Abstract
In utero androgen excess reliably induces polycystic ovary syndrome (PCOS)-like reproductive and metabolic traits in female monkeys, sheep, rats and mice. In humans, however, substantial technical and ethical constraints on fetal sampling have curtailed safe, pathogenic exploration during gestation. Evidence consistent with in utero origins for PCOS in humans has thus been slow to amass, but the balance now leans towards developmental fetal origins. Given that PCOS is familial and highly heritable, difficulty in discerning genetic contributions to PCOS pathogenesis is puzzling and, to date, accounts for <10% of PCOS presentations. Unaccounted heritability notwithstanding, molecular commonality in pathogenic mechanism is emerging, suggested by co-occurrence of replicated PCOS genetic variants and epigenetic alterations in DNA methylation at the same replicated, PCOS risk loci with bioinformatics-identified gene loci within monkey epigenetic alterations in DNA methylation array-determined gene networks from females exposed to testosterone (T) in utero. In addition, naturally occurring female hyperandrogenism in monkeys singles out females with PCOS-like reproductive and metabolic traits accompanied by somatic biomarkers of in utero T exposure. Such phenotypic and molecular convergence between highly related species, suggests not only dual genetic and epigenetic contributions to PCOS, as well as a developmental origin, but also common molecular pathogenesis extending beyond humans.
Keywords: fetal programming, developmental origins of adult disease, nonhuman primate, animal model
Introduction
With a staggering 10–20% prevalence among premenopausal women, polycystic ovary syndrome (PCOS) is the most common endocrine and metabolic women’s health disorder [1]. Its diagnosis by Rotterdam criteria requires at least two of the following three: high testosterone (T) levels or hirsutism, intermittent or absent menstrual cycles, and ultrasonography-visualized polycystic ovaries, excluding endocrine mimics such as congenital adrenal hyperplasia [1]. Luteinizing hormone (LH) hypersecretion commonly accompanies hyperandrogenism likely due to T-diminished, estradiol (E2) induction of neural progesterone receptors in the mediobasal hypothalamus (MBH) [2,3], resulting in diminished E2-progesterone negative feedback regulation of gonadotropin-releasing hormone (GnRH) episodic release from the MBH [4]. Elevated ovarian granulosa cell anti-mullerian hormone (AMH) commonly accompanies polycystic ovary morphology and provides an accurate biomarker for increased numbers of growing follicles [5].
Clinical sequelae for PCOS include acne, infertility, endometrial hyperplasia and malignancy, obesity, type 2 diabetes (T2D), sleep apnea, cardiovascular disease, mood disorders and sexual dysfunction [1,6,7]. PCOS is thus a uniquely challenging, multi-faceted disorder with several phenotypes, in which progressive obesity enhances severity of phenotype, diminishes wellbeing and quality of life [8]. Overt signs and symptoms of PCOS usually manifest at puberty, progressing with age from issues related to appearance and reproduction, to weight gain accompanying metabolic sequelae and diminished wellbeing [1].
Given its complexity, PCOS is highly heritable and familial, and hyperandrogenism is its most heritable trait [1]. Pathogenic mechanisms, however, have remained elusive, hindering progress towards a cure. In this regard, in utero findings of hyperandrogenic origins for PCOS-like traits in nonhuman primates [9,10], provided a novel, single pathogenic origin for PCOS that mimicked PCOS phenotypic diversity in women (hence PCOS-like traits), and provided a potential epigenetic amplification mechanism [11] effectively reprogramming diverse genetic backgrounds into PCOS-like individuals [12]. Confirmatory findings soon followed in sheep [13], mice [14] and rats[15], expanding into molecular insight of developmental pathogenesis [16–18]. While clinical acceptance of in utero hyperandrogenic origins for PCOS is not universal [19], accumulating supportive evidence from human studies is shifting the balance of evidence towards pathogenic onset for PCOS during fetal life [1,12,20]. This brief review will focus on current understanding emerging from human and animal model studies concerning in utero androgen excess contributing to PCOS and its potential pathogenic mechanisms, with an emphasis on nonhuman primate findings.
In utero androgen excess: human studies
During early-to-mid gestation, ~40% of girls experience elevated circulating concentrations of unbound, bioavailable, testosterone (T) levels in the fetal male range [21]. This is relevant to PCOS since comparable circulating T excursions into the fetal male range generate PCOS-like traits in gestational T-exposed female rhesus monkeys [22] and sheep [23], revealing the devasting, life-long impact on females of in utero androgen excess. In short-gestation rodents, late gestation and the immediate post-partum period provide a comparable developmentally vulnerable period for females [12]. Perhaps not surprisingly, therefore, fetal male-similar T levels are found in mid-gestation amnionic fluid from daughters of women with PCOS, well exceeding those in mid-gestation daughters of women without PCOS [24]. As mid-gestation amnionic fluid T originates from the fetus [25], elevated T levels suggest hyperandrogenism in fetal daughters of women with PCOS during a crucial, developmental window when female nonhuman primates and sheep are vulnerable to PCOS-like reprogramming [12,16]. Approximately 50% of daughters born to PCOS women develop signs and symptoms of PCOS by adolescence [26], indicating the substantial risk for PCOS phenotype accompanying female in utero androgen excess in humans [12].
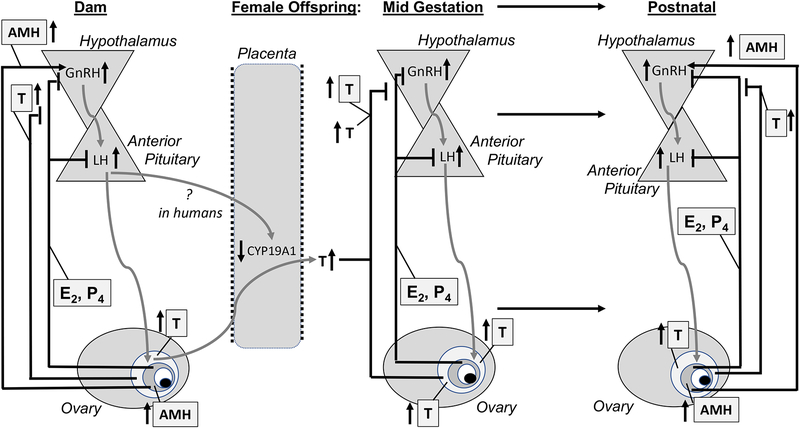
Pregnant women with PCOS retain hyperandrogenism throughout pregnancy [27], together with elevated AMH levels [28] and reduced placental aromatase expression [29]. Despite population differences [30], ~40% of PCOS women experience gestational diabetes [31] and other pregnancy complications [32], with maternal diabetes predisposing offspring to metabolic dysfunction in later life through fetal hyperinsulinemia [33]. Alternatively, a recent mouse model suggests that AMH expression in pregnancy (as seen in PCOS women) can promote both LH-mediated T excess and reduced placental aromatization of maternal androgens [28], thereby contributing to fetal hyperandrogenism in their daughters (Figure 1), although such a mechanism in humans is uncertain.
Figure 1.
Hypothetical maternal and fetal contributions to in utero female androgen excess reprogramming for a hyperandrogenic female offspring.
Post-natal consequences of in utero androgen excess are found as early as the newborn for women with PCOS. Infant daughters not only exhibit transient facial sebum [34], a biomarker of prior T exposure, but also demonstrate an elongated anogenital distance [35], a reliable biomarker for early-to-mid gestation androgen excess [36]. Newborn daughters of women with PCOS also exhibit elevated AMH levels indicative of increased numbers of ovarian antral follicles, a PCOS trait. In adulthood, women with PCOS retain an elongated anogenital distance ([37,38], typical of in utero, T-exposed, PCOS-like female monkeys [36] and sheep [23]. A diminished or exaggerated 2D:4D finger length ratio is also associated with both in utero androgen excess and PCOS, in women [39], their prepubertal daughters [24] and adult, early-to-mid gestation, T-exposed PCOS-like monkeys [36], since similar T- and E2-regulated genes control differentiation of gonads, hands and feet. Prepubertal daughters of women with PCOS also excrete increased concentrations of dihydrotestosterone (DHT) metabolites in their urine compared to prepubertal girls of women without PCOS [40], indicating increased 5-alpha reductase activity, and perhaps amplified target tissue androgen action, well before the onset of PCOS signs and symptoms at puberty.
Mixed umbilical cord blood androgen levels from human female fetuses at term, however, have yielded inconsistent results in support of late gestation fetal hyperandrogenism in daughters of PCOS women, regardless of whether “gold standard” liquid chromatography-tandem mass spectrometry (LC-MS/MS) assays are employed. Increased T or androstenedione levels are reported in two studies [41,42], equivalent levels in one [43], and diminished levels in a further two [29,44]. Labor onset and duration, together with increasing term gestational age, however, diminish umbilical cord androgen levels and likely often confound understanding of late gestation female androgenic state [45]. Moreover, no sex differences remain between circulating T levels in male and female human fetuses by late gestation [21], suggesting term birth is inauspicious for explore developmental hyperandrogenism.
Genetic origins: PCOS risk genes are compatible with in utero hyperandrogenic pathogenesis for PCOS
Family-based and extensive genome-wide association studies have yielded 17 replicated PCOS risk genes, regulating gonadotropin secretion (FSHB), gonadotropin action and ovarian function (LHCGR, FSHR, DENND1A, RAB5/SUOX, HMGA2, C9orf3, YAP1, TOX3, RAD50, FBN3), and various metabolic functions (THADA, GATA4/NEIL2, ERBB4, SUMO1P1, INSR and KRR1) [46–48]. Of the genes regulating gonadotropin and ovarian function, a substantial number have been proposed as enabling ovarian hyperandrogenism [46,49]. A recent alternative approach, employing rare gene variant association testing, followed by targeted resequencing of AMH in a replication cohort, identified an additional 17 PCOS-specific, rare coding and splice-site variants in AMH that diminish AMH signaling [49]. Ovarian hyperandrogenism is a potential outcome of reduced ovarian AMH inhibition of CYP17A1 expression [49]. While progress towards understanding gene variant-based PCOS heritability has clearly advanced, a heritability gap between low incidence of PCOS risk genes (~10%) and the high heritability of PCOS (~70%) [50], indicates a pressing need to identify (1) more PCOS risk genes, as each may confer a small degree of disease risk, (2) rare gene variants, as each may confer unduly large degrees of PCOS risk, and/or (3) epigenetic mechanisms altering a wide range of gene expression that confer considerable risk for PCOS. Current thinking embraces a combination of polygenic, epigenetic and developmental contributions to PCOS pathogenesis that are ameliorated or exaggerated by lifestyle [1,47].
Epigenetic origins: developmental contribution to PCOS
T and its biopotent metabolites are highly effective regulators of DNA methylation during fetal development. They enable the majority of phenotypic sexual differentiation in multiple organ systems and tissues, including brain [51]. Increased or decreased DNA methylation can diminish or enhance, respectively, mRNA transcription of inherited gene variants [52]. Different pattern and amount of DNA methylation at any single gene locus, however, are specific to each organ system or cell type in each individual. Unlike GWAS, therefore, there is less certainty as to how genome-wide methylation studies generalize beyond an organ system or cell type. DNA is differentially methylated in a variety of organ systems in women with PCOS [53,54]. Gene-targeted DNA methylation studies of LHCGR have reported its hypomethylation in blood cells and subcutaneous (SC) adipose of women with PCOS, concurrent with increased LHCGR mRNA expression [55–57]. If comparable DNA hypomethylation of LHCGR occurs in PCOS ovarian theca cells, it would likely increase androgenic responses to LH pulses, causing or amplifying ovarian hyperandrogenism. GWMS and bioinformatic pathway analyses have identified clusters of differentially methylated genes in PCOS women that may alter a variety of cellular functions and processes, including immune response pathways (including autoimmunity), ovarian steroidogenic and metabolic functions, and cancer-related pathways [56,58,59]. Notably, there are commonalties between identified PCOS risk genes and differentially methylated genes in PCOS women, including LHCGR, RAB5/SUOX, AMH/AMHR2 and INSR, suggesting convergence of molecular pathogenic mechanisms around the same critical genes. Do similar mechanistic insights emerge from animal models of in utero female hyperandrogenism?
Mechanistic PCOS insight from nonhuman primate models of in utero female hyperandrogenism
To induce fetal male levels of T in fetal female rhesus monkeys, monkey dams require daily SC injections of 10–15mg T propionate to generate circulating T levels equivalent to nighttime adult male levels of T (~20 ng/ml). Such high maternal levels are needed to exceed the primate liver’s ability to produce sex hormone binding globulin (SHBG), rendering >90% of circulating T non-bioavailable, as well as the primate placenta’s avid capacity to aromatize, conjugate and metabolize T, thus delivering a small fraction (1–2%) of dam T concentrations to a female monkey fetus [22], as determined by LC-MS/MS. Why start with such a challenging animal model? Until PCOS-like traits are reliably replicated in a nonhuman primate with >90% of its genome shared with humans, and with highly similar neuroendocrine, reproductive, metabolic, developmental, aging and behavioral attributes to humans [60], animal models are always open to criticism.
As illustrated in Table 1, T exposure in monkeys during early-to-mid gestation is more effective at inducing reproductive and metabolic PCOS-like signs and symptoms in female offspring than T exposure during late gestation, reinforcing the concept of a particularly vulnerable, mid-gestation developmental window for in utero T reprogramming of females. Mid-gestation excessive maternal weight gain and transient hyperglycemia, accompanied by fetal hyperglycemia, are all T-induced metabolic sequelae contributing additional reprogramming to exposed female fetuses [61]. As late gestation T exposure-induced PCOS-like traits demonstrate, however, a degree of fetal female monkey vulnerability to T reprogramming (and its gestational metabolic sequelae) remains beyond mid-gestation (Table 1). Increased incidence of gestational diabetes, as well as increased or diminished birthweight [54], accompanying PCOS gestations, closely emulating metabolic compromise of hyperandrogenic gestation in monkeys. Metabolically compromised gestation, alone, including obese monkeys and women [62], and women with T2D, is insufficient to cause PCOS. Increased T in utero, however, may reprogram female neurocircuitry controlling energy balance and increase vulnerability to in utero metabolic compromise [62].
Table 1.
PCOS-like reproductive and metabolic traits exhibited by in utero androgen excess animal models in adulthood, and by naturally occurring hyperandrogenic adult female monkeys.
| Species | Gestation staae | Route, treatment | Hyper-androaenism | Intermittent, absent cycles | Polycystic ovaries | Diminished fertility | Increased LH | Increased adiposity | Insulin resistance | Pancreatic defect |
|---|---|---|---|---|---|---|---|---|---|---|
| Monkey | Early-to-mid | M, TP | + | + | + | + | + | + | + | + |
| Late | M, TP | + | + | ? | − | − | + | − | − | |
| Late | M, letrozole | ? | + | ? | ? | − | − | ? | ? | |
| Naturally occurring | None | + | − | + | + | + | ? | + | ? | |
| Sheep | Early-to-late | M, T | +/− | + | + | + | + | + | + | ? |
| Early-to-late | M, DHT | − | − | + | ? | + | ? | + | ? | |
| Mid | M, T | − | + | ? | + | − | ? | ? | ? | |
| Mid-to-late | M, T | − | + | − | + | + | − | + | ? | |
| Mid-to-late | F, T | − | ? | ? | ? | ? | − | + | ? | |
| Rat | Late | M, T | + | + | − | + | + | + | − | ? |
| Late | M, DHT | + | + | Cystic | + | + | + | ? | ? | |
| Mouse | Late | M, T | ? | − | − | ? | ? | ? | ? | ? |
| Late | M, DHT | + | + | − | + | + | − | − | + | |
| Late | M, AMH | + | + | Cystic | + | + | − | − | − | |
| All | ARKO | − | + | − | + | − | ? | ? | ? | |
| All | NeuroArko | ? | + | + | − | + | ? | ? | ? | |
| All | AromKO | + | + | Cystic | + | + | + | − | ? | |
| All | ERaKO | + | + | Cystic | + | + | ? | ? | ? | |
| All | LHR excess | + | + | Cystic | + | + | ? | ? | ? |
M, administered to dam; F, administered to fetus; P, propionate; DHT, dihydrotestosterone; letrozole, aromatase inhibitor; ARKO, androgen receptor knockout; AromKO, aromatase knockout; ERaKO, estrogen receptor alpha knockout; LHR, LH receptor; Cystic, large follicle cysts, not more numerous, non-dominant antral follicles; ?, unknown.
Phenotypic manifestation of early-to-mid gestation T reprogramming of female monkeys begins with mid-gestation increase in fetal head size, followed in late gestation by hypolipidemia and fetal LH hypersecretion [22,61]. LH hypersecretion persists into early infancy, accompanied by modest hyperandrogenism [22], reflecting precocious development of insensitive negative feedback regulation of gonadotropin-releasing hormone (GnRH)/LH in the absence of mature ovarian hormone levels providing homeostatic constraint. While birthweight is normal, accompanying metabolic dysfunctions includes newborn hypoglycemia, accelerated infant weight gain and relative hyperinsulinemia related to defective pancreatic beta cell compensation for insulin sensitivity and excessive beta-to-alpha cell ratio in infant pancreatic islets [61,63]. Increased fetal growth, neonatal hypoglycemia and subsequent accelerated postnatal growth are typical of human pregnancies encountering excessive maternal weight gain and hyperglycemia [64], and such gestations greatly increase the risk of developing T2D in adulthood.
During adolescence, menarche is delayed by ~6 months in both early-to-mid and late gestation T-exposed female monkeys [65], but such pubertal delay is absent when lower amounts of T are administered to monkey dams [66]. Subsequent onset of menstrual cycles in early-to-mid gestation T-exposed monkeys is accompanied by a prolonged succession of luteal insufficiency [65], demonstrating adolescent origins of ovulatory cycle dysfunction, an attribute of adolescent girls presenting with PCOS [54].
By their reproductive years, early-to-mid gestation T-exposed female monkeys become comprehensive counterparts of women with PCOS. Ovarian and adrenal hyperandrogenism co-occur with intermittent and absent menstrual cycles, as well as large, polyfollicular ovaries [9,22]. Elevated LH levels are omnipresent, driven by increased hypothalamic GnRH pulse frequency and increased pituitary gonadotrope response to GnRH, both likely resulting from diminished sensitivity to E2- and progesterone-mediated negative feedback regulation [67], all neuroendocrine traits found in women with PCOS [68,69]. Oocyte developmental competence is compromised in these monkeys [70], and may reflect contributions from increased adiposity that accompanies diminished oocyte quality in women with PCOS [71]. Circulating AMH levels in T-exposed monkeys, however, do not exceed those of controls, and exhibit premature, aging-related decline [72]. While absence of AMH excess is not typical of women with PCOS, PCOS women over 30 years of age demonstrate a steeper decline in circulating AMH levels than their non-PCOS counterparts [73]. Early-to-mid gestation T reprogramming of ovarian follicle granulosa cells may therefore be less pronounced than ovarian theca, stroma or oocytes, and a maternal source of fetal hyperandrogenism, alone, may be insufficient to reprogram granulosa cell AMH hypersecretion or increased follicle number and proliferation.
Accompanying metabolic dysfunction is just as pronounced in adult T-exposed monkeys as in PCOS women, despite the monkeys’ non-obesogenic diet. Monkey visceral fat accumulation is increased (“metabolic obesity”), likely arising from PCOS-like hyperandrogenic adipogenic constraint, limiting SC adipocyte maturation and safe lipid storage [74]. Such pro-lipotoxic traits may contribute to hyperlipidemia-associated insulin resistance, impaired pancreatic beta cell compensation and compromised islet size [63] enabling increased progression to T2D [12]. Consistent with gestational origins of PCOS-like metabolic dysfunction, increased postnatal weight gain is associated with increased risk of PCOS in women [54], as well as T2D [64]. Interestingly, DNA methylation array analysis of visceral adipose identifies transforming growth factor beta (TGF-beta) signaling as the most significantly altered pathway in adult, T-exposed female monkeys [11], implicating an influential signaling pathway regulating adipocyte catabolism (brown or beige adipose, BAT) and adipocyte accumulation of lipid (white adipose, WAT) that potentially enables positive energy balance [75,76] favoring weight gain.
When T treatment of monkey dams begins peri-pubertally (renewable SC capsules generating circulating total T levels of ~ 1.5 ng/ml), and 50% are additionally switched to a “western-style diet” (T+WSD), age at first pregnancy is later, blood flow in the primary lobe of the placenta is diminished by early gestation, and these pregnant, T-treated females demonstrate glucose intolerance, hyperinsulinemia and insulin resistance, while female fetuses harvested during late gestation exhibit increased abdominal circumference suggestive of fetal adiposity [77]. Fetal female concentrations of T and other reproduction-related hormones, as well as postnatal phenotypes, have yet to be reported for female offspring of these T-treated female monkeys.
In utero androgen excess and female behavioral reprogramming
Sexual dysfunction [7] and depression [78] co-occur with PCOS in women. In this regard, and in addition to reproductive and metabolic dysfunction, early-to-mid gestation T exposure reprograms (“organizes”) prepubertal and adult female behavior. T-exposed female monkeys exhibit increased male-typical infant vocalizations, diminished intimate social grooming of mothers and interest in infants, increased mounting of peers, and diminished, but not absent, engagement in female-typical sexual interactions with males [79], all independent of circulating sex hormone levels. Such behavioral reprogramming is difficult to reconcile with traditional female gender roles in human societies, potentially leading to sexual dysfunction and depression. Recent reports from in utero androgen excess rodent models clearly demonstrate anxiety-like behavior in female offspring accompanied by upregulation of amygdala gene expression, including corticotropin-releasing hormone (CRH) [80], an identical neural site and neuropeptide system implicated in the pathogenesis of anxious phenotype in monkeys and humans [81], leading to depression.
Mechanistic PCOS insight from non-primate animal models of in utero female hyperandrogenism
Non-primate models of in utero androgen excess emulate many of the reproductive and metabolic traits found in PCOS women and T-exposed monkeys (Table 1). With their relative in expense and ease of manipulation, these models have generated a plethora of incisive pharmacological and molecular manipulations that provide additional insight into pathogenic mechanisms engaged by in utero androgen excess (recently reviewed by [16–18]). Differential gestational timing or duration of T exposure in female sheep (Table 1), illustrate the skew in gestational vulnerability to PCOS-like reprogramming of both reproductive and metabolic traits reported in female monkeys, and suggest that longer durations of T exposure commencing before mid-gestation induce a more pronounced PCOS-like phenotype. By late gestation, following cessation of maternal early-to-late T administration, increased ovarian theca cell expression of CYP17A1 and increased release of androstenedione are already present [82]. Postnatally, however, circulating androgen levels in such T-exposed sheep are not elevated, but ovarian androgen receptor expression is increased suggesting “functional hyperandrogenism” within an androgen target organ [83]. Early-to-late DHT exposure, while recapitulating T reprogramming of reproductive traits and insulin resistance, does not disrupt maturation of regular ovarian cycles or ovarian morphology (Table 1), suggesting limits to androgen receptor-mediated PCOS-like reprogramming. Maternal co-administration of the androgen receptor antagonist, flutamide, along with T during early-to-late gestation prevents early puberty, and likely LH hypersecretion, as well as onset of ovulatory dysfunction, PCOS-like ovarian morphology and ovarian steroidogenic abnormalities [84]. Gestational flutamide co-treatment, however, fails to prevent metabolic phenotype, including insulin resistance, adipogenic constraint, hyperlipidemia and fatty liver [16], again demonstrating limits to androgen receptor-mediated, PCOS-like reprogramming. In this regard, flutamide treatment of adult female mice previously exposed to fetal T reverses their acyclicity [14], and in some PCOS women, improves fertility, menstrual cyclicity and LH levels [85], as well as normalizing progesterone negative feedback regulation of episodic GnRH/LH release [69].
Gestational co-administration of the peroxisome proliferator-activated receptor gamma (PPARG or NR1C3), a nuclear transcription factor crucial for adipocyte maturation, along with T during early-to-late gestation, prevents insulin resistance and early puberty onset, and likely LH hypersecretion, in T-exposed female lambs, but does not prevent adipogenic dysfunction, hyperlipidemia and fatty liver [16]. In this regard, it is interesting that treatment of late gestation DHT-exposed female offspring as adults with the insulin sensitizer, metformin, restores normal cyclicity, as well as normalizing androgen and LH levels. Taken together, these findings suggest that while reprogramming of a variety of PCOS-like reproductive traits involves androgen receptor and/or insulin-mediated actions, adipogenic and lipogenic traits may involve additional reprogramming, perhaps engaging estrogenic T metabolites.
Mouse models have predominantly used the more biopotent androgenic T metabolite of DHT to induce late gestation in utero androgen excess and PCOS-like reprogramming (e.g., [14,17,18]). Late gestation DHT administered to female GnRH-green fluorescent protein (GFP)-transgenic mouse dams (derived from CBB6/F1 strain) produces PCOS-like female offspring exhibiting hyperandrogenism, intermittent/absent cycles, aberrant ovarian follicle morphology, LH hypersecretion derived from accelerated episodic GnRH release, fatty liver and enlarged adipocytes, without accompanying increased adiposity and insulin resistance (Table 1). Elegant use of genetic manipulation to globally delete androgen receptor (ARKO) protects fetal female mice (>98% C57BL/6J strain) from fetal DHT-induced, PCOS-like reprogramming, including absence of intermittent/absent cycles, aberrant follicle morphology and enlarged adipocytes [86]. The wild type mice from which ARKO females are derived, nevertheless, do not exhibit fetal DHT-induced hyperandrogenism or LH hypersecretion, possibly reflecting GnRH-GFP and ARKO mouse strain differences in fetal female susceptibility to DHT fetal reprogramming. Neural androgen receptor expression may be particularly crucial for DHT-mediated, PCOS-like reprogramming since selective deletion of neuronal androgen receptor expression, NeuroARKO, provides the best protection against peri-pubertal onset, DHT induction of PCOS-like traits [87]. NeuroARKO mice, however, have not yet been challenged with late gestation DHT to ascertain whether absence of neuronal androgen receptor abrogates in utero PCOS-like reprogramming. With less resemblance to in utero androgen excess in sheep, androgen receptors in in utero DHT-exposed female mice mediate the majority of PCOS-like reprogramming.
GnRH-GFP transgenic mice have also enabled neuro-immunohistochemical delineation of hypothalamic changes that may underlie PCOS-like reprogramming of GnRH release and its negative feedback regulation. Late gestation DHT increases anatomical and functional gamma-aminobutyric acid (GABA) neuronal connectivity to GnRH neurons, generating increased episodic release of GnRH, as well as LH hypersecretion, related to diminished progesterone negative feedback regulation [14,88]. Such enhanced GABA excitatory connectivity, originating from the hypothalamic arcuate nucleus, is established well before puberty, when circulating androgen levels are low [88]. Since GABA neurons express progesterone, estrogen and androgen receptors, and GnRH neurons do not express the former, aberrant GABA excitatory connectivity may mediate diminished progesterone (and E2) negative feedback regulation demonstrated by in utero androgen excess female mice, and potentially rats, sheep and monkeys.
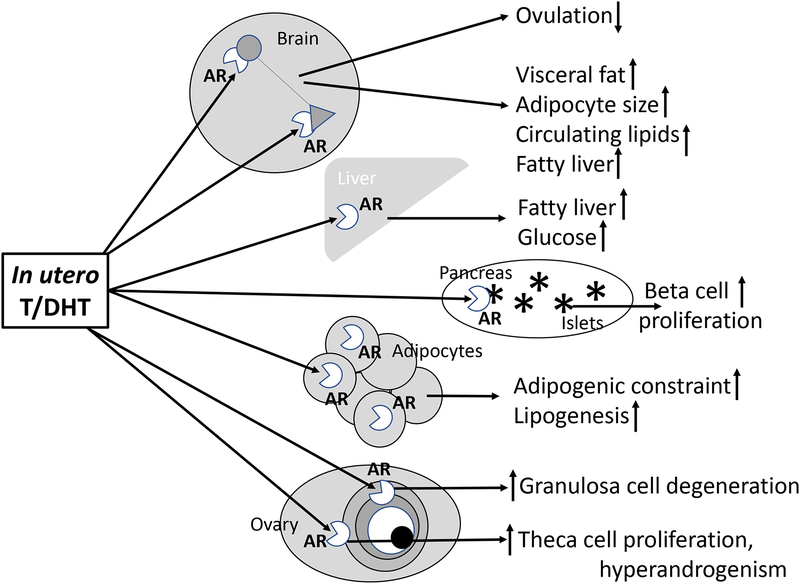
Unexpectedly, long-term administration of flutamide to adult, DHT-treated female mice normalizes neuronal connectivity between GABA and GnRH neurons, as well as episodic release of GnRH, circulating LH levels, negative feedback regulation of GnRH/LH, ovarian cyclicity and follicle morphology [88], suggesting extant, extra-ovarian androgen excess is crucial to maintain neuronal reprogramming and its PCOS-like sequelae. Figure 2 illustrates hypothetical sites, implicated by animal models, for specific, androgen receptor-mediated, in utero programming of PCOS-like traits. The implication, emphasized by sheep and transgenic mouse studies, is that abrogation of androgen production and action in specific organ systems may effectively normalize PCOS traits.
Figure 2.
Hypothetical target sites for androgen receptor mediated in utero androgen excess female fetal reprogramming for reproductive and metabolic PCOS-like traits.
Naturally occurring in utero androgen excess and female hyperandrogenism: Origins of PCOS beyond humans?
Naturally occurring high levels of T confer athletic advantage in women [89] and accompany female behavioral dominance in some, but not all, nonhuman primates [90,91]. About 15–25% of women in their reproductive years exhibit androgen excess, but most have PCOS [92]. Naturally occurring, hyperandrogenic (high T) female macaque monkeys emulate PCOS women in the co-occurrence of PCOS-like traits [93,94]. In a multi-Primate Research Center study, adult female macaques with average BMI and in prime reproductive years, were identified with high levels of T, 1SD above each population mean, in three separate laboratory populations of macaques. In the Wisconsin rhesus monkey population [94], PCOS-like traits of high T females exhibited generalized hyperandrogenism and increased steroidogenesis, including elevated circulating levels of unbound, “free” testosterone (unpublished results), hypersecretion of both LH and AMH, as well as uterine endometrial hyperplasia and infertility (Table 1). Intriguingly, a predominance of infertility and insulin resistance are found among high T monkeys with the most extreme elevations of T, 2SD above the monkey population mean (unpublished results), and equivalent to hyperandrogenic criteria required for peer-reviewed clinical studies of PCOS [95].
Thus, within limits, high T may not impair either fertility or metabolic function. Subtle changes in anatomical biomarkers of prior T exposure suggest fetal origins for naturally hyperandrogenic female rhesus monkeys [94], analogous to findings in behaviorally dominant female Malagasy lemurs [91], a more ancient branch of nonhuman primates. Interestingly, mild-to-moderate PCOS-like phenotypes formed the majority of those identified among naturally hyperandrogenic female monkeys, emulating PCOS phenotype prevalence in studies of women recruited from local populations, and not from clinical referrals (Table 2). The contrast of predominantly mild-to-moderate phenotypes in naturally occurring PCOS-like monkeys and PCOS women recruited from local human populations to the predominantly severe and classic phenotypes of early-to-mid gestation T-exposed monkeys and clinically referred PCOS women suggests commonality in PCOS phenotype may include duration or degree of gestational T exposure, and age and BMI (younger age and normal BMI with more mild-to-moderate phenotype). Naturally occurring PCOS-like phenotypes beyond humans certainly supports increasing speculation of survival and reproductive advantages from hyperandrogenic, energy-conserving, insulin resistant, delayed fecundity, female phenotypes [1,96].
Table 2.
Classification of PCOS-Like Phenotypes in PCOS-like Adult Female Rhesus Monkeys and in women with PCOS
| Female Population | PCOS-Like/PCOS Phenotype1 | |||
|---|---|---|---|---|
| (% of PCOS-like and PCOS individuals) | ||||
| Type A | Type B | Type C | Type D | |
| Early-to-mid gestation T-exposed female monkeys2 | 38 | 25 | 12 | 25 |
| PCOS women3 (from clinical referrals) | 49 | 13 | 14 | 17 |
| Naturally occurring PCOS-like female monkeys4 | 25 | 8 | 42 | 25 |
| PCOS women5 (from unselected human populations) | 25 | 19 | 35 | 20 |
Type A: hyperandrogenism or hirsutism (HA) + intermittent/absent cycles (OD) + (polycystic ovary morphology) (PCOM); Type B: HA + OD; Type C: HA + PCOM; Type D: OD + PCOM, as described in Abbott, 2017
Derived from Abbott et al., 2013
Derived from Norman et al., 2007 Lancet 370:685; Wild et al., 2010 J Clin Endocrinol Metab 95:2038; Dumesic et al., 2015 Endocr Rev. 36:487
Derived from Abbott, 2017
In utero Androgen Excess and Androgen Receptor: Developmental Commonality and Molecular Gateway to PCOS?
Mounting evidence from human and animal studies repeatedly implicates appropriately timed in utero androgen excess, from either maternal and/or fetal sources, as high risk for PCOS emerging at adolescence. Figure 1 provides a diagrammatic representation of maternal and fetal sources of gestational androgen excess, together with relevant PCOS risk genes, programming for ovarian androgen excess. Figure 2 illustrates hypothetical sites for female reprogramming mediated by androgen receptor, as identified by genetically manipulated mouse studies [17,18]. Such a unified hypothetical model is compatible with postnatal androgen activating reprogrammed function, such that anti-androgens or androgen-diminishing consequences of weight loss interventions, including lifestyle, diet, bariatric surgery, and insulin sensitizing treatments, ameliorate PCOS traits in adulthood. Increasing sophistication of bioinformatics to assess risk for functional outcome of genomic and epigenomic variants vulnerable to in utero androgen excess, hold promise for identification of PCOS risk in newborn, enabling early intervention.
Acknowledgements
This work was funded, in part, by NIH grants P50 HD028934 (PI: Marshall), P50 HD044405 (PI: Dunaif), and P50 HD071836 (PI: Stouffer). The authors have no potential conflicts of interest.
References
- 1.Escobar-Morreale HF. Polycystic ovary syndrome: definition, aetiology, diagnosis and treatment. Nat Rev Endocrinol. 2018;14;270. [DOI] [PubMed] [Google Scholar]
- 2.Foecking EM, McDevitt MA, Acosta-Martínez M, Horton TH, Levine JE. Neuroendocrine consequences of androgen excess in female rodents. Horm Behav. 2008;53;673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moore AM, Campbell RE. Polycystic ovary syndrome: Understanding the role of the brain. Front Neuroendocrinol. 2017;46;1. [DOI] [PubMed] [Google Scholar]
- 4.Burt Solorzano CM, Beller JP, Abshire MY, Collins JS, McCartney CR, Marshall JC. Neuroendocrine dysfunction in polycystic ovary syndrome. Steroids. 2012;77;332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lie Fong S, Laven JSE, Duhamel A, Dewailly D. Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Müllerian hormone using cluster analysis. Hum Reprod. 2017;32;1723. [DOI] [PubMed] [Google Scholar]
- 6.Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, Lobo R, Norman RJ, Talbott E, Dumesic DA. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome: a consensus statement by the Androgen Excess and Polycystic Ovary Syndrome (AE-PCOS) Society. J Clin Endocrinol Metab. 2010;95;2038. [DOI] [PubMed] [Google Scholar]
- 7.Nasiri Amiri F, Ramezani Tehrani F, Esmailzadeh S, Tohidi M, Azizi F, Basirat Z. Sexual function in women with polycystic ovary syndrome and their hormonal and clinical correlations. Int J Impot Res. 2018;30;54. [DOI] [PubMed] [Google Scholar]
- 8.Teede HJ, Joham AE, Paul E, Moran LJ, Loxton D, Jolley D, Lombard C. Longitudinal weight gain in women identified with polycystic ovary syndrome: results of an observational study in young women. Obesity (Silver Spring). 2013;21;1526. [DOI] [PubMed] [Google Scholar]
- 9.Abbott DH, Dumesic DA, Eisner JR, Kemnitz JW, Goy RW. The prenatally androgenized female rhesus monkey as a model for polycystic ovarian syndrome In: Azziz R, Nestler JE and Dewailly D (eds). Androgen Excess Disorders in Women. Philadelphia, Lippencott-Raven Press, 1997, pp. 369–382. [Google Scholar]
- 10.Dumesic DA, Abbott DH, Eisner JR, Goy RW. Prenatal exposure of female rhesus monkeys to testosterone propionate increases serum luteinizing hormone levels in adulthood. Fertil Steril. 1997;67;155. [DOI] [PubMed] [Google Scholar]
- 11.Xu N, Kwon S, Abbott DH, Geller DH, Dumesic DA, Azziz R, Guo X, Goodarzi MO. Epigenetic mechanism underlying the development of polycystic ovary syndrome(PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One. 2011;6;e27286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbott DH, Levine JE, Dumesic DA. Translational Insight Into Polycystic Ovary Syndrome (PCOS) From Female Monkeys with PCOS-like Traits. Curr Pharm Des. 2016;22;5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.West C, Foster DL, Evans NP, Robinson J, Padmanabhan V. Intra-follicular activin availability is altered in prenatally-androgenized lambs. Mol Cell Endocrinol. 2001;185;51. [DOI] [PubMed] [Google Scholar]
- 14.Sullivan SD, Moenter SM. Prenatal androgens alter GABAergic drive to gonadotropin-releasing hormone neurons: implications for a common fertility disorder. Proc Natl Acad Sci U S A. 2004;101;7129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foecking EM, Szabo M, Schwartz NB, Levine JE. Neuroendocrine consequences of prenatal androgen exposure in the female rat: absence of luteinizing hormone surges, suppression of progesterone receptor gene expression, and acceleration of the gonadotropin-releasing hormone pulse generator. Biol Reprod. 2005;72;1475. [DOI] [PubMed] [Google Scholar]
- 16.Padmanabhan V, Cardoso RC, Puttabyatappa M. Developmental Programming, a Pathway to Disease. Endocrinology. 2016;157;1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walters KA. Androgens in polycystic ovary syndrome: lessons from experimental models. Curr Opin Endocrinol Diabetes Obes. 2016;23;257. [DOI] [PubMed] [Google Scholar]
- 18.Moore AM, Campbell RE. Polycystic ovary syndrome: Understanding the role of the brain. Front Neuroendocrinol. 2017;46:1. [DOI] [PubMed] [Google Scholar]
- 19.de Zegher F, Ibáñez L. Early Origins of polycystic ovary syndrome: hypotheses may change without notice. J Clin Endocrinol Metab. 2009;94;3682. [DOI] [PubMed] [Google Scholar]
- 20.Filippou P, Homburg R. Is foetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update. 2017;23;421. [DOI] [PubMed] [Google Scholar]
- 21.Beck-Peccoz P, Padmanabhan V, Baggiani AM, Cortelazzi D, Buscaglia M, Medri G, Marconi AM, Pardi G, Beitins IZ. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73;525. [DOI] [PubMed] [Google Scholar]
- 22.Abbott DH, Barnett DK, Levine JE, Padmanabhan V, Dumesic DA, Jacoris S, Tarantal AF. Endocrine antecedents of polycystic ovary syndrome in fetal and infant prenatally androgenized female rhesus monkeys. Biol Reprod. 2008;79;154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veiga-Lopez A, Steckler TL, Abbott DH, Welch KB, MohanKumar PS, Phillips DJ, Refsal K, Padmanabhan V. Developmental programming: impact of excess prenatal testosterone on intrauterine fetal endocrine milieu and growth in sheep. Biol Reprod. 2011;84;87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palomba S, Marotta R, Di Cello A, Russo T, Falbo A, Orio F, Tolino A, Zullo F, Esposito R, La Sala GB. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol (Oxf). 2012;77;898. [DOI] [PubMed] [Google Scholar]
- 25.Schindler AE. Hormones in human amniotic fluid. Monogr Endocrinol. 1982;21;1. [PubMed] [Google Scholar]
- 26.Cesta CE, Månsson M, Palm C, Lichtenstein P, Iliadou AN, Landén M. Polycystic ovary syndrome and psychiatric disorders: Co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. 2016;73;196. [DOI] [PubMed] [Google Scholar]
- 27.Sir-Petermann T, Maliqueo M, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17;2573. [DOI] [PubMed] [Google Scholar]
- 28.Tata B, Mimouni NEH, Barbotin AL, Malone SA, Loyens A, Pigny P, Dewailly D, Catteau-Jonard S, Sundström-Poromaa I, Piltonen TT, Dal Bello F, Medana C, Prevot V, Clasadonte J, Giacobini P. Elevated prenatal anti-Müllerian hormone reprograms the fetus and induces polycystic ovary syndrome in adulthood. Nat Med. 2018. May 14. doi: 10.1038/s41591-018-0035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166;151. [DOI] [PubMed] [Google Scholar]
- 30.Fux-Otta C, Maliqueo M, Echiburú B, Rosato O, Crisosto N, Iraci GS, Fiol de Cuneo M, Szafryk de Mereshian P, Sir-Petermann T. Pregnancy outcomes in women with polycystic ovary syndrome in two Latin American populations. J Obstet Gynaecol. 2018. March 14:1 doi: 10.1080/01443615.2017.1410532. [DOI] [PubMed] [Google Scholar]
- 31.Palm CVB, Glintborg D, Kyhl HB, McIntyre HD, Jensen RC, Jensen TK, Jensen DM, Andersen M. Polycystic ovary syndrome and hyperglycaemia in pregnancy. A narrative review and results from a prospective Danish cohort study. Diabetes Res Clin Pract. 2018. April 22 pii: S0168–8227(18)30554–0. Doi: 10.1016/j.diabres.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Bahri Khomami M, Boyle JA, Tay CT, Vanky E, Teede HJ, Joham AE, Moran LJ. Polycystic ovary syndrome and adverse pregnancy outcomes: Current state of knowledge, challenges and potential implications for practice. Clin Endocrinol (Oxf). 2018. February 20. doi: 10.1111/cen.13579. [DOI] [PubMed] [Google Scholar]
- 33.Weiss PAM, Scholz HS, Haas J, Tamussino KF, Seissler J, Borkenstein MH. Long-term follow-up of diabetes of mothers with type I diabetes. Evidence for hereditary and nonhereditary transmission of diabetes and precursors. Diabetes Care 2000;23;905. [DOI] [PubMed] [Google Scholar]
- 34.Homburg R, Gudi A, Shah A, M Layton A. A novel method to demonstrate that pregnant women with polycystic ovary syndrome hyper-expose their fetus to androgens as a possible stepping stone for the developmental theory of PCOS. A pilot study. Reprod Biol Endocrinol. 2017. August 8;15(1):61. doi: 10.1186/s12958-017-0282-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barrett ES, Hoeger KM, Sathyanarayana S, Abbott DH, Redmon JB, Nguyen RHN, Swan SH. Anogenital distance in newborn daughters of women with polycystic ovary syndrome indicates fetal testosterone exposure. J Dev Orig Health Dis. 2018. January 9:1–8. doi: 10.1017/S2040174417001118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abbott AD, Colman RJ, Tiefenthaler R, Dumesic DA, Abbott DH. Early-to-mid gestation fetal testosterone increases right hand 2D:4D finger length ratio in polycystic ovary syndrome-like monkeys. PLoS One. 2012;7(8):e42372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Ferrer ML, Mendiola J, Hernández-Peñalver AI, Corbalán-Biyang S, Carmona-Barnosi A, Prieto-Sánchez MT, Nieto A, Torres-Cantero AM. Presence of polycystic ovary syndrome is associated with longer anogenital distance in adult Mediterranean women. Hum Reprod. 2017;32;2315. [DOI] [PubMed] [Google Scholar]
- 38.Wu Y, Zhong G, Chen S, Zheng C, Liao D, Xie M. Polycystic ovary syndrome I associated with anogenital distance, a marker of prenatal androgen exposure. Hum Reprod. 2017;32;937. [DOI] [PubMed] [Google Scholar]
- 39.Lujan ME, Bloski TG, Chizen DR, Lehotay DC, Pierson RA. Digit ratios do not serve as anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Hum Reprod. 2010;25;204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Torchen LC, Idkowiak J, Fogel NR, O’Neil DM, Shackleton CH, Arlt W, Dunaif A. Evidence for Increased 5α-Reductase Activity During Early Childhood in Daughters of Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2016;101;2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barry JA, Kay AR, Navaratnarajah R, Iqbal S, Bamfo JE, David AL, Hines M, Hardiman PJ. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30;444. [DOI] [PubMed] [Google Scholar]
- 42.Daan NM, Koster MP, Steegers-Theunissen RP, Eijkemans MJ, Fauser BC. Endocrine and cardiometabolic cord blood characteristics of offspring born to mothers with and without polycystic ovary syndrome. Fertil Steril. 2017;107;261. [DOI] [PubMed] [Google Scholar]
- 43.Caanen MR, Kuijper EA, Hompes PG, Kushnir MM, Rockwood AL, Meikle WA, Homburg R, Lambalk CB. Mass spectrometry methods measured androgen and estrogen concentrations during pregnancy and in newborns of mothers with polycystic ovary syndrome. Eur J Endocrinol. 2016;174;25. [DOI] [PubMed] [Google Scholar]
- 44.Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL, Dunaif A. Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95;2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollier LP, Keelan JA, Hickey M, Maybery MT, Whitehouse AJ. Measurement of androgen and estrogen concentrations in cord blood: accuracy, biological interpretation, and applications to understanding human behavioral development. Front Endocrinol (Lausanne). 2014;5;64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McAllister JM, Legro RS, Modi BP, Strauss JF 3rd. Functional genomics of PCOS: from GWAS to molecular mechanisms. Trends Endocrinol Metab. 2015;26;118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dunaif A Perspectives in Polycystic Ovary Syndrome: From Hair to Eternity. J Clin Endocrinol Metab. 2016;101;759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pau CT, Mosbruger T, Saxena R, Welt CK. Phenotype and Tissue Expression as a Function of Genetic Risk in Polycystic Ovary Syndrome. PLoS One. 2017;12;e0168870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gorsic LK, Kosova G, Werstein B, Sisk R, Legro RS, Hayes MG, Teixeira JM, Dunaif A, Urbanek M. Pathogenic Anti-Müllerian Hormone Variants in Polycystic Ovary Syndrome. J Clin Endocrinol Metab. 2017;102;2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91;2100. [DOI] [PubMed] [Google Scholar]
- 51.Nugent BM, Bale TL. The omniscient placenta: Metabolic and epigenetic regulation of fetal programming. Front Neuroendocrinol. 2015;39;28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corso-Díaz X, Jaeger C, Chaitankar V, Swaroop A. Epigenetic control of gene regulation during development and disease: A view from the retina. Prog Retin Eye Res. 2018. March 12 pii: S1350–9462(17)30104–0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li S, Zhu D, Duan H, Tan Q. The epigenomics of polycystic ovarian syndrome: from pathogenesis to clinical manifestations. Gynecol Endocrinol. 2016;32;942. [DOI] [PubMed] [Google Scholar]
- 54.Ibáñez L, Oberfield SE, Witchel S, Auchus RJ, Chang RJ, Codner E, Dabadghao P, Darendeliler F, Elbarbary NS, Gambineri A, Garcia Rudaz C, Hoeger KM, López-Bermejo A, Ong K, Peña AS, Reinehr T, Santoro N, Tena-Sempere M, Tao R, Yildiz BO, Alkhayyat H, Deeb A, Joel D, Horikawa R, de Zegher F, Lee PA. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolescence. Horm Res Paediatr. 2017;88;371. [DOI] [PubMed] [Google Scholar]
- 55.Wang P, Zhao H, Li T, Zhang W, Wu K, Li M, Bian Y, Liu H, Ning Y, Li G, Chen ZJ. Hypomethylation of the LH/choriogonadotropin receptor promoter region is a potential mechanism underlying susceptibility to polycystic ovary syndrome. Endocrinology. 2014;155;1445. [DOI] [PubMed] [Google Scholar]
- 56.Jones MR, Brower MA, Xu N, Cui J, Mengesha E, Chen YD, Taylor KD, Azziz R, Goodarzi MO. Systems Genetics Reveals the Functional Context of PCOS Loci and Identifies Genetic and Molecular Mechanisms of Disease Heterogeneity. PLoS Genet. 2015;11;e1005455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Song J, Luo S, Li SW. miRNA-592 is downregulated and may target LHCGR in polycystic ovary syndrome patients. Reprod Biol. 2015;15;229. [DOI] [PubMed] [Google Scholar]
- 58.Wang XX, Wei JZ, Jiao J, Jiang SY, Yu DH, Li D. Genome-wide DNA methylation and gene expression patterns provide insight into polycystic ovary syndrome development. Oncotarget. 2014;5;6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu YY, Sun CX, Liu YK, Li Y, Wang L, Zhang W. Genome-wide screen of ovary-specific DNA methylation in polycystic ovary syndrome. Fertil Steril. 2015;104;145. [DOI] [PubMed] [Google Scholar]
- 60.Stouffer RL, Woodruff TK. Nonhuman Primates: A Vital Model for Basic and Applied Research on Female Reproduction, Prenatal Development, and Women’s Health. ILAR J. 2017;58;281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Abbott DH, Bruns CR, Barnett DK, Dunaif A, Goodfriend TL, Dumesic DA, Tarantal AF. Experimentally induced gestational androgen excess disrupts glucoregulation in rhesus monkey dams and their female offspring. Am J Physiol Endocrinol Metab. 2010;299;E741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sullivan EL, Rivera HM, True CA, Franco JG, Baquero K, Dean TA, Valleau JC, Takahashi DL, Frazee T, Hanna G, Kirigiti MA, Bauman LA, Grove KL, Kievit P. Maternal and postnatal high-fat diet consumption programs energy balance and hypothalamic melanocortin signaling in nonhuman primate offspring. Am J Physiol Regul Integr Comp Physiol. 2017;313;R169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nicol LE, O’Brien TD, Dumesic DA, Grogan T, Tarantal AF, Abbott DH. Abnormal infant islet morphology precedes insulin resistance in PCOS-like monkeys. PLoS One. 2014;9;e106527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martin B, Sacks DA. The global burden of hyperglycemia in pregnancy – Trends from studies in the last decade. Diabetes Res Clin Pract. 2018. April 19 pii: S0168–8227(18)30490-X. doi: 10.1016/j.diabres.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Goy RW, Robinson JA. Prenatal exposure of rhesus monkeys to patent androgens: morphological, behavioral and physiological consequences. Banbury Report 11;355 [Google Scholar]
- 66.Zehr JL, Van Meter PE, Wallen K. Factors regulating the timing of puberty onset in female rhesus monkeys (Macaca mulatta): role of prenatal androgens, social rank, and adolescent body weight. Biol Reprod. 2005;72;1087. [DOI] [PubMed] [Google Scholar]
- 67.Abbott DH, Hoffman SE Horton TH, Terasawa E, Levine JE. Accelerated episodic LH release accompanies blunted progesterone regulation in PCOS-like female rhesus monkeys (Macaca mulatta) exposed to testosterone during early-to-mid gestation. Neuroendocrinology 2018;00;000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Daniels TL, Berga SL. Resistance of gonadotropin releasing hormone drive to sex steroid-induced suppression in hyperandrogenic anovulation. J Clin Endocrinol Metab. 1997;82;4179. [DOI] [PubMed] [Google Scholar]
