Abstract
Refractory idiopathic orbital inflammation (IOI) represents a management challenge due to its significant proportion of treatment failures. Currently, there are no established guidelines for recalcitrant IOI and majority of immunosuppressive agents have resulted in variable outcomes. Advancements of plasmapheresis therapy can play a significant role in ophthalmological diseases. This treatment has shown to produce a satisfactory therapeutic response in immune-mediated neurological conditions. In this case report, we share our successful experience with the use of plasmapheresis therapy in a patient with recalcitrant IOI.
Keywords: eye, haematology (incl blood transfusion)
Background
Idiopathic orbital inflammation (IOI) is defined as idiopathic tumor-like inflammation, consisting of a pleomorphic cellular response and reactive fibrovascular tissue.1 This disease can affect all ages without gender or racial predilection. Symptoms can be quite varied ranging from pain, diplopia, swelling, proptosis to, in severe cases, optic nerve swelling. IOI is a diagnosis of exclusion and therefore, local and systemic diseases should be considered including lymphoproliferative diseases, IgG4-related disease, thyroid disease and sarcoidosis.2 Given the wide array of potential differential diagnosis, radiological studies and orbital biopsy often assist in attaining a definitive diagnosis.
First-line treatment for IOI is high-dose corticosteroids that are often tapered off slowly over months. Literature reports an estimate of 30% to 50% inflammation recurrence despite use of corticosteroids.3 4 Immunosuppressive agents such as mycophenolate mofetil, cyclosporine, cyclophosphamide, methotrexate and azathioprine have been somewhat effective in the steroid-resistant patients.1 5 6
Plasmapheresis is a therapeutic procedure that uses the centrifugation apheresis to separate and takes away the plasma components. This therapy has been shown to a satisfactory response in immune-mediated neurological conditions such as myasthenia gravis, Guillain–Barré syndrome and multiple sclerosis.7 Currently, there has been no report in the scientific literature regarding the use of plasmapheresis as a treatment for IOI. In this case report, we describe a steroid and immunosuppressive resistant case of recalcitrant IOI that was successfully treated following plasmapheresis therapy.
Case presentation
A 49-year-old female presented to our clinic with symptoms of recurrent headaches and sharp left orbital pain for 2 weeks. On exam, there was a mild proptosis of left eye with swelling of the left lid but no restriction of eye movements. Her visual acuity was 20/20 in the right eye and 20/30 in the left eye. Her intraocular pressure was 17 on the right eye and 18 on the left eye. Her pupils were round and reactive, with a normal confrontational visual field. General anterior examination under the slit lamp was unremarkable. Retinal scleral depressed exam showed the left optic nerve grossly elevated with a Frisen grade 2 papilloedema. Unremarkable fundus exam of the right eye was noted. Due to significant swelling of the left optic nerve and high risk of vision loss in that eye, patient was sent to the emergency department for an emergent CT of the orbit with contrast. The image showed a significant increase in soft tissue prominence involving the left bony with replacement of the post septal fat (figure 1A,B). There was mild protrusion of the optic nerve head along with diffuse infiltrative appearance of the retrobulbar fat and overall worsening of oedema and inflammation compared with previous imaging. Additionally, there was diminished opacification of the left ophthalmic artery compared with the right which may be have been associated with compression.
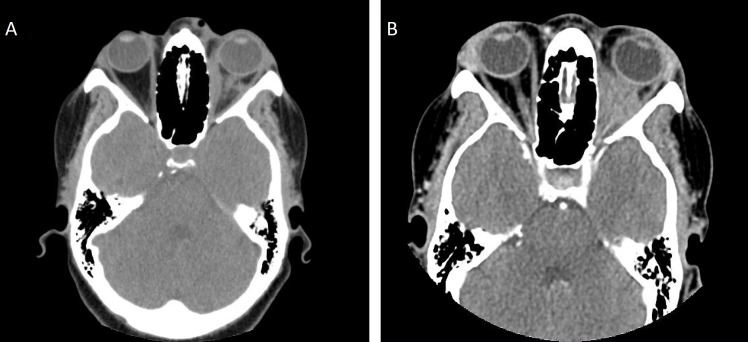
Figure 1.
