Abstract
Individuals who carry a premutation (PM) allele on the FMR1 gene may experience executive limitations associated with their genetic status, including inhibition deficits. However, poor understanding of individualized risk factors has limited clinical management of this group, particularly in mothers who carry the PM allele who have children with fragile X syndrome (FXS). The present study examined CGG repeat length and age as factors that may account for variable expressivity of inhibition deficits. Participants were 134 carriers of the PM allele who were mothers of children with FXS. Inhibition skills were measured using both self-report and direct behavioral assessments. Increased vulnerability for inhibition deficits was observed at mid-range CGG lengths of approximately 80–100 repeats, with some evidence of a second zone of vulnerability occurring at approximately 130–140 CGG repeats. Risk associated with the genotype also became more pronounced with older age. This study identifies personalized risk factors that may be used to tailor the clinical management of executive deficits in carriers of the PM allele. Inhibition deficits may contribute to poor outcomes in carriers of the PM allele and their families, particularly in midlife and early old age, and clinical monitoring may be warranted.
Keywords: fragile X premutation carrier, executive dysfunction, age effects, FMR1 premutation, mid-range CGG
1. Introduction
Over one million individuals in the United States are genetic carriers of a premutation (PM) allele on the Fragile X Mental Retardation-1 (FMR1) gene (Maenner et al., 2013). Carriers of the PM allele have 55–200 CGG repeats on the FMR1 gene on the X chromosome, which represents an expansion beyond the normal range of 6–54 CGG repeats (Maddalena et al., 2001). PM expansion of the FMR1 CGG sequence is associated with molecular genetic abnormalities, such as decreased production of the protein encoded by FMR1, Fragile X Mental Retardation Protein (FMRP; Kenneson, Zhang, Hagedorn, & Warren, 2001; Oh et al., 2015; Primerano et al., 2002) and elevated levels of FMR1 mRNA (Garcia-Arocena & Hagerman, 2010; Kenneson et al., 2001; Tassone et al., 2000). Accumulating evidence now supports the PM allele as a genotype that is associated with clinical risk, although varying symptom severity and age of symptom onset, as well as incomplete penetrance, has made it difficult to pinpoint the full range of associated clinical features (Movaghar et al., 2019; Wheeler, Raspa, Hagerman, Mailick, & Riley, 2017; Wheeler et al., 2014).
Delineating the phenotype as manifested in female carriers of the PM allele is of particular importance given that PM alleles are about twice as likely to occur in females compared to males (Hunter et al., 2014). Moreover, female carriers of the PM allele can pass an expanded version of the affected gene to their children, causing fragile X syndrome (FXS)—an inherited form of intellectual disability (Fu et al., 1991). Understanding the presentation of PM symptoms in mothers of children with FXS is critically important because the clinical effects of the PM allele may have implications not only for the mother’s health but also for her ability to care and advocate for her children living with FXS. Understanding the clinical phenotype of carriers of the PM allele as manifested in mothers of children with FXS is essential to the development of family-centered treatments that target the specific needs of carriers of the PM allele and their families. Moreover, carriers of the PM allele who are mothers of children with FXS may be more vulnerable to poor outcomes in general, due to the combined effects of genetic risk coupled with the added environmental stressors associated with parenting a child with a disability (e.g., Seltzer et al., 2012). In sum, the clinical significance of the PM allele may be elevated among mothers of children with FXS due to the broader family context in which it occurs.
The FMR1 PM allele confers risk for two well-established genotype-specific conditions: Fragile X-Associated Primary Ovarian Insufficiency (FXPOI) and Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). FXPOI affects approximately 20% of female carriers of the PM allele and is characterized by early menopause, hormonal disruption, and fertility problems (Sullivan, Welt, & Sherman, 2011). FXTAS is a late-onset neurodegenerative disorders that occurs in approximately 17% of female carriers of the PM allele and is characterized by tremor, ataxia, cognitive decline, and associated neuropathological features that include white matter degeneration, global brain atrophy, and intranuclear inclusions throughout the cortex and brainstem (Greco et al., 2005; Halket, Wang, Hessl, & Rivera, 2016; Rodriguez-Revenga et al., 2009). Other documented clinical consequences of the FMR1 PM genotype in women who have children with fragile X include elevated risk for depression, anxiety, and physical health problems such as hypertension and migraines (Bourgeois et al., 2011; Hagerman & Hagerman, 2013; Roberts et al., 2016). Mild autism-related features, including difficulties with eye contact and the social use of language, are also elevated in this group (Klusek, Fairchild, & Roberts, 2019; Klusek, Ruber, & Roberts, 2018; Losh et al., 2012).
Executive deficits in working memory, attention, and inhibition have been reported in female carriers of the PM allele, although the executive phenotype in females is controversial due to inconsistencies across reports (e.g., Hunter et al., 2008). Neuroimaging evidence from carriers of the PM allele lends support for executive deficits associated with this genotype, as evidenced by structural, microstructural, and functional differences in brain regions important for executive functions. For example, white matter abnormalities have been implicated in attenuated performance on a range of executive tasks (Gunning-Dixon & Raz, 2000), and are observed in frontal, parietal, and cerebellar pathways in both male and female carriers of the PM allele who do not have FXTAS (Battistella et al., 2013; Hashimoto, Srivastava, Tassone, Hagerman, & Rivera, 2011; Leow et al., 2014). Some reports have detected associations between white matter abnormalities in female carriers of the PM allele and FMR1-related molecular indices (mRNA levels and methylation of the FMR1 exon 1/intron 1 boundary), suggesting that these differences in brain structure may arise due to genetic risk associated with the PM allele (Shelton et al., 2017). White matter microstructure in the corpus callosum and cerebellar peduncles, and disruption of diffusion network measures in the left superior parietal cortex, have been reported as correlates of performance on executive functioning measures in female carriers of the PM allele who were asymptomatic for FXTAS (Leow et al., 2014; Shelton et al., 2017). Moreover, reduced activation in pre-frontal and frontal-parietal regions—areas important for executive function—has been observed in female carriers of the PM allele during completion of a various executive tasks and are associated with specific FMR1 molecular genetic markers (Hashimoto, Backer, Tassone, Hagerman, & Rivera, 2011; Kim, Hashimoto, Tassone, Simon, & Rivera, 2013). Overall, neuroimaging studies of female carriers of the PM allele suggest the presence of structural, microstructural, and functional differences in brain regions that are implicated in executive functions, and which appear to be linked to FMR1-related molecular genetic alterations.
Behavioral studies of executive skills in female carriers of the PM allele suggest that deficits in inhibition, or the suppression of automatic or preplanned responses (Aron, 2011), are perhaps the most widely documented executive deficit. This body of evidence suggests poorer performance than matched controls on tasks requiring verbal, visual, or motor suppression of prepotent responses (Kraan, Hocking, Georgiou-Karistianis, et al., 2014; Shelton et al., 2014; Yang et al., 2013). Yet, findings have been mixed, with some studies failing to detect inhibition differences in female carriers of the PM allele (see Hunter et al., 2008; Wheeler et al., 2017). Clarifying these discrepancies is important given that inhibition deficits can impact performance in social and occupational contexts, and are linked with a host of maladaptive behaviors including substance abuse, suicidal ideation, criminal conduct, and antisocial behavior (Feilhauer, Cima, Korebrits, & Kunert, 2012; Meza, Owens, & Hinshaw, 2016; Rubio et al., 2008; Tremblay, Pihl, Vitaro, & Dobkin, 1994; Verdejo-Garcia, Lawrence, & Clark, 2008).
