Abstract
Background
A routine mandibular fracture matures in about 12 weeks and hence hinders normal function significantly. Alternatives are being researched to hasten the healing and allow early function. This prospective study aimed to assess the osteogenic potential of the drug Cissus quadrangularis (CQ) on the mandibular fracture healing.
Design
Prospective randomized study.
Results
The study between the groups revealed a statistically significant increase in the alkaline phosphatase levels in CQ group in comparison with control group. The radiographic findings (increment in density and rate of change of density), clinical findings (mobility, swelling, reduction in pain) and other biochemical findings (serum calcium, serum phosphorous) did not differ statistically between the CQ and control groups.
Conclusion
Based on the lack of a statistically significant improvement in almost all parameters except for the alkaline phosphatase levels, we believe that a larger sample size is required to ascertain the absolute value of CQ before adding it to the mandibular fracture regimen.
Keywords: Mandibular fracture, Fracture healing, Cissus quadrangularis, Ayurveda
Introduction
Mandibular fractures are among the most commonly encountered maxillofacial injuries [1]. These fractures, even after appropriate surgical interventions, are associated with postop discomfort and prolonged rehabilitation. Unconventional herbal remedies have thus been used in an attempt to hasten the healing and reduce the period of rehabilitation [2–5].
Cissus quadrangularis (CQ), a succulent plant of the Vitaceae family, is used frequently in Ayurveda for healing fractures [6]. The processed stem is ingested for early fracture union [7]. The efficacy of CQ on early ossification and remodelling of bones have been reported to be, due to its ability to stimulate metabolism and increase uptake of the minerals by the osteoblasts [3].
The current study was aimed to understand if CQ provides a quantifiably early healing and to understand the value of adding CQ to a regular regimen of mandibular fracture treatment. In this study, two similarly treated sets of mandibular fracture patients have been studied: one set that had been treated surgically with an addition of oral CQ and another set that had only been treated surgically.
Methodology
The study was conducted on 30 consecutive consenting patients who presented with isolated traumatic mandibular fractures who came to the Department of Maxillofacial Surgery in 2014–2015. Patients of either sex between the ages of 20–40 years, who presented within 24 h of the injury—isolated mandibular fractures: symphyseal, parasymphyseal or body fractures, were included. Patients who had mandibular condyle or angle fractures, other facial fractures, comminuted fractures, edentulous arches and metabolic or endocrine disorders were excluded. The patients in the study were randomly allocated to the CQ group and control group by using a computer generated software. The CQ group included 15 patients who were prescribed capsules of C. quadrangularis (250 mg each)—two capsules B.D. for 42 days post-trauma by an Ayurvedic practitioner. The control group was treated without the Ayurvedic drug. Over the counter, CQ capsules were used for this study (Himalaya Hadjod tablets 250 mg). Apart from CQ, all patients were given injectable antibiotics and analgesics for 5 days postoperatively.
The fractured mandible was treated intraorally with open reduction and internal fixation (ORIF) with miniplates under general anaesthesia. Surgical treatment was carried out within 24 h of the trauma. The following parameters were recorded preoperatively, on the 7th, the 21st and the 42nd day post-trauma: biochemical parameters—serum calcium, serum phosphorous, serum alkaline phosphatase, clinical parameters—pain, swelling, mobility and radiographic parameter on CBCT exposures: increment in density and rate of change of density.
Pain was measured with a visual analogue scale, mobility was analysed by clinical manipulation of the fractured fragments, and the swelling was measured using a thread to measure the largest dimension vertical and horizontal dimensions. The CBCT scans in this study were acquired using a Kodak CBCT unit. The patients were positioned seated, and the head was secured with a chin support, a head support, and a forehead strap. The CBCT scans were saved as digital imaging and communications in medicine (DICOM) files. The radiographic images were set up for analysis similar to the study conducted by Bujtár et al. [8]; 1-mm axial sections along the fracture line were chosen. Twenty-five circular 4 mm2 areas (a single pixel is 0.20 mm × 0.20 mm) were chosen on the Image J software to obtain a mean radiodensity along the fracture line. The areas chosen were devoid of metallic artefacts and the consequent noise. The increment in density and rate of change of density were calculated using the mean radiodensity values as per the formulae provided by Villarreal et al. [9] (Tables 1, 2).
Table 1.
Formula for increment in density
| Increment in density (ID) | |
|---|---|
| ID 7 | RESULT 2 − RESULT 1/25 |
| ID 21 | RESULT 3 − RESULT 1/25 |
| ID 42 | RESULT 4 − RESULT 1/25 |
ID 7, 21 and 42 were the increments in density at 7th, 21st and 42nd days, respectively
RESULT 1: the mean radiodensity at the fracture site preoperatively, RESULT 2: the mean radiodensity value at the fracture site on the seventh day postoperatively, RESULT 3: the mean radiodensity value at the fracture site at 21 days, RESULT 4: the mean radiodensity value at the fracture site at 42 days
Table 2.
Rate of change of density (RD)
| Rate of change of density (RD) | |
|---|---|
| RD 7 | RESULT 2 − RESULT 1/7 |
| RD 21 | RESULT 3 − RESULT 2/14 |
| RD 42 | RESULT 4 − RESULT 3/21 |
RD 7, 14, 21 and 42 were the rate of change in density during the first 7 days, between 7th and 21st days and between the 21st and 42nd days
RESULT 1: the mean radiodensity at the fracture site preoperatively, RESULT 2: the mean radiodensity value at the fracture site on the seventh day postoperatively, RESULT 3: the mean radiodensity value at the fracture site at 21 days, RESULT 4: the mean radiodensity value at the fracture site at 42 days
Statistical Analysis
The approximation to normality of each quantitative variable was checked by means of the Shapiro–Wilk test. The multiple intergroup comparisons of each of the radiographic, biochemical and pain parameters were performed using a Greenhouse–Geisser analysis. Intergroup comparisons for mobility and swelling were analysed with Fisher’s exact test and Chi-square tests, respectively. The statistical level of significance was set at p < 0.05.
Results
In this prospective randomized trial, two groups of mandibular fracture patients were studied. Each group consisted of 13 male and 2 female patients. The control group consisted of 13 parasymphyseal and 2 symphyseal fractures. The CQ group consisted of 11 parasymphyseal, 3 symphyseal and 1 body fracture. Both the CQ group and the control group had similar treatment of mandibular fracture barring the intake of CQ capsules by the CQ group. About 13 cases in each group were treated with open reduction and internal fixation with titanium miniplates, and two cases in each group were treated with closed reduction and arch bar fixation. The results are described under the following headings—biochemical, clinical and radiographic parameters.
Biochemical findings In both the CQ and control groups, the serum calcium and serum phosphorous levels showed no statistically significant differences during the follow-up period. The alkaline phosphatase levels showed a statistically significant increase in CQ group in comparison with the control group at all the follow-up intervals (p = 0.01*) (Table 3; Figs. 1, 2, 3).
Table 3.
Inter-comparison group of the various biochemical parameters
| Biochemical parameters | Time period | Groups | Mean | SD | Level of significance |
|---|---|---|---|---|---|
| Serum calcium (SC) | SC preoperatively | CQ group | 9.13 | 0.34 | 0.86 |
| Control group | 9.21 | 0.61 | |||
| SC 7th day | CQ group | 8.66 | 1.35 | ||
| Control group | 8.92 | 0.53 | |||
| SC 21st day | CQ group | 9.04 | 0.54 | ||
| Control group | 9.15 | 0.45 | |||
| SC 42nd day | CQ group | 9.14 | 0.42 | ||
| Control group | 9.15 | 0.26 | |||
| Serum phosphorous (SP) | SP preoperatively | CQ group | 3.47 | 0.79 | 0.806 |
| Control group | 3.49 | 0.90 | |||
| SP 7th day | CQ group | 3.79 | 1.84 | ||
| Control group | 3.53 | 0.70 | |||
| SP 21st day | CQ group | 4.06 | 0.40 | ||
| Control group | 3.92 | 0.63 | |||
| SP 42nd day | CQ group | 4.20 | 0.38 | ||
| Control group | 4.06 | 0.55 | |||
| Serum alkaline phosphatase (SAP) | SAP preoperative | CQ group | 65.80 | 15.58 | 0.01* |
| Control group | 81.33 | 25.71 | |||
| SAP 7th day | CQ group | 60.61 | 20.30 | ||
| Control group | 76.08 | 23.51 | |||
| SAP 21st day | CQ group | 91.13 | 19.49 | ||
| Control group | 87.83 | 19.86 | |||
| SAP 42nd day | CQ group | 80.60 | 18.88 | ||
| Control group | 98.25 | 21.14 |
SC serum calcium, SP serum phosphorous, SAP serum alkaline phosphatase
Fig. 1.
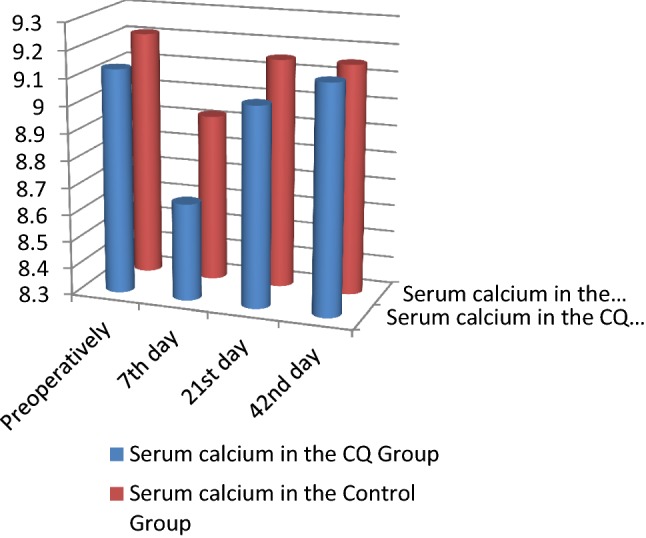
Serum calcium comparison between the CQ and control groups
Fig. 2.
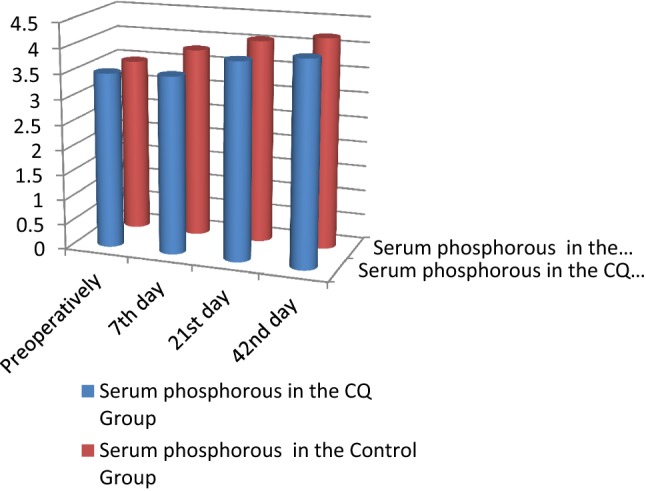
Serum phosphorous comparison between the CQ and control groups
Fig. 3.
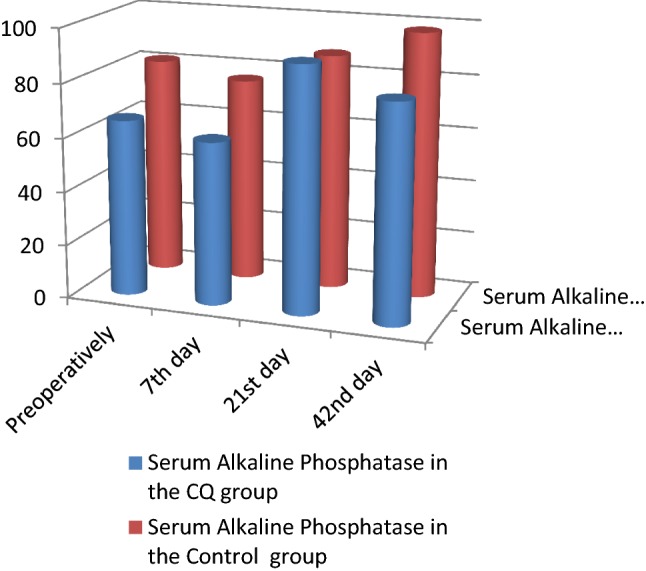
Serum alkaline phosphatase comparison between the CQ and control groups
Clinical findings and radiographic findings There was no statistically significant difference between the two groups across all clinical parameters and radiographic parameters. No side effects to the CQ drug were recorded (Tables 4, 5; Figs. 4, 5, 6, 7, 8).
Table 4.
