Abstract
Purpose
The purpose of this retrospective study was to identify preoperative inflammatory biomarkers and clinical parameters and evaluate their prognostic significance in patients with spinal metastasis from clear cell renal cell carcinoma (CCRCC).
Patients and methods
Correlations of overall survival (OS) with traditional clinical parameters and inflammatory indicators including the neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), lymphocyte–monocyte ratio (LMR), albumin–globulin ratio (AGR), and C-reactive protein to albumin ratio (CRP/Alb ratio) were analyzed in 95 patients with spinal metastasis from CCRCA using the Kaplan–Meier method to identify potential prognostic factors. Factors with P values ≤ 0.1 were subjected to multivariate analysis by Cox regression analysis. P values ≤ 0.05 were considered statistically significant.
Results
The 95 patients included in this study were followed up by a mean of 48.8 months (median 51 months; range 6–132 months), during which 21 patients died, with a death rate of 22.1%. The statistical results indicated that patients with total piecemeal spondylectomy (TPS), targeted therapy, NLR < 3.8 and PLR < 206.9 had a significantly longer OS rate.
Conclusion
TPS and targeted therapy could significantly prolong the OS of patients with spinal metastasis from CCRCC. In addition, NLR and PLR are robust and convenient prognostic indicators that have a discriminatory ability superior to other inflammatory biomarkers.
Keywords: clear cell renal cell carcinoma, spinal metastasis, overall survival, prognosis, inflammatory biomarkers
Introduction
Prognostic significance of preoperative inflammatory biomarkers and traditional clinical parameters in patients with spinal metastasis from clear cell renal cell carcinoma: a retrospective study of 95 patients in a single center.
Clear cell renal cell carcinoma (CCRCC) is the most common subtype of renal cell carcinoma (RCC), accounting for approximately 70% of all RCC cases.1–3 Nearly 20–30% patients of CCRCC presented bone metastasis at the time of diagnosis, while 20% patients with localized CCRCC finally progressed into metastatic CCRCC, with the spine common site.4,5 Spinal metastasis causes malignant spinal cord compression (MSCC), defined as compression of the spinal cord or cord equina by metastatic or direct spread to the vertebrae that may cause neurological disability.6 MSCC exhibits paralysis, intractable pain, spinal instability, and hypercalcemia, increased morbidity and decreased quality of life of the patients.7,8 In our previous study,9 we reported surgical intervention and survival outcomes of 30 patients with spinal metastasis from CCRCC, in which the statistical results revealed that Tokuhashi score was the only independent prognostic indicator.9 However, the outcome obtained from that study is not convincing enough due to the small sample size. In addition, clinical practices have demonstrated that the conventional prognostic factors including sex, treatment history, surgical modality and Tomita score lack accuracy and adequacy in predicting the prognosis; therefore, more accessible and reliable prognostic indicators need to be explored to identify high-risk patients and allocate personalized treatment for the sake of improving the therapeutic and clinical outcome.
Increasing evidence has demonstrated that inflammatory mediators and cytokines produced by tumor inflammatory cells in the tumor microenvironment are important factors contributing to cancer progression by promoting proliferation, angiogenesis, and metastasis, reducing response to hormones and chemotherapeutic agents, and subverting adaptive immunity.10–14 Targeting of inflammatory pathways has been indicated as a novel way to further enhance the therapeutic efficacy.15,16 Therefore, preoperative inflammatory biomarkers may be potential prognostic factors for patients with spinal metastasis from CCRCC. Accordingly, serum white blood cells, neutrophils, lymphocytes, platelets and acute-phase proteins, such as C-reactive (CRP) protein and albumin (ALB), have been evaluated in different tumors and found to predict the prognosis and response to treatment.17,18 The appearance of Circulating tumor cells (CTCs) in the peripheral blood indicated active disease, proliferation, and metastatic potency, and is followed by genomic analyses that provide data in terms of the tumor biology and real-time monitoring of the therapeutic efficacy.19 Given the association between inflammatory response and tumor progression, the prognostic significance of several preoperative inflammatory biomarkers has been suggested, including the neutrophil/lymphocyte ratio (NLR), platelet/lymphocyte ratio (PLR), lymphocyte/monocyte ratio (LMR), ALB/globulin ratio (AGR), and CRP/ALB ratio (CAR). However, to the best of our knowledge, no publications in the medical literature have taken inflammatory factors as prognostic predictors of patients with spinal metastasis from CCRCC.
The aim of the present retrospective study was to evaluate the prognostic significance of several preoperative inflammatory biomarkers including the NLR, PLR, LMR, AGR, and CAR by comparing them with the conventional prognostic factors.
Materials and Methods
Patients
Medical records of 157 patients with spinal metastasis from CCRCC who received surgical treatment in our tumor center between August 2005 and September 2016 were analyzed retrospectively. This research project was examined and approved by the medical ethics committee of Changzheng hospital before commencing this study, and written informed consent was obtained from all patients or their legal guardians. The study was in compliance with the Declaration of Helsinki.
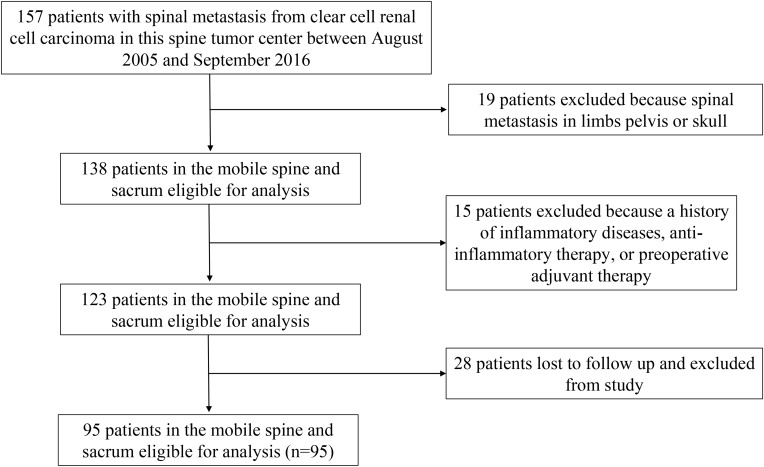
The inclusion criteria were as follows: 1) spinal metastasis from CCRCC confirmed by clinical manifestations, radiological presentations, and postoperative pathology; 2) patients who underwent surgical treatment in our tumor center; 3) patients had not taken anti-inflammatory medicines or received immunosuppressive therapy including recent steroid exposure, or with chronic inflammatory diseases including autoimmune diseases and infections before operation; 4) patients without receiving preoperative adjuvant therapy; and 5) the laboratory test results obtained before surgery. Finally, 95 patients who met with these inclusion criteria were enrolled in this study, and the flow diagram is shown in Figure 1. The material of a typical case underwent total en bloc spondylectomy (TES) is shown in Figure 2.
Figure 1.
Patient flow diagram.
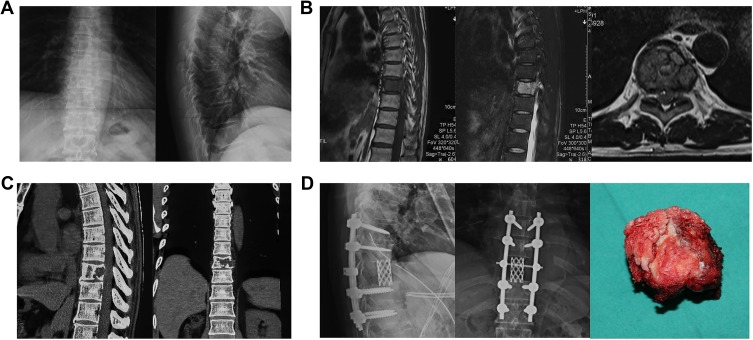
Figure 2.
A typical case underwent the removal of tumor by total en bloc spondylectomy in our center and was diagnosed as spinal metastasis from CCRCC. (A) Preoperative X-rays of anteroposterior and lateral spine demonstrated wedge deformation and osseous destruction in ninth thoracic spine. (B) Preoperative magnetic resonance imaging (MRI) indicated that the lesion showed low-intensity signal on T1-weighted image and high-intensity signal on T2-weighted image. (C) Preoperative CT showed osteolytic destruction in first lumbar vertebrae and its posterior elements, paravertebral soft tissue mass, and compression of spinal cord. (D) Total en bloc spondylectomy was conducted, and the ninth thoracic vertebral body was removed. The postoperative X-rays showed the ninth thoracic spine was removed and replaced by titanium mesh, with solid internal-fixation.
Abbreviation: CT, computed tomography.
The clinical and operative notes, radiographic images, and pathological reports of all patients who received surgery in our department for RCC spinal metastases were reviewed retrospectively. The preoperative neurological status was evaluated according to the Frankel score.20 The spinal tumors were classified based on Tomita classification21 and Tokuhashi score.22 Only overall survival (OS) was considered as the endpoint in this study. Event times were defined as the interval from the date of surgery to death, or until September 2016 for living patients. All patients were followed up on an outpatient basis at 3, 6, and 12 months after surgical treatment, every 6 months for the second year, and then annually for life.
Statistical Method
X-tile 3.6.1 software 20 (Yale University, New Haven, CT, USA) was used to determine the optimal cut-off values for NLR, PLR, LMR, and CAR. Quantitative data are described by median (range), and qualitative data are described as counts and percentages. Statistical calculations were analyzed using SPSS version 19.0 (SPSS, Inc. Chicago, IL, USA). Univariate and multivariate analyses were used to identify the independent significant factors especially preoperative inflammatory biomarkers that could predict prognosis for OS. Log-rank tests were performed for univariate analysis, and Cox proportional hazards model was used for multivariate analysis. OS were calculated by the Kaplan–Meier method. Factors with P value ≤ 0.10 in univariate analysis were subjected to multivariate analysis. Factors with P values ≤ 0.05 in multivariate analysis were considered statistically significant.
Results
Patients’ Baseline Characteristics and Optimal Cutoff Values of Inflammatory Biomarkers
The characteristics of all patients are detailed in Table 1. The population comprised 84 males and 11 females with a mean age of 54.2 years (median, 56 years; range, 16–75 years). The mean follow-up time was 48.8 months (median, 51 months; range, 6–132 months). Of the 95 included patients, 21 died during the postoperative follow-up period, with death rate of 22.1%. The mean duration from initial surgery to death was 17.9 months (median, 15 months; range, 6–45 months). Of these patients, 80 patients were admitted for primary spinal metastasis from CCRCC, and the remaining 15 patients underwent surgical treatment for recurrences.
Table 1.