One source of inconsistency in the extant literature may be variation due to measurement techniques. It has been argued that tasks that require rapid, on-line recruitment of executive skills have increased sensitivity to PM-associated executive difficulties relative to tasks that do not impose time constraints, underscoring the need for multimodal assessment in future work (e.g., Kraan, Hocking, Bradshaw, et al., 2014; Shelton et al., 2016). Discrepant findings may also be due, in part, to differing sample characteristics across studies, such as the age of the sample members. Many prior reports have focused on samples consisting of young-adult and middle-aged women, while evidence suggests that PM-associated symptoms may become more prominent at older ages. Age-related decline in cognitive-executive functioning has been suggested in some emerging reports. For example, older age is correlated with reduced language fluency and lower scores on magnitude comparison and numerical enumeration tasks in female carriers of the PM allele, but not among age-matched controls (Goodrich-Hunsaker, Wong, McLennan, Srivastava, et al., 2011; Goodrich-Hunsaker, Wong, McLennan, Tassone, et al., 2011; Sterling, Mailick, Greenberg, Warren, & Brady, 2013).
However, few prior investigations have examined the impact of aging on inhibition skills specifically. In one report by Kraan, Hocking, Georgiou-Karistianis, et al. (2014), a correlation between older age and verbal inhibition errors was detected in female carriers of the PM allele (n=35), although the association did not survive correction for multiple comparisons. Few other investigations have focused on females, although age-inhibition associations have also been noted in male carriers of the PM allele (Hunter, Sherman, Grigsby, Kogan, & Cornish, 2012). Thus, the extant literature is inconclusive and follow-up is needed to better understand the profile of age-related vulnerability in female carriers of the PM allele.
The length of the PM CGG repeat expansion is another factor that may relate to variability in the expression of inhibition deficits. PM expansion of the CGG sequence leads to molecular genetic alterations such as elevated mRNA and decreased FMRP (Kenneson et al., 2001; Oh et al., 2015; Primerano et al., 2002). These changes in gene function are thought to underlie phenotypic expression (Hagerman & Hagerman, 2013), and may vary according to the length of the CGG repeat expansion within the PM range (Chen, Tassone, Sahota, & Hagerman, 2003; Kenneson et al., 2001; Loesch et al., 2007; Sellier et al., 2014). Thus, the size of the CGG expansion may provide important information regarding individualized risk profiles, including risk for inhibition difficulties. One report by Shelton et al. (2014) reported a correlation between ocular-motor inhibition errors and CGG repeat length, where errors were the highest at the lower end of the PM range and decreased linearly as the length of the CGG expansion increased. However, evidence is mixed, as not all reports have detected CGG-related variation in inhibition skills (Kraan, Hocking, Georgiou-Karistianis, et al., 2014; Yang et al., 2013). Notably, most prior studies have been limited by small samples that were characterized by poor representation at the low and high ends of the PM CGG range, and as a result were unable to test for nonlinear associations. This is critical because curvilinear CGG relationships have been reported relative to other aspects of PM phenotype, with risk for clinical involvement the highest at mid-range PM repeat lengths (approximately 80–110) and lower at the tails (Ennis, Ward, & Murray, 2006; Klusek, Porter, et al., 2018; Mailick, Hong, Greenberg, Smith, & Sherman, 2014; Roberts et al., 2009; Seltzer et al., 2012). Only one prior report has probed for nonlinear CGG-inhibition relationships, with inconclusive results. In a study of 37 female carriers of the PM allele, Klusek et al. (2018) detected a trend where verbal inhibition errors were the most pronounced in carriers with approximately 60–80 CGG repeats, decreased by degree from approximately 80–120 CGG repeats, and began to increase again at CGG repeat sizes greater than approximately 120. Follow-up studies incorporating larger sample sizes are needed to clarify potential CGG-related modulation of inhibition skills in female carriers of the PM allele.
The present study represents the largest investigation to date of inhibition skills in female carriers of the PM allele. Including a sample of over 130 carriers of the PM allele, we aim to clarify the effect of age and CGG repeat length on the expression of inhibition deficits in this group. We focused on carriers of the PM allele who are mothers of a child with FXS (hereafter referred to as “PM mothers of children with FXS”)—a subgroup of women who may be particularly vulnerable to poor outcomes due to the coupling of genetic risk with elevated stress exposure associated with parenting a child with a disability (e.g., Seltzer et al., 2012). The study of the clinical phenotype of PM mothers of children with FXS is of particular importance because of broader family context in which it occurs; mothers are often the primary caregiver for their children and the clinical effects of the PM allele in mothers can impact health and quality of life for both the mother as well as her children with FXS. To address potential measurement effects that may account for discrepancies across prior reports, we adopt a multimodal approach that incorporates both self-report and direct assessment indices of inhibitory control, including measurement of response latency as well as errors. The effort to identify individual risk factors that relate to inhibition deficits in carriers of the PM allele is consistent with a personalized medicine approach and may have implications for understanding individual risk profiles and the eventual application of tailored counseling and prevention efforts. Our specific research questions were: (1) Are inhibition skills associated with FMR1 CGG repeat length in PM mothers of children with FXS?; (2) Is older age associated with poorer inhibition skills in PM mothers of children with FXS? Consistent with prior reports of vulnerability at mid-range PM CGG repeat lengths, we expected inhibition skills to be most impaired in mothers with CGG repeat lengths of approximately 80–110. We also hypothesized that older age would relate to greater inhibition difficulties.
2. Methods
2.1. Participants
Participants in the present study included 134 female carriers of PM allele who were biological mothers of a child with FXS. Participants were drawn from a larger ongoing research study, Family Adaptation to Fragile X Syndrome, which focused on the adaptation of families of adolescents and adults with FXS (Mailick et al., 2018; Seltzer et al., 2012; Smith, Hong, Greenberg, & Mailick, 2016). One hundred of the present sample of 134 participants were recruited in 2008 and have been studied four times since then. Data for the present analysis were drawn from the study’s fourth wave of data collection (2016–2018). These participants resided in 38 U.S. states and one Canadian province, and were recruited through fragile X support groups, university-based disability research registries, and advertisement at clinics, service agencies, and national disability organizations. An additional 34 participants were enrolled in the study through a supplementary recruitment effort begun in 2016; their children were patients at the fragile X clinic at the Rush University Medical Center.
To be included in the present analysis, the mother’s CGG repeat number had to be between 55 and 200 (i.e., within the PM range) and she had to be the biological mother of a child with FXS who was confirmed to have the full mutation (>200 CGG repeats in 5’UTR of FMR1) via review of medical records. The mean age of the children with FXS was 27 years (SD = 9, range = 12–62 years). Consistent with the broader goals of the Family Adaptation to Fragile X Syndrome project, our focus was on mothers of adolescent and adult-aged children. FXS is a lifelong disability and the caregiving responsibilities of parents of children with intellectual and developmental disabilities often span several decades, persisting even as parents reach midlife and early old age (Namkung, Greenberg, Mailick, & Floyd, 2018; Seltzer, Floyd, Song, Greenberg, & Hong, 2011). Thus, the need for family-centered interventions to support individuals with FXS and their caregivers continues across the life course. Descriptive and demographic characteristics of the sample are presented in Table 1.
Table 1.
Demographics and Descriptives
| Variable | n = 134 |
|---|---|
| Age in years | |
| M (SD) | 56.79 (8.65) |
| Range | 39.00–88.00 |
| Household Income | |
| <40k | 12% |
| 40–59k | 12% |
| 60–79k | 12% |
| 80–99k | 19% |
| 100–139k | 19% |
| >139k | 26% |
| Highest Education Level | |
| High school graduate or less | 10% |
| Some college | 24% |
| Bachelor’s degree | 28% |
| Some graduate school or higher | 38% |
| Race | |
| White | 94% |
| Black or African American | 3% |
| Asian, Pacific Islander or Native Hawaiian | 1% |
| American Indian or Alaskan Native | 1% |
| Other | 1% |
2.2. Procedures
Participants completed paper-and-pencil questionnaires and provided buccal swabs for DNA analyses, which were returned to the researchers via the mail. The assessment also consisted of an audio-recorded telephone interview that lasted about an hour. The Hayling Sentence Completion Test (Burgess & Shallice, 1997) was administered over the phone as part of the telephone interview. The Hayling has been widely used as a sensitive index of inhibition deficits in studies of carriers of the PM allele (Cornish et al., 2015; Hunter et al., 2012; Kraan, Hocking, Georgiou-Karistianis, et al., 2014). While this task has not been standardized for phone administration, neuropsychological assessment via the phone has been supported as a valid and reliable mode of evaluation for other cognitive-executive batteries that have been administered to the general population, such as the Brief Test of Adult Cognition by Telephone (BTACT; (Lachman, Agrigoroaei, Tun, & Weaver, 2014). Raw Hayling scores are used in analysis and standard scores are interpreted with caution given the non-standard administration. All subjects provided written informed consent in accordance with the Declaration of Helsinki. Procedures were approved by the Institutional Review Boards of the University of Wisconsin-Madison and the Marshfield Clinic Research Institute.