Intergroup comparisons of clinical parameters
| Clinical parameters | Time period | Groups | Mean | SD | Level of significance |
|---|---|---|---|---|---|
| Visual analogue scale (VAS) | VAS preoperatively | CQ group | 7.40 | 1.35 | 0.471 |
| Control group | 8.58 | 1.31 | |||
| VAS 7th day | CQ group | 6.67 | 2.32 | ||
| Control group | 7.42 | 1.62 | |||
| VAS 21st day | CQ group | 1.47 | 1.46 | ||
| Control group | 1.83 | 1.03 | |||
| VAS 42nd day | CQ group | 0.20 | 0.41 | ||
| Control group | 0.33 | 0.89 |
| Parameter | Time period | Groups | Present (in %) | Absent (in % of cases) | Level of significance |
|---|---|---|---|---|---|
| Mobility | Mobility preoperatively | CQ group | 100.00 | 0.00 | 1.000 |
| Control group | 80.00 | 20.00 | |||
| Mobility 7th day | CQ group | 13.30 | 86.70 | 0.224 | |
| Control group | 20.00 | 80.00 | |||
| Mobility 21st day | CQ group | 0.00 | 100.00 | – | |
| Control group | 0.00 | 100.00 | |||
| Mobility 42nd day | CQ group | 0.00 | 100.00 | – | |
| Control group | 0.00 | 100.00 | |||
| Swelling | Swelling preoperatively | CQ group | 73.30 | 26.70 | 0.330 |
| Control group | 93.30 | 6.70 | |||
| Swelling 7th day | CQ group | 6.70 | 93.30 | 0.785 | |
| Control group | 0.00 | 100.00 | |||
| Swelling at 21st day | CQ group | 0.00 | 100.00 | 0.147 | |
| Control group | 0.00 | 100.00 | |||
| Swelling at 42nd day | CQ group | 0.00 | 100.00 | 0.343 | |
| Control group | 0.00 | 100.00 |
Table 5.
Intergroup comparisons of radiographic parameters
| Clinical parameters | Time period | Groups | Mean | SD | Level of significance |
|---|---|---|---|---|---|
| Increment in density | ID 7 | CQ group | 0.15 | 0.75 | 0.667 |
| Control group | − 0.09 | 0.66 | |||
| ID 21 | CQ group | 0.37 | 0.74 | ||
| Control group | − 0.04 | 0.67 | |||
| ID 6 | CQ group | 0.37 | 0.71 | ||
| Control group | − 0.06 | 0.79 | |||
| Rate of change of density | RD 7 | CQ group | 0.45 | 2.70 | 0.591 |
| Control group | − 0.38 | 2.30 | |||
| RD 21 | CQ group | 0.35 | 0.93 | ||
| Control group | 0.21 | 1.07 | |||
| RD 42 | CQ group | − 0.07 | 0.83 | ||
| Control group | − 0.07 | 0.82 |
Fig. 4.
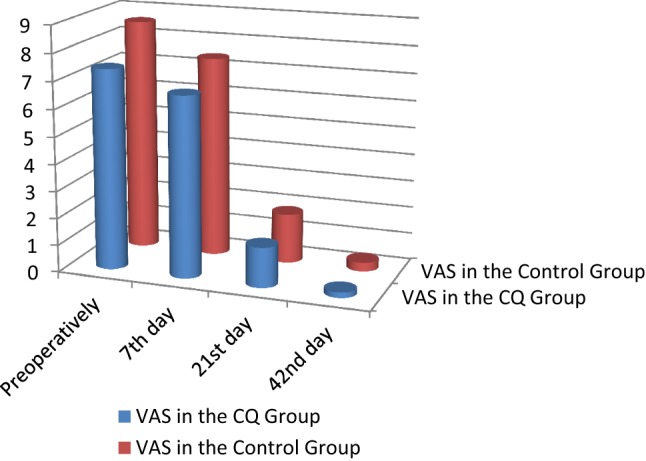
Comparison of visual analogue scale (VAS) between the CQ and control groups
Fig. 5.
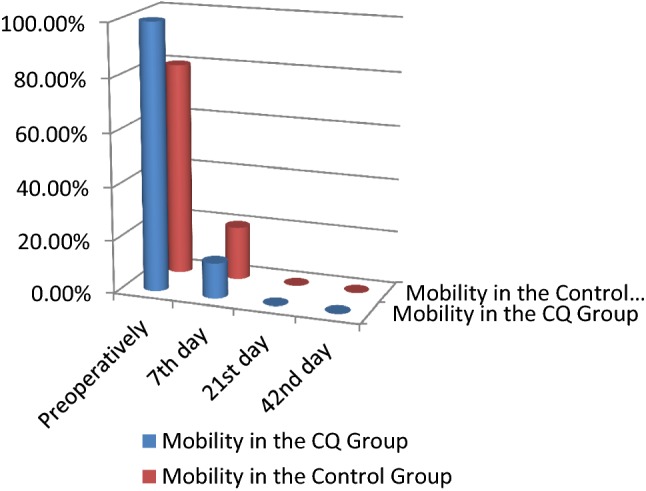
Comparison of the fracture mobility between the CQ and control groups
Fig. 6.
