Abstract
Introduction
Occult neck node metastasis in head and neck squamous cell carcinoma (HNSCC) in the form of micrometastasis and isolated tumour cell (ITC) often goes unnoticed in the routine pathological examination. This limitation can be overcome by using serial sectioning and immunohistochemistry for detection of micrometastasis and ITC in clinically and pathologically node-negative neck. The primary objective was to determine the incidence of micrometastasis and ITC in the selective neck dissection specimen, whereas to determine the levels of lymph nodes involved, depending upon the site of primary tumour, was the secondary objective.
Materials and Methods
Lymph nodes from selective neck dissection specimen were subjected to serial sectioning and immunohistochemistry with pan-cytokeratin marker. Incidence of micrometastasis and ITC, site and stage of primary tumours and level of lymph nodes involved were determined.
Results
In total, 8.8% patients in the study got upstaged after serial sectioning and immunohistochemistry. Tongue and lower alveolar primaries showed the presence of micrometastasis and ITC in neck nodes. All the primary tumours were of pT1 stage. Level IB and II lymph nodes were primarily involved.
Conclusion
Micrometastasis and isolated tumour cells are found in approximately 9% of cases of early-stage oral cavity squamous cell carcinoma. The predictive factors and clinical significance are still unknown. More prospective trials are required to solve this evolving aspect of HNSCC.
Keywords: Head and neck cancer, Oral cancer, Neck node metastasis, Micrometastasis, Isolated tumour cell, Immunohistochemistry, Pan-cytokeratin
Introduction
It is a well known fact by now that cervical nodal status is the single most important prognostic factor in head and neck squamous cell carcinoma (HNSCC). The presence of nodal metastasis decreases 5-year survival by 50% [1]. The active management for metastatic neck disease in HNSCC includes elective neck dissection or elective neck irradiation [2, 3]. There are two arguments in favour of treating neck metastasis surgically. First, neck dissection helps in staging the disease, and thus, helps to determine the adjuvant treatment [4]. Second, it improves the locoregional control [5]. But the real area of controversy is the management of clinically node-negative (cN0) neck. The overall risk of occult metastasis in cN0 neck is 10–30% depending on the tumour characteristics [6, 7]. But occult metastasis in neck nodes does not always necessitate the presence of a macrometastasis. It might be in the form of micrometastasis and isolated tumour cell (ITC) which often goes unnoticed in the routine pathological examination. This limitation can be overcome by using serial sectioning and immunohistochemistry (IHC) for the detection of micrometastasis and ITC in clinically and pathologically node-negative neck [8].
Materials and Methods
This prospective longitudinal study was done at a tertiary level healthcare centre in Bengaluru with prior approval from the institutional ethics committee (EC/329/17/03). Informed consent was obtained from all patients enrolled in the study. The primary objective was to determine the incidence of micrometastasis and ITC in the selective neck dissection specimen with serial sectioning and IHC, whereas to determine the levels of lymph nodes involved, depending upon the site of primary tumour, was the secondary objective.
Patients with HNSCC with clinically and/or ultrasound-guided fine needle aspiration cytology (FNAC) proven node-negative neck who underwent selective neck dissection and postoperative histopathology confirming the absence of neck node metastasis were included in the study. On the contrary, any head neck cancer other than SCC, clinically and/or histopathologically proven node-positive (N+) neck, the presence of distant metastasis, residual disease after definitive chemoradiation, recurrent tumours and the presence of synchronous primary tumour were excluded. Management of the enrolled patients was not altered for this prospective study.
Forty-two consecutive patients with HNSCC presenting to the head and neck clinic of the institution and scheduled for a neck dissection were asked to participate. All the patients underwent ipsilateral selective neck dissection (SND) as all the primary tumours were lateralized lesions. Once the neck specimen was removed, lymph nodes were immediately dissected from each level of the neck. The lymph nodes were bivalved, then ‘breadloafed’, and submitted for standard paraffin embedding and haematoxylin and eosin (H&E) studies by the Department of Pathology. After standard pathological analysis, paraffin blocks of nodes deemed negative for metastatic SCC were exhaustively sectioned. Serial sections, each measuring 3–5 μm, were retained every 100 μm throughout the block. The number of sections obtained varied according to the size of lymph nodes. At each level, alternate sections were stained with H&E and pan-cytokeratin (pan-CK). All stained slides were examined under a light microscope independently by a single pathologist for expression of the positive immunostaining. Only those cells with a strong, globoid cytoplasmic reaction were considered metastatic.
Data analysis was done using SPSS version 21. Data were mainly descriptive in nature.
Results
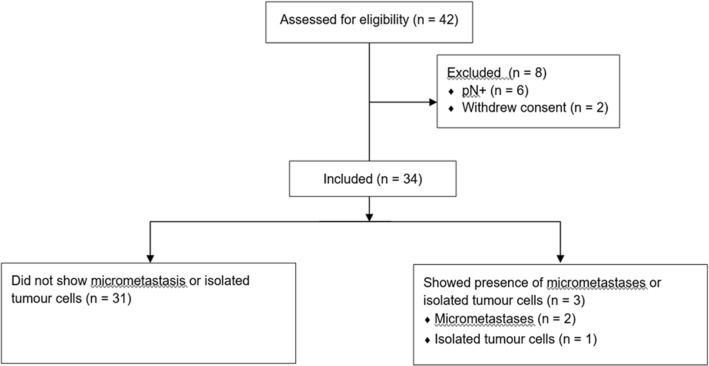
Forty-two patients were enrolled in the study initially. Two patients withdrew consents successively, and in six patients, the H&E examination showed the presence of metastatic SCC which was undetected preoperatively (Fig. 1).
Fig. 1.
The flow diagram of the study
Thirty-four patients, therefore, were available for analysis. Majority (n = 24, 71%) of them were male, with a mean and median age of 50.7 years and 51 years, respectively. All 34 cases were of oral cavity primary, as all the patients with oropharyngeal, laryngeal and hypopharyngeal cancer who underwent surgery were due to the presence of residual or recurrent tumours post-chemoradiation, and, thus, excluded. No case of SCC involving nose or nasopharynx was operated during the study period. The subsite distribution for these 34 oral cavity cancers is detailed in Fig. 2.
Fig. 2.
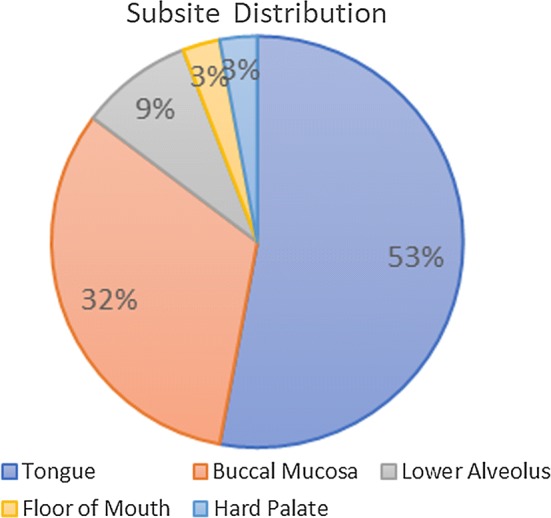
Subsite distribution of the primary tumours. Most common subsite was tongue (n = 18), followed by buccal mucosa (n = 11), lower alveolus (n = 3), floor of mouth (n = 1) and hard palate (n = 1)
Twenty-seven patients presented in early stage (pT1 and pT2), and six patients, with advanced primary disease (pT3 and pT4). In one case, T staging could not be determined as the patient presented after undergoing an excisional biopsy for a tongue lesion at an outside centre, which came positive for malignancy, and successive surgery (partial glossectomy) did not reveal any residual tumour (Fig. 3).
Fig. 3.