2.3. Evaluation of Inhibition Skills
Hayling Sentence Completion Test (Burgess & Shallice, 1997).
The Hayling was administered as a direct-assessment measure of response inhibition. In this assessment, participants are read 15 sentences that have the final word of the sentence missing. Participants are asked to provide a word that is unconnected to the meaning of the sentence as quickly as possible, requiring participants to suppress a prepotent verbal response. Performance was indexed in terms of two variables: response latency and errors. Response latency was quantified as the time in seconds to inhibit the prepotent verbal response and provide an alternative response. Longer latency reflects poorer inhibition skills. Errors on the Hayling were recorded as category A errors (responses closely connected to the sentence) and category B errors (responses loosely connected to the sentence). The converted A+B error score was computed as described by the test developers; this score weights errors for frequency and severity. Converted error scores can range from 0–78, with higher scores indicating that errors were more frequent or more severe. Errors on the Hayling have been shown to differentiate female carriers of the PM allele from neurotypical controls in prior work (Cornish et al., 2015; Kraan, Hocking, Georgiou-Karistianis, et al., 2014), whereas Hayling latency scores have not been thoroughly examined in this population. In this study we focused on both error and latency scores, in line with the recent argument that temporal response time has a substantial influence on the executive performance of female carriers of the PM allele (Shelton et al., 2016).
Response latency was recorded by the examiner live during the telephone interview, and later checked off-line by an independent coder who reviewed the audio recordings and made corrections to the live scoring as necessary. Errors were coded off-line by the independent coder. Twenty percent of samples were randomly selected and scored by a second independent coder for reliability. Inter-rater reliability was substantial for both latency scores (κ = 0.91) and error classification (ICC [3,1] = 0.99). Hayling data were missing for one participant who did not complete the phone interview portion of the study.
Behavior Rating Inventory of Executive Function- Adult Version (BRIEF-A; Roth & Gioia, 2005).
The Inhibit subscale of the BRIEF-A provided a self-report measure of inhibition. The Inhibit subscale consists of 8 items that capture impulse control and the ability to appropriately stop one’s own behavior at the proper time, such as waiting for one’s turn. Items are scored on a 3-point scale denoting the extent to which each problem is experienced in daily life. T-scores were computed; scores at or above 65 are considered clinically significant. Following the verification procedures outlined in the BRIEF-A manual, data were considered invalid if high scores were obtained on either the negativity or infrequency scales. This resulted in missing BRIEF-A data for four participants.
2.4. Evaluation of FMR1 CGG Repeat Length
Buccal swabs were analyzed for DNA using standard methods. FMR1 CGG genotyping was conducted with the Asuragen AmplideX® Kit (Chen et al., 2010; Grasso et al., 2014). Analyses were conducted at Rush University Medical Center in the laboratory of Dr. Elizabeth Berry-Kravis.
2.5. Analysis Plan
Analyses were conducted in SAS v9.4 (SAS Institute, 2013). Descriptive statistics were computed and the variables were examined for distribution. Normal distribution was observed for all inhibition variables except the Hayling error score which showed significant right skewing. A BoxCox power transformation (Box & Cox, 1964) of λ= −0.25 was applied to correct the Hayling error score. However, analyses conducted on the original vs. transformed variable yielded identical inferential results so only analyses based on the untransformed data are presented. The distribution of CGG repeat length was characterized by an extreme outlier that fell more than three times outside the interquartile range above the third quartile. This data point had a CGG repeat length of 186, which was considerably higher than the CGG lengths of all other cases (67–138 repeats). In all statistical models including CGG repeat length as a variable of interest, regression diagnostics indicated that this case had an unduly large influence on the regression coefficients, as indicated by a Cook’s Distance value that was considerably larger than the Distance value of all other cases and far exceeded the recommended cut-off criteria of Di >4/n-k-1 (Cook, 1977). Given its undue influence on the model results, this highly influential point was excluded from all analyses. Dropping this outlier was viewed as a conservative approach that avoided estimating patterns occurring at high CGG repeat lengths based on information gleaned from a single data point.
Next, preliminary analyses were conducted to inform model specification. A Pearson’s correlation matrix was computed to inform the intercorrelation among variables of interest. Recruitment source (i.e., whether participants were drawn from the longitudinal family adaptation study that began in 2008 or as part of the supplemental recruitment in 2016) was also explored as a potential confound. Participants recruited in 2016 were younger, on average, than the participants recruited in 2008. No other differences were detected across the two samples on any of the inhibition or genetic variables. Based on these preliminary analyses, recruitment source was not covaried in the final models to preserve degrees of freedom.
To test the first research question regarding the effect of CGG repeat length on inhibition skills, a series of general linear models were fit to test CGG repeat length on the PM allele as a predictor of each of the inhibition variables. Education level was included as a covariate, coded as a four-level categorical variable (high school graduate or less, some college, bachelor’s degree, some graduate school or higher). Given prior reports of curvilinear risk patterns (Klusek, Porter, et al., 2018; Mailick et al., 2014; Roberts et al., 2009; Seltzer et al., 2012), quadratic and cubic CGG terms were probed to test for nonlinear relationships. Higher-order polynomial terms were successively added to the model and retained if they accounted for significant variance and visual examination of fit plots indicated improved model fit. Effect sizes were computed for all general linear models. In general, partial eta squared (η2p) values of 0.01, 0.06, and 0.14 are interpreted as “small”, “medium”, and “large” in size (Cohen, 1988).
Next, analyses were conducted to address the second research question regarding the effect of age on inhibition. General linear models tested age as a predictor of each of the inhibition variables, controlling for education level. To determine if age was an independent predictor beyond the effect of CGG repeat length, age was also added as a predictor to the curvilinear CGG models specified for the first research question. In addition, preliminary analyses tested for possible interactions between age and nonlinear CGG, to determine whether the effect of age on inhibition varied nonlinearly at different levels of CGG repeat lengths. For this analysis CGG repeat length was re-coded into categories of low (55–89), mid-size (90–110), and high (111–200) CGG repeats, consistent with the approximate zone of mid-size vulnerability identified in prior reports (Allen et al., 2007; Mailick et al., 2014; Sullivan et al., 2005). General linear models were fit testing age, the categorical CGG variable, and their interaction, as predictors of inhibition skills, controlling for education level. Categorical modeling of CGG repeat length was used in this analysis in order to preserve statistical power (modeling age interactions across the polynomial CGG terms would have required the inclusion of multiple interaction terms, limiting degrees of freedom).
3. Results
3.1. Descriptive Statistics
Descriptive statistics are presented in Table 2. The average CGG repeat length on the PM allele was 93, with a range of 67–138. Eight percent of the sample obtained Hayling response latency scores falling at or below the 5th percentile, which corresponds to scaled scores classified within the “Impaired”, “Abnormal”, or “Poor” ranges, as described in the test manual (Burgess & Shallice, 1997). Twenty-five percent obtained Hayling error scores falling at or below the 5th percentile. Five percent of participants obtained Inhibit subscale scores on the BRIEF-A that fell within the clinically significant range (i.e., t-score ≥65). A Pearson’s correlation matrix is presented in Table 3.
Table 2.
Descriptive statistics
| Descriptive Statistic | |||
|---|---|---|---|
| Variable | N | M (SD) | Range |
| Hayling Latency Score | 132 | 49 (33) | 1–184 |
| Hayling Error Score | 132 | 10 (13) | 0–56 |
| BRIEF-A Inhibit Subscale t-score | 129 | 51 (8) | 38–82 |
| CGG Repeat Length | 133 | 93 (15) | 67–138 |
Table 3.