Univariate and Multivariate Analysis of Clinical and Inflammatory Indicators for Overall Survival of Patients with Spinal Metastasis from CCRCC
| Variables | Overall Survival | |||
|---|---|---|---|---|
| Univariate Analysis | P value | Multivariate Analysis HR(95%) | P value | |
| Age, year | 0.331 | |||
| <40 | 11(11.6%) | |||
| ≥40 | 84(88.4%) | |||
| Gender | 0.277 | |||
| Male | 84(88.4%) | |||
| Female | 11(11.6%) | |||
| Treatment history | <0.001* | |||
| Primary | 80(84.2%) | |||
| Recurrent | 15(15.8%) | |||
| Duration time, month | ||||
| <6 | 73(76.8%) | 0.610 | ||
| ≥6 | 22(23.2%) | |||
| Visceral metastasis | 0.211 | |||
| Yes | 19(20.0%) | |||
| No | 76(80.0%) | |||
| PS | 0.421 | |||
| 0–2 | 52(54.7%) | |||
| 3–4 | 43(45.3%) | |||
| PFS | 0.197 | |||
| A–C | 35(36.8%) | |||
| D–E | 60(63.2%) | |||
| Location | 0.789 | |||
| Cervical spine | 23(24.2%) | |||
| Thoracic spine | 34(35.8%) | |||
| Lumber spine | 31(32.6%) | |||
| Sacral spine | 7(7.4%) | |||
| Involved segment | 0.871 | |||
| Monosegment | 51(53.7%) | |||
| Multisegment | 44(46.3%) | |||
| Tomita score | 0.713 | |||
| III–V | 69(72.6%) | |||
| VI–VIII | 26(27.4%) | |||
| Tokuhashi score | <0.001* | |||
| 4–9 | 41(43.2%) | |||
| 10–12 | 54(56.8%) | |||
| Preoperative embolism | 0.871 | |||
| Yes | 44(46.3%) | |||
| No | 51(53.7%) | |||
| Surgical approach | 0.462 | |||
| Anterior | 4(4.2%) | |||
| Posterior | 77(91.6%) | |||
| Combined | 4(4.2%) | |||
| Resection mode | <0.001* | |||
| Subtotal | 12(12.6%) | 1.000 | ||
| Piecemeal | 73(76.8%) | 0.319(0.119–0.859) | 0.024☨ | |
| En bloc | 10(10.6%) | 0.196(0.024–1.631) | 0.132 | |
| Intraoperative chemotherapy | 0.292 | |||
| Yes | 68(71.6%) | |||
| No | 27(28.4%) | |||
| Postoperative radiotherapy | 0.329 | |||
| Yes | 16(16.8%) | |||
| No | 79(83.2%) | |||
| Postoperative chemotherapy | 0.612 | |||
| Yes | 7(7.4%) | |||
| No | 88(92.6%) | |||
| Targeted therapy | 0.001* | 0.016☨ | ||
| Yes | 56(59.0%) | 1.000 | ||
| No | 39(41.0%) | 3.471(1.260–9.563) | ||
| Bisphosphonates | 0.327 | |||
| Yes | 32(33.7%) | |||
| No | 63(66.3%) | |||
| Blood loss | 0.381 | |||
| Yes | 49(51.6%) | |||
| No | 46(48.4%) | |||
| NLR | <0.001* | <0.001☨ | ||
| <3.8 | 76(80.0%) | 1.000 | ||
| ≥3.8 | 19(20.0%) | 8.332(2.74–24.938) | ||
| PLR | <0.001* | 0.010☨ | ||
| <206.9 | 81(85.3%) | 1.000 | ||
| ≥206.9 | 14(14.7%) | 3.808(1.370–10.584) | ||
| AGR | 0.566 | |||
| <1.5 | 41(43.2%) | |||
| ≥ 1.5 | 54(56.8%) | |||
| LMR | 0.310 | |||
| <2.3 | 51(53.7%) | |||
| ≥2.3 | 44(46.3%) | |||
| CAR | 0.230 | |||
| <1.2 | 32(33.7%) | |||
| ≥1.2 | 63(66.3%) | |||
Notes: *P value < 0.1; ☨P value <0.05.
Abbreviations: HR, hazard ratio; PS, Performance Status; PFS, preoperative Frankel score; NLR, neutrophil-to-lymphocyte ratio; PLR, Platelet-to-lymphocyte ratio; AGR, albumin/globulin ratio; LMR, lymphocyte-to-monocyte ratio; CAR, C-reactive protein to albumin ratio.
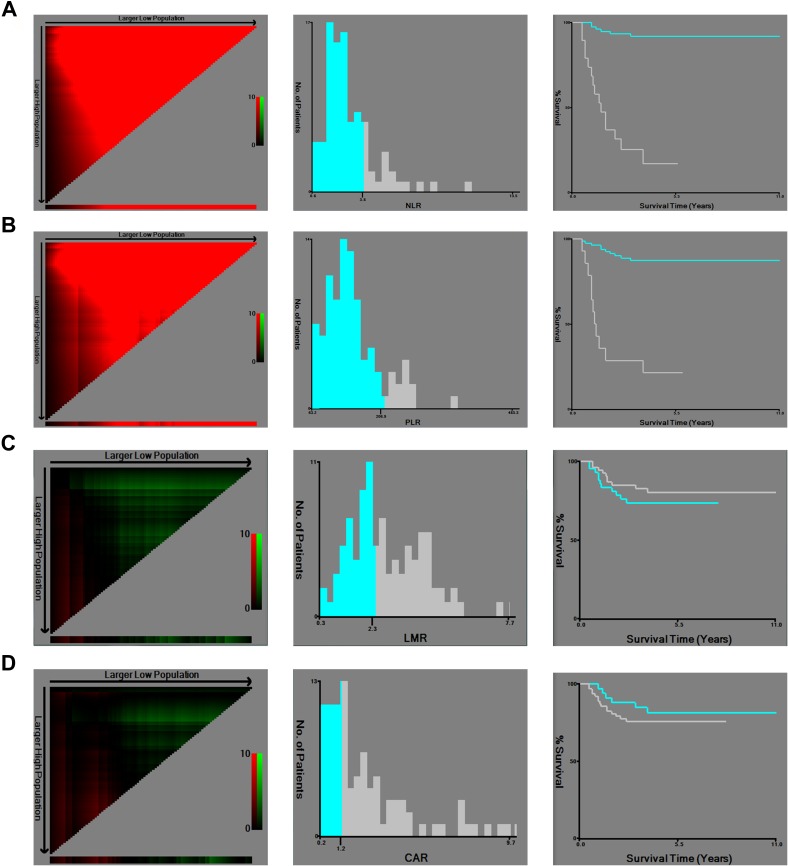
The optimal cut-off value of NLR, PLR, LMR, and CAR was determined by the X-tile program, which was 3.8, 206.9, 2.3, and 1.2, respectively (Figure 3). The log-rank value for NLR, PLR, LMR, and CAR was 63.5, 47.0, 51.0, and 8.2, respectively. According to the standard value reported by the Clinical Laboratory Department in Changzheng Hospital (Shanghai, China), the cut-off value for AGR was 1.5. Therefore, all patients were divided into two groups for further analysis (NLR<3.8 and NLR≥3.8; PLR<206.9 and PLR≥206.9; AGR<1.5 and AGR≥1.5; LMR<2.3 and LMR≥2.3; CAR<1.2 and CAR≥1.2).
