Abstract
A 48-year-old male patient requiring extracorporeal membrane oxygenation (ECMO) support for hypoxaemic respiratory failure failed to achieve therapeutic anticoagulation with bivalirudin after continuous dose escalations, and continued to have recurrent fibrin stranding in the circuit over a 6-day course of treatment. Suspecting bivalirudin resistance, the patient was transitioned to argatroban and achieved a therapeutic response in less than 24 hours. The case describes the challenges of anticoagulation in ECMO supported patients. The interplay between bivalirudin metabolism, renal replacement therapy, and immunological effects leading to a heparin-like-effect, inflammatory mediators, and thrombotic burdens may all impact the clinical effect during bivalirudin therapy. The structural biochemistry of thrombin and bivalirudin likely plays a role in the presented patient’s successful response to argatroban. Bivalirudin may fail at achieving therapeutic anticoagulation in patients with genetic thrombin mutations or structural defects that alter the binding pockets at the thrombin exosites.
Keywords: mechanical ventilation, venous thromboembolism, adult intensive care, pharmacology and therapeutics, therapeutic indications
Background
During extracorporeal membrane oxygenation (ECMO) support, patients are continuously at risk for developing thromboembolic complications particularly due to the direct contact of their blood with the non-biological surfaces of the ECMO circuit tubing and membrane filter. This may ultimately trigger the activation of the clotting cascade to regenerate clotting factors increasing the thrombotic risk. Furthermore, the process of ECMO weaning is associated with the risk of thrombotic episodes due to blood stagnation during times of reduced flows. Therefore, optimal anticoagulation is essential for successful outcomes in patients on ECMO support. The Extracorporeal Life Support Organization has published guidelines regarding antithrombotic therapy in patients on ECMO.1
Unfractionated heparin (UFH) continuous infusion is the preferred anticoagulant in ECMO given its ease of administration and availability of protamine as a heparinoid reversal agent. However, UFH infusions bear an intrinsic risk for heparin-induced thrombocytopenia (HIT). Additionally, heparin anticoagulation chronically consumes antithrombin, activates platelets, and may result in non-immune-mediated thrombocytopenia as well as in HIT, which is quite a common feature in ECMO patients, with reported rates of 10%–15% and is of course a catastrophic complication of this procedure.2 In patients with contraindications or resistance to heparin, or to minimise the previously mentioned concerns (as was the decision in this case), alternative approaches for anticoagulation have been used. Bivalirudin is a direct thrombin inhibitor (DTI) that can be used as a primary agent or secondary in patients with HIT. There are few reports of resistance to DTIs. This case report describes a 48-year-old male patient requiring veno-venous ECMO support for hypoxaemic respiratory failure who failed to achieve therapeutic anticoagulation with bivalirudin therapy. Due to the possibility of evolving resistance to bivalirudin, the patient was switched to argatroban, an alternative DTI agent, and was successfully anticoagulated over a short period of time.
Case presentation
A 48-year-old male patient with a medical history significant for smoking and alcohol abuse, chronic obstructive pulmonary disorder, anxiety and bipolar disorder was admitted to the intensive care unit (ICU) as a hospital transfer for escalation in management of acute hypoxic respiratory failure secondary to influenza A pneumonia acute respiratory distress syndrome. The patient had progressively worsening respiratory failure despite being on maximal lung protective ventilatory support including prone positioning and neuromuscular blockade, antiviral therapy with oseltamivir, and a fluid restrictive strategy. He underwent veno-venous ECMO placement with the drainage and return cannulas to the right femoral vein and right internal jugular vein, respectively.
Treatment
The patient was initiated on bivalirudin 0.15 mg/kg/hour as part of the ECMO anticoagulation protocol at our institution. In this case, when resistance to anticoagulation therapy was suspected and increased in-circuit fibrin stranding was noted in the setting of suboptimal anticoagulation, the activated partial thromboplastin time (aPTT) therapeutic range was escalated from 50–70 to 60–90 after 4 days. The course of therapy, he required continuous bivalirudin dose escalation up to 0.54 mg/kg/hour (maximum dose according to our protocol is 0.6mg/kg/hour) with only a mild elevation of his aPTT ranging between 44.2 and 62.8 s with a required goal of 60–90 s (table 1). Following day 4 of therapy, the route of administration was changed and the drug was infused directly into the ECMO circuit preoxygenator. On day 6 of bivalirudin therapy, the ECMO circuit developed fibrin strands despite increasing the dose of bivalirudin from 0.34 mg/kg/hour to 0.54 mg/kg/hour within a 24-hour window (table 1). With concerns for bivalirudin resistance, the patient was transitioned to argatroban therapy and was initiated on a dose of 1.25 μg/kg/min). The argatroban dose was escalated to 2.2 μg/kg/min with an aPTT range of 72–85.1 s and no fibrin stranding in the ECMO circuit. All aPTT values were derived from blood sampled from an arterial line.
