Abstract
Purpose:
Extracellular vesicles (EVs) can mediate long-distance communication in polarized RPE monolayers. Specifically, EVs from oxidatively stressed donor cells (stress EVs) rapidly reduced barrier function (transepithelial resistance, TER) in naïve recipient monolayers, when compared to control EVs. This effect on TER was dependent on dynamin-mediated EV uptake, which occurred rapidly with EVs from oxidatively stressed donor cells. Here, we further determined molecular mechanisms involved in uptake of EVs by naïve RPE cells.
Methods:
RPE cells were grown as monolayers in media supplemented with 1% FBS followed by transfer to FBS-free media. Cultures were used to collect control or stress EVs upon treatment with H2O2, others served as naïve recipient cells. In recipient monolayers, TER was used to monitor EV-uptake-based activity, live-cell imaging confirmed uptake. EV surface proteins were quantified by protein chemistry.
Results:
Clathrin-independent, lipid raft-mediated internalization was excluded as an uptake mechanism. Known ligand-receptor interactions involved in clathrin-dependent endocytosis include integrins and proteoglycans. Desialylated glycans and integrin-receptors on recipient cells were necessary for EV uptake and subsequent reduction of TER in recipient cells. Protein quantifications confirmed elevated levels of ligands and neuraminidase on stress EVs. However, control EVs could confer activity in the TER assay if exogenous neuraminidase or additional ligand was provided.
Conclusions:
In summary, while EVs from both stressed cells and control contain cargo to communicate stress messages to naive RPE cells, stress EVs contain surface ligands that confer rapid uptake by recipient cells. We propose that EVs potentially contribute to RPE dysfunction in aging and disease.
Keywords: retinal pigment epithelium, extracellular vesicles, uptake mechanisms, ligands, neuraminidase, annexin A2, age-related macular degeneration
INTRODUCTION
Age-related macular degeneration (AMD) is the leading cause of visual impairment and blindness in the elderly worldwide. The retinal pigment epithelium (RPE), which is part of the blood retina barrier, is a primary site of damage. Persistent oxidative stress is recognized as one of the important underlying risk factors that leads to AMD pathology. Importantly, RPE pathology in AMD does not initiate in a single location but occurs in multiple patches that slowly coalesce. This pattern of pathology might involve both short- and long-distance communication between diseased and healthy parts of the RPE. We previously examined both the role of gap-junction communication as part of the short-distance communication mechanism [1, 2] and the role of extracellular vesicles (EVs) as part of the long-distance communication mechanism [3] in RPE pathology.
EVs are small vesicles that are released from different types of cells with an approximate size of 40-150 nm, and are present in almost all body fluids. EVs contain various molecular constituents including RNA, proteins and miRNA and play a crucial role in intercellular communication [4].
We previously characterized the release of EVs from ARPE-19 and primary porcine RPE cells grown as stable monolayers. When polarized, RPE cells release EVs towards both the apical and basal side. Previously, we confirmed, using five independent assays that these EVs released by these cells and isolated by Exoquick-TC represent mainly exosomes [3]; but will be referred to here as EVs. Using these EVs from either control or H2O2-stimulated monolayers, we established two assays to analyze EV uptake. First, we established a transfer assay, determining the effect of EVs on the barrier function (transepithelial resistance [TER]) of naïve recipient cells of the same maturity (i.e., cultured in parallel) over time. The effect of EVs from H2O2-stimulated cells on barrier function was rapid, depended on exosome uptake in a dynamin-dependent manner, and was mediated by HDAC6 activity present in EVs. In contrast, EVs from control monolayers failed to enter the recipient cells [3]. Second, we analyzed internalization of EVs by RPE monolayers by live cell imaging, which revealed two differences between stress and control EVs. Stress EVs were rapidly internalized by recipient cells (~10-20 mins), whereas control EVs were internalized slowly (taking ~100 mins), and while the majority of stress EVs were internalized, ~80% of the control EVs remained on the plasma membrane [3].
Finally, we reported dynamin-dependent endocytosis as one of the mechanism of vesicle uptake, involving the ligand annexin A2 present on exosome surfaces [3]. Our results in RPE were similar to those reported by Zhang and coworkers, who reported that photo-oxidative stress results in the release of EVs that trigger stress in naïve recipient cells [5]. What is missing in our published set of data is the underlying mechanism by which stress EVs are taken up into recipient cells, or on the flip-side by which control exosomes are excluded.
Endocytosis is the primary mechanism of EV uptake and includes caveolae-dependent endocytosis [6], clathrin-dependent endocytosis [7], caveolae and clathrin independent endocytosis, lipid raft-mediated endocytosis [8], macropinocytosis and phagocytosis [9], each requiring different ligands [7, 10, 11]. In this study, we further characterize the general EV uptake mechanisms in RPE cells and identify molecular mechanisms by which uptake of stress EVs is mediated by employing selective inhibitors and molecular tools.
MATERIAL AND METHODS
Cell culture
ARPE-19 cells (ATCC® CRL-2302™; American Type Culture Collection, Manassas VA), between 20-35 passages, were expanded in 100 mm dishes (Thermo Fisher) with Dulbecco's modified Eagle's medium (DMEM), 10% fetal bovine serum (FBS), and 1× antibiotic-antimycotic for 7 days or until 100% confluent. Once confluent, ARPE-19 cells were washed with 1× Phosphate-buffered saline (Gibco), trypsinized with 0.05% trypsin (Gibco), seeded at 3.0 × 105 cells on 6-well transwell filters (Costar), and grown for 4 weeks as a polarized monolayer. Primary porcine RPE (pRPE) were cultured according to a published protocol [3], using eyes obtained from a local abattoir. Pooled cells from 10 eyes were suspended in 10% FBS Growth Medium (high glucose DMEM with l-glutamine, sodium pyruvate, 1% nonessential amino acids, 10% FBS and Penicillin and Streptomycin), expanded in T25 cell culture flasks until 90% confluency followed by induction of differentiation in the presence of 1% FBS for 7 days. Cells are then transferred to 12-well plates at 100% confluency to allow for immediate monolayer formation (6 × 105 cells/well) and continued to grow in 1% FBS.
For ARPE-19 cells, upon reaching confluency, tight junction formation was enabled by step-wise FBS reduction to 1%. Specifically, during the first week, cells were grown in the presence of 10% FBS in the top and 2% on the bottom of transwells, followed by a weekly step-wise reduction of FBS from 2% to 1%. Finally, FBS was removed completely for the final 2–3 media changes prior to the experiments to remove any FBS-derived EVs. For pRPE, which were plated at confluency, tight junction formation was supported by 1% FBS for 2 weeks, or until the desired TER values are reached (see below). Since pRPE do not tolerate serum-free conditions, for the final 2 media changes prior to the experiments exosome-depleted FBS (ThermoFisher) was used to remove any FBS-derived EVs.
Monolayer integrity was assessed by transepithelial resistance (TER) measurements using an EVOM volt-ohmmeter (World Precision Instruments) as described [12]. Monolayers are considered stable when TER was repeatedly measured as ~40–45 Ω cm2 (ARPE-19 cells) or ~150-200 Ω cm2 (pRPE cells).
Isolation of EVs
To trigger EV release, ARPE-19 monolayers were stimulated apically with 0.5 mM H2O2 (Sigma Aldrich) or pRPE with 0.3 mM once a day for 3 days without changing the media. Supernatants were collected from the apical side (2 ml per well), spun at 3000g, and submitted for EV isolation using Exoquick-TC (Systems Biosciences) according to the manufacturer's instructions. Specifically, Exoquick-TC (10%) was added to the culture media collected from the cells, incubated overnight at 4 °C, centrifuged at 1600g for 35 min to collect the EV pellet, and resuspended into the amount of fresh media needed for the specific experiments (0.5 ml or 2 ml for 6- and 12-well plates, respectively).
Zetaview nanoparticle tracking analysis (NTA)
EV concentrations were determined using the ZetaView PMX 110 (Particle Metrix, Meerbusch, Germany) and its corresponding software (ZetaView 8.02.28). For each sample, 1 μl of the resuspended EV pellet isolated by Exoquick-TC was diluted into 1 mL of 1× PBS, and loaded into the NTA cell for analysis to obtain the EV particle concentration. The instrument measures each sample at 11 different positions throughout the cell, with two cycles of readings at each position. The instrument pre-acquisition parameters were set to: temperature of 23 °C, sensitivity of 85, frame rate of 30 frames per second (fps), shutter speed of 100, and laser pulse duration equal to that of shutter duration. Automated analysis of all 11 positions and removal of any outlier positions, the mean, median, mode sizes, and concentration of the sample, were calculated by the optimized machine software.
Transfer assays
Transfer assays were performed to study cell-cell communication using purified EVs from donor RPE cells. Equal amounts of EVs corresponding to the average amount of EVs released from cells from a single well isolated by Exoquick-TC (1×109) were diluted into fresh media (2 mL or 0.5 mL for 6- and 12-well plates, respectively) and transferred to naïve recipient monolayers of the same age and TER as donor cells. TER measurements were performed prior to the transfer (designated as 0 hr) and after incubation of 4 hrs for each treatment.
Treatment of Cells or EVs
Some recipient monolayers or some donor EVs were pre-treated with compounds known to inhibit or accelerate EV uptake. This included the following compounds: filipin which binds to cholesterol and blocks lipid-based interactions; RGD (arginylglycylaspartic acid) peptide which blocks the interaction between integrin and its ligands; heparinase to remove surface proteoglycans; oseltamivir phosphate which inhibits the enzyme neuraminidase (a sialidase) and prevents the removal of sialic acids; and neuraminidase which removes sialic acids. All compounds were used for the pretreatment of cells or exosomes. Pretreatment of cells were performed with RGD peptide for 1 hr (10 μg/ml, Sigma Aldrich), heparinase for 30 min (10 μg/ml, Sigma Aldrich), filipin for 30 min (250 μg/ml, Sigma Aldrich), oseltamivir phosphate for 1 hr (400 μM, Sigma Aldrich) or neuraminidase for 1 hr (from Vibrio cholera, 15 μU, Sigma-Aldrich). Pretreatment of EVs included incubating EVs with heparinase for 30 min (10 μg/ml) or neuraminidase for 1 hr (15 μU) was followed by cleanup with Exoquick-TC to remove any unbound compounds. Finally, to assess the involvement of HDAC6 in TER reduction, cells or EVs were preincubated with the class I and II mammalian HDAC family inhibitor trichostatin A (TSA, 100 nM, Sigma) [3].
Western Analysis
Protein was extracted by solubilizing EVs (1.7 × 1010) in RIPA buffer (10 mM Tris-HCl, pH 7.5, 300 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% SDS, and 0.1% sodium deoxycholate; Fisher Scientific) containing a cocