Abstract
Activation of costimulatory receptor 4-1BB enhances T helper 1 (Th1) and CD8 T cell responses in protective immunity, and prevents or attenuates several autoimmune diseases by increasing Treg numbers and suppressing Th17 or Th2 effector response. We undertook this study to elucidate the impact of enforced 4-1BB activation on the development of SS-like sialadenitis in non-obese diabetic (NOD) model of this disease. An anti-4-1BB agnostic antibody was intraperitoneally injected to female NOD mice aged 7 weeks, prior to the disease onset that occurs around 10-11 weeks of age, 3 times weekly for 2 weeks, and the mice were analyzed for SS pathologies at age 11 weeks. The salivary flow rate was markedly higher in the anti-4-1BB-treated NOD mice compared to the IgG-treated controls. Anti-4-1BB treatment significantly reduced the leukocyte infiltration of the submandibular glands (SMGs) and the levels of serum antinuclear antibodies. Flow cytometric analysis showed that the percentages of CD4 T cells, Th17 cells and plasmacytoid dendritic cells among SMG leukocytes were markedly reduced by anti-4-1BB treatment, in conjunction with a reduction in SMG IL-23p19 mRNA levels and serum IL-17 concentrations. Although the proportion of Tregs and IL-10 mRNA levels in SMGs were not altered by 4-1BB activation, IL-10 mRNA levels in submandibular lymph nodes and serum IL-10 concentrations were both markedly increased. While anti-4-1BB treatment did not affect the amount of Th1 cells and IFNγ mRNA, it increased these measurables in submandibular lymph nodes. Hence, agonistic activation of 4-1BB impedes the development of SS-like sialadenitis and hyposalivation.
Keywords: Salivary gland, autoimmune exocrinopathy, CD137, costimulatory molecule, T helper 17, plasmacytoid dendritic cells
1. Introduction
Sjögren’s syndrome (SS) is a chronic autoimmune disease that affects an estimated 4 million Americans (1). SS is characterized by leukocyte infiltration of salivary and lacrimal glands and autoantibody production, which lead to destruction and dysfunction of these exocrine glands and xerostomia (dry mouth) and keratoconjunctivitis (dry eyes) as key clinical manifestations. SS also frequently affects various other organs, causing sytemic problems including fibromyogia, fatigue, neuro-cognitive impairment and B cell lymphoma (1–3). Aberrant and coordinated activation of innate immune cells and autoreactive effector T cells and B cells drives the development, onset and progression of SS-like exocrinopathy (4, 5) (3, 6, 7) (8, 9). Cytokines IFNγ, TNFα, IL-4 and IL-17, derived from T helper (Th) 1, Th2 and Th17 effector cells, and autoantibodies produced by B cells, all play indispensable roles in SS pathogenesis (6, 7, 10–18) (8, 9, 19). Hence, strategies that can attenuate the activity and effect of the pathogenic innate and adaptive immune cells, including the endogenous immune regulators, have the potential to combat this autoimmune disorder.
4–1BB, also termed CD137, is a cell surface receptor that belongs to the TNF receptor superfamily and the T cell costimulatory family (20–23). 4–1BB-mediated signaling in CD4 T cells attenuates effector Th1 and Th17 response while enhancing the expansion and function of Tregs (20, 21, 24). Indeed, enforced activation of 4–1BB signaling with an agonistic antibody ameliorates EAE and T1D in experimental models (22, 23). Moreover, 4–1BB signaling has well-documented promoting-effect on CD8 T cell-mediated long-term protective immunity, hence bearing the potential to attenuate pathogenic Th response and autoimmune disease while preserving protective CD8 T cell function (25–27). In addition to its role in the modulation of T cell activity, 4–1BB is also expressed by and modulates/stimulates the function of various innate immune cells, including monocytes/macrophages, NK cells, follicular dendritic cells, other myeloid cells, and non-immune tissue cells such as osteoclasts and endothelial cells (25, 26, 28). In SS, 4–1BB deficiency exacerbates dacryoadenitis in a mouse model (29), suggesting that 4–1BB-mediated signals may have a similar immune-regulatory and disease-inhibiting function in SS-like sialadenitis. We hence undertook the current study to determine if enhancing 4–1BB activation with an agonistic antibody can prevent or impede the development of SS-like sialadenitis and hyposalivation in the NOD model of this disease.
2. Materials and Methods
2.1. Mice
Female non-obese diabetic (NOD) mice were purchased from the Jackson Laboratory and were housed in the specific pathogen-free animal facility at the Forsyth Institution. All the experimental protocols and procedures were approved by the Institutional Animal Care and Use Committee of the Forsyth Institute and implemented in compliance with the National Institutes of Health guidelines for the care and use of laboratory animals.
2.2. Antibodies
Purified monoclonal rat-anti-mouse 4–1BB (TKS-1) and its isotype control rat-IgG2a (2A3) used for injection were obtained from BioXCell. For flow cytometry, fluorescence conjugated antibodies specific for CD45, CD4, CD8, CD19, Foxp3, IFN-γ, IL-17, B220, CD11c, CD11b, Siglec-H, BST2 and CD16/32 antibodies were purchased from BioLegend.
2.3. In vivo administration of anti-4–1BB antibody
7 week-old female NOD mice received intraperitoneal (i.p.) administration of 200 μg anti-mouse 4–1BB antibody or control rat IgG2a 3 times weekly for two weeks. All the analyses were performed 2 weeks after the last injection.
2.4. Histological analysis
SMG tissues were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned to 5 μm thickness. Routine histology was carried out on 3 non-consecutive SMG sections for each mouse with hematoxylin and eosin (H&E) to determine the degree of inflammation. The highest focus number as well as the average focus number of the 3 sections were used for further calculation and statistical analysis.
2.5. Flow cytometry
Single cells from SMGs or salivary gland-draining lymph nodes (SGLNs) were freshly isolated and incubated with anti-CD16/32 antibody to prevent unspecific binding. The cells were then stained with a combination of fluorescence-conjugated antibodies against surface immune cell markers at 4°C for 30 min. Where indicated, cells were further fixed and permeabilized and subjected to intracellular or nuclear staining for cytokines and Foxp3. These stained cells were analyzed on a FACS Arial II flow cytometer (BD) and the data are processed using the FlowJo V10 software.
2.6. Detection of serum antinuclear antibodies (ANA)
Sera collected from the mice were diluted 1:40 and subjected to ANA measurements using HEp-2 human epithelial cell substrate slides (INOVA Diagnostics), according to the manufacturer’s instructions. After staining, the samples were examined and imaged under an inverted wide-field fluorescence microscope (Zeiss) at 400× magnification. The images were further processed with the Zeiss software (ZEN blue edition), and the fluorescence intensity of the staining was quantified with the ImageJ 1.50i software.
2.7. Measurement of stimulated salivary flow rate
Female NOD mice received i.p.-administration of 100 μl PBS-based secretagogue solution containing pilocarpine (1 mg/ml) and isoproterenol (2 mg/ml). One min after the injection, saliva was collected continuously for 5 min and the volume of the saliva from each mouse was measured and normalized to the body weight.
2.8. Real-time PCR
Total RNA was purified using RNeasy Micro kit (Qiagen) and reverse transcribed into cDNA with MLV reverse transcriptase (Promega). SYBR Green-based real-time PCR amplification (Qiagen) was conducted for 40 cycles with annealing and extension temperature at 60°C on a LightCycler 480 Real-Time PCR System (Roche). Primer sequences are available upon request. The gene expression level was normalized to β-actin.
2.9. Multiplex assay of serum cytokine concentrations
Serum levels of cytokines including IFN-γ, IL-4, IL-17, IL-10, and TNF-α were determined using a magnetic bead-based multiplex assay kit (LXSAMSM-06, R&D Systems) following the manufacturer’s instructions. Data were collected on a Bio-Plex®200 (Luminex) and further analyzed using Bio-Plex Manager software v6.0.
2.10. Statistical analysis
Statistical significance was determined by two-tailed Student’s t-test (for normally distributed data sets, as determined using the SPSS software) or Mann-Whitney U test (for data that were not normally distributed). P values smaller than 0.05 were considered as statistically significant.
3. Results:
Administration of an agonistic anti-4–1BB antibody impeded the development of SS-like sialadenitis and hyposalivation in female NOD mice
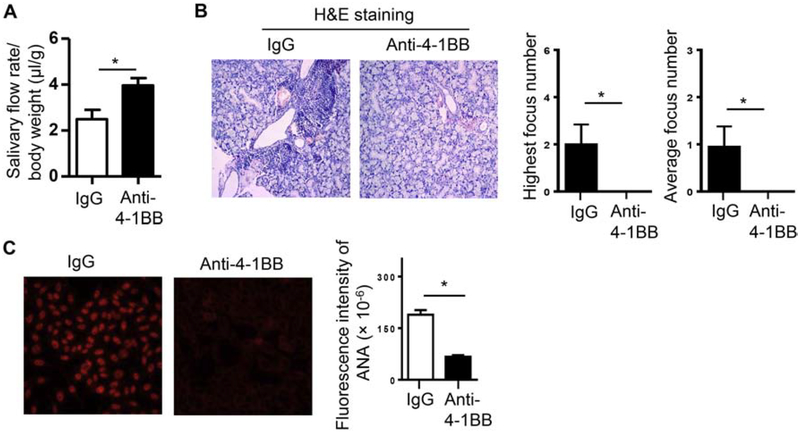
We have previously shown that female NOD mice have the initial onset of SS-like clinical disease around 10 weeks of age (30, 31). To determine the effect of 4–1BB activation on the development of SS, we i.p.-administered an agonistic anti-4–1BB antibody (200 μg) to the NOD mice aged 7 weeks, prior to the clinical disease onset, 3 times weekly for two weeks. Mice were then analyzed for characteristic SS pathologies at age 11 weeks. Activation of 4–1BB markedly improved the salivary flow rate (Fig. 1A), and reduced salivary gland inflammation as determined by H&E staining of the submandibular gland (SMG) sections (Fig. 1B). The numbers of leukocyte foci in SMG sections, both the average number and the highest number of the three non-consecutive sections from each SMG sample, were reduced significantly by anti-4–1BB treatment (Fig. 1B, middle and right panels). The levels of serum antinuclear antibody (ANA) were also substantially reduced by anti-4–1BB treatment (Fig. 1C).