(A. left): Previous CT imaging of the orbit and sella with intravenous contrast from a year ago indicating medial rectus oedema and inflammatory infiltration along with protrusion of the optic nerve. (B. right): Current CT imaging at the time of the first visit showing progression of retro-orbital inflammation and medical rectus oedema compared with previous year’s imaging.
Investigations
Patient had a past medical history significant for hypertension on losartan 25 mg and orbital inflammatory syndrome diagnosed in 2017. Initial orbital biopsy at an outside hospital revealed chronic inflammation with fibrotic tissue. Investigative laboratory workup showed non-detective levels of antinuclear antibody, anti-ds DNA antibody, C-reactive protein (<5), antineutrophil cytoplasmic antibodies (ANCA) IFA, myeloperoxidase antibody, serine protease 3 antibody, extractable nuclear antigen (ENA) screening and glucose-6-phosphate dehydrogenase (G6PD). Comprehensive metabolic workup revealed normal levels of metabolites, renal and liver function. Angiotensin converting enzyme level was within normal range. Complete blood count and complete blood workout was performed with normal haemoglobin, white cell counts, unremarkable peripheral smear and thyroid function. Infectious workup for hepatitis B antigen and C antibody, HIV and QuantiFERON gold all came back negative.
Since onset of diagnosis, patient had experienced multiple relapses. She responded well to 80 mg of oral prednisone but recurred when tapered to 40 mg daily despite adjuvant therapy of 3 g daily of mycophenolate mofetil. Given her multiple relapses, a repeat biopsy was performed to rule out systemic associated diseases. The results of the biopsy revealed a fibroadipose tissue infiltration (figure 2A) with lymphocytic dominance (figure 2B) suggesting a chronic inflammatory process. Immunohistochemical study with anti-IgG4 antibody showed no immunoreactivity, ruling out an IgG4 sclerosing disease (figure 2C). Following the biopsy and rheumatology consultation, a second immunomodulatory agent rituximab was suggested, yet was not started due to pending health insurance approval.
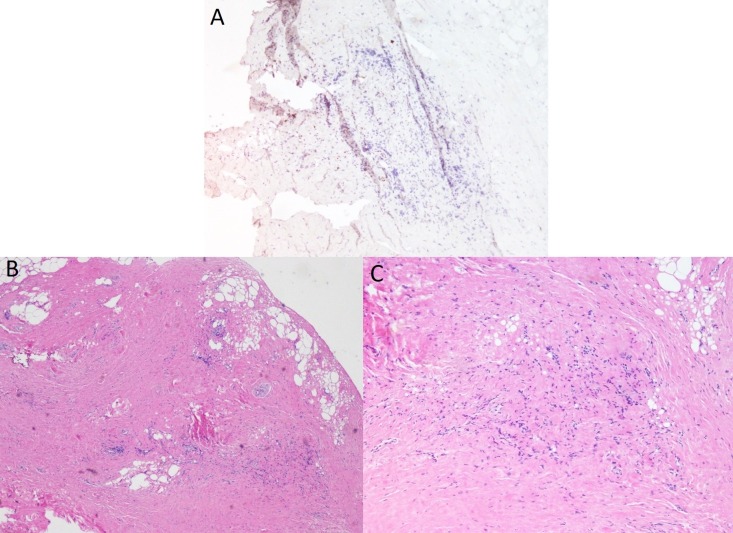
Figure 2.
(A. bottom, left) Fibroadipose tissue with scattered inflammation. Note variation in adipocyte size at the periphery and extent of fibrous tissue replacing the adipose. (B. bottom, right) Chronic, lymphocytic inflammation. (C. top): Immunohistochemistry study with negative anti-IgG4 antibody, ruling out IgG4 sclerosing diseases.