Correlation matrix
| Variable | 1. | 2. | 3. | 4. | 5. |
|---|---|---|---|---|---|
| 1. Hayling Latency Score | 1.00 | ||||
| 2. Hayling Error Score | .43* | 1.00 | |||
| 3. BRIEF-A Inhibit Subscale t-score | .00 | .05 | 1.00 | ||
| 4. CGG Repeat Length | −.22* | −.07 | −.06 | 1.00 | |
| 5. Age | .26* | .11 | −.02 | −.19* | 1.00 |
p < .050
3.2. Relationship between CGG Repeat Length and Inhibition Skills
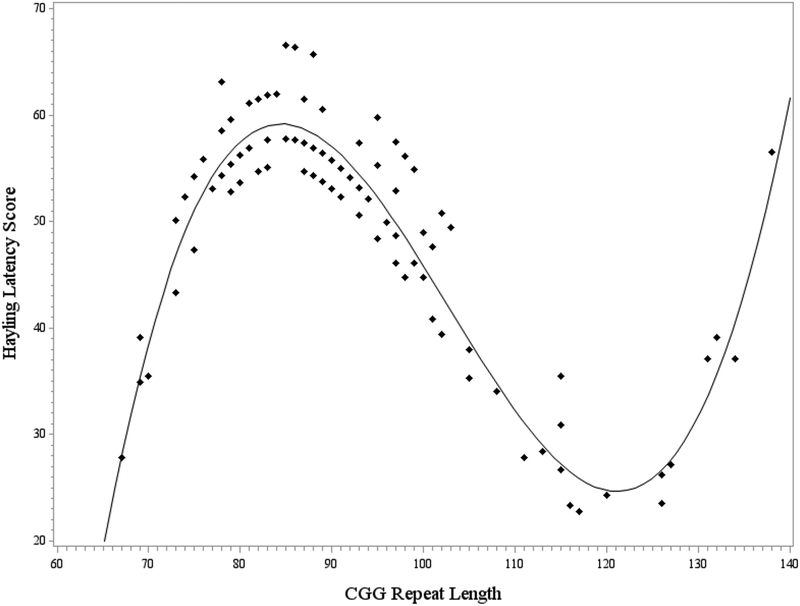
The overall model testing CGG repeat length as a predictor of the Hayling error score was not significant (F [6, 125] = 1.41, p = .214, R2 = .06). However, the overall model testing CGG repeat length as a predictor of Hayling latency was significant with the cubic model demonstrating the best fit, F (6, 125) = 2.73, p = .016, R2 = .11. The linear (p =.010, η2p =0.05), quadratic (p =.010, η2p =0.05), and cubic terms (p =.011, η2p =0.05) for CGG length accounted for significant variance in Hayling latency, with small-to-medium effect sizes. Education level did not contribute significantly to the model (p =.651, η2p =0.01). The curvilinear association between CGG length and latency is illustrated in Figure 1. Response latency was increased at approximately 80–90 CGG repeats. Latency decreased as CGG length increased from approximately 90–115 repeats, and then began to increase again at CGG repeat lengths above approximately 130. See Table 4 for model results.
Figure 1. Curvilinear association between CGG repeat length and the Hayling latency score.
Note. Model-predicted values are presented, controlling for education level. Longer latency on the Hayling reflects poorer inhibition skills.
Table 4.
Model results: Effect of CGG repeat length on the Hayling inhibition variables
| Predictor | Type III Sum of Squares | |||||
|---|---|---|---|---|---|---|
| Hayling Latency Score | Hayling Error Score | |||||
| F | p | η2p | F | p | η2p | |
| Education level | 0.55 | .651 | 0.01 | 2.49 | .064 | 0.06 |
| Linear CGG | 6.86 | .009* | 0.05 | 0.09 | .761 | 0.00 |
| Quadratic CGG | 6.79 | .010* | 0.05 | 0.10 | .751 | 0.00 |
| Cubic CGG | 6.53 | .012* | 0.05 | 0.10 | .747 | 0.00 |
p < .050
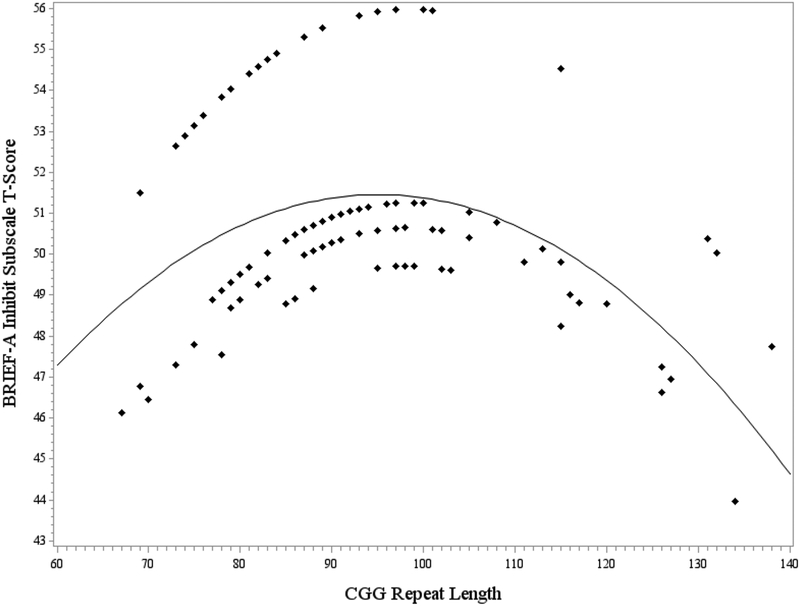
Next, a general linear model was fit to test CGG repeat length as a predictor of performance on the BRIEF-A Inhibit subscale. For this model, the addition of the cubic CGG term did not result in improved model fit and therefore the quadratic model was selected as the final model. Together, the terms for education level, linear CGG, and quadratic CGG accounted for significant variance in performance on the BRIEF-A Inhibit subscale (F [5, 123] = 2.30, p = .049, R2 = .09). Significant effects were observed for both the linear CGG term (p =.047, η2p = 0.03) and the quadratic CGG term (p =.042, η2p = 0.03). Education level also accounted for significant variance in the model, with a medium effect size (p =.030, η2p =0.07). Participants who had completed some college scored higher on the Inhibit subscale (signifying greater impairments in inhibition) than participants in any other education level category (p’s < .027). Figure 2 demonstrates the quadratic association between CGG length and scores on the Inhibit subscale. Inhibition deficits were most pronounced at mid-size CGG lengths of approximately 90–110 repeats, with better inhibition skills reported at lower and higher CGG repeat sizes.
Figure 2. Curvilinear association between CGG repeat length and the BRIEF-A Inhibit subscale.
Note. Model-predicted values are presented, controlling for education level. Higher t-scores on the Inhibit subscale reflect poorer inhibition skills.
3.3. Impact of Older Age on Inhibition
Linear age effects were tested as a predictor of each of the inhibition measures, controlling for education level. Model results are presented in Table 5. Older age was a significant predictor of longer response latency on the Hayling, with a medium effect size (p =.003, η2p = 0.07), but did not predict Hayling errors or scores on the BRIEF-A Inhibit subscale.
Table 5.
Model results: Effect of age on the inhibition measures
| Predictor | Type III Sum of Squares | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Hayling Latency Score | Hayling Error Score | BRIEF-A Inhibit Subscale | |||||||
| F | p | η2p | F | p | η2p | F | p | η2p | |
| Education level | 0.73 | .535 | 0.02 | 2.61 | .054 | 0.06 | 2.38 | .073 | 0.05 |
| Age | 9.00 | .003* | 0.07 | 1.44 | .234 | 0.01 | 0.20 | .660 | 0.00 |
p < .050
3.4. The Combined Effect of Older Age and CGG Repeat Length on Inhibition Skills
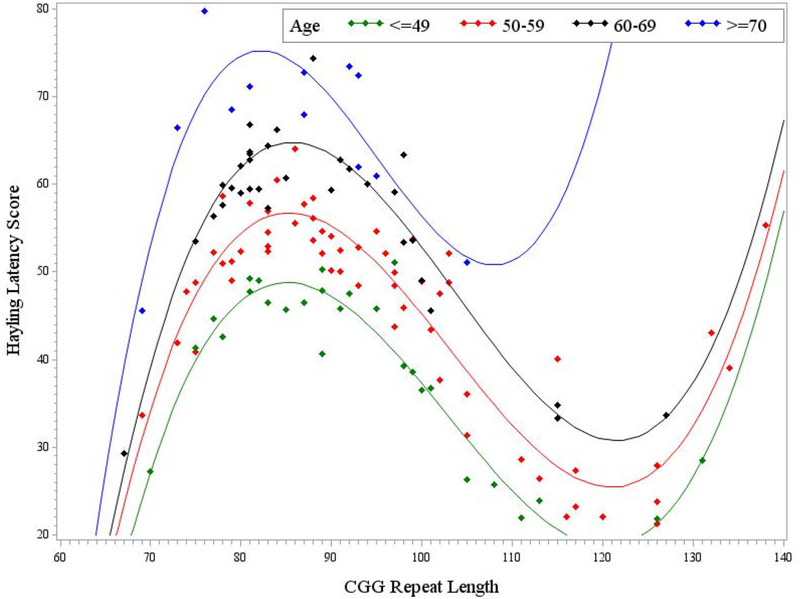
In order to test the combined effect of age and CGG repeat length on inhibition skills, age was added as a predictor to models testing the effect of CGG repeat length on the inhibition outcomes. First, age was added as a predictor, along with education level and the linear, quadratic, and cubic CGG terms, in the general linear model predicting Hayling latency. The overall model was significant, F (7, 124) = 3.45, p = .002, R2 = .16. Age accounted for significant variance in response latency with a small-medium effect size, even after education level and the curvilinear effect of CGG repeat length was accounted for in the model (p = .009, η2p =0.05). Model results are provided in Table 6 and graphically illustrated in Figure 3. Older age was associated with longer response latency. Probing of the interaction between age and CGG repeat length category (low, mid, high) did not yield evidence that the effect of age differed at different levels of CGG repeat length (p = .391, η2p =0.02). Thus, age and CGG repeat number had independent effects on Hayling latency, where older age and mid-range CGG repeats both predicted longer latency.
Table 6.
Model results: Combined effect of age and CGG repeat length on the Hayling latency score
| Variable | Type III Sum of Squares | ||
|---|---|---|---|
| F | p | η2p | |
| Education level | 0.52 | .669 | 0.01 |
| Linear CGG | 6.87 | .009* | 0.05 |
| Quadratic CGG | 6.77 | .010* | 0.05 |
| Cubic CGG | 6.50 | .012* | 0.05 |
| Age | 6.97 | .009* | 0.05 |
p < .050
Figure 3. Effect of age on the Hayling latency score.
Note. Model-predicted values are presented, controlling for education level. Longer latency on the Hayling reflects poorer inhibition skills.
Age did not account for significant variance when added to the model testing CGG as a predictor of the Hayling error score outcome (p = .279, η2p < 0.01) or BRIEF-A Inhibit subscale outcomes (p = .621, η2p < 0.01). Probing of the interaction between age and CGG repeat length category did not yield evidence that the effect of age differed according to CGG repeat length for either the Hayling error outcome (p = .796, η2p < 0.01) or the BRIEF-A Inhibit subscale (p = .326, η2p = 0.02)
4. Discussion
This study represents the largest multimodal investigation conducted to date of inhibition skills in PM mothers of children with FXS. We detected increased vulnerability for inhibition deficits among older mothers and those who carried mid-range CGG lengths of approximately 80–100 repeats. Results also suggest a possible increase in risk occurring at approximately 130–140 repeats. This study enhances understanding of older age and mid-range PM CGG repeat length as personalized risk factors that may inform the clinical management of the FMR1 PM in mothers of children with FXS. Clinical monitoring of the progression of PM-associated executive symptoms may be warranted during the transition from middle to older age, particularly for those carrying mid-range CGG repeat sizes. Future study of the functional consequences of inhibition deficits across mid-to-late adulthood will further clarify the clinical significance of this work for PM mothers and their children with FXS.
4.1. Vulnerability at Mid-range CGG Repeat Lengths
To our knowledge, this is the first study to detect increased vulnerability for executive deficits among individuals with mid-range CGG repeat lengths (approximately 80–100), relative to lower or higher PM repeat sizes. This mid-range vulnerability was evident across both self-report and direct-assessment indices of inhibition. It was particularly striking on the Hayling Sentence Completion Test where individuals with mid-range CGG repeats took approximately 30 seconds longer to inhibit a prepotent verbal response and provide an alternative response relative to individuals with lower or higher repeat sizes. Thus, we add inhibition deficits to the growing list of PM-associated symptoms that are more pronounced among individuals carrying mid-range repeat sizes. This list also includes mental health involvement, early reproductive aging, and problems with language production (Allen et al., 2007; Ennis et al., 2006; Klusek, Porter, et al., 2018; Mailick et al., 2014; Roberts et al., 2016; Seltzer et al., 2012; Sterling et al., 2013). The fact that the relationship between CGG repeat length and inhibition skills was curvilinear may also account for some inconsistencies in prior investigations of CGG-dependent variation in executive skills, which have relied primarily on tests of linear association (e.g., Kraan, Hocking, Georgiou-Karistianis, et al., 2014; Shelton et al., 2014; Yang et al., 2013).
Despite growing evidence from this study and prior reports of increased risk of certain phenotypes at mid-range CGG repeat sizes, the mechanisms underlying this vulnerability are unclear. CGG-dependent changes in gene expression could play a role; FMR1 transcript and protein levels have been shown to vary across the continuum of CGG repeat sizes (Chen et al., 2003; Kenneson et al., 2001; Loesch et al., 2007; Sellier et al., 2014), with some evidence of increased protein production at mid-range repeat sizes relative to other repeat sizes in the PM range (Peprah et al., 2010). However, this possible explanation cannot be investigated in the present study due to the lack of FMRP and mRNA data, which is a limitation. It is also possible that mid-range vulnerability is related to hormonal dysfunction associated with the increased risk for FXPOI that occurs within the CGG range (Allen et al., 2007; Lekovich et al., 2018); future investigations may explore this hypothesis. Another consideration is the potential for gene-environment interactions. There is good evidence that women with mid-range CGG repeat sizes are more vulnerable to the negative effects of environmental stress exposure (Hartley et al., 2012; Seltzer et al., 2012) and less able to derive benefit from positive environmental factors (Hartley, DaWalt, Hong, Greenberg, & Mailick, in press). The women in our study all had a child with FXS and may have been more vulnerable to poor outcomes due to parenting stress exposure. Future studies may explore whether similar patterns of phenotypic expression are observed among women who do not have children with FXS.
4.2. Increased Inhibition Deficits Occurring at >130 CGG Repeats
Knowledge of CGG-dependent phenotypes occurring at “high” PM repeat lengths (approximately 110–200) is relatively limited, given that these repeat sizes occur more rarely in the population and are poorly represented in research samples. The current study was also limited by poor representation across 140–200 CGG repeats. However, we did have good representation across 110–140 repeats, which is a strength that may have allowed us to detect cubic associations when others have not. In addition to detecting increased inhibition deficits at mid-range CGG repeat sizes, we also observed a second rise in Hayling latency scores as CGG’s increased from approximately 130–140 repeats. It is unclear whether this rise would continue beyond 140 repeats, as we cannot infer patterns beyond the limits of our data. Ours is not the first report to suggest a rise in inhibition deficits occurring at greater than approximately 130 CGG repeats. In an independent sample, Klusek, Porter, et al. (2018) detected a trend-level cubic association between CGG length and Hayling errors where symptoms increased at mid-range CGG sizes and again at greater than 120 repeats. Taken together, findings suggest complex nonlinear risk patterns for certain PM phenotypes, characterized by peaks and valleys of relative risk occurring across the range of CGG repeat lengths. The reasons for increased risk at greater than 130 repeats are unclear, although decreased FMRP production related to ribosomal stalling that occurs in the upper PM CGG range could be implicated (Kenneson et al., 2001; Tassone et al., 2000). Delineating zones of phenotypic vulnerability may translate back to basic science research aimed at identifying the molecular genetic properties that distinguish CGG repeat size ranges.