Table 1.
Bivalirudin anticoagulation management flowsheet tracked over course of treatment
| Date | Time | aPTT (s) |
aPTT goal (s) |
Dosing (mg/kg/hour) |
∆ in dosing | TEG | INR | |
| R (min) |
K (min) |
|||||||
| 25 April | 18:32 | 23.3 | 0.15 | 4.2 | 1.6 | 1 | ||
| 26 April | 01:51 | 62.6 | 40–60 | 0.15 | ↓ 20% | |||
| 26 April | 05:19 | 50–70 | 0.12 | ↑ 20% | ||||
| 26 April | 09:12 | 44.2 | 50–70 | 0.15 | ↑ 20% | |||
| 26 April | 15:42 | 45.6 | 50–70 | 0.18 | 0% | |||
| 26 April | 17:32 | 45.5 | 50–70 | 0.18 | 0% | |||
| 26 April | 21:49 | 48 | 50–70 | 0.234 | 0% | |||
| 27 April | 01:00 | 49.8 | 50–70 | 0.234 | ↑ 20% | |||
| 27 April | 03:54 | 9.5 | 1.1 | 1.6 | ||||
| 27 April | 09:55 | 51 | 50–70 | 0.28 | 0% | |||
| 27 April | 16:04 | 57 | 50–70 | 0.28 | 0% | |||
| 28 April | 03:39 | 49.1 | 50–70 | 0.28 | ↑ 20% | 8 | 1.3 | |
| 28 April | 10:00 | 51.5 | 50–70 | 0.34 | 0% | |||
| 28 April | 14:00 | 54 | 50–70 | 0.34 | 0% | |||
| 28 April | 21:47 | 54 | 50–70 | 0.34 | 0% | |||
| Bivalirudin to be infused into the circuit pre oxygenator | ||||||||
| 29 April | 03:26 | 60–90 | 0.34 | ↑ 20% | 9.4 | 1.2 | ||
| 29 April | 05:15 | 54.1 | 60–90 | 0.41 | ↑ 20% | |||
| 29 April | 09:53 | 62.8 | 60–90 | 0.49 | 0% | |||
| 29 April | 15:17 | 56.6 | 60–90 | 0.49 | ↑ 10% | |||
| 29 April | 19:50 | 61.1 | 60–90 | 0.54 | 0% | |||
| 29 April | 01:24 | 60.4 | 60–90 | 0.54 | 0% | 1.8 | ||
| 30 April | 03:57 | 58.8 | 60–90 | 0.54 | 0% | |||
| Multiple fibrin strands noted; bivalirudin to be infused via central line | ||||||||
| 30 April | 11:47 | 62.7 | 60–90 | 0.54 | 0% | |||
| 30 April | 13:53 | 61.7 | 60–90 | 0.54 | ↑ 20% | |||
| Switched anticoagulation therapy to argatroban due to possible resistance to bivalirudin | ||||||||
| 1 May | 04:54 | 64.3 | 60–90 | 1.76 | 0% | 11.6 | 2.8 | |
| 1 May | 09:55 | 56.6 | 60–90 | 2.2 | ↑ 20% | |||
| 1 May | 16:03 | 84.5 | 60–90 | 2.2 | 0% | |||
| 1 May | 21:39 | 72.6 | 60–90 | 2.2 | 0% | |||
aPTT, activated partial thromboplastin time; INR, international normalised ratio; TEG, thromboelastography.
Outcome and follow-up
The patient underwent the placement of a tracheostomy on day 12 of ECMO, and was successfully decannulated following 35 days of ECMO support. He developed thromboses in the right internal jugular and right femoral vein at the cannulation sites diagnosed 1 day after decannulation. He was weaned from mechanical ventilation 1 week later, and was transitioned to apixaban at the time of discharge.
Discussion
This case describes a patient with resistance to bivalirudin anticoagulation therapy while on veno-venous ECMO. At our institution, anticoagulation is typically started within the first 24–48 hours of cannulation, as to not compromise the circuit and the membrane. Additionally, the initial infusion rate of bivalirudin depends on the patient’s renal function; in a patient without renal dysfunction with a creatinine clearance greater than 60 mL/min, the rate is 0.15 mg/kg/hour. The aPTT therapeutic range is set at approximately 1.5–2.5 times the baseline aPTT value prior to initiation of therapy. Per institutional protocol the upper dosing limit for bivalirudin is 0.6 mg/kg/hour which is built as a hard stop in the pump guardrails for safety. The target aPTT (60–90) was just met with a maximum value of 62.8 despite 6 days of bivalirudin therapy with a maximum dose of 0.54 mg/kg/hour (table 1). It is important to note that higher doses of bivalirudin are used in other scenarios such as percutaneous coronary intervention, percutaneous transluminal coronary angioplasty, and the doses used here were high for their respective indication. On review of the literature, there are several reports of DTI resistance, requiring atypically large doses of bivalirudin, or complete resistance to argatroban and bivalirudin alike.3 4 To our knowledge, this is the first description of complete failure to achieve clinically significant anticoagulation in response to bivalirudin, with a successful response to argatroban therapy.
ECMO has been regarded as resource intensive, associated with prolonged duration of ventilator support, ICU length of stay and high mortality.5 Haemorrhagic and thromboembolic complications remain the major adverse consequences of ECMO treatment, being cited as the most frequent cause of death.6 Anticoagulation is essential for smooth functioning of the system to maximise the intended benefits.7
Systemic anticoagulation with UFH is the standard of care for patients on extracorporeal life support. When alternative regimens are necessary, bivalirudin has been demonstrated to be a potential option, but has been associated with a wide variation of dosing requirements (range 0.04–0.26 mg/kg/hour).4 8–11 DTIs bind directly to thrombin, independent of antithrombin, and there is no reversal agent currently available. Several proposed mechanisms for resistance include the complex interplay between bivalirudin metabolism (20% renal), continuous renal replacement therapy requiring higher doses, the presence of sepsis resulting in the release of heparinoids from the glycocalyx and the mast cells due to the systemic inflammatory response, and variable acute phase reactants that shorten the aPTT.3 9 12
In the case presented here, the highest aPTT on bivalirudin was 62.7, with a goal of 60–90 s (table 1). At a near maximal dose of bivalirudin running peripherally, and a less than expected aPTT for the given dose, along with fibrin buildup in the circuit and oxygenator, bivalirudin was administered directly into the ECMO circuit, with the intention of reducing fibrin stranding. The authors did not anticipate a change in the aPTT as a result of this, and were mindful of the lack of evidence. With little improvement a decision was made to transition to argatroban. After 1 day of argatroban therapy, an aPTT of 84.5 was achieved. The differential response between the DTIs suggests that bivalirudin resistance may involve additional factors, different from those that play a role in resistance to argatroban. Bivalirudin differs from argatroban in that it binds to the thrombin active site, as well as an additional exosite 1 on the thrombin molecule that is not bound by argatroban.13 These structural differences may explain why bivalirudin might fail at achieving therapeutic anticoagulation in patients with genetic thrombin mutations or structural defects that alter the binding pockets at the thrombin exosites. It is important to mention that there are limitations when using the aPTT (extrinsic pathway) to monitor the effectiveness of DTIs (common pathway, see figure 1) due to the non-linear dose response curve especially at higher doses.14 Alternatives including dilute thrombin time, ecarin thrombin time and chromogenic substrate based assays are under investigation.15–17 Nonetheless, the patient did develop fibrin strands within the ECMO circuit, consistent with the largely subtherapeutic response to bivalirudin therapy. To our knowledge, there are no objective criteria that recommend transitioning to another anticoagulant when suspecting DTI resistance. The authors propose that an inappropriately small increase in aPTT levels despite significant increases in the administered dose, in combination with clinical signs of thrombosis such as fibrin stranding within the circuit may serve as a queue to switch to another anticoagulant. In conclusion, bivalirudin may share some mechanisms that are similar to those described previously in patients on heparin or argatroban, and further research is necessary given the variable response between two drugs of the same class as highlighted in this report.
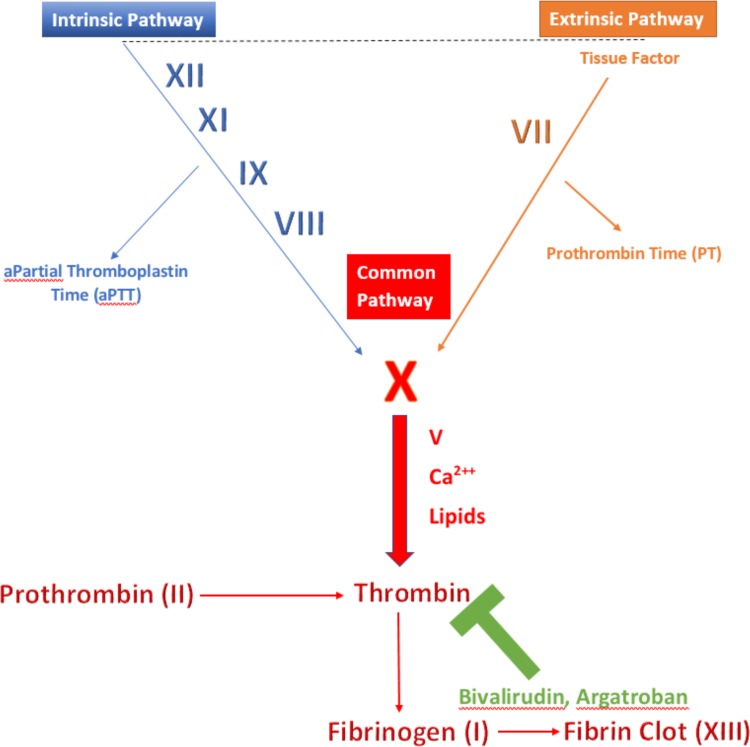
Figure 1.
Coagulation cascade. The figure points out the key components of the independent pathways within the coagulation cascade that join at a common pathway where bivalirudin binds to thrombin. The activated partial thromboplastin time is a laboratory test that characterises coagulation of blood with respect to the extrinsic pathway.
Patient’s perspective.
I really have a hard time remembering the events of my hospitalisation. The whole thing was a blur to me, particularly my time spent in the intensive care unit. I do remember the initial part of the flu, and how that made me feel prior to being admitted to the hospital. The next thing you know, I am short of breath and having a breathing tube placed. I’ll tell you, waking up without that breathing tube in place, and being alive has made me so grateful.
When I woke up with the tracheostomy, and unable to eat, I was shocked. My mother had told me what had happened, and that I was placed on a life-saving bypass like machine. I only had a 5% chance to live! I would recommend this life-saving measure to anybody! During the hospitalisation, I failed my speech and swallow examination, and had to have a feeding tube placed at the time of my discharge from the hospital. That was a pretty large hit for me, because sometimes I feel so incapable. Right now, I am working with my speech therapist and I have my next swallow evaluation coming up soon. I think this next one is going to be the charm, and I will be able to start eating food in my normal way again.
I have also been working pretty consistently with physical therapy, and I have been walking for quite some time. I have a plan to get back to work sometime in late August or early September. The journey was long, but I am very hopeful that things will turn out well. If it wasn’t for the lord himself, I would not be here. He gave the doctors, the wisdom and knowledge to take care of me. I know this. I know how sick I was, and without you guys I would have died.
Learning points.
There are few documented cases of direct thrombin inhibitor resistance in the extracorporeal membrane oxygenation (ECMO) population. To our knowledge, resistance to bivalirudin, with a successful response to argatroban therapy, has not yet been documented in the literature.
This case highlights a differential response between two drugs in the direct thrombin inhibitor class highlighting the complexities present when managing critically ill ECMO patients.
Resistance to bivalirudin remains unknown. There may be several mechanisms involved including: drug metabolism in renal failure, the presence of systemic inflammatory markers, acute phase reactants, thromboembolic burden and genetic predispositions.
As utilisation of ECMO increases, a multimodal approach to anticoagulation will be required.
Acknowledgments
We thank Daniel A Jackson, PharmD for his clinical expertise.