Figure 1. Administration of an agonistic anti-4–1BB antibody impeded the development of SS-like sialadenitis and hyposalivation in female NOD mice.
Anti-4–1BB antibody or isotype IgG was i.p.-administered to 7-week-old female NOD mice 3 times weekly for 2 weeks, and the mice were analyzed for SS pathologies two weeks after the last injection. (A) Stimulated salivary flow rate normalized to body weight. (B) Left panels, images of H&E staining of SMG sections (200X magnification); right panels, statistical analysis of the highest focus number and the average focus number of the 3 non-consecutive SMG sections from each mouse in the control and anti-4–1BB-treated groups. (C) Serum ANA assay. Left panels, images of the immunofluorescence staining of ANA (200X magnification). Right panels, fluorescence intensity of ANA staining. Data are representative or the average of 8 mice per group. Error bars represent the SEM.* p<0.05
Activation of 4–1BB reduced the proportion of CD4 T cells and Th17 cells in the SMGs
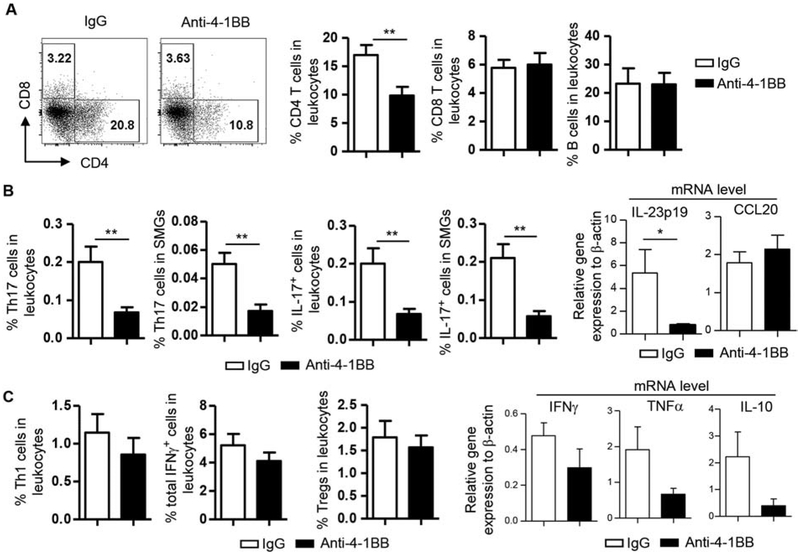
Flow cytometric analysis of the SMG cells showed that the percentage of CD4 T cells among leukocytes (defined as CD45+ cells) was markedly reduced, whereas those of CD8 T cells and B cells were not altered by anti-4–1BB treatment (Fig. 2A). The proportion of Th17 cells among leukocytes (defined based on the forward scatter-side scatter profile) and that among total SMG cells were both strikingly decreased in anti-4–1BB-treated mice (Fig. 2B, left panels). Similarly, the percentages of total IL-17-producing cells (all cells that were IL-17+, including Th17 cells and all other IL-17-producing populations/subsets) among leukocytes and those among total SMG cells were also lowered by anti-4–1BB treatment (Fig. 2B, left panels). In exploring the potential mechanisms underlying the Th17 reduction in SMGs, we found that the expression levels of IL-23p19, a subunit of Th17-promoting cytokine IL-23, in the SMGs was substantially reduced in anti-4–1BB-treated group (Fig. 2B, right panels). Expression of CCL20, a major chemoattractant of Th17 cells, was not affected by anti-4–1BB treatment (Fig. 2B, right panels). In comparison, the proportion of Th1 cells, total IFNγ -producing cells (all cells that were IFNγ+, including Th1, IFNγ+ CD8 T/Tc1 cells, and all other IFNγ-producing populations/subsets) and Foxp3+CD4 T cells (Tregs) among leukocytes (defined based on the forward scatter-side scatter profile) did not show a statistically significant change in the anti-4–1BB-treated group (Fig. 2C, left panels). In accordance, mRNA levels of Th1 cytokines IFNγ, TNFα and the immune-regulatory cytokine IL-10 were not affected in a statistically significant degree by anti-4–1BB as determined by real-time qPCR analysis (Fig. 2C, right panels). In addition, the expression of Th2 cytokine IL-4 was not altered (data not shown).