4.3. Premature Cognitive Aging in Carriers of the PM Allele
Older age was associated with slower Hayling responses in PM mothers of children with FXS. Age-related slowing in Hayling latency has also been observed in normal aging, with age accounting for about 10% of variation in performance (Andrés & Van der Linden, 2000; Bielak, Mansueti, Strauss, & Dixon, 2006; Treitz, Heyder, & Daum, 2007). However, in normal aging, scores tend to be relatively stable through middle adulthood and pronounced slowing is not evident until the 60’s (Treitz et al., 2007). In contrast, we observed age-related decline in PM mothers of children with FXS beginning as early as the fourth decade of life and continuing with each passing decade. Older mothers who carried mid-range CGG repeat sizes were most at risk, given that both age and CGG repeat exerted an independent effect on inhibitory control. These results may inform a personalized medicine approach to counseling, prevention, and treatment of PM mothers of children with FXS.
It will be informative in future work to address how age-related decline in executive skills may correspond to age-related neuroanatomical changes in PM mothers of children with FXS. In studies focused on male carriers of the PM allele, there is emerging evidence of atypical age-related changes in brain structure and function, specifically degeneration in functional activation in the temporoparietal area that is associated with aging (Brown, Basu, Whalley, Kind, & Stanfield, 2018) and accelerated age-related decreases in the volume of the cerebellum and whole brain (Wang et al., 2017). Elucidating neural correlates of age-related executive deficits in PM mothers of children with FXS will inform gene-brain-behavior pathways implicated in the expression of neurodegenerative phenotypes associated with the PM genotype.
4.4. Measurement Effects
Although mid-range CGG repeat vulnerability was observed across both inhibition indices, results did not always align across the Hayling and BRIEF-A. For example, only the Hayling was sensitive to age effects and to the increase in symptoms occurring at >130 CGG repeat lengths. Additionally, education effects were observed on the BRIEF-A but not the Hayling, with patterns suggesting that individuals who started college but did not finish were more likely to report executive limitations. Notably, the BRIEF-A inhibit subscale was not correlated with the Hayling latency and error scores, suggesting that these indices may tap different aspects of inhibitory control. It is possible that the Hayling latency score, which captures rapid, time-dependent responses, may be more sensitive to PM-associated executive deficits than are non-timed measures, as has been suggested by Shelton et al. (2016). Shelton et al. (2016) also discussed the importance of white matter integrity in the speed of neural processing (e.g., Turken et al., 2008) and proposed that specific difficulties on timed executive tasks may be associated with the reduced white matter integrity within the neural tracts connecting the prefrontal, parietal, and cerebellar regions that has been documented in male and female carriers of the PM allele (e.g., Battistella et al., 2013; Hashimoto, Srivastava, et al., 2011; Leow et al., 2014). Consistent with the findings of Shelton et al. (2016), our results across the two Hayling indices also suggest that timing is important; response latency was more sensitive to PM-associated executive deficits than were response errors. Latency may better reflect the efficiency of executive responses, as captured by the time required to suppress an irrelevant competing response and generate a better response. In contrast, errors indicate whether successful inhibition was achieved, but not the effort required to achieve it. In future work, the adoption of multimodal assessment that includes measures of response efficiency as well as errors will further our understanding of the nature of executive deficits in PM mothers of children with FXS.
4.5. Strengths and Limitations
Strengths of the present study include wide age range represented in the large sample, which was drawn from a variety of recruitment sources including the community as well as a fragile X clinic. Though the sample was diverse in terms of education and household income, it was limited by a lack of racial diversity. The wide range of CGG repeats represented is also a strength, particularly at higher repeat lengths of 100–138 which are often poorly represented in studies employing smaller samples. However, no participant included in analysis had a repeat number of over 138 (we excluded one outlier of 186 repeats), which limited inferences that could be drawn across the full range of PM CGG repeat sizes. Our sample also consisted of PM mothers of children with FXS; future studies should explore whether patterns extend to carriers of the PM allele who do not have children with FXS.
Our inclusion of both self-reports and behavioral tests to index inhibition is also a strength and may shed light on some mixed findings in previous reports. However, it is unclear how our results may have been influenced by phone administration of the Hayling. Although phone administration limits our ability to compare our data to published Hayling norms, our use of phone administration may also be viewed as a strength in terms of laying the groundwork for future phone-based executive behavioral assessments in studies of carriers of the PM allele. This method also makes it possible to include women for whom travel to the testing site is not possible due to their own or their child’s limitations. Despite our use of phone administration, performance on the Hayling was highly sensitive to age and CGG-related modulation of inhibition deficits in the present sample of PM mothers of children with FXS.
4.6. Conclusions
Patterns of mid-range CGG repeat size vulnerability have been reported relative to other aspects of the PM phenotype, and this report is the first to extend our understanding of mid-range vulnerability to the executive deficits experienced by PM mothers of children with FXS. Across both self-report and direct assessment measures of inhibitory control, women carrying mid-range CGG lengths of approximately 80–100 repeats exhibited increased inhibition deficits. Results also suggested a possible second zone of CGG-repeat-size vulnerability occurring at 130–140 repeats. Risk associated with the genotype became more pronounced with older age, resulting in the most severe deficits in older individuals who carried CGG repeat sizes of approximately 80–100 and 130–140. Inhibition deficits can impact performance in everyday social and occupational settings. Our results suggest that inhibition deficits may contribute to poor outcomes in PM mothers of children with FXS and their families, particularly in midlife and the early years of old age. This study supports older age and mid-range CGG repeat length as personalized risk factors that may be used to tailor the clinical monitoring and management of executive deficits in PM mothers of children with FXS.
Highlights.
We examined inhibition skills in mother carriers of the FMR1 premutation.
Mid-range CGG repeats of ~80–100 were associated with greater inhibition deficits.
Older age was also linked with inhibition deficits, beyond the effect of CGG size.
Mid-range CGG’s and older age could be personalized risk factors for carriers.
Acknowledgments
We are grateful to the women and families who participated in this research.
Funding: This work was supported by the National Institutes of Health [grant numbers R01HD082110, P30 HD003100-S1, U54 HD090256].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Statement
The dataset analyzed in this study is not publicly available. Due to the sensitive nature of the study, participants were assured data would be released in aggregate form only and raw data would not be shared.