- 69.Eagleson CA, Gingrich MB, Pastor CL, Arora TK, Burt CM, Evans WS, Marshall JC. Polycystic ovarian syndrome: evidence that flutamide restores sensitivity of the gonadotropin-releasing hormone pulse generator to inhibition by estradiol and progesterone. J Clin Endocrinol Metab. 2000;85;4047. [DOI] [PubMed] [Google Scholar]
- 70.Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH. Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87;1111. [DOI] [PubMed] [Google Scholar]
- 71.Broughton DE, Moley KH. Obesity and female infertility: potential mediators of obesity’s impact. Fertil Steril. 2017;107;840. [DOI] [PubMed] [Google Scholar]
- 72.Dumesic DA, Patankar MS, Barnett DK, Lesnick TG, Hutcherson BA, Abbott DH. Early prenatal androgenization results in diminished ovarian reserve in adult female rhesus monkeys. Hum Reprod. 2009;24;3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ahmad AK, Kao CN, Quinn M, Lenhart N, Rosen M, Cedars MI, Huddleston H. Differential rate in decline in ovarian reserve markers in women with polycystic ovary syndrome compared with control subjects: results of a longitudinal study. Fertil Steril. 2018;109;526. [DOI] [PubMed] [Google Scholar]
- 74.Keller E, Chazenbalk GD, Aguilera P, Madrigal V, Grogan T, Elashoff D, Dumesic DA, Abbott DH. Impaired preadipocyte differentiation into adipocytes in subcutaneous abdominal adipose of PCOS-like female rhesus monkeys. Endocrinology. 2014;155;2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pervin S, Singh V, Tucker A, Collazo J, Singh R. Modulation of transforming growth factor-β/follistatin signaling and white adipose browning: therapeutic implications for obesity related disorders. Horm Mol Biol Clin Investig. 2017. September 9;31(2). pii: /j/hmbci.2017.31.issue-2/hmbci-2017–0036/hmbci-2017–0036.xml. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sam S Differential effect of subcutaneous abdominal and visceral adipose tissue on cardiometabolic risk. Horm Mol Biol Clin Investig. 2018. March 9;33(1). pii: /j/hmbci.2018.33.issue-1/hmbci-2018–0014/hmbci-2018–0014.xml. [DOI] [PubMed] [Google Scholar]
- 77.Bishop CV, Stouffer RL, Takahashi DL, Mishler EC, Wilcox MC, Slayden OD, True CA. Chronic hyperandrogenemia and western-style diet beginning at puberty reduces fertility and increases metabolic dysfunction during pregnancy in young adult, female macaques. Hum Reprod. 2018;33;694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Berni TR, Morgan CL, Berni ER, Rees DA. Polycystic ovary syndrome is associated with adverse mental health and neurodevelopmental outcomes. J Clin Endocrinol Metab. 2018. April 10. doi: 10.1210/jc.2017-02667. [DOI] [PubMed] [Google Scholar]
- 79.Thornton J, Zehr JL, Loose MD. Effects of prenatal androgens on rhesus monkeys: a model system to explore the organizational hypothesis in primates. Horm Behav. 2009;55;633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manti M, Fornes R, Qi X, Folmerz E, Lindén Hirschberg A, de Castro Barbosa T, Maliqueo M, Benrick A, Stener-Victorin E. Maternal androgen excess and obesity induce sexually dimorphic anxiety-like behavior in the offspring. FASEB J. 2018. March 22:fj201701263RR. doi: 10.1096/fj.201701263RR. [DOI] [PubMed] [Google Scholar]
- 81.Rogers J, Raveendran M, Fawcett GL, Fox AS, Shelton SE, Oler JA, Cheverud J, Muzny DM, Gibbs RA, Davidson RJ, Kalin NH. CRHR1 genotypes, neural circuits and the diathesis for anxiety and depression. Mol Psychiatry. 2013;18;700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hogg K, Young JM, Oliver EM, Souza CJ, McNeilly AS, Duncan WC. Enhanced thecal androgen production is prenatally programmed in an ovine model of polycystic ovary syndrome. Endocrinology. 2012;153;450. [DOI] [PubMed] [Google Scholar]
- 83.Ortega HH, Salvetti NR, Padmanabhan V. Developmental programming: prenatal androgen excess disrupts ovarian steroid receptor balance. Reproduction. 2009;137;865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Veiga-Lopez A, Moeller J, Abbott DH, Padmanabhan V. Developmental programming: rescuing disruptions in preovulatory follicle growth and steroidogenesis from prenatal testosterone disruption. J Ovarian Res. 2016;9;39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.De Leo V, Lanzetta D, D’Antona D, la Marca A, Morgante G. Hormonal effects of flutamide in young women with polycystic ovary syndrome. J Clin Endocrinol Metab. 1998;83;99. [DOI] [PubMed] [Google Scholar]
- 86.Caldwell AS, Eid S, Kay CR, Jimenez M, McMahon AC, Desai R, Allan CM, Smith JT, Handelsman DJ, Walters KA. Haplosufficient genomic androgen receptor signaling is adequate to protect female mice from induction of polycystic ovary syndrome features by prenatal hyperandrogenization. Endocrinology. 2015;156;1441. [DOI] [PubMed] [Google Scholar]
- 87.Caldwell ASL, Edwards MC, Desai R, Jimenez M, Gilchrist RB, Handelsman DJ, Walters KA. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci U S A. 2017;114;E3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Silva MS, Prescott M, Campbell RE. Ontogeny and reversal of brain circuit abnormalities in a preclinical model of PCOS. JCI Insight. 2018;3(7). pii: 99405. doi: 10.1172/jci.insight.99405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Allen DB. Hormonal Eligibility Criteria for ‘Includes Females’ Competition: A Practical but Problematic Solution. Horm Res Paediatr. 2016;85;278. [DOI] [PubMed] [Google Scholar]
- 90.Batty KA, Herbert J, Keverne EB, Vellucci SV. Differences in blood levels of androgens in female talapoin monkeys related to their social status. Neuroendocrinology. 1986;44;347. [DOI] [PubMed] [Google Scholar]
- 91.Petty JM, Drea CM. Female rule in lemurs is ancestral and hormonally mediated. Sci Rep. 2015;5;9631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azziz R, Sanchez LA, Knochenhauer ES, Moran C, Lazenby J, Stephens KC, Taylor K, Boots LR. Androgen excess in women: experience with over 1000 consecutive patients. J Clin Endocrinol Metab. 2004;89;453. [DOI] [PubMed] [Google Scholar]
- 93.Arifin E, Shively CA, Register TC, Cline JM. Polycystic ovary syndrome with endometrial hyperplasia in a cynomolgus monkey (Macaca fascicularis). Vet Pathol. 2008;45;512. [DOI] [PubMed] [Google Scholar]
- 94.Abbott DH, Rayome BH, Dumesic DA, Lewis KC, Edwards AK, Wallen K, Wilson ME, Appt SE, Levine JE. Clustering of PCOS-like traits in naturally hyperandrogenic female rhesus monkeys. Hum Reprod. 2017;32;923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Foong SC, Abbott DH, Zschunke MA, Lesnick TG, Phy JL, Dumesic DA. Follicle luteinization in hyperandrogenic follicles of polycystic ovary syndrome patients undergoing gonadotropin therapy for in vitro fertilization. J Clin Endocrinol Metab. 2006;91;2327. [DOI] [PubMed] [Google Scholar]
- 96.Azziz R, Dumesic DA, Goodarzi MO. Polycystic ovary syndrome: an ancient disorder? Fertil Steril. 2011;95;1544. [DOI] [PMC free article] [PubMed] [Google Scholar]