Figure 2. Activation of 4–1BB reduced the proportion of CD4 T cells and Th17 cells in the SMGs.
Anti-4–1BB antibody or IgG was i.p.-administered to 7-week-old female NOD mice 3 times weekly for 2 weeks, and the mice were analyzed 2 weeks after the last injection. (A) Dot plots, flow cytometry profile of lymphocyte populations; bar graphs, average percentage of lymphocyte populations in SMG leukocytes. (B) Left 4 panels, average percentage of Th17 and IL-17+ cells; right panels, relative gene expression of IL-23p19 and CCL20 levels in the SMG tissues, normalized to β-actin. (C) Left 3 panels, average percentage of Th1 cells, total IFNγ+ cells (including Th1, IFNγ + CD8 T/Tc1 cells, and all other IFNγ+ populations/subsets) and Tregs (Foxp3+CD4 T cells) among leukocytes; right panels, relative gene expression of IFNγ, TNFα and IL-10 levels in the SMGs, normalized to β-actin. Data are representative or the average of analyses of 8 mice per group. * p<0.05; **p<0.01.
Activation of 4–1BB enhanced IFNγ production and did not affect IL-17 production in SMG-draining lymph nodes and blood
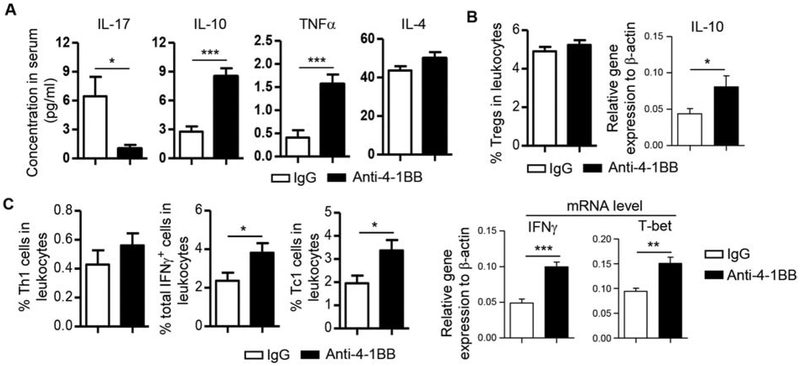
We next assessed the change in cytokine levels in the blood by multiplex assay, which showed that IL-17 concentration in sera was substantially reduced by anti-4–1BB, mimicking the decrease in the SMG tissues (Fig. 3A). Serum IL-4 concentration was not affected by anti-4–1BB treatment (Fig. 3A), paralleling the unaltered IL-4 expression in SMGs. Interestingly, although IL-10 and TNFα mRNA amounts in SMG tissues were not altered, the serum concentration of both cytokines was significantly elevated by anti-4–1BB treatment (Fig. 3A). Accordingly, IL-10 mRNA amounts in SMG-draining lymph nodes (SGLNs) was also markedly increased by anti-4–1BB treatment, although the proportion of Tregs was not changed as determined by flow cytometric analysis (Fig. 3B). The serum IFNγ concentration was below detection level (Fig. 3A), but flow cytometric analysis of SGLNs showed that the proportions of Th1, Tc1 and total IFNγ-producing cells were increased by anti-4–1BB treatment (Fig. 3C, left panels), accompanied by an increase in IFNγ and T-bet mRNA levels based on real-time qPCR analysis (Fig. 3C, right panels). These results are consistent with the reported Th1/Tc1-enhancing effect of 4–1BB signaling in various immunological settings. Hence, systemic administration of the agonistic anti-4–1BB attenuated local and systemic IL-17 responses and enhanced systemic IL-10 production, both of which may contribute to 4–1BB-mediated suppression of SS-like disease.
Figure 3. Activation of 4–1BB enhanced IFNγ production without affecting IL-17 production in SMG-draining lymph nodes and blood.
Anti-4–1BB antibody or IgG was i.p.-administered to 7-week-old female NOD mice 3 times weekly for 2 weeks. All the analyses were performed 2 weeks after the last injection. (A) Multiplex analysis of serum cytokine concentrations. (B) Left panel, average percentage of Tregs (Foxp3+CD4 T cells) in SGLNs as determined by flow cytometry; right panel, relative gene expression of IL-10 in SGLNs, normalized to β-actin. (C) Left 3 panels, average percentage of Th1, IFNγ+ and Tc1 cells in SGLNs; right panels, relative gene expression of IFNγ and T-bet in the SGLNs, normalized to β-actin. Data are the average of analyses of 8 mice per group. * p<0.05; **p<0.01; ***p<0.001.