Treatment
In the hospital, patient was started on Solu-Medrol 1 g/day while awaiting plasmapheresis treatment. She completed 3 days of intravenous steroids. Under ultrasound and fluoroscopic guidance, a right internal jugular vein 11.5-Fr x 15 cm non-tunnelled phoresies catheter was placed. Five consecutive plasmapheresis sessions were completed successfully with 2.5 litre volume exchange and 5% albumin as replacement solution. The Solu-Medrol was stopped during plasmapheresis treatment. Following completion of the five sessions, she had clinically significant improvement of her symptoms and reduction of her ocular pain and headaches. The swelling was improved. She was discharged on prednisone 50 mg orally with follow-up examination in 2 weeks.
Outcome and follow-up
Outpatient follow-up 9 days post-plasmapheresis therapy, reported absence of recurrence of symptoms (orbital pain, headache, swelling). Ophthalmological exam was unremarkable with fundus examination showing resolution of optic nerve swelling in her left eye, with no macular or retinal oedema. Patient is currently on rituximab therapy, off oral prednisone and has remained without recurrences for six consecutive months.
Discussion
Currently there are no established guidelines for recalcitrant IOI. The spectrum of adjuvant treatment in IOI is broad and expanding. Immunosuppressive agents such as mycophenolate mofetil, cyclosporine, cyclophosphamide, methotrexate and azathioprine have been tried with variable results.1 5 6 More recently, development of novel biological agents such as rituximab and infliximab increase the prospect of targeted immunosuppression. These have been shown to play a role in IOI recalcitrant cases.8–10 The use of these agents mandates frequent clinic visits, close haematological monitoring and liver and renal function testing. All of these factors significantly add to patients’ financial burden.11
Despite advances in diagnosis and treatment, the pathogenesis of IOI is poorly understood. However, there is a strong evidence for a humoral-cellular mediated immune complex process.12 Therefore, the first-line treatment to consider would be plasmapheresis. This therapy allows for removal of the implicated antibodies that have a significant role in the pathogenesis of this autoimmune disease. Current ocular pathology that is amenable to plasmapheresis therapy include neuromyelitis optica (NMO) aquaporin-4 autoantibody, Miller Fisher GQ1b antibody, paraneoplastic antiretinal antibodies in bilateral diffuse uveal melanocytic proliferation and myasthenia gravis crisis.13–15
In theory, elimination of antibodies and other humoral factors through plasmapheresis should reduce IOI relapses. B-cell targeted therapy such as rituximab or cytotoxic therapy should be started after plasmapheresis since the pathogenic B cells are thought to be most active at this point. We initially planned to initiate rituximab after the fifth session, but she acquired a hospital associated staphylococcus infection, leading to a delay in receiving rituximab therapy. This experience emphasises the need for close monitoring as multiple plasmapheresis sessions increase the risks of patients to the morbidities of immunosuppression.16
In summary, refractory IOI represents a management challenge due to the significant number of treatment failures. In such recalcitrant cases, plasmapheresis offers a safe and effective treatment alternative.
Learning points.
Idiopathic inflammatory pseudotumor of the orbit is a benign, non-infective inflammatory condition of the orbit with no exact local or systemic causes.
This condition is based on diagnosis of exclusion following evaluation directed to exclude neoplasms, infections and systemic disorders.
Idiopathic orbital inflammation can be refractory to both systemic corticosteroids and immunomodulatory therapy poses a major therapeutic challenge.
Plasmapheresis has never been used as a potential option for treatment of refractory idiopathic orbital inflammation.
In such recalcitrant cases, plasmapheresis offers a safe and effective treatment alternative.