Figure 3.
X-tile analysis of OS was performed using patients’ data to determine the optimal cut-off values for D-dimer, NLR, PLR, LMR, and CAR. The sample of spinal metastatic CCRCC patients was equally divided into training and validation sets. X-tile plots of training sets are shown in the left panels, with plots of matched validation sets shown in the small inset. The optimal cut-off values highlighted by the black circles in left panels are shown in histograms of the entire cohort (middle panels), and Kaplan-Meier plots are displayed in right panels. P values were determined by using the cut-off values defined in training sets and applying them to validation sets. The optimal cut-off values for NLR, PLR, LMR, and CAR were 3.8, 206.9, 2.3, and 1.2, respectively. (A) NLR, (B) PLR, (C) LMR, and (D) CAR.
Univariate and Multivariate Analyses of Prognostic Factors for OS
Twenty-one patients died during the follow-up period, thus the OS rate of patients with spinal metastasis from CCRCC was 77.9%, with a median OS of 57.6 months (range, 24–132 months). The results of univariate and multivariate analyses of possible prognostic factors are shown in Table 1. Univariate analysis revealed that significant difference was obtained in patients with treatment history (P < 0.001), Tokuhashi score (P < 0.001), resection mode (P < 0.001), targeted therapy (P = 0.001), NLR (P < 0.001), and PLR (P < 0.001).
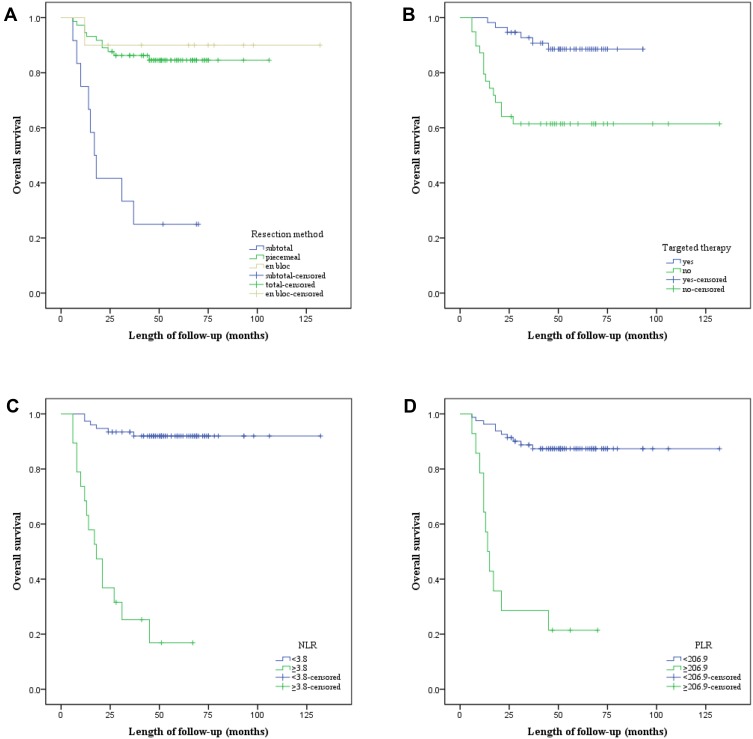
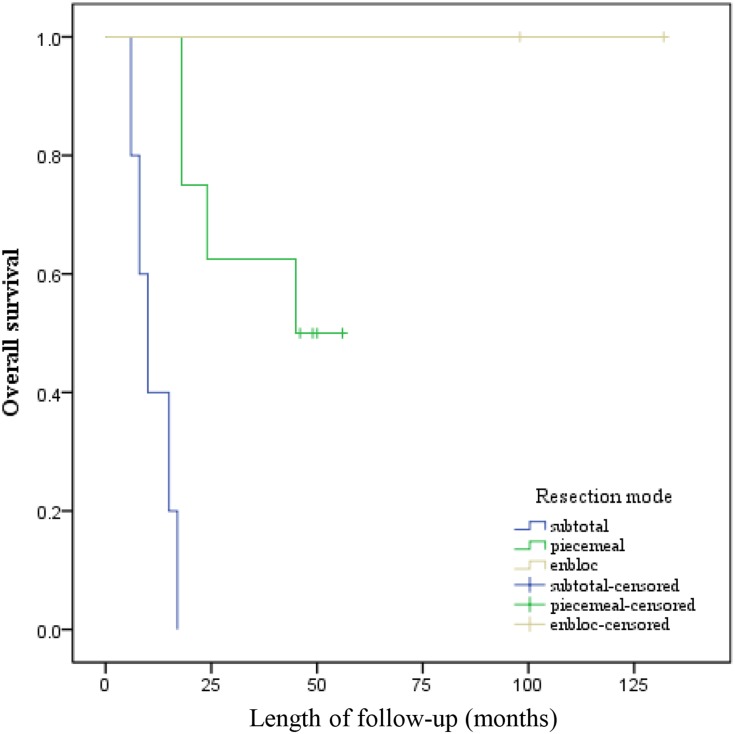
All potential prognostic factors obtained by univariate analysis were extracted into Cox proportional hazard for multivariate analysis (Table 1). Patients who underwent TPS had longer OS than those who received TES or subtotal resection (TPS [HR, 0.319, P = 0.024] versus TES [HR, 0.196; P = 0.132]). Targeted therapy significantly increased chance of OS (HR, 3.471; P = 0.016). Patients with preoperative NLR < 3.8 had longer OS than those with preoperative NLR≥ 3.8 (HR, 8.332; P < 0.001). Simultaneously, preoperative PLR value≥206.9 significantly suggested poorer OS for patients with spinal metastasis from CCRCC (HR, 3.808; P = 0.010). The Kaplan–Meier curves of OS for resection mode, targeted therapy, NLR, and PLR are presented in Figure 4A–D. Therefore, resection mode, targeted therapy, NLR, and PLR were independent prognostic factors for OS.
Figure 4.
Kaplan–Meier curves of overall survival for (A) Resection mode, (B) Targeted therapy, (C) NLR, and (D) PLR.
Clinical Management of Patients with Recurrent Spinal Metastasis from CCRCC
Based on the clinical manifestations and radiological presentations, 15 patients who received curettage in other hospitals were identified as having local recurrence of spinal lesions and chosen to undergo a second operation in our spinal tumor center. The characteristics of these recurrent patients are detailed in Tables 2 and 3. Interestingly, all patients were male with a mean age of 58.8 (43–68) years. The spinal lesions were classified by Tomita classification, and the condition of the patients was assessed by the Tokuhashi score. All spinal lesions were classified as Tomita IV-VI in accordance with Tomita classification. In three patients (Case # 10–12) with Tokuhashi score < 9, TPS or subtotal resection was performed TPS or subtotal resection under the robust request of patients or their guardians. Unfortunately, all of them died during the follow-up period. Case # 2 with the lesion classified as Tomita IV only received TES, and Case # 1 with the tumor evaluated as Tomita VI underwent TES and postoperative targeted therapy. All patients who received TES were alive during the follow-up period. TPS was performed in eight patients, three of whom (Case # 8,10,12) died of tumor recurrence, progression, and metastasis within 2 years, respectively. One patient (case # 10) dead 45 months after surgical treatment. Meanwhile, all patients (case # 9,11,13–15) who received subtotal resection died of tumor progression and metastasis. Case # 3 and Case # 7 received repeated operations for tumor recurrences in our spinal tumor center. To better understand the outcomes of different surgical methods, we conducted the Kaplan-Meier survival curve and Log-rank test and the result showed p value = 0.045 (Figure 5).
Table 2.
Clinical Management of Recurrent Patients with Spinal Metastasis from CCRCC
| No | Age (years)/Gender | DS(M) | VM | Pre F-S | LC | Tomita Classification/Tokuhashi Score | Surgical Approach | Resection Mode | Adjuvant Therapy | OS (m) | Last Status |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 45/M | 1.5 | No | D | L3 | VI/12 | P | TES | TT | 132 | Alive |
| 2 | 55/M | 0.3 | No | D | L3 | IV/13 | P | TES | None | 98 | Alive |
| 3* | 59/M | 2 | No | D | S1-2 | IV/11 | P | Piecemeal | None | 56 | Alive |
| 4 | 64/M | 10 | No | E | S1-2 | VI/10 | P | Piecemeal | TT | 50 | Alive |
| 5 | 64/M | 10 | No | C | T1-2 | VI/9 | P | Piecemeal | RT | 49 | Alive |
| 6 | 68/M | 1 | No | C | L1,3 | V/9 | P | Piecemeal | TT | 46 | Alive |
| 7* | 55/M | 24 | Yes | D | T6-8 | VI/11 | P | Piecemeal | RT+TT | 45 | Dead |
| 8 | 62/M | 8 | No | D | T8 | V/10 | P | Piecemeal | TT | 18 | Dead |
| 9 | 49/M | 3 | No | E | L7-8 | V/9 | P | Subtotal | None | 15 | Dead |
| 10 | 60/M | 4 | No | B | L4-S1 | IV/7 | P | Piecemeal | None | 18 | Dead |
| 11 | 65/M | 2 | No | E | C6 | V/7 | A+P | Subtotal | None | 10 | Dead |
| 12 | 43/M | 0.5 | No | C | T8-10 | IV/8 | P | Piecemeal | TT | 24 | Dead |
| 13 | 64/M | 8 | No | B | T9 | V/9 | P | Subtotal | None | 6 | Dead |
| 14 | 64/M | 0.2 | No | C | C5 | V/9 | P | Subtotal | None | 8 | Dead |
| 15 | 65/M | 3 | No | D | L1 | V/9 | P | Subtotal | None | 17 | Dead |
Note: *Two cases with local recurrence were retreated in our spinal tumor center.
Abbreviations: DS, duration of symptom; m, month; VM, visceral metastasis; pre F-S, preoperative Frankel score; LC, location; OS, overall survival; M, male; P, posterior; A, anterior; TES, total en bloc spondylectomy; CT, chemotherapy; TT, targeted therapy; RT, radiotherapy.
Table 3.
Treatment Protocols and Outcomes of Recurrent Spinal Metastasis from CCRCC
| Treatment Protocol | N | Local Recurrence | Dead | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Surgery | |||||
| Subtotal resection | 5 | 4 | 80 | 5 | 100 |
| Total piecemeal spondylectomy | 8 | 3 | 37.5 | 4 | 50 |
| Total en bloc spondylectomy | 2 | 0 | 0 | 0 | 0 |
Figure 5.
Kaplan–Meier curves of overall survival of recurrent patients for resection mode.
Discussion
To the best of our knowledge, this is the largest monocentric retrospective study regarding the prognostic significance of clinical factors and inflammatory biomarkers. About 30% CCRCC patients developed bone metastasis, especially in the spine.23
With the advance in surgical treatment, targeted therapy, and precise radiotherapy, the OS of spinal metastasis from CCRCC has improved remarkably in recent years.24–26
In this research, clinical factors and inflammatory biomarkers were analyzed to identify convenient and reliable prognostic biomarkers for identifying high-risk patients and improve the clinical outcome of spinal metastasis from CCRCC. Simultaneously, we attempted to find out answers to the following questions:
Which parameters have the prognostic value for predicting OS for spinal metastasis from CCRCC?
Do the inflammatory factors affect disease progression and OS?
Do the widely used adjuvant therapies, such as adjuvant radiotherapy, intraoperative local treatment, and targeted therapy have a therapeutic effect?
