbiosis with Th17 cells in severe asthma. Huang et al. found in severe asthma patients that Th17 cells are positively correlated with a number of taxa, mainly Proteobacteria like Pasteurellaceae, Enterobacteriaceae and Bacillaceae.93 Interestingly, a proportion of taxa showing correlation with expression of FKBP5, an indicator of steroid responsiveness, also belong to Proteobacteria but not the same families as that correlated with Th17 cells,93 suggesting a multifactorial effect of dysbiosis on allergic inflammatory responses. Moreover, although lung microbiota from pneumotypeSPT individuals blunts alveolar macrophages TLR response as mentioned above, it is positively correlated with a proinflammatory phenotype characterized by multiple Th17‐related cytokines like IL‐1β, IL‐6 and Th17 lymphocytes.17
Regulatory T (Treg) cells are critical for maintenance of pulmonary immunological tolerance to airborne allergens and for restrain of deleterious immune responses to self and non‐self antigens.13, 94 A limited number of lung microbiota in neonatal mice render the host susceptible to airway allergic inflammation, and the increased bacterial load after birth could promote development of Helios‐ Treg cells, which decrease the hyperallergic responsiveness.13 The positively correlated increase of lung microbiota and Treg cells is mainly mediated by the induced expression of PD‐L1 on DCs,13 indicating a mechanism through which lung commensal microbes modulate Treg cells development and allergic responses. At the same time, Treg cells are downregulated in many chronic inflammatory pulmonary diseases, including CF 95 and COPD,96 and their decrease correlates with the disease progression. Maybe it is the lung microbiota dysbiosis that leads to the impaired Treg cells, resulting in deficient capability to restrain the local inflammatory responses. One recent study shows that aerosol administration of vancomycin and neomycin for 5 days significantly affects lung microbiota diversity, displaying reduced population of the genus Streptococcus, which are common Gram‐positive Firmicutes commensal of the lung, and increased bacterial genera belonging to less represented members of Proteobacteria and Actinobacteria.55 The compositional changes are accompanied with reduced Treg cells, which contribute to a significant reduction of melanoma B16 lung metastasis.55
The causal microbes that contribute to pulmonary diseases
Dysregulated lung microbiota is associated with the pathogenesis of nearly all kinds of chronic inflammatory pulmonary diseases, which has been discussed in many reviews. However, whether the dysbiosis is a consequence or cause of these diseases remains largely unknown. Here we briefly categorize recent progresses exploring the causal microbes of these diseases.
Our recent work shows that lung microbiota is dysregulated in Bleomycin‐induced fibrosis mice, and the fibrotic phenotypes are reduced in either antibiotics‐pretreated or germ‐free mice.19 In order to identify whether there’s specific commensal microbes contributing to fibrosis, individually intranasal inoculation of several bacterial species, including the most significantly expanded bacteria Bacteroides ovatus, Bacteroides stercoris, Prevotella melaninogenica and Ruminococcus gnavus in Bleomycin‐treated lungs, and the most abundant bacteria Streptococcus sp. and Lactobacillus murinus in healthy lungs, into antibiotic‐pretreated mice and germ‐free mice shows that B. ovatus, B. stercoris and P. melaninogenica remarkably enhanced the fibrotic responses.19 Mechanistically, these profibrotic bacteria can secret outer membrane vesicles (OMVs), containing ligands for TLR2/4, to stimulate alveolar macrophages, which then produce IL‐17B to promote fibrosis through inducing Th17 cells and neutrophils.19 Therefore, specific microbes could modulate disease progression by producing OMVs.
Another recent study shows that pulmonary local microbiota is disordered along with the development of lung adenocarcinoma, and most importantly the dysbiosis can provoke local inflammation by inducing the proliferation and activation of γδT cells.27 Kras mutation and p53 loss‐induced lung cancer development is dramatically ameliorated in germ‐free mice or antibiotics‐pretreated mice.27 To further confirm the important role of lung microbiota in promoting tumour development, they isolated and cultured several bacterial species including Lactobacillus, Streptococcus and Staphylococcus from the late‐stage lung tumours and then intratracheally inoculated into another cohort of mice shortly after tumour initiation. The inoculation of these bacteria cocktails dramatically enhanced the expansion of IL‐17‐producing γδT cells in a TLR‐Myd88‐dependent manner and accelerated tumour development.27 Consistently, local administration of TLR ligands like LPS and peptidoglycan also triggered the expansion of IL‐17‐producing γδT cells in the lung.27 However, the specific commensal microbes contributing to tumour development remain to be defined.