Activation of 4–1BB decreased the proportion of plasmacytoid DCs (pDCs) in SMGs
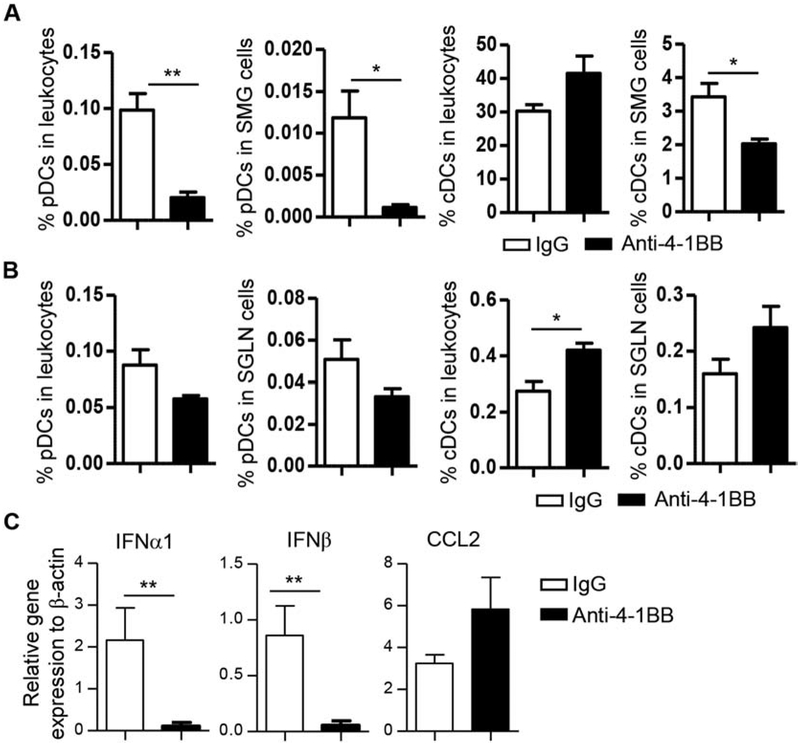
Flow cytometric analysis of the SMG cells showed that the percentage of pDCs (defined as CD11b−CD11cmidB220+ Siglec-H+BST2+) among leukocytes and that among total SMG cells were both significantly reduced by anti-4–1BB treatment (Fig. 4A). In comparison, the percentage of conventional DCs (cDCs, defined as CD45+CD11b+CD11c+B220−) among leukocytes was not significantly altered by anti-4–1BB treatment, but that among total SMG cells was decreased as a result of a reduction in total leukocytes in the SMGs (Fig. 4A). Consistent with a decrease in pDCs in the SMG tissues, the mRNA levels of IFNα and IFNβ were both markedly reduced (Fig. 4C). Similar to its effect on lymphocytes, anti-4–1BB treatment differentially affected DCs in salivary glands versus SGLNs as it did not decrease the proportion of pDCs and cDCs in the SGLNs (Fig. 4B). To explore if such differential impact can be explained by impaired recruitment of pDCs and cDCs to the SMGs, we examined the expression levels of two major chemoattractants of pDCs, CCL2 and CCL20, and found that they were not altered by anti-4–1BB treatment (Fig. 4C and 2B). Hence, anti-4–1BB treatment reduced the amount of pDCs and type 1 IFNs in the SMGs without affecting those in the SGLNs.
Figure 4. Activation of 4–1BB decreased the proportion of pDCs in SMGs.
Anti-4–1BB antibody or IgG was i.p.-administered to 7-week-old female NOD mice 3 times weekly for 2 weeks, and mice were analyzed 2 weeks after the last injection. (A) Flow cytometry of pDC and cDC populations in SMGs. (B) Flow cytometry of pDC and cDC populations in SGLNs. (C) Real-time qPCR analysis of relative expression of IFNα, IFNβ and CCL2 genes in the SMGs, normalized to β-actin. Data are the average of analyses of 8 mice per group. * p<0.05; **p<0.01
4. Discussion
The key finding of this study is that agonistic antibody-mediated 4–1BB activation impedes the development and onset of SS-like sialadenitis and hyposalivation, with the effect associated with a reduction in Th17 cells and other IL-17-producting cells and a decrease in pDCs specifically in salivary gland tissues.
Our findings that activation of 4–1BB reduced Th17 response in salivary gland tissues in SS disease setting is consistent to what was reported in the EAE model (23). Since the decrease in Th17 and other IL-17-producing cells in SMGs are not accompanied by a reduction of these cells in the SGLNs, we examined the level of Th17-chemoattractant CCL20 in the SMGs and found that it was not reduced by anti-4–1BB treatment. The result suggests that the decrease in Th17 cells in SMGs is not a consequence of a defective CCL20-mediated recruitment of these cells. Instead, we found that the amount of IL-23p19 transcripts in SMGs was significantly decreased, which likely contributes to the local reduction of Th17 and other IL-17-producing cells by impairing the differentiation/stabilization of these cells. The inhibitory effect of 4–1BB activation on IL-23 production has not been previously reported, and the cellular producers of IL-23 responding to anti-4–1BB treatment remain to be identified, with dendritic cells, macrophages as well as salivary gland epithelial cells being possible candidates.