References
- Allen E, Sullivan A, Marcus M, Small C, Dominguez C, Epstein M, et al. (2007). Examination of reproductive aging milestones among women who carry the FMR1 premutation. Human reproduction, 22(8), 2142–2152. [DOI] [PubMed] [Google Scholar]
- Andrés P, & Van der Linden M (2000). Age-related differences in supervisory attentional system functions. The Journals of Gerontology: Series B, 55(6), P373–P380. [DOI] [PubMed] [Google Scholar]
- Aron AR (2011). From reactive to proactive and selective control: Developing a richer model for stopping inappropriate responses. Biological Psychiatry, 69(12), e55–e68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battistella G, Niederhauser J, Fornari E, Hippolyte L, Gronchi Perrin A, Lesca G, et al. (2013). Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiology of Aging, 34(6), 1700–1707. [DOI] [PubMed] [Google Scholar]
- Bielak AA, Mansueti L, Strauss E, & Dixon RA (2006). Performance on the Hayling and Brixton tests in older adults: Norms and correlates. Archives of Clinical Neuropsychology, 21(2), 141–149. [DOI] [PubMed] [Google Scholar]
- Bourgeois JA, Seritan AL, Casillas EM, Hessl D, Schneider A, Yang Y, et al. (2011). Lifetime prevalence of mood and anxiety disorders in fragile X premutation carriers. Journal of Clinical Psychiatry, 72(2), 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Box GE, & Cox DR (1964). An analysis of transformations. Journal of the Royal Statistical Society. Series B (Methodological), 211–252. [Google Scholar]
- Brown SSG, Basu S, Whalley HC, Kind PC, & Stanfield AC (2018). Age-related functional brain changes in FMR1 premutation carriers. NeuroImage: Clinical, 17, 761–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P, & Shallice T (1997). Hayling sentence completion test. Suffolk, England: Thames Valley Test Company. [Google Scholar]
- Chen L, Hadd A, Sah S, Filipovic-Sadic S, Krosting J, Sekinger E, et al. (2010). An information-rich CGG repeat primed PCR that detects the full range of fragile X expanded alleles and minimizes the need for southern blot analysis. Journal of Molecular Diagnostics, 12(5), 589–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L-S, Tassone F, Sahota P, & Hagerman PJ (2003). The (CGG) n repeat element within the 5′ untranslated region of the FMR1 message provides both positive and negative cis effects on in vivo translation of a downstream reporter. Human molecular genetics, 12(23), 3067–3074. [DOI] [PubMed] [Google Scholar]
- Cohen J (1988). Statistical power analysis for the behavioral sciences. Hillsdale, NJ: L. Erlbaum Associates. [Google Scholar]
- Cook RD (1977). Detection of influential observation in linear regression. Technometrics, 19(1), 15–18. [Google Scholar]
- Cornish KM, Kraan CM, Bui QM, Bellgrove MA, Metcalfe SA, Trollor JN, et al. (2015). Novel methylation markers of the dysexecutive-psychiatric phenotype in FMR1 premutation women. Neurology, 84(16), 1631–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ennis S, Ward D, & Murray A (2006). Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. European Journal of Human Genetics 14(2), 253–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feilhauer J, Cima M, Korebrits A, & Kunert HJ (2012). Differential associations between psychopathy dimensions, types of aggression, and response inhibition. Aggressive behavior, 38(1), 77–88. [DOI] [PubMed] [Google Scholar]
- Fu YH, Kuhl DP, Pizzuti A, Pieretti M, Sutcliffe JS, Richards S, et al. (1991). Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell, 67. [DOI] [PubMed] [Google Scholar]
- Garcia-Arocena D, & Hagerman PJ (2010). Advances in understanding the molecular basis of FXTAS. Human Molecular Genetics, 19(R1), R83–R89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Srivastava S, Tassone F, Harvey D, et al. (2011). Young adult female fragile X premutation carriers show age- and genetically-modulated cognitive impairments. Brain and Cognition. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodrich-Hunsaker NJ, Wong LM, McLennan Y, Tassone F, Harvey D, Rivera SM, et al. (2011). Adult female fragile X premutation carriers exhibit age- and CGG repeat length-related impairments on an attentionally-based enumeration task. Frontiers in Human Neuroscience, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasso M, Boon EMJ, Filipovic-Sadic S, van Bunderen PA, Gennaro E, Cao R, et al. (2014). A novel methylation PCR that offers standardized determination of FMR1 methylation and CGG repeat length without southern blot analysis. The Journal of Molecular Diagnostics, 16(1), 23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. (2005). Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain, 129(1), 243–255. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, & Raz N (2000). The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology, 14(2), 224. [DOI] [PubMed] [Google Scholar]
- Hagerman R, & Hagerman P (2013). Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. The Lancet Neurology, 12(8), 786–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halket ES, Wang JY, Hessl D, & Rivera SM (2016). Neuroimaging Findings in FXTAS. In FXTAS, FXPOI, and Other Premutation Disorders (pp. 71–85): Springer. [Google Scholar]
- Hartley SL, DaWalt LS, Hong J, Greenberg J, & Mailick MR (in press). Positive emotional support in premutation carrier mothers of adolescents and adults with fragile X syndrome: Gene by environment interactions. American Journal on Intellectual and Developmental Disabilities [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley SL, Seltzer MM, Hong J, Greenberg JS, Smith L, Almeida D, et al. (2012). Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. International Journal of Behavioral Development, 36, 53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Backer KC, Tassone F, Hagerman RJ, & Rivera S (2011). An fMRI study of the prefrontal activity during the performance of a working memory task in premutation carriers of the fragile X mental retardation 1 gene with and without fragile X-associated tremor/ataxia syndrome (FXTAS). Journal of Psychiatric Research, 45(1), 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto R, Srivastava S, Tassone F, Hagerman RJ, & Rivera S (2011). Diffusion tensor imagin in male premutation carriers of the fragile X mental retardation gene. Movement Disorders, 26(7), 1329–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter J, Rivero-Arias O, Angelov A, Kim E, Fotheringham I, & Leal J (2014). Epidemiology of fragile X syndrome: A systematic review and meta-analysis. American Journal of Medical Genetics Part A, 164(7), 1648–1658. [DOI] [PubMed] [Google Scholar]
- Hunter JE, Allen EG, Abramowitz A, Rusin M, Leslie M, Novak G, et al. (2008). No evidence for a difference in neuropsychological profile among carriers and noncarriers of the FMR1 premutation in adults under the age of 50. American Journal of Human Genetics, 83(6), 692–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter JE, Sherman S, Grigsby J, Kogan C, & Cornish K (2012). Capturing the fragile X premutation phenotypes: a collaborative effort across multiple cohorts. Neuropsychology, 26(2), 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenneson A, Zhang F, Hagedorn CH, & Warren ST (2001). Reduced FMRP and increased FMR1 transcription is proportionally associated with CGG repeat number in intermediate-length and premutation carriers. Human Molecular Genetics, 10(14), 1449–1454. [DOI] [PubMed] [Google Scholar]
- Kim S-Y, Hashimoto R.-i., Tassone F, Simon TJ, & Rivera SM (2013). Altered neural activity of magnitude estimation processing in adults with the fragile X premutation. Journal of Psychiatric Research, 47(12), 1909–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Fairchild AJ, & Roberts JE (2019). Vagal tone as a putative mechanism for pragmatic competence: An investigation of carriers of the FMR1 premutation Journal of Autism and Developmental Disorders, 49, 197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Porter A, Abbeduto L, Adayev T, Tassone F, Mailick MR, et al. (2018). Curvilinear association between language disfluency and FMR1 CGG repeat size across the normal, intermediate, and premutation range. Frontiers in Genetics, 9(344). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klusek J, Ruber A, & Roberts JE (2018). Impaired eye contact in the FMR1 premutation is not associated with social anxiety or the broad autism phenotype. The Clinical Neuropsychologist, 32, 1337–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraan CM, Hocking DR, Bradshaw JL, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, et al. (2014). Symbolic sequence learning is associated with cognitive–affective profiles in female FMR1 premutation carriers. Genes, Brain and Behavior, 13(4), 385–393. [DOI] [PubMed] [Google Scholar]
- Kraan CM, Hocking DR, Georgiou-Karistianis N, Metcalfe SA, Archibald AD, Fielding J, et al. (2014). Impaired response inhibition is associated with self-reported symptoms of depression, anxiety, and ADHD in female FMR1 premutation carriers. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 165(1), 41–51. [DOI] [PubMed] [Google Scholar]