T staging (AJCC/UICC 8th edition) of the primary tumours [T1 = 38%, T2 = 29%, T3 = 6%, T4a = 21%; *Tx = primary tumour staging could not be elicited due to excision biopsy as a diagnostic procedure]
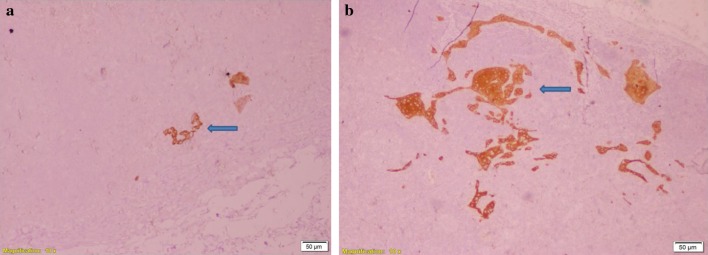
A total of 1126 lymph nodes were examined with serial section and pan-CK immunohistochemistry from 34 neck dissection specimens, with an average lymph nodal yield of 33 per patient, which is at par with the standard mentioned in the AJCC/UICC 8th edition of TNM staging. Three lymph nodes from three different individuals showed the presence of micrometastasis and ITC (Fig. 4). The primary tumour subsite, stage and the level of lymph node found to be involved in these three cases are highlighted in Fig. 5.
Fig. 4.
Immunohistochemistry with pan-cytokeratin showing the presence of micrometastasis and isolated tumour cells under × 10 magnification. a IHC highlights micrometastasis in subcapsular area of lymph node (arrow); b IHC highlights ITC in subcapsular area of lymph node (arrow)
Fig. 5.
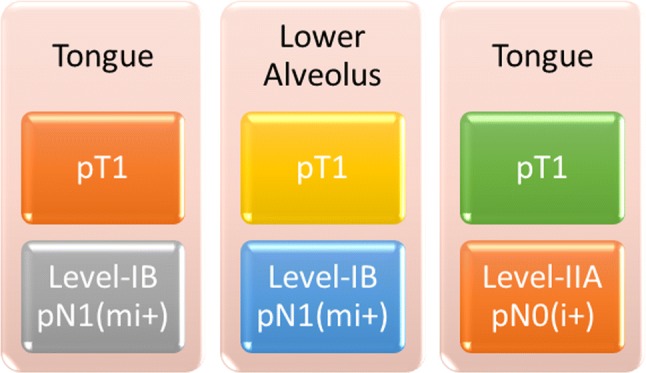
The site and stage of the primary tumour and level of lymph node showing the presence of micrometastasis and isolated tumour cells
Discussion
In May, 2002, the 6th edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual was published [9], and the new staging system was officially adopted for use in tumour registries in January, 2003. According to it, ITCs are defined as metastatic lesions no larger than 0.2 mm in dimension and classified as pN0, whereas micrometastasis is defined as metastatic lesions larger than 0.2 mm in dimension but no larger than 2.0 mm in dimension and classified as pN1mi (Table 1).
Table 1.
Difference between isolated tumour cells and micrometastasis. Adapted from: Hermanek P, Hutter RVP, Sobin LH, Wittekind C. Classification of isolated tumour cells and micrometastasis. Cancer 1999; 86: 2668–2673
| Isolated tumour cells | Micrometastasis | |
|---|---|---|
| Size | Single tumour cells or small clusters ≤ 0.2 mm or < 200 cellsa | ≤ 0.2 cm in greatest dimension |
| Contact with vessel or lymphatic wall | No | Yes |
| Invasion and penetration of vessel or lymphatic wall | No | Yes |
| Extravascular stromal reaction | No | Usually yes |
| Extravascular tumour cell proliferation | No | Yes |
aUpdated from AJCC/UICC TNM cancer staging 8th edition
All the cases in the present study were of oral cavity as all the oropharyngectomy, laryngectomy and partial or total pharyngectomies were performed as a salvage procedure, and no nasopharyngectomy was done during the whole study period. This goes with the established international and institutional protocol.
Three patients showed the presence of micrometastasis and ITC after being declared pN0 with conventional histopathologic examination. This resulted in pathologic upstaging in 8.8% of our cases. This result is supported by studies done by Ambrosch et al. [10], Enepekides et al. [11] and Hamakawa et al. [12], where pathological upstaging was recorded in 10%, 5% and 15.9% of patients, respectively. But the incidence is quite low when compared to Yoshida et al.’s study [13], where 58% patients got upstaged after AE1/AE3 IHC (Table 2). But all the cases in their study were T2N0 tongue carcinoma, and the fact that the risk of metastasis in tongue and lower alveolar primaries are higher than other subsites of oral cavity, is well established [14, 15]. This is in concordance with the finding of the present study where two cases were pT1N0 tongue carcinoma and the remaining one was pT1N0 lower alveolar cancer. In all three cases, metastasis was found in level IB and IIA lymph nodes, which are well known as the sentinel nodes for oral cavity cancer [14, 15].
Table 2.
Incidence of occult micrometastasis detected by immunohistochemistry in patients with HNSCC in different studies
| Studies | Year | Sample size | Total lymph nodal yield | Patients showed the presence of MM/ITC in IHC (%) |
|---|---|---|---|---|
| Ambrosch et al. [10] | 1995 | 60 | 1020 | 6 (10%) |
| Enepekides et al. [11] | 1999 | 40 | 419 | 2 (5%) |
| Hamakawa et al. [12] | 2000 | 44 | 339 | 7 (15.9%) |
| Yoshida et al. [13] | 2005 | 24 | 401 | 14 (58%) |
| Present study | 2018 | 34 | 1126 | 3 (8.8%) |
One interesting fact that was noted in this study was that all the three patients were pathologically staged as T1. But whether pT staging (or any other histological feature) can be definitely concluded as a pathologic predictor of micrometastasis or ITC is beyond the scope of this article at present. We hope, with further accrual, we will be able to solve this shortcoming.
The present study, being a pilot project, bears a few limitations. First, only oral cavity cancers may not highlight the overall metastatic potential of HNSCC. With the recruitment of primary oropharyngectomy, laryngectomy and laryngopharyngectomies, the true incidence of micrometastasis and ITC will be more clearly defined. Second, the clinical significance of micrometastasis and ITC in HNSCC is still evolving. The effect on locoregional control, survival and role of adjuvant therapy are still unknown. We hope further follow-up with more number of cases will be able to determine the clinical significance of these two lesser known dimensions of HNSCC.
Conclusion
Micrometastasis and isolated tumour cells are found in 9% of cases of early-stage oral cavity squamous cell carcinoma. The predictive factors and their clinical significance on disease control, survival and role of adjuvant therapy are still unknown. More prospective trials with longer follow-up are required to solve this evolving aspect of head and neck squamous cell carcinoma.
Acknowledgements
We thank Professor Ravi C Nayar, Dean Academics, HCG Cancer Centre, Bengaluru, and all the members of Institutional Scientific Research Committee for comments on design and methodology of the study that greatly improved the manuscript.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional ethics committee (EC/329/17/03) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants enrolled in the study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kinjal Shankar Majumdar, Email: drkinjalmajumdar@gmail.com.
Vishal U. S. Rao, Email: drvishalrao@yahoo.com
Rachana Prasad, Email: rachana.shilpi@gmail.com.
Veena Ramaswamy, Email: drveena.r@strandls.com.
Piyush Sinha, Email: sinhapiyush30@gmail.com.
Anand Subash, Email: dranandsubash@gmail.com.
References
- 1.Johnson JT, Barnes EL, Myers EN, Schramm VL, Borochovitz D, Sigler BA. The extracapsular spread of tumors in cervical node metastasis. Arch Otolaryngol. 1981;107:725–729. doi: 10.1001/archotol.1981.00790480001001. [DOI] [PubMed] [Google Scholar]
- 2.Moore MG, Bhattacharyya N. Effectiveness of chemotherapy and radiotherapy in sterilizing cervical nodal disease in squamous cell carcinoma of the head and neck. Laryngoscope. 2005;115(4):570–573. doi: 10.1097/01.mlg.0000161359.58567.0e. [DOI] [PubMed] [Google Scholar]
- 3.Hosal AS, Carrau RL, Johnson JT, Myers EN. Selective neck dissection in the management of the clinically node-negative neck. Laryngoscope. 2009;110(12):2037–2040. doi: 10.1097/00005537-200012000-00011. [DOI] [PubMed] [Google Scholar]
- 4.Jose J, Coatesworth AP, MacLennan K. Cervical metastases in upper aerodigestive tract squamous cell carcinoma: histopathologic analysis and reporting. Head Neck. 2003;25(3):194–197. doi: 10.1002/hed.10194. [DOI] [PubMed] [Google Scholar]
- 5.Clark J, Li W, Smith G, Shannon K, Clifford A, McNeil E, et al. Outcome of treatment for advanced cervical metastatic squamous cell carcinoma. Head Neck. 2005;27(2):87–94. doi: 10.1002/hed.20129. [DOI] [PubMed] [Google Scholar]
- 6.Andry G, Hamoir M, Leemans CR. The evolving role of surgery in the management of head and neck tumors. Curr Opin Oncol. 2005;17(3):241–248. doi: 10.1097/01.cco.0000160277.86386.dc. [DOI] [PubMed] [Google Scholar]
- 7.Sparano A, Weinstein G, Chalian A, Yodul M, Weber R. Multivariate predictors of occult neck metastasis in early oral tongue cancer. Otolaryngol Head Neck Surg. 2004;131(4):472–476. doi: 10.1016/j.otohns.2004.04.008. [DOI] [PubMed] [Google Scholar]
- 8.Dhawan I, Sandhu SV, Bhandari R, Sood N, Bhullar RK, Sethi N. Detection of cervical lymph node micrometastasis and isolated tumor cells in oral squamous cell carcinoma using immunohistochemistry and serial sectioning. J Oral Maxillofac Pathol. 2016;20:436–444. doi: 10.4103/0973-029X.190946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, Morrow M, et al. AJCC cancer staging manual. 6. New York: Springer; 2002. [Google Scholar]
- 10.Ambrosch P, Kron M, Fischer G, et al. Micrometastases in carcinoma of the upper aerodigestive tract: detection, risk of metastasizing, and prognostic value of depth of invasion. Head Neck. 1995;17:473–479. doi: 10.1002/hed.2880170604. [DOI] [PubMed] [Google Scholar]
- 11.Enepekides DJ, Sultanem K, Nguyen C, Shenouda G, Black MJ, Rochon L. Occult cervical metastases: immunoperoxidase analysis of the pathologically negative neck. Otolaryngol Head Neck Surg. 1999;120:713–717. doi: 10.1053/hn.1999.v120.a91761. [DOI] [PubMed] [Google Scholar]
- 12.Hamakawa H, Takemura K, Sumida T, Kayahara H, Tanioka H, Sogawa K. Histological study on pN upgrading of oral cancer. Virchows Arch. 2000;437:116–121. doi: 10.1007/s004280000199. [DOI] [PubMed] [Google Scholar]
- 13.Yoshida K, Kashima K, Suenaga S, Nomi N, Shuto J, Suzuki M. Immunohistochemical detection of cervical lymph node micrometastases from T2N0 tongue cancer. Acta Otolaryngol. 2005;125:654–658. doi: 10.1080/00016480410025252. [DOI] [PubMed] [Google Scholar]
- 14.Lindberg R. Distribution of cervical lymph node metastases from squamous cell carcinoma of the upper respiratory and digestive tracts. Cancer. 1972;29(6):1446–1449. doi: 10.1002/1097-0142(197206)29:6<1446::AID-CNCR2820290604>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Shah JP. Patterns of cervical lymph node metastasis from squamous carcinomas of the upper aerodigestive tract. Am J Surg. 1990;160(4):405–409. doi: 10.1016/S0002-9610(05)80554-9. [DOI] [PubMed] [Google Scholar]