Footnotes
Contributors: YR, XL: planning, design and conception. YR, FA: data acquisition and analysis. YR, XL: interpretation of data. YR, FA: drafting the manuscript. YR, FA, XL: revising the manuscript critically for important intellectual content. YR, FA, XL: final approval of the version published.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Espinoza GM. Orbital inflammatory pseudotumors: etiology, differential diagnosis, and management. Curr Rheumatol Rep 2010;12:443–7. 10.1007/s11926-010-0128-8 [DOI] [PubMed] [Google Scholar]
- 2. Hersh D, Rose GE. Idiopathic orbital inflammatory disease. Emergencies of the Orbit and Adnexa: Springer, 2017: 225–33. [Google Scholar]
- 3. Yuen SJA, Rubin PAD. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Arch Ophthalmol 2003;121:491–9. 10.1001/archopht.121.4.491 [DOI] [PubMed] [Google Scholar]
- 4. Braich PS, Kuriakose RK, Khokhar NS, et al. Factors associated with multiple recurrences of nonspecific orbital inflammation AKA orbital pseudotumor. Int Ophthalmol 2018;38:1485–95. 10.1007/s10792-017-0610-7 [DOI] [PubMed] [Google Scholar]
- 5. Hatton MP, Rubin PAD, Foster CS. Successful treatment of idiopathic orbital inflammation with mycophenolate mofetil. Am J Ophthalmol 2005;140:916–8. 10.1016/j.ajo.2005.04.053 [DOI] [PubMed] [Google Scholar]
- 6. Gordon LK. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye 2006;20:1196–206. 10.1038/sj.eye.6702383 [DOI] [PubMed] [Google Scholar]
- 7. Meneses de Oliveira FT, Corrêa De Luca N, Peter Tilbery C. Plasmapheresis therapy for immune-mediated diseases in neurology: literature review. Glob Vaccines Immunol 2016;1:29–32. 10.15761/GVI.1000108 [DOI] [Google Scholar]
- 8. Garrity JA, Coleman AW, Matteson EL, et al. Treatment of recalcitrant idiopathic orbital inflammation (chronic orbital myositis) with infliximab. Am J Ophthalmol 2004;138:925–30. 10.1016/j.ajo.2004.06.077 [DOI] [PubMed] [Google Scholar]
- 9. Schafranski MD. Idiopathic orbital inflammatory disease successfully treated with rituximab. Clin Rheumatol 2009;28:225–6. 10.1007/s10067-008-1040-8 [DOI] [PubMed] [Google Scholar]
- 10. Abell RG, Patrick A, Rooney KG, et al. Complete resolution of idiopathic sclerosing orbital inflammation after treatment with rituximab. Ocul Immunol Inflamm 2015;23:176–9. 10.3109/09273948.2013.863943 [DOI] [PubMed] [Google Scholar]
- 11. Rubin PAD, Foster CS. Etiology and management of idiopathic orbital inflammation. Am J Ophthalmol 2004;138:1041–3. 10.1016/j.ajo.2004.09.032 [DOI] [PubMed] [Google Scholar]
- 12. Harris GJ. Idiopathic orbital inflammation: a pathogenetic construct and treatment strategy: the 2005 ASOPRS Foundation lecture. Ophthalmic Plast Reconstr Surg 2006;22:79–86. 10.1097/01.iop.0000203734.52333.93 [DOI] [PubMed] [Google Scholar]
- 13. Wingerchuk DM. Diagnosis and treatment of neuromyelitis optica. Neurologist 2007;13:2–11. 10.1097/01.nrl.0000250927.21903.f8 [DOI] [PubMed] [Google Scholar]
- 14. Jaben EA, Pulido JS, Pittock S, et al. The potential role of plasma exchange as a treatment for bilateral diffuse uveal melanocytic proliferation: a report of two cases. J Clin Apher 2011;26:356–61. 10.1002/jca.20310 [DOI] [PubMed] [Google Scholar]
- 15. Barth D, Nabavi Nouri M, Ng E, et al. Comparison of IVIg and PLEX in patients with myasthenia gravis. Neurology 2011;76:2017–23. 10.1212/WNL.0b013e31821e5505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ebadi H, Barth D, Bril V. Safety of plasma exchange therapy in patients with myasthenia gravis. Muscle Nerve 2013;47:510–4. 10.1002/mus.23626 [DOI] [PubMed] [Google Scholar]