Footnotes
Twitter: @pkguru10
Contributors: DKS, BB and HSK conceived of the presented idea. BB took the lead in writing the manuscript, acquired the patient’s perspective and conducted the primary literature review. HSK conducted the initial chart review and tabulated the pharmacologic data. HSK contributed to the literature review with respect to pharmacology. DKS supervised the project and conducted a revision of the initial draft. PKG was editor to the final draft, providing valuable information. All authors provided critical feedback and helped.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Extracorporeal Life Support Organization - ECMO and ECLS ELSO anticoagulation guideline, 2014. Available: https://www.elso.org/Resources/Guidelines.aspx
- 2. Ranucci M, Ballotta A, Kandil H, et al. Bivalirudin-based versus conventional heparin anticoagulation for postcardiotomy extracorporeal membrane oxygenation. Crit Care 2011;15 10.1186/cc10556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cardinale M, Ha M, Liu MH, et al. Direct thrombin inhibitor resistance and possible mechanisms. Hosp Pharm 2016;51:922–7. 10.1310/hpj5111-922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Walker EA, Roberts AJ, Louie EL, et al. Bivalirudin dosing requirements in adult patients on extracorporeal life support with or without continuous renal replacement therapy. Asaio J 2019;65:134–8. 10.1097/MAT.0000000000000780 [DOI] [PubMed] [Google Scholar]
- 5. Bostwick A. Direct thrombin inhibitor resistance. Abstract published at hospital medicine 2015, March 29-April 1, National harbor, MD. Abstract 461. J Hospital Med 2015;10. [Google Scholar]
- 6. Sukhal S, Sethi J, Ganesh M, et al. Extracorporeal membrane oxygenation in severe influenza infection with respiratory failure: a systematic review and meta-analysis. Ann Card Anaesth 2017;20:14 10.4103/0971-9784.197820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cho HJ, Kim DW, Kim GS, et al. Anticoagulation therapy during extracorporeal membrane oxygenator support in pediatric patients. Chonnam Med J 2017;53:110 10.4068/cmj.2017.53.2.110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sanfilippo F, Asmussen S, Maybauer DM, et al. Bivalirudin for alternative anticoagulation in extracorporeal membrane oxygenation: a systematic review. J Intensive Care Med 2017;32:312–9. 10.1177/0885066616656333 [DOI] [PubMed] [Google Scholar]
- 9. Ranucci M, Baryshnikova E, Isgrò G, et al. Heparin-Like effect in postcardiotomy extracorporeal membrane oxygenation patients. Crit Care 2014;18 10.1186/s13054-014-0504-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee CJ, Ansell JE. Direct thrombin inhibitors. Br J Clin Pharmacol 2011;72:581–92. 10.1111/j.1365-2125.2011.03916.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weitz JI, Buller HR. Direct thrombin inhibitors in acute coronary syndromes: present and future. Circulation 2002;105:1004–11. 10.1161/hc0802.104331 [DOI] [PubMed] [Google Scholar]
- 12. Hellwig TR, Peitz GJ, Gulseth MP. High-Dose Argatroban for treatment of heparin-induced thrombocytopenia with thrombosis: a case report and review of laboratory considerations. Am J Health Syst Pharm 2012;69:490–5. 10.2146/ajhp110147 [DOI] [PubMed] [Google Scholar]
- 13. Weitz JI, Middeldorp S, Geerts W, et al. Thrombophilia and new anticoagulant drugs. Hematology 2004;2004:424–38. 10.1182/asheducation-2004.1.424 [DOI] [PubMed] [Google Scholar]
- 14. Hafner G, Roser M, Nauck M. Methods for the monitoring of direct thrombin inhibitors. Semin Thromb Hemost 2002;28:425–30. 10.1055/s-2002-35282 [DOI] [PubMed] [Google Scholar]
- 15. Curvers J, van de Kerkhof D, Stroobants AK, et al. Measuring direct thrombin inhibitors with routine and dedicated coagulation assays. Am J Clin Pathol 2012;138:551–8. 10.1309/AJCPQOD9WFPEYY0H [DOI] [PubMed] [Google Scholar]
- 16. Carroll RC, Chavez JJ, Simmons JW, et al. Measurement of patients' bivalirudin plasma levels by a thrombelastograph ecarin clotting time assay: a comparison to a standard activated clotting time. Anesth Analg 2006;102:1316–9. 10.1213/01.ane.0000205746.50440.98 [DOI] [PubMed] [Google Scholar]
- 17. Skrupky LP, Smith JR, Deal EN, et al. Comparison of bivalirudin and Argatroban for the management of heparin-induced thrombocytopenia. Pharmacotherapy 2010;30:1229–38. 10.1592/phco.30.12.1229 [DOI] [PubMed] [Google Scholar]