Moreover, intranasal inoculation of microbiota isolated from LPS‐treated mice intensifies IL‐6‐induced lung inflammation in naive mice,61 while intranasal transfer of fluid enriched with the pulmonary microbiota isolated from LPS/Elastase‐treated mice, a mouse model to mimic human COPD diseases, enhances IL‐17A production in the lungs of antibiotics‐treated or germ‐free mice.91 Despite that the specific composition of lung microbiota needs further exploration, these studies elucidate that lung microbiota, especially the set of inborn opportunistic pathogens outgrew in the environment of inflamed lungs but not the external infectious pathogens, play causal roles in many pulmonary diseases.
Conclusions and future perspectives
Significant progresses have been made in the past several years, which elucidate the critical roles of the lung microbiota in establishing local immune homeostasis and protection against external threats. The bidirectional and geographically isolated lung structure endows lung microbiota with fragility and dynamic, therefore dysbiosis occurs under various deleterious factors, which once established will lead to acceleration of pulmonary diseases. On the other hand, the relatively low abundance of lung microbiota makes it a challenge to isolate, culture and identify the functional microbes. However, the low biomass does not undermine its potential critical roles in shaping local lung immunity.
A deep understanding of the interactions between lung microbiota dysbiosis and the local immune system makes modulation of pulmonary microbiota an insightful therapeutic strategy to treat lung diseases just like already suggested in the intestine like Bifidobacterium bifidum, which has been demonstrated to activate TLR2 and endow DC cells with the capability to induce Treg cells thereby protecting against experimental colitis,97 and Lactobacillus plantarum, which combined with fructo‐oligosaccharide enables long‐term colonization and protection against sepsis in neonates.98 Interestingly, aerosolization with Lactobacillus rhamnosus or B. bifidum was recently proved to be able to decrease the immunosuppressive cells and increase the activity of effector cells, thereby preventing tumour implantation in the lungs.55 Moreover, intranasal administrations of several different bacterial species of the genus Lactobacillus have also been reported to protect the host against the influenza infections.99, 100, 101
Compared with the research of microbiota in other mucosal tissues, especially the intestine, our understanding of lung microbiota is still limited and a set of conceptual questions still need to be answered. One question is what are the nonbacterial components of lung microbiota like fungi and virus? Fungal102, 103 and viral 65, 79 infections are common in many lung diseases, deciphering their composition and interaction with the local immune system will help us understand the primary cause of particular diseases and develop more efficient therapies. The second question is how to deeply identify the causal commensals of a lung disease. Chronic lung diseases are associated with lung microbiota dysbiosis, allowing for the complexity of the composition, searching for the real ‘assailant’ remains a challenge. Triangulation of microbe–phenotype relationships has been shown to be an effective method for reducing the noise inherent in microbiota studies and enabling identification of causal microbes in colitis.104 However, there is still no such efficient method as to lung research except for testing the candidate bacteria by inoculation of them individually into bacteria‐depleted mice. Another question is how these causal bacteria exert their detrimental effects. To answer this question, we need to combine the genomic, transcriptomic and metabolomic analysis to identify the mechanisms by which the dysregulated microbiota promotes the pathogenesis of lung diseases. The last question is how to restore the normal lung microbiota after dysbiosis. The prerequisite of restoring the dysregulated microbiota is finding out the main triggers among the various deleterious factors and developing specific targeting therapies. Extensive fecal microbiota transplantation trials aimed at restoring intestinal microbiota diversity have been conducted in an attempt to combat the associated diseases, showing success rates of over 80% in treating infection with Clostridium difficile, a nosocomial pathogen often recalcitrant to treatment with antibiotics.105 Likewise, intranasal or aerosol administration of normal microbiota into patient lungs may be a potential choice to restore lung microbiota to treat the associated pulmonary disorders.
Disclosure
The authors declare no competing financial interests.
Author contributions
D.Y. wrote the manuscript; Y.Q., X.S. and Y.X. edited the manuscript.
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (91842306, 81830018 and 81430036), National Key R&D Program of China (2018YFA0507402), and the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No. XDB19000000).