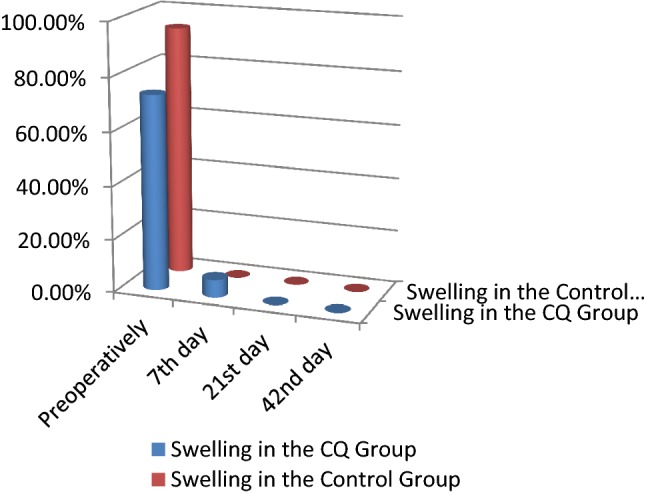
Comparison of the swelling between the CQ and control groups
Fig. 7.
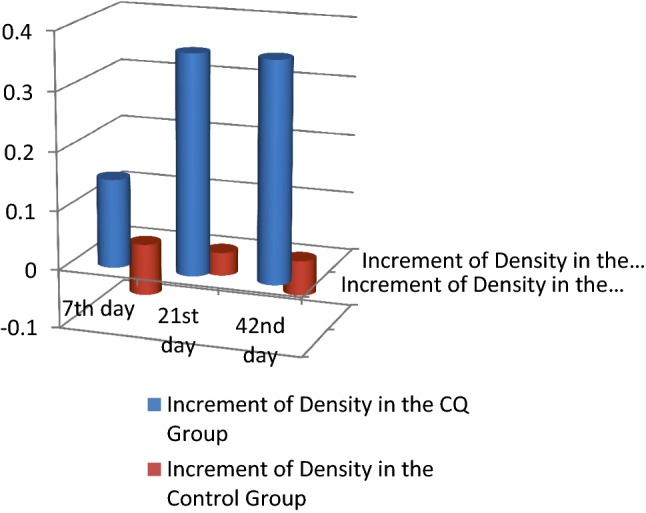
Comparison of the increment in density between the CQ and control groups
Fig. 8.
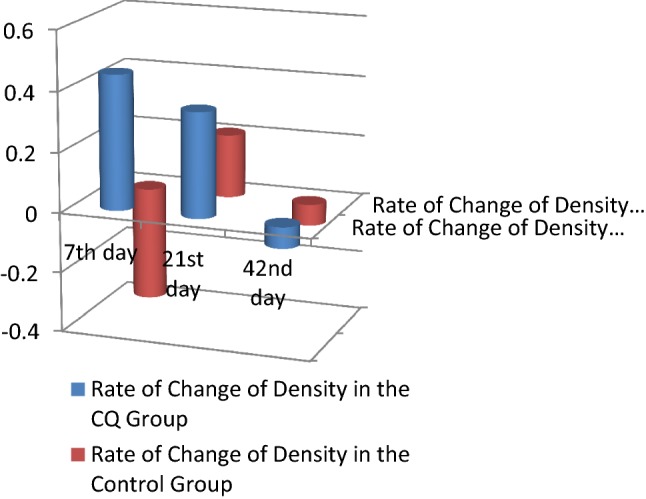
Comparison of the rate of change of density between the CQ and control groups
Discussion
The healing in mandibular fractures is often prolonged and leads to loss of productivity in young adults in whom this fracture is commonly seen [1]. The fracture stabilizes in 4–6 weeks and matures at 12 weeks [10]. This study aimed to evaluate the efficacy of CQ in hastening mandibular fracture healing and gauge the possibility of adding the drug as an adjuvant to regular mandibular fracture treatment.
The value of C. quadrangularis on early ossification and remodelling of bones have been reported by various authors in animal models [7, 11–13] and human patients [2, 3, 14]. Pharmacologically, C. quadrangularis contains high amount of carotene A, anabolic steroidal substances and calcium. It also contains ascorbic acid and calcium oxalate, two asymmetric tetracyclic triterpenoids, onocer-7-ene-3α,21β-diol (C30H52O2 m.p. 200–202 °C) and onocer-7-ene-3β,21α-diol (C30H52O2, m.p. 233–234 °C). The presence of β-sitosterol, δ-amyrin and δ-amyrone has also been reported. The aerial parts of C. quadrangularis is found to contain a new asymmetric tetracyclic triterpenoid, 7-oxo-onocer-8-ene-3β-21α-diol (C30H50 O3, m.p. 235–237 °C), 4-hydroxy-2-methyl-tricos-2-ene-22-one, 9-methyloctadec-9-ene, heptadecyl-octadecanoate, icosanylicosanoate, 31-methyl tritiacontan-1-ol, 7-hydroxy20-oxo-docosanyl cyclohexane and 31-methyl tritiacontanoic acid. It also conatins small amounts of taraxeryl acetate, friedelan-3-one, taraxerol and isopentacosanoic acid [15]. Though the exact mechanism of action has not been identified, a radioactive study conducted with calcium (Ca45) indicated that CQ stimulated cells of mesenchymal origin—the fibroblasts, the chondroblasts and osteoblasts at early stage and hastened the healing at the fracture site by about 10–14 days in the treated group [15]. Other studies have also found that a phytogenic steroid isolated from CQ stimulates osteoblasts and leads to early fracture healing [4, 6]. The other purported cause is that C. quadrangularis builds up the chemical composition of the fractured bone, namely its mucopolysaccharides, collagen, calcium, phosphorus and others as well as its functional efficiency [4]. During fracture healing, alkaline phosphatase—an enzyme secreted by the osteoblast, increases, indicating callus formation [6]. The volume and mineralization of the callus are proportional to the increase in the levels of alkaline phosphatase [16]. In the current study, we found a statistically increased level of alkaline phosphatase in the CQ group in comparison with the control group at all the intervals but no statistically significant difference across all the radiographic, clinical and other biochemical parameters between the two groups. These findings were in contrast to the findings of Deka et al. [7], who showed an early reduction in serum calcium and increased alkaline phosphatase indicating a faster callus formation in the CQ treated sample. Singh et al. [2, 3], on the other hand, showed an increase in the levels of calcium, phosphorous and alkaline phosphatase and a faster rate of reduction in pain, mobility and swelling in the CQ treated group.
Though there have been efforts to evaluate the efficacy of CQ, no absolute conclusion has been derived due a lack of adequate number of human studies and systematic reviews or meta-analysis of the medicinal use of this plant [17]. The other deterrent is the possibility of toxicity which has been associated with ingestion of various Ayurvedic drugs [18]. The study population had no side effects to the drug similar to the findings of all the previously mentioned studies.
Conclusion
Herbal remedies like C. quadrangularis have been propagated to hasten the fracture healing process. To be used as an adjunct, we feel that the healing needs to be clinically significant and radiographically quantifiable. In this study, we found biochemical findings which inferred a faster callus formation in patients who had consumed CQ; however, there was no significant radiographic or clinical improvement when compared to the control group. Based on this pilot study, we believe adding CQ as an adjunct to the regular regimen of mandibular fracture treatment may not have much merit and recommend that a larger population be tested to generalize its value.
Acknowledgements
We would like to thank Dr. Lalitha BR, Professor, Government Ayurveda College, Bangalore, who provided insight and expertise that greatly assisted in the current research.
Compliance with Ethical Standards
Ethical Clearance
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. Reference No. 02_D010_43915.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koshy JC, Feldman EM, Chike-Obi CJ, Bullocks JM. Pearls of mandibular trauma management. Semin Plast Surg. 2010;24(4):357–374. doi: 10.1055/s-0030-1269765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh V, Singh N, Pal US, Dhasmana S, Mohammad S, Singh N. Clinical evaluation of Cissus quadrangularis and Moringa oleifera and osteoseal as osteogenic agents in mandibular fracture. Natl J Maxillofac Surg. 2011;2(2):132–136. doi: 10.4103/0975-5950.94466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singh N, Singh V, Singh RK, et al. Osteogenic potential of Cissus quadrangularis assessed with osteopontin expression. Natl J Maxillofac Surg. 2013;4(1):52–56. doi: 10.4103/0975-5950.117884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mohammad S, Pal US, Pradhan R, Singh N. Herbal remedies for mandibular fracture healing. Natl J Maxillofac Surg. 2014;5(1):35–38. doi: 10.4103/0975-5950.140167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmkshatriya HR, Shah KA, Ananthkumar GB, Brahmkshatriya MH. Clinical evaluation of Cissus quadrangularis as osteogenic agent in maxillofacial fracture: a pilot study. Ayu. 2015;36(2):169–173. doi: 10.4103/0974-8520.175542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Udupa KN, Prasad G, Sen SP. The effect of phytogenic steroid in the acceleration of fracture repair. Life Sci. 1965;4:317–327. doi: 10.1016/0024-3205(65)90148-7. [DOI] [PubMed] [Google Scholar]
- 7.Deka DK, Lohan LC, Saikia J, Mukti A. Effect of Cissus quadrangularis in accelerating healing process of experimentally fractured radius ulna of dog: a preliminary study. Indian J Pharmacol. 1994;26:44–45. [Google Scholar]
- 8.Bujtár P, Simonovics J, Zombori G, Fejer Z, Szucs A, Bojtos A, et al. Internal or in-scan validation: a method to assess CBCT and MSCT gray scales using a human cadaver. Oral Surg Oral Med Oral Pathol Oral Radiol. 2014;117(6):768–779. doi: 10.1016/j.oooo.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Villarreal PM, Junquera LM, Martínez A, García-Consuegra L. Study of mandibular fracture repair using quantitative radiodensitometry: a comparison between maxillomandibular and rigid internal fixation. J Oral Maxillofac Surg. 2000;58(7):776–781. doi: 10.1053/joms.2000.7264. [DOI] [PubMed] [Google Scholar]
- 10.Chiodo TA, Milles M. Use of monocortical miniplates for the intraoral treatment of mandibular fractures. Atlas Oral Maxillofac Surg Clin N Am. 2009;17(1):19–25. doi: 10.1016/j.cxom.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 11.Shirwaikar A, Khan S, Malini S. Antiosteoporotic effect of ethanol extract of Cissus quadrangularis Linn. on ovariectomized rat. J Ethnopharmacol. 2003;89(2–3):245–250. doi: 10.1016/j.jep.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 12.Sanyal A, Ahmad A, Sastry M. Calcite growth in Cissus quadrangularis plant extract, a traditional Indian bone-healing aid. Curr Sci. 2005;89(10):1742–1745. [Google Scholar]
- 13.Maiti SK, Saravanan B, Singh GR, Kumar N, Hoque M, Lal J, et al. Evaluation of the Herb, Cissus quadrangularis in accelerating the healing process of femur osteotomies in dogs. J Appl Anim Res. 2007;31(1):47–52. doi: 10.1080/09712119.2007.9706628. [DOI] [Google Scholar]
- 14.Sharma N, Nathawat RS, Gour R, Patni V. Establishment of callus tissue and effect of growth regulators on enhanced sterol production in Cissus quadrangularis L. Int J Pharmacol. 2011;7(5):653–658. doi: 10.3923/ijp.2011.653.658. [DOI] [Google Scholar]
- 15.Mishra G, Srivastava S, Nagori BP. Pharmacological and therapeutic activity of Cissus quadrangularis: an overview. Int J PharmTech Res. 2010;2(2):1298–1310. [Google Scholar]
- 16.Muljačić A, Poljak-Guberina R, Turčić J, Živković O, Guberina M, et al. The changes of bone-specific alkaline phosphatase (BsALP) associated with callus formation and rate of bone healing. Croat Chem Acta. 2010;83(3):315–321. [Google Scholar]
- 17.Sawangjit R, Puttarak P, Saokaew S, Chaiyakunapruk N. Efficacy and safety of Cissus quadrangularis L. in clinical use: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2017 doi: 10.1002/ptr.5783. [DOI] [PubMed] [Google Scholar]
- 18.Datta-Mitra A, Ahmed O., Jr Ayurvedic medicine use and lead poisoning in a child: a continued concern in the United States. Clin Pediatr (Phila) 2015;54(7):690–692. doi: 10.1177/0009922814553397. [DOI] [PubMed] [Google Scholar]