The results obtained from this study suggest that resection mode, targeted therapy, NLR and PLR are significantly correlated with OS. Total resection is superior to subtotal resection for OS of patients with spinal metastasis from CCRCC. However, statistical analysis revealed that TPS could prolong the OS of patients compared with TES. With the development of targeted agents that inhibit the vascular endothelial grow factor (VEGF) and the mammalian target of rapamycin (mTOR) signal transduction pathways (7, 8), patients administered with targeted therapy had longer OS than those without it. Our finding demonstrated that NLR and PLR versus other indicators had a discriminatory ability as novel and promising inflammatory biomarkers.
Surgical treatment is the standard treatment based on Tomita classification for spinal metastasis with respect to preserving neurological functionality, relieving pain and promising prolonged survival.27–29 Petteys et al25 reported that resection and fixation provided pain relief and neurological stabilization in patients with spinal metastasis arising from RCC, and aggressive intervention was beneficial to younger patients. Tatsui et al6 also supported surgical treatment as aggressive intervention on the basis of identifying several factors influencing survival. Our former study suggested that surgical treatment was not the independent prognostic factor for patients with spinal metastasis from CCRCC, but the sample size of that study is small.9 In the present study, we for the first time demonstrated that TPS, rather than TES and subtotal resection, was the most beneficial surgical approach. As spinal metastatic lesions usually exhibit paraspinal extensions involving even the soft tissue, TES is not feasible. Extensive tumor resection may cause loss of stability of the spine, which was a major concern. This risk has been minimized by the anterior interbody fusion with or without instrumentation.30 Simultaneously, blood bleeding in the procedure of surgical treatment was high owing to rich blood supply in metastasis from RCC, which requires the surgeons to complete the operation as soon as possible. Subtotal resection produces tumor residuals, which is likely to cause local recurrence and tumor progression. In such a case, TPS seems the most suitable of the three surgical approaches.
Although chemotherapy and radiotherapy as adjuvant therapies have been applied to the treatment of spinal metastasis from CCRCC, the therapeutic outcome remains controversial.31 Tao et al32 reported that the combination of surgery with stereotactic body radiation therapy could offer patients with spinal metastatic a chance of durable tumor control with minimal toxicity. However, chemotherapy and postoperative radiotherapy are not significant prognostic factors for OS. With the understanding of molecular biology of RCC, several targeted agents inhibiting VEGF and mTOR signal transduction pathways such as Sunitinib, Sorafenib, Temsirolimus, and Pazopanib have been put into clinical practice.33–36 Meanwhile, statistical results in our study revealed that targeted therapy significantly prolonged the OS of patients with spinal metastasis from CCRCC. Increasing clinical evidence has proven that targeted therapy can downsize the tumor size, control tumor progression, and improve OS, in view of Sunitinib and Sorafenib as the first-line or second-line treatment.37,38 Furthermore, surgery in combination with targeted therapy is useful for patients with spinal metastasis from CCRCC in that it provides a new therapeutic strategy for such patients.
In addition, our results suggested that NLR and PLR could serve as reliable and novel inflammatory prognostic biomarkers superior to other indicators for spinal metastasis from CCRCC. The relationship between chronic inflammation and RCC has been investigated, and it is increasingly recognized that systemic inflammatory response plays a crucial role in RCC development and progression.39,40 However, there is no published information regarding the prognostic significance of preoperative inflammatory indicators for spinal metastasis from CCRCC. Zhun et al41 reported that a high preoperative PLR was correlated with poor prognosis in RCC patients, and Hu et al42 demonstrated that pre-operative NLR could be considered as a potential prognostic biomarker in RCC patients who underwent surgical resection. Recent studies43–45 have demonstrated that neutrophils interact with tumor cells via secretion of cytokines, thus promoting tumor development, lymphocytes mediate immunological destruction of cancer cells; and platelets serve as chemoattractants to promote migration of tumor cells. These inflammatory backgrounds at least theoretically support the use of several inflammatory biomarkers as prognostic indicators of spinal metastasis from CCRCC. Tokuhashi score and Tomita classification are known as the most popular prognostic scores for patients with spinal metastasis.21,22 In our previous study,9 we found that only the Tokuhashi score was an independent prognostic factor for patients with CCRCC. Pettey et al46 reported that Tokuhashi score could be used for selecting surgical patients with RCC spinal metastases, and maybe more relevant as compared with its effectiveness in patients with spinal metastases from other cancers. However, our results showed that the Tokuhashi score was not a suitable prognostic factor for OS. In our opinion, targeted therapy may be able to prolong the survival of patients with a low Tokuhashi score. In recurrent cases, nine of the 15 recurrent patients in our series died during the follow-up period, suggesting that local recurrence maybe an adverse prognostic factor for OS. Our subtype analysis suggested that TES significantly prolonged the OS of recurrent patients.
The present study has some limitations. First, this is a retrospective single-center study. In addition, the follow-up duration is not long enough. Finally, the study only focused on the OS of patients who underwent surgical treatment.
Conclusion
Our findings demonstrate that TPS and targeted therapy can significantly prolong the OS of patients with spinal metastasis from CCRCC. Besides, NLR and PLR are robust and convenient prognostic indicators whose discriminatory ability is superior to that of other preoperative inflammatory biomarkers. However, further multi-center studies are required to validate our results and conclusions.
Ethics Approval and Consent
All procedures involving human participants in this study were approved by the ethics committee of Changzheng Hospital (Shanghai, China), and written informed consent was obtained from all patients or their legal guardians. The study was in compliance with the Declaration of Helsinki. Patients or their legal guardians know of and approved the publication.
Disclosure
The authors report no funding and no conflicts of interest in this work.
References
- 1.Goodwin CR, Ahmed AK, Boone C, et al. The challenges of renal cell carcinoma metastatic to the spine: a systematic review of survival and treatment. Global Spine J. 2018;8(5):517–526. doi: 10.1177/2192568217737777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirsch MS, Signoretti S, Dal Cin P. Adult renal cell carcinoma: a review of established entities from morphology to molecular genetics. Surg Pathol Clin. 2015;8(4):587–621. doi: 10.1016/j.path.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 3.Tan PH, Cheng L, Rioux-Leclercq N, et al. Renal tumors: diagnostic and prognostic biomarkers. Am J Surg Pathol. 2013;37(10):1518–1531. doi: 10.1097/PAS.0b013e318299f12e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guida A, Escudier B, Albiges L. Treating patients with renal cell carcinoma and bone metastases. Expert Rev Anticancer Ther. 2018;5:1–9. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi M, Sun M, Jeldres C, et al. Distribution of metastatic sites in renal cell carcinoma: a population-based analysis. Ann Oncol. 2012;23(4):973–980. doi: 10.1093/annonc/mdr362 [DOI] [PubMed] [Google Scholar]
- 6.Boussios S, Cooke D, Hayward C, et al. Metastatic spinal cord compression: unraveling the diagnostic and therapeutic challenges. Anticancer Res. 2018;38(9):4987–4997. doi: 10.21873/anticanres.12817 [DOI] [PubMed] [Google Scholar]
- 7.Higuchi T, Yamamoto N, Hayashi K, et al. Long-term patient survival after the surgical treatment of bone and soft-tissue metastases from renal cell carcinoma. Bone Joint J. 2018;100-B(9):1241–1248. doi: 10.1302/0301-620X [DOI] [PubMed] [Google Scholar]
- 8.Alamanda VK, Robinson MM, Kneisl JS, et al. Functional and survival outcomes in patients undergoing surgical treatment for metastatic disease of the spine. J Spine Surg. 2018;4(1):28–36. doi: 10.21037/jss [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S, Wang T, Jiang D, et al. Surgery and survival outcomes of 30 patients with neurological deficit due to clear cell renal cell carcinoma spinal metastases. Eur Spine J. 2015;24(8):1786–1791. doi: 10.1007/s00586-015-3912-3 [DOI] [PubMed] [Google Scholar]
- 10.Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–e503. doi: 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 11.Candido J, Hagemann T. Cancer-related inflammation. J Clin Immunol. 2013;33(Supply 1):S79–S84. doi: 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- 12.Hirahara N, Matsubara T, Kawahara D, et al. Prognostic significance of preoperative inflammatory response biomarkers in patients undergoing curative thoracoscopic esophagectomy for esophageal squamous cell carcinoma. Eur J Surg Oncol. 2017;43(2):493–501. doi: 10.1016/j.ejso.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 13.Cerón-Carrasco JP, Pérez-Sánchez H, Zúñiga J, et al. Antibodies as carrier molecules: encapsulating anti-inflammatory drugs inside herceptine. J Phys Chem B. 2018;122(7):2064–2072. doi: 10.1021/acs.jpcb.7b10749 [DOI] [PubMed] [Google Scholar]
- 14.Diakos CI, Charles KA, McMillan DC, et al. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15:e493–e503. doi: 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 15.Xu K, Lou Y, Sun R, et al. Establishment of a nomogram-based model for predicting the prognostic value of inflammatory biomarkers and preoperative D-Dimer level in spinal Ewing’s sarcoma family tumors: a retrospective study of 83 patients. World Neurosurg. 2019;121(Suppl 1):e104–e112. doi: 10.1007/s10875-012-9847-0 [DOI] [PubMed] [Google Scholar]
- 16.Savant SS, Sriramkumar S, O’Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel). 2018;10(8):E251. doi: 10.3390/cancers10080251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vivar Chevez AR, Finke J, Bukowski R. The role of inflammation in kidney cancer. Adv Exp Med Biol. 2014;8:197–234. doi: 10.1007/978-3-0348-0837-8_9 [DOI] [PubMed] [Google Scholar]
- 18.Lee CS, Ryan EJ, Doherty GA. Gastro-intestinal toxicity of chemotherapeutics in colorectal cancer: the role of inflammation. World J Gastroenterol. 2014;20(14):3751–3761. doi: 10.3748/wjg.v20.i14.3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boussios S, Ozturk MA, Moschetta M, et al. The developing story of predictive biomarkers in colorectal cancer. J Pers Med. 2019;9(1):E12. doi: 10.3390/jpm9010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frankel HL, Hancock DO, Hyslop G, et al. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7(3):179–192. [DOI] [PubMed] [Google Scholar]
- 21.Tomita K, Kawahara N, Kobayashi T, et al. Surgical strategy for spinal metastases. Spine. 2001;26(3):298–306. doi: 10.1097/00007632-200102010-00016 [DOI] [PubMed] [Google Scholar]
- 22.Tokuhashi Y, Matsuzaki H, Toriyama S, et al. Scoring system for the preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976). 1990;15:1110–1113. doi: 10.1097/00007632-199011010-00005 [DOI] [PubMed] [Google Scholar]
- 23.Ruatta F, Derosa L, Escudier B, et al. Prognosis of renal cell carcinoma with bone metastases: experience from a large cancer center. Eur J Cancer. 2018;107:79–85. doi: 10.1016/j.ejca.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 24.Miller JA, Balagamwala EH, Angelov L, et al. Spine stereotactic radiosurgery with concurrent tyrosine kinase inhibitors for metastatic renal cell carcinoma. J Neurosurg Spine. 2016;25(6):766–774. doi: 10.3171/2016.4.SPINE16229 [DOI] [PubMed] [Google Scholar]
- 25.Petteys RJ, Spitz SM, Goodwin CR, et al. Factors associated with improved survival following surgery for renal cell carcinoma spinal metastases. Neurosurg Focus. 2016;41(2):E13. doi: 10.3171/2016.5.FOCUS16145 [DOI] [PubMed] [Google Scholar]