- Lachman ME, Agrigoroaei S, Tun PA, & Weaver SL (2014). Monitoring cognitive functioning: psychometric properties of the brief test of adult cognition by telephone. Assessment, 21(4), 404–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekovich J, Man L, Xu K, Canon C, Lilienthal D, Stewart JD, et al. (2018). CGG repeat length and AGG interruptions as indicators of fragile X–associated diminished ovarian reserve. Genetics in Medicine, 20(9), 957. [DOI] [PubMed] [Google Scholar]
- Leow A, Harvey D, Goodrich-Hunsaker NJ, Gadelkarim J, Kumar A, Zhan L, et al. (2014). Altered structural brain connectome in young adult fragile X premutation carriers. Human brain mapping, 35(9), 4518–4530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui QM, Huggins RM, Mitchell RJ, Hagerman RJ, & Tassone F (2007). Transcript levels of the intermediate size or grey zone fragile X mental retardation 1 alleles are raised, and correlate with the number of CGG repeats. Journal of Medical Genetics, 44(3), 200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Klusek J, Martin GE, Sideris J, Parlier M, & Piven J (2012). Defining genetically meaningful language and personality traits in relatives of individuals with fragile X syndrome and relatives of individuals with autism. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 159B(6), 660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddalena A, Richards CS, McGinniss MJ, Brothman A, Desnick RJ, Grier RE, et al. (2001). Technical standards and guidelines for fragile X: the first of a series of disease-specific supplements to the Standards and Guidelines for Clinical Genetics Laboratories of the American College of Medical Genetics. Genetics in Medicine, 3(3), 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner MJ, Baker MW, Broman KW, Tian J, Barnes JK, Atkins A, et al. (2013). FMR1 CGG expansions: prevalence and sex ratios. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 162(5), 466–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailick MR, Hong J, Greenberg J, Smith L, & Sherman S (2014). Curvilinear association of CGG repeats and age at menopause in women with FMR1 premutation expansions. American Journal of Medical Genetics: Part B, 165(8), 705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailick MR, Movaghar A, Hong J, Greenberg JS, DaWalt LS, Zhou L, et al. (2018). Health profiles of mosaic versus mon-mosaic FMR1 premutation carrier mothers of children with fragile X syndrome. Frontiers in Genetics, 9(173). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meza JI, Owens EB, & Hinshaw SP (2016). Response inhibition, peer preference and victimization, and self-harm: Longitudinal associations in young adult women with and without ADHD. Journal of Abnormal Child Psychology, 44(2), 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movaghar A, Page D, Brilliant M, Baker MW, Greenberg J, Hong J, et al. (2019). Data-driven phenotype discovery of FMR1 premutation carriers in a population-based sample. Science Advances, 5(8), eaaw7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namkung EH, Greenberg JS, Mailick MR, & Floyd FJ (2018). Lifelong parenting of adults with developmental disabilities: Growth trends over 20 years in midlife and later life. American Journal on Intellectual and Developmental Disabilities, 123(3), 228–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh SY, He F, Krans A, Frazer M, Taylor JP, Paulson HL, et al. (2015). RAN translation at CGG repeats induces ubiquitin proteasome system impairment in models of fragile X-associated tremor ataxia syndrome. Human molecular genetics, 24(15), 4317–4326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peprah E, He W, Allen E, Oliver T, Boyne A, & Sherman SL (2010). Examination of FMR1 transcript and protein levels among 74 premutation carriers. Journal of Human Genetics, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primerano B, Tassone F, Hagerman RJ, Hagerman P, Amaldi F, & Bagni C (2002). Reduced FMR1 mRNA translation efficiency in fragile X patients with premutations. Rna, 8(12), 1482–1488. [PMC free article] [PubMed] [Google Scholar]
- Roberts JE, Bailey DB, Mankowski J, Ford A, Weisenfeld LA, Heath TM, et al. (2009). Mood and anxiety disorders in females with the FMR1 premutation. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics, 150B(1), 130–139. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Tonnsen BL, McCary LM, Ford AL, Golden RN, & Bailey DB (2016). Trajectory and Predictors of Depression and Anxiety Disorders in Mothers with the FMR1 Premutation. Biological psychiatry, 79(10), 850–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Madrigal I, Pagonabarraga J, Xuncla M, Badenas C, Kulisevsky J, et al. (2009). Penetrance of FMR1 premutation associated pathologies in fragile X syndrome families. Eur J Hum Genet, 17(10), 1359–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth RM, & Gioia GA (2005). Behavior rating inventory of executive function--adult version: Psychological Assessment Resources; Lutz, FL. [Google Scholar]
- Rubio G, Jiménez M, Rodríguez-Jiménez R, Martínez I, Avila C, Ferre F, et al. (2008). The role of behavioral impulsivity in the development of alcohol dependence: a 4-year follow-up study. Alcoholism, Clinical And Experimental Research, 32(9), 1681–1687. [DOI] [PubMed] [Google Scholar]
- SAS Institute. (2013). SAS Institute version 9.4): Cary NC. [Google Scholar]
- Sellier C, Usdin K, Pastori C, Peschansky VJ, Tassone F, & Charlet-Berguerand N (2014). The multiple molecular facets of fragile X-associated tremor/ataxia syndrome. Journal of Neurodevelopmental Disorders, 6(1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe C, & Almeida D (2012). Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychology, 31(5), 612–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Floyd F, Song J, Greenberg J, & Hong J (2011). Midlife and Aging Parents of Adults With Intellectual and Developmental Disabilities: Impacts of Lifelong Parenting. American Journal on Intellectual and Developmental Disabilities, 116(6), 479–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton AL, Cornish K, Kraan C, Georgiou-Karistianis N, Metcalfe SA, Bradshaw JL, et al. (2014). Exploring inhibitory deficits in female premutation carriers of fragile X syndrome: Through eye movements. Brain and cognition, 85, 201–208. [DOI] [PubMed] [Google Scholar]
- Shelton AL, Cornish KM, Godler D, Bui QM, Kolbe S, & Fielding J (2017). White matter microstructure, cognition, and molecular markers in fragile X premutation females. Neurology, 88(22), 2080–2088. [DOI] [PubMed] [Google Scholar]
- Shelton AL, Cornish KM, Kraan CM, Lozano R, Bui M, & Fielding J (2016). Executive dysfunction in female FMR1 premutation carriers. The Cerebellum, 15(5), 565–569. [DOI] [PubMed] [Google Scholar]
- Smith LE, Hong J, Greenberg JS, & Mailick MR (2016). Change in the behavioral phenotype of adolescents and adults with FXS: role of the family environment. Journal of Autism and Developmental Disorders, 46(5), 1824–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling AM, Mailick M, Greenberg J, Warren SF, & Brady N (2013). Language dysfluencies in females with the FMR1 premutation. Brain and Cognition, 82(1), 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, et al. (2005). Association of FMR1 repeat size with ovarian dysfunction. Human Reproduction,20(2), 402–412. [DOI] [PubMed] [Google Scholar]
- Sullivan SD, Welt C, & Sherman S (2011). FMR1 and the continuum of primary ovarian insufficiency. Paper presented at the Seminars in reproductive medicine. [DOI] [PubMed] [Google Scholar]
- Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, & Hagerman PJ (2000). Elevated levels of FMR1 messenger RNA in carrier males: A new mechanism of involvement in the fragile X syndrome. American Journal of Human Genetics, 66, 6–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treitz FH, Heyder K, & Daum I (2007). Differential course of executive control changes during normal aging. Aging, Neuropsychology, and Cognition, 14(4), 370–393. [DOI] [PubMed] [Google Scholar]
- Tremblay RE, Pihl RO, Vitaro F, & Dobkin PL (1994). Predicting early onset of male antisocial behavior from preschool behavior. Archives of general psychiatry, 51(9), 732–739. [DOI] [PubMed] [Google Scholar]
- Turken U, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, & Gabrieli JD (2008). Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage, 42(2), 1032–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdejo-García A, Lawrence AJ, & Clark L (2008). Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience & Biobehavioral Reviews, 32(4), 777–810. [DOI] [PubMed] [Google Scholar]
- Wang JY, Hessl D, Hagerman RJ, Simon TJ, Tassone F, Ferrer E, et al. (2017). Abnormal trajectories in cerebellum and brainstem volumes in carriers of the fragile X premutation. Neurobiology of Aging, 55, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler A, Raspa M, Hagerman R, Mailick M, & Riley C (2017). Implications of the FMR1 premutation for children, adolescents, adults, and their families. Pediatrics, 139, S172–S182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler AC, Bailey DB Jr, Berry-Kravis E, Greenberg J, Losh M, Mailick M, et al. (2014). Associated features in females with an FMR1 premutation. Journal of Neurodevelopmental Disorders, 6(30). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-C, Simon C, Niu Y-Q, Bogost M, Schneider A, Tassone F, et al. (2013). Phenotypes of hypofrontality in older female fragile X premutation carriers. Annals of neurology, 74(2), 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]