The autoimmune disease-alleviating function of anti-4–1BB treatment demonstrated for type 1 diabetes and EAE is associated with increased Treg cell numbers. In NOD mice, we did not detect a significant alteration of the number of Foxp3+ Tregs in either SMGs or SGLNs upon anti-4–1BB treatment, nor the level of immune-regulatory cytokine IL-10 in the SMGs. However, the IL-10 mRNA levels in SGLNs and the IL-10 concentration in sera were both upregulated by anti-4–1BB treatment, suggesting that 4–1BB activation could use IL-10 induction as a mechanism to limit effector T cell response. The cellular producers of IL-10 in response to anti-4–1BB stimulation require further characterization. Apart from Tregs, multiple immune cell populations/subsets, including Th1 and Tc1 cells, can also produce IL-10 under various immunological conditions. We showed that upon anti-4–1BB treatment, the amount of Th1 and Tc1 cells was increased in SGLNs but not in SMGs, which paralleled the pattern of change in IL-10 levels. Hence, the elevated IL-10 and TNFα levels in SGLNs and sera induced by anti-4–1BB may largely result from the increase in Th1 and Tc1 cells. In addition, it is also possible that although anti-4–1BB treatment did not affect the number of Tregs, it enhanced the IL-10-producing ability of Tregs in SGLNs and blood. Future studies will test these hypotheses, defining the specific cellular producers of IL-10 and TNFα upon anti-4–1BB treatment and also comprehensively examining the impact of anti-4–1BB stimulation on Treg immune-suppressive activity, including the production of IL-10, IL-35 and TGFβ.
A key novel finding in this study is the inhibitory effect of anti-4–1BB treatment on the number of pDCs and the level of type 1 IFNs, likely a consequence of pDC reduction, in the target salivary gland tissues in the SS disease setting. In addition to its role in the modulation of T cell activity, 4–1BB is also expressed by and modulates/stimulates the function of various innate immune cells, myeloid cells and non-immune tissue cells (25, 26, 28). This study is the first that identifies an impact of 4–1BB activation on pDCs, which has not been reported in any other autoimmune diseases or immunological conditions. We found that the effect of anti-4–1BB treatment specifically reduces the number of pDCs in the target salivary glands but not the salivary gland-draining lymph nodes. In comparison, anti-4–1BB treatment affects cDCs to a much lower degree in salivary glands. Our finding is further supported by a substantial reduction in type 1 IFNs, for which pDCs are the main producers. Since type 1 IFNs have been shown to be critical for the development and pathogenesis of SS (32, 33), the inhibitory effect of anti-4–1BB treatment on pDCs and type 1 IFNs in the salivary gland tissues could critically contribute to its SS-restraining effect. Examination of two critical pDC-chemoattractants, CCL2 and CCL20, showed that their expression in salivary glands is not altered upon anti-4–1BB treatment. The key mechanisms by which systemic 4–1BB triggering decreases pDC amount in the salivary gland tissues without affecting that in SGLNs awaits further elucidation.
In accordance with the reported Th1-and Tc1-promoting effect of 4–1BB activation (25, 26), our study showed that the proportions of Th1 and Tc1 cells in the SGLNs are both increased by anti-4–1BB treatment, while those in the SMG tissues were not significantly altered. This discrepant outcome could be attributed to a failure of Th1 and Tc1 cells to migrate from SGLNs to the SMGs tissues, or inefficient local activation/expansion of these effector cells in the SMGs. Regardless, the ability of 4–1BB-triggering to attenuate Th17 response in SMGs and to enhance Th1/Tc1 responses in lymphoid tissues but not in SMGs likely underpins its effect to attenuate SS pathologies while preserving/boosting protective immunity against tumor and viral infections. Such unique feature also makes 4–1BB an attractive therapeutic candidate for combatting SS and other autoimmune diseases.
Highlights.
Anti-4–1BB treatment significantly reduced the leukocyte infiltration of the submandibular glands and the levels of serum antinuclear antibodies in NOD mice
Anti-4–1BB treatment reduced the percentages of CD4 T cells, Th17 cells and plasmacytoid dendritic cells and the amount of IL-23 transcripts in submandibular glands
Anti-4–1BB treatment significantly upregulated IL-10 mRNA levels in submandibular lymph nodes and IL-10 concentrations in the blood
Anti-4–1BB treatment did not alter the amount of Th1 cells and IFNγ mRNA in submandibular glands, but increased these measurables in submandibular lymph nodes
Acknowledgements
We thank Danielle Stephens, Michele Patel and Dr. Alpdogan Kantarci of the Forsyth Multiplex Core for performing the multiplex assay of serum cytokines, and thank Forsyth animal facility staff for the animal care.