- 26.Mosele GR, Caggiari G, Scarpa RM, et al. The treatment of vertebral metastases from renal cell carcinoma: a retrospective study. Minerva Urol Nefrol. 2017;69(2):166–172. doi: 10.23736/S0393-2249.16.02809-5 [DOI] [PubMed] [Google Scholar]
- 27.Ma Y, He S, Liu T, et al. Quality of life of patients with spinal metastasis from cancer of unknown primary origin: a longitudinal study of surgical management combined with postoperative radiation therapy. J Bone Joint Surg Am. 2017;99(19):1629–1639. doi: 10.2106/JBJS.16.00286 [DOI] [PubMed] [Google Scholar]
- 28.Kakutani K, Sakai Y, Maeno K, et al. Prospective cohort study of performance status and activities of daily living after surgery for spinal metastasis. Clin Spine Surg. 2017;30(8):E1026–E1032. doi: 10.1097/BSD.0000000000000456 [DOI] [PubMed] [Google Scholar]
- 29.Toquart A, Graillon T, Mansouri N, et al. Management of spinal metastasis by minimal invasive surgery technique: surgical principles, indications: a literature review. Neurochirurgie. 2016;62(3):157–164. doi: 10.1016/j.neuchi.2015.12.007 [DOI] [PubMed] [Google Scholar]
- 30.Boussios S, Hayward C, Cooke D, et al. Spinal ewing sarcoma debuting with cord compression: have we discovered the thread of ariadne? Anticancer Res. 2018;38(10):5589–5597. doi: 10.21873/anticanres.12893 [DOI] [PubMed] [Google Scholar]
- 31.Stenman M, Sinclair G, Paavola P, et al. Overall survival after stereotactic radiotherapy or surgical metastasectomy in oligometastatic renal cell carcinoma patients treated at two Swedish centres 2005-2014. Radiother Oncol. 2018;127:501–506. doi: 10.1016/j.radonc.2018.04.028 [DOI] [PubMed] [Google Scholar]
- 32.Tao R, Bishop AJ, Brownlee Z, et al. Stereotactic body radiation therapy for spinal metastases in the postoperative setting: a secondary analysis of mature phase 1-2 trials. Int J Radiat Oncol Biol Phys. 2016;95(5):1405–1413. doi: 10.1016/j.ijrobp.2016.03.022 [DOI] [PubMed] [Google Scholar]
- 33.Staehler M, Motzer RJ, George DJ, et al. Adjuvant sunitinib in patients with high-risk renal cell carcinoma: safety, therapy management, and patient-reported outcomes in the S-TRAC trial. Ann Oncol. 2018;29(10):2098–2104. doi: 10.1093/annonc/mdy329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Q, Wu G, Che X, et al. Sorafenib induces renal cell carcinoma apoptosis via upregulating activating transcription factor 4. Pharmazie. 2018;73(3):156–160. doi: 10.1691/ph.2018.7855 [DOI] [PubMed] [Google Scholar]
- 35.Satoh T, Koie T, Horiguchi H, et al. Longer recurrence-free survival in a patient with metastatic renal cell carcinoma treated with temsirolimus. Clin Case Rep. 2017;5(12):1950–1953. doi: 10.1002/ccr3.1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Méndez-Vidal MJ, Molina Á, Anido U, et al. Pazopanib: evidence review and clinical practice in the management of advanced renal cell carcinoma. BMC Pharmacol Toxicol. 2018;19(1):77. doi: 10.1186/s40360-018-0264-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cai W, Huang J, Yuan Y, et al. Sunitinib or sorafenib as neoadjuvant therapy may not improve the survival outcomes of renal cell carcinoma with tumor thrombus. Urol Int. 2018;101:391–399. doi: 10.1159/000492723 [DOI] [PubMed] [Google Scholar]
- 38.Maroun R, Mitrofan L, Benjamin L, et al. Real life patterns of care and progression free survival in metastatic renal cell carcinoma patients: retrospective analysis of cross-sectional data. BMC Cancer. 2018;18(1):214. doi: 10.1186/s12885-018-4117-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mizuno R, Kimura G, Fukasawa S, et al. Angiogenic, inflammatory and immunologic markers in predicting response to sunitinib in metastatic renal cell carcinoma. Cancer Sci. 2017;108(9):1858–1863. doi: 10.1111/cas.13320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu L, Ma X, Wang L, et al. Prognostic value of a systemic inflammatory response index in metastatic renal cell carcinoma and construction of a predictive model. Oncotarget. 2016;8(32):52094–52103. doi: 10.18632/oncotarget.10626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z, Peng S, Wang A, et al. Platelet-lymphocyte ratio acts as an independent predictor of prognosis in patients with renal cell carcinoma. Clin Chim Acta. 2018;480:166–172. doi: 10.1016/j.cca.2018.02.014 [DOI] [PubMed] [Google Scholar]
- 42.Hu H, Yao X, Xie X, et al. Prognostic value of preoperative NLR, dNLR, PLR and CRP in surgical renal cell carcinoma patients. World J Urol. 2017;35(2):261–270. doi: 10.1007/s00345-016-1864-9 [DOI] [PubMed] [Google Scholar]
- 43.Swierczak A, Mouchemore KA, Hamilton JA, et al. Neutrophils: important contributors to tumor progression and metastasis. Cancer Metastasis Rev. 2015;34(4):735–751. doi: 10.1007/s10555-015-9594-9 [DOI] [PubMed] [Google Scholar]
- 44.Lissoni P, Brivio F, Fumagalli L, et al. Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers. 2004;19:135–140. doi: 10.1177/172460080401900208 [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa S, Miyashita T, Inokuchi M, et al. Platelets surrounding primary tumor cells are related to chemoresistance. Oncol Rep. 2016;36(6):787–794. doi: 10.3892/or.2016.4898 [DOI] [PubMed] [Google Scholar]
- 46.Petteys RJ, Spitz SM, Rhee J, et al. Tokuhashi score is predictive of survival in a cohort of patients undergoing surgery for renal cell carcinoma spinal metastases. Eur Spine J. 2015;24(10):2142–2149. doi: 10.1007/s00586-015-3862-9 [DOI] [PubMed] [Google Scholar]