References
- 1. Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell 2014; 157:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Turnbaugh PJ, Ley RE, Hamady M, Fraser‐Liggett CM, Knight R, Gordon JI. The Human Microbiome Project. Nature 2007; 449:804–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Integrative HMP (iHMP) Research Network Consortium . The Integrative human microbiome project. Nature 2019; 569:641–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dickson RP, Erb‐Downward JR, Martinez FJ, Huffnagle GB. The Microbiome and the respiratory tract. Annu Rev Physiol 2016; 78:481–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Erb‐Downward JR, Thompson DL, Han MK, Freeman CM, McCloskey L, Schmidt LA et al Analysis of the lung microbiome in the "healthy" smoker and in COPD. PLoS ONE 2011; 6:e16384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C et al Disordered microbial communities in asthmatic airways. PLoS ONE 2010; 5:e8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charlson ES, Bittinger K, Haas AR, Fitzgerald AS, Frank I, Yadav A et al Topographical continuity of bacterial populations in the healthy human respiratory tract. Am J Respir Crit Care Med 2011; 184:957–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marsland BJ, Gollwitzer ES. Host‐microorganism interactions in lung diseases. Nat Rev Immunol 2014; 14:827–35. [DOI] [PubMed] [Google Scholar]
- 9. Remot A, Descamps D, Noordine ML, Boukadiri A, Mathieu E, Robert V et al Bacteria isolated from lung modulate asthma susceptibility in mice. ISME J 2017; 11:1061–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Macpherson AJ, de Aguero MG, Ganal‐Vonarburg SC. How nutrition and the maternal microbiota shape the neonatal immune system. Nat Rev Immunol 2017; 17:508–17. [DOI] [PubMed] [Google Scholar]
- 11. Lal CV, Travers C, Aghai ZH, Eipers P, Jilling T, Halloran B et al The airway microbiome at birth. Sci Rep 2016; 6:31 023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu DM, Ma J, Prince AL, Antony KM, Seferovic MD, Aagaard KM. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat Med 2017; 23:314–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gollwitzer ES, Saglani S, Trompette A, Yadava K, Sherburn R, McCoy KD et al Lung microbiota promotes tolerance to allergens in neonates via PD‐L1. Nat Med 2014; 20:642–7. [DOI] [PubMed] [Google Scholar]
- 14. Trompette A, Gollwitzer ES, Yadava K, Sichelstiel AK, Sprenger N, Ngom‐Bru C et al Gut microbiota metabolism of dietary fiber influences allergic airway disease and hematopoiesis. Nat Med 2014; 20:159–66. [DOI] [PubMed] [Google Scholar]
- 15. Dickson RP, Erb‐Downward JR, Huffnagle GB. Towards an ecology of the lung: new conceptual models of pulmonary microbiology and pneumonia pathogenesis. Lancet Respir Med 2014; 2:238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Segal LN, Alekseyenko AV, Clemente JC, Kulkarni R, Wu B, Gao Z et al Enrichment of lung microbiome with supraglottic taxa is associated with increased pulmonary inflammation. Microbiome 2013; 1:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG et al Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol 2016; 1:16 031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dickson RP, Erb‐Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB et al Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc 2015; 12:821–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang D, Chen X, Wang J, Lou Q, Lou Y, Li L et al Dysregulated lung commensal bacteria drive interleukin‐17B production to promote pulmonary fibrosis through their outer membrane vesicles. Immunity 2019 50:692–706.e7 [DOI] [PubMed] [Google Scholar]
- 20. O'Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR et al Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med 2019; 199:1127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Han MK, Zhou Y, Murray S, Tayob N, Noth I, Lama VN et al Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med 2014; 2:548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coburn B, Wang PW, Diaz Caballero J, Clark ST, Brahma V, Donaldson S et al Lung microbiota across age and disease stage in cystic fibrosis. Sci Rep 2015; 5:10 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Guss AM, Roeselers G, Newton IL, Young CR, Klepac‐Ceraj V, Lory S et al Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. ISME J 2011; 5:20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Huang YJ, Sethi S, Murphy T, Nariya S, Boushey HA, Lynch SV. Airway microbiome dynamics in exacerbations of chronic obstructive pulmonary disease. J Clin Microbiol 2014; 52:2813–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mathieu E, Escribano‐Vazquez U, Descamps D, Cherbuy C, Langella P, Riffault S et al Paradigms of lung microbiota functions in health and disease, particularly, in asthma. Front Physiol 2018; 9:1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mao Q, Jiang F, Yin R, Wang J, Xia W, Dong G et al Interplay between the lung microbiome and lung cancer. Cancer Lett 2018; 415:40–8. [DOI] [PubMed] [Google Scholar]
- 27. Jin C, Lagoudas GK, Zhao C, Bullman S, Bhutkar A, Hu B et al Commensal microbiota promote lung cancer development via gammadelta T cells. Cell 2019; 176:998–1013.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Holt PG, Strickland DH, Wikstrom ME, Jahnsen FL. Regulation of immunological homeostasis in the respiratory tract. Nat Rev Immunol 2008; 8:142–52. [DOI] [PubMed] [Google Scholar]
- 29. Fahy JV, Dickey BF. Airway mucus function and dysfunction. N Engl J Med 2010; 363:2233–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Roy MG, Livraghi‐Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM et al Muc5b is required for airway defence. Nature 2014; 505:412–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hancock LA, Hennessy CE, Solomon GM, Dobrinskikh E, Estrella A, Hara N et al Muc5b overexpression causes mucociliary dysfunction and enhances lung fibrosis in mice. Nat Commun 2018; 9:5363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S et al MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med 2018; 379:2209–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Aristoteli LP, Willcox MD. Mucin degradation mechanisms by distinct Pseudomonas aeruginosa isolates in vitro . Infect Immun 2003; 71:5565–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dennesen P, Veerman E, van Nieuw Amerongen A, Jacobs J, Kessels A et al High levels of sulfated mucins in bronchoalveolar lavage fluid of ICU patients with ventilator‐associated pneumonia. Intensive Care Med 2003; 29:715–9. [DOI] [PubMed] [Google Scholar]
- 35. Ohneck EJ, Arivett BA, Fiester SE, Wood CR, Metz ML, Simeone GM et al Mucin acts as a nutrient source and a signal for the differential expression of genes coding for cellular processes and virulence factors in Acinetobacter baumannii . PLoS ONE 2018; 13:e0190599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pilette C, Ouadrhiri Y, Godding V, Vaerman JP, Sibille Y. Lung mucosal immunity: immunoglobulin‐A revisited. Eur Respir J 2001; 18:571–88. [DOI] [PubMed] [Google Scholar]
- 37. Polosukhin VV, Cates JM, Lawson WE, Zaynagetdinov R, Milstone AP, Massion PP et al Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2011; 184:317–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Richmond BW, Brucker RM, Han W, Du RH, Zhang Y, Cheng DS et al Airway bacteria drive a progressive COPD‐like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat Commun 2016; 7:11 240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prussin AJ 2nd, Marr LC. Sources of airborne microorganisms in the built environment. Microbiome 2015; 3:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Adams RI, Bateman AC, Bik HM, Meadow JF. Microbiota of the indoor environment: a meta‐analysis. Microbiome 2015; 3:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol 2010; 10:861–8. [DOI] [PubMed] [Google Scholar]
- 42. Stein MM, Hrusch CL, Gozdz J, Igartua C, Pivniouk V, Murray SE et al Innate immunity and asthma risk in Amish and Hutterite farm children. N Engl J Med 2016; 375:411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Birzele LT, Depner M, Ege MJ, Engel M, Kublik S, Bernau C et al Environmental and mucosal microbiota and their role in childhood asthma. Allergy 2017; 72:109–19. [DOI] [PubMed] [Google Scholar]
- 44. Kirjavainen PV, Karvonen AM, Adams RI, Taubel M, Roponen M, Tuoresmaki P et al Farm‐like indoor microbiota in non‐farm homes protects children from asthma development. Nat Med 2019; 25:1089–95. [DOI] [PubMed] [Google Scholar]
- 45. Zhang R, Chen L, Cao L, Li KJ, Huang Y, Luan XQ et al Effects of smoking on the lower respiratory tract microbiome in mice. Respir Res 2018; 19:253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Panzer AR, Lynch SV, Langelier C, Christie JD, McCauley K, Nelson M et al Lung microbiota is related to smoking status and to development of acute respiratory distress syndrome in critically Ill trauma patients. Am J Respir Crit Care Med 2018; 197:621–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morris A, Beck JM, Schloss PD, Campbell TB, Crothers K, Curtis JL et al Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am J Respir Crit Care Med 2013; 187:1067–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu GD, Chen J, Hoffmann C, Bittinger K, Chen YY, Keilbaugh SA et al Linking long‐term dietary patterns with gut microbial enterotypes. Science 2011; 334:105–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE et al Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kim S, Covington A, Pamer EG. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev 2017; 279:90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Becattini S, Taur Y, Pamer EG. Antibiotic‐induced changes in the intestinal microbiota and disease. Trends Mol Med 2016; 22:458–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA 2011; 108(Suppl 1):4554–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Russell SL, Gold MJ, Reynolds LA, Willing BP, Dimitriu P, Thorson L et al Perinatal antibiotic‐induced shifts in gut microbiota have differential effects on inflammatory lung diseases. J Allergy Clin Immunol 2015; 135:100–9. [DOI] [PubMed] [Google Scholar]
- 54. Obiakor CV, Tun HM, Bridgman SL, Arrieta MC, Kozyrskyj AL. The association between early life antibiotic use and allergic disease in young children: recent insights and their implications. Expert Rev Clin Immunol 2018; 14:841–55. [DOI] [PubMed] [Google Scholar]
- 55. Le Noci V, Guglielmetti S, Arioli S, Camisaschi C, Bianchi F, Sommariva M et al Modulation of pulmonary microbiota by antibiotic or probiotic aerosol therapy: a strategy to promote immunosurveillance against lung metastases. Cell Rep 2018; 24:3528–38. [DOI] [PubMed] [Google Scholar]
- 56. Cervantes‐Barragan L, Chai JN, Tianero MD, DiLuccia B, Ahern PP, Merriman J et al Lactobacillus reuteri induces gut intraepithelial CD4+CD8alphaalpha+ T cells. Science 2017; 357:806–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Macaluso C, Furcada JM, Alzaher O, Chaube R, Chua F, Wells AU et al The potential impact of azithromycin in idiopathic pulmonary fibrosis. Eur Respir J 2019; 53:1800628. [DOI] [PubMed] [Google Scholar]
- 58. Hammond M, Clark AB, Cahn AP, Chilvers ER, Fraser WD, Livermore DM et al The efficacy and mechanism evaluation of treating idiopathic pulmonary fibrosis with the addition of co‐trimoxazole (EME‐TIPAC): study protocol for a randomised controlled trial. Trials 2018; 19:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Shenoy MK, Lynch SV. Role of the lung microbiome in HIV pathogenesis. Curr Opin HIV AIDS 2018; 13:45–52. [DOI] [PubMed] [Google Scholar]
- 60. Lozupone C, Cota‐Gomez A, Palmer BE, Linderman DJ, Charlson ES, Sodergren E et al Widespread colonization of the lung by Tropheryma whipplei in HIV infection. Am J Respir Crit Care Med 2013; 187:1110–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Poroyko V, Meng F, Meliton A, Afonyushkin T, Ulanov A, Semenyuk E et al Alterations of lung microbiota in a mouse model of LPS‐induced lung injury. Am J Physiol Lung Cell Mol Physiol 2015; 309:L76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Dang AT, Marsland BJ. Microbes, metabolites, and the gut‐lung axis. Mucosal Immunol 2019; 12:843–50. [DOI] [PubMed] [Google Scholar]
- 63. Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P et al Emerging pathogenic links between microbiota and the gut‐lung axis. Nat Rev Microbiol 2017; 15:55–63. [DOI] [PubMed] [Google Scholar]
- 64. Anand S, Diet Mande SS. Microbiota and gut‐lung connection. Front Microbiol 2018; 9:2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang J, Li F, Wei H, Lian ZX, Sun R, Tian Z. Respiratory influenza virus infection induces intestinal immune injury via microbiota‐mediated Th17 cell‐dependent inflammation. J Exp Med 2014; 211:2397–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hanada S, Pirzadeh M, Carver KY, Deng JC. Respiratory Viral infection‐induced microbiome alterations and secondary bacterial pneumonia. Front Immunol 2018; 9:2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science 2012; 336:1268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Yun Y, Srinivas G, Kuenzel S, Linnenbrink M, Alnahas S, Bruce KD et al Environmentally determined differences in the murine lung microbiota and their relation to alveolar architecture. PLoS ONE 2014; 9:e113466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sabatel C, Radermecker C, Fievez L, Paulissen G, Chakarov S, Fernandes C et al Exposure to bacterial CpG DNA protects from airway allergic inflammation by expanding regulatory lung interstitial macrophages. Immunity 2017; 46:457–73. [DOI] [PubMed] [Google Scholar]
- 70. Corfield AP. The interaction of the gut microbiota with the mucus barrier in health and disease in human. Microorganisms 2018;6:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. McGeachy MJ, Cua DJ, Gaffen SL. The IL‐17 family of cytokines in health and disease. Immunity 2019; 50:892–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Song X, Dai D, He X, Zhu S, Yao Y, Gao H et al Growth factor FGF2 cooperates with interleukin‐17 to repair intestinal epithelial damage. Immunity 2015; 43:488–501. [DOI] [PubMed] [Google Scholar]
- 73. de Almeida Nagata DE, Demoor T, Ptaschinski C, Ting HA, Jang S, Reed M et al IL‐27R‐mediated regulation of IL‐17 controls the development of respiratory syncytial virus‐associated pathogenesis. Am J Pathol 2014; 184:1807–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sharkhuu T, Matthaei KI, Forbes E, Mahalingam S, Hogan SP, Hansbro PM et al Mechanism of interleukin‐25 (IL‐17E)‐induced pulmonary inflammation and airways hyper‐reactivity. Clin Exp Allergy 2006; 36:1575–83. [DOI] [PubMed] [Google Scholar]
- 75. Fujisawa T, Velichko S, Thai P, Hung LY, Huang F, Wu R. Regulation of airway MUC5AC expression by IL‐1beta and IL‐17A; the NF‐kappaB paradigm. J Immunol 2009; 183:6236–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hussell T, Bell TJ. Alveolar macrophages: plasticity in a tissue‐specific context. Nat Rev Immunol 2014; 14:81–93. [DOI] [PubMed] [Google Scholar]
- 77. Roberts AW, Lee BL, Deguine J, John S, Shlomchik MJ, Barton GM. Tissue‐resident macrophages are locally programmed for silent clearance of apoptotic cells. Immunity 2017; 47:913–27.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Westphalen K, Gusarova GA, Islam MN, Subramanian M, Cohen TS, Prince AS et al Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature 2014; 506:503–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang J, Li F, Sun R, Gao X, Wei H, Li LJ et al Bacterial colonization dampens influenza‐mediated acute lung injury via induction of M2 alveolar macrophages. Nat Commun 2013; 4:2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bernasconi E, Pattaroni C, Koutsokera A, Pison C, Kessler R, Benden C et al Airway microbiota determines innate cell inflammatory or tissue remodeling profiles in lung transplantation. Am J Respir Crit Care Med 2016; 194:1252–63. [DOI] [PubMed] [Google Scholar]
- 81. Lambrecht BN, Hammad H. Lung dendritic cells in respiratory viral infection and asthma: from protection to immunopathology. Annu Rev Immunol 2012; 30:243–70. [DOI] [PubMed] [Google Scholar]
- 82. Dua B, Tang W, Watson R, Gauvreau G, O'Byrne PM. Myeloid dendritic cells type 2 after allergen inhalation in asthmatic subjects. Clin Exp Allergy 2014; 44:921–9. [DOI] [PubMed] [Google Scholar]
- 83. Lommatzsch M, Bratke K, Knappe T, Bier A, Dreschler K, Kuepper M et al Acute effects of tobacco smoke on human airway dendritic cells in vivo . Eur Respir J 2010; 35:1130–6. [DOI] [PubMed] [Google Scholar]
- 84. Froidure A, Vandenplas O, D'Alpaos V, Evrard G, Pilette C. Defects in plasmacytoid dendritic cell expression of inducible costimulator ligand and IFN‐alpha are associated in asthma with disease persistence. Am J Respir Crit Care Med 2015; 192:392–5. [DOI] [PubMed] [Google Scholar]
- 85. Larsen JM, Steen‐Jensen DB, Laursen JM, Sondergaard JN, Musavian HS, Butt TM et al Divergent pro‐inflammatory profile of human dendritic cells in response to commensal and pathogenic bacteria associated with the airway microbiota. PLoS ONE 2012; 7:e31976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Van Kaer L, Parekh VV, Wu L. Invariant natural killer T cells: bridging innate and adaptive immunity. Cell Tissue Res 2011; 343:43–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nieuwenhuis EE, Matsumoto T, Lindenbergh D, Willemsen R, Kaser A, Simons‐Oosterhuis Y et al Cd1d‐dependent regulation of bacterial colonization in the intestine of mice. J Clin Invest 2009; 119:1241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A et al Microbial exposure during early life has persistent effects on natural killer T cell function. Science 2012; 336:489–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk‐Hasdemir D et al Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell 2014; 156:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Cheng M, Hu S. Lung‐resident gammadelta T cells and their roles in lung diseases. Immunology 2017; 151:375–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Yadava K, Pattaroni C, Sichelstiel AK, Trompette A, Gollwitzer ES, Salami O et al Microbiota promotes chronic pulmonary inflammation by enhancing IL‐17A and autoantibodies. Am J Respir Crit Care Med 2016; 193:975–87. [DOI] [PubMed] [Google Scholar]
- 92. Alcorn JF, Crowe CR, Kolls JK. TH17 cells in asthma and COPD. Annu Rev Physiol 2010; 72:495–516. [DOI] [PubMed] [Google Scholar]
- 93. Huang YJ, Nariya S, Harris JM, Lynch SV, Choy DF, Arron JR et al The airway microbiome in patients with severe asthma: associations with disease features and severity. J Allergy Clin Immunol 2015; 136:874–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Abdel‐Gadir A, Massoud AH, Chatila TA. Antigen‐specific Treg cells in immunological tolerance: implications for allergic diseases. F1000Res 2018; 7:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. McGuire JK. Regulatory T cells in cystic fibrosis lung disease. More answers, more questions. Am J Respir Crit Care Med 2015; 191:866–8. [DOI] [PubMed] [Google Scholar]
- 96. Lane N, Robins RA, Corne J, Fairclough L. Regulation in chronic obstructive pulmonary disease: the role of regulatory T‐cells and Th17 cells. Clin Sci 2010; 119:75–86. [DOI] [PubMed] [Google Scholar]
- 97. Verma R, Lee C, Jeun EJ, Yi J, Kim KS, Ghosh A et al Cell surface polysaccharides of Bifidobacterium bifidum induce the generation of Foxp3(+) regulatory T cells. Sci Immunol 2018;3:eaat6975. [DOI] [PubMed] [Google Scholar]
- 98. Panigrahi P, Parida S, Nanda NC, Satpathy R, Pradhan L, Chandel DS et al A randomized synbiotic trial to prevent sepsis among infants in rural India. Nature 2017; 548:407–12. [DOI] [PubMed] [Google Scholar]
- 99. Belkacem N, Serafini N, Wheeler R, Derrien M, Boucinha L, Couesnon A et al Lactobacillus paracasei feeding improves immune control of influenza infection in mice. PLoS ONE 2017; 12:e0184976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Park MK, Ngo V, Kwon YM, Lee YT, Yoo S, Cho YH et al Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013; 8:e75368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Youn HN, Lee DH, Lee YN, Park JK, Yuk SS, Yang SY et al Intranasal administration of live Lactobacillus species facilitates protection against influenza virus infection in mice. Antiviral Res 2012; 93:138–43. [DOI] [PubMed] [Google Scholar]
- 102. Charlson ES, Diamond JM, Bittinger K, Fitzgerald AS, Yadav A, Haas AR et al Lung‐enriched organisms and aberrant bacterial and fungal respiratory microbiota after lung transplant. Am J Respir Crit Care Med 2012; 186:536–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Jhingran A, Kasahara S, Shepardson KM, Junecko BA, Heung LJ, Kumasaka DK et al Compartment‐specific and sequential role of MyD88 and CARD9 in chemokine induction and innate defense during respiratory fungal infection. PLoS Pathog 2015; 11:e1004589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Surana NK, Kasper DL. Moving beyond microbiome‐wide associations to causal microbe identification. Nature 2017; 552:244–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM et al Duodenal infusion of donor feces for recurrent Clostridium difficile . N Engl J Med 2013; 368:407–15. [DOI] [PubMed] [Google Scholar]