Funding: This study was supported by grants from NIH/NIDCR (R01 DE023838) and NIH/NIAID (R03 AI142273) to QY, and NIH/NIDCR (R03 DE028033) to JZ.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure
The authors have no competing financial interests.
References:
- 1.Fox PC. Autoimmune diseases and Sjogren’s syndrome: an autoimmune exocrinopathy. Ann N Y Acad Sci 2007;1098:15–21. Epub 2007/03/03. doi: annals.1384.003 [pii] 10.1196/annals.1384.003. [DOI] [PubMed] [Google Scholar]
- 2.Segal B, Bowman SJ, Fox PC, Vivino FB, Murukutla N, Brodscholl J, et al. Primary Sjogren’s Syndrome: health experiences and predictors of health quality among patients in the United States. Health Qual Life Outcomes 2009;7:46 Epub 2009/05/29. doi: 1477-7525-7-46 [pii] 10.1186/1477-7525-7-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voulgarelis M, Tzioufas AG. Pathogenetic mechanisms in the initiation and perpetuation of Sjogren’s syndrome. Nat Rev Rheumatol 2010;6(9):529–37. doi: 10.1038/nrrheum.2010.118. [DOI] [PubMed] [Google Scholar]
- 4.Kiripolsky J, McCabe LG, Kramer JM. Innate immunity in Sjogren’s syndrome. Clinical immunology 2017;182:4–13. doi: 10.1016/j.clim.2017.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kramer JM. Early events in Sjogren’s Syndrome pathogenesis: the importance of innate immunity in disease initiation. Cytokine. 2014;67(2):92–101. doi: 10.1016/j.cyto.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Nguyen CQ, Cha SR, Peck AB. Sjogren’s syndrome (SjS)-like disease of mice: the importance of B lymphocytes and autoantibodies. Front Biosci 2007;12:1767–89. Epub 2006/11/28. doi: 2187 [pii]. [DOI] [PubMed] [Google Scholar]
- 7.Katsifis GE, Moutsopoulos NM, Wahl SM. T lymphocytes in Sjogren’s syndrome: contributors to and regulators of pathophysiology. Clin Rev Allergy Immunol 2007;32(3):252–64. Epub 2007/11/10. doi: 10.1007/s12016-007-8011-8. [DOI] [PubMed] [Google Scholar]
- 8.Dean S, Wang CS, Nam K, Maruyama CL, Trump BG, Baker OJ. Aspirin Triggered Resolvin D1 reduces inflammation and restores saliva secretion in a Sjogren’s syndrome mouse model. Rheumatology (Oxford) 2019;58(7):1285–92. doi: 10.1093/rheumatology/kez072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang CS, Maruyama CL, Easley JT, Trump BG, Baker OJ. AT-RvD1 Promotes Resolution of Inflammation in NOD/ShiLtJ mice. Scientific reports 2017;7:45525. doi: 10.1038/srep45525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cha S, Brayer J, Gao J, Brown V, Killedar S, Yasunari U, et al. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol 2004;60(6):552–65. Epub 2004/12/09. doi: SJI1508 [pii] 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 11.Brayer JB, Cha S, Nagashima H, Yasunari U, Lindberg A, Diggs S, et al. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD.IL-4-gene knockout mouse model of Sjogren’s syndrome. Scand J Immunol 2001;54(1–2):133–40. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen CQ, Yin H, Lee BH, Chiorini JA, Peck AB. IL17: potential therapeutic target in Sjogren’s syndrome using adenovirus-mediated gene transfer. Lab Invest 2011;91(1):54–62. doi: 10.1038/labinvest.2010.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin X, Rui K, Deng J, Tian J, Wang X, Wang S, et al. Th17 cells play a critical role in the development of experimental Sjogren’s syndrome. Ann Rheum Dis 2015;74(6):1302–10. doi: 10.1136/annrheumdis-2013-204584. [DOI] [PubMed] [Google Scholar]
- 14.Youinou P, Pers JO. Disturbance of cytokine networks in Sjogren’s syndrome. Arthritis Res Ther 2011;13(4):227. doi: 10.1186/ar3348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chiorini JA, Cihakova D, Ouellette CE, Caturegli P. Sjogren syndrome: advances in the pathogenesis from animal models. J Autoimmun 2009;33(3–4):190–6. doi: S0896–8411(09)00123–1 [pii] 10.1016/j.jaut.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nikolov NP, Illei GG. Pathogenesis of Sjogren’s syndrome. Curr Opin Rheumatol 2009;21(5):465–70. Epub 2009/07/02. doi: 10.1097/BOR.0b013e32832eba21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jin JO, Yu Q. T Cell-Associated Cytokines in the Pathogenesis of Sjogren’s Syndrome. Journal of clinical & cellular immunology 2013;S!(9). doi: 10.4172/2155-9899.S1-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee BH, Tudares MA, Nguyen CQ. Sjogren’s syndrome: an old tale with a new twist. Arch Immunol Ther Exp (Warsz) 2009;57(1):57–66. Epub 2009/02/17. doi: 10.1007/s00005-009-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker OJ, Camden JM, Redman RS, Jones JE, Seye CI, Erb L, et al. Proinflammatory cytokines tumor necrosis factor-alpha and interferon-gamma alter tight junction structure and function in the rat parotid gland Par-C10 cell line. American journal of physiology Cell physiology 2008;295(5):C1191–201. doi: 10.1152/ajpcell.00144.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee SW, Croft M. 4–1BB as a therapeutic target for human disease. Adv Exp Med Biol 2009;647:120–9. doi: 10.1007/978-0-387-89520-8_8. [DOI] [PubMed] [Google Scholar]
- 21.Sun Y, Chen JH, Fu Y. Immunotherapy with agonistic anti-CD137: two sides of a coin. Cellular & molecular immunology 2004;1(1):31–6. [PubMed] [Google Scholar]
- 22.Irie J, Wu Y, Kachapati K, Mittler RS, Ridgway WM. Modulating protective and pathogenic CD4+ subsets via CD137 in type 1 diabetes. Diabetes 2007;56(1):186–96. doi: 10.2337/db06-0793. [DOI] [PubMed] [Google Scholar]
- 23.Sun Y, Lin X, Chen HM, Wu Q, Subudhi SK, Chen L, et al. Administration of agonistic anti-4–1BB monoclonal antibody leads to the amelioration of experimental autoimmune encephalomyelitis. J Immunol 2002;168(3):1457–65. [DOI] [PubMed] [Google Scholar]
- 24.Elpek KG, Yolcu ES, Franke DD, Lacelle C, Schabowsky RH, Shirwan H. Ex vivo expansion of CD4+CD25+FoxP3+ T regulatory cells based on synergy between IL-2 and 4–1BB signaling. J Immunol 2007;179(11):7295–304. [DOI] [PubMed] [Google Scholar]
- 25.Bartkowiak T, Curran MA. 4–1BB Agonists: Multi-Potent Potentiators of Tumor Immunity. Frontiers in oncology 2015;5:117. doi: 10.3389/fonc.2015.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang C, Lin GH, McPherson AJ, Watts TH. Immune regulation by 4–1BB and 4–1BBL: complexities and challenges. Immunological reviews 2009;229(1):192–215. doi: 10.1111/j.1600-065X.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- 27.Rahman K, Iyer SS. Costimulatory molecules as vaccine adjuvants: to 4–1BB or not to 4–1BB? Cellular & molecular immunology 2015;12(4):508–9. doi: 10.1038/cmi.2014.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bartkowiak T, Jaiswal AR, Ager CR, Chin R, Chen CH, Budhani P, et al. Activation of 4–1BB on Liver Myeloid Cells Triggers Hepatitis via an Interleukin-27-Dependent Pathway. Clinical cancer research : an official journal of the American Association for Cancer Research 2018;24(5):1138–51. doi: 10.1158/1078-0432.CCR-17-1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vinay DS, Kim JD, Asai T, Choi BK, Kwon BS. Absence of 4 1BB gene function exacerbates lacrimal gland inflammation in autoimmune-prone MRL-Faslpr mice. Investigative ophthalmology & visual science 2007;48(10):4608–15. doi: 10.1167/iovs.07-0153. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Jin JO, Kawai T, Yu Q. Endogenous programmed death ligand-1 restrains the development and onset of Sjgren’s syndrome in non-obese diabetic mice. Scientific reports 2016;6:39105. doi: 10.1038/srep39105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhou J, Kawai T, Yu Q. Pathogenic role of endogenous TNF-alpha in the development of Sjogren’s-like sialadenitis and secretory dysfunction in non-obese diabetic mice. Lab Invest 2017;97(4):458–67. doi: 10.1038/labinvest.2016.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szczerba BM, Rybakowska PD, Dey P, Payerhin KM, Peck AB, Bagavant H, et al. Type I interferon receptor deficiency prevents murine Sjogren’s syndrome. Journal of dental research 2013;92(5):444–9. doi: 10.1177/0022034513483315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nandula SR, Dey P, Corbin KL, Nunemaker CS, Bagavant H, Deshmukh US. Salivary gland hypofunction induced by activation of innate immunity is dependent on type I interferon signaling. J Oral Pathol Med 2013;42(1):66–72. doi: 10.1111/j.1600-0714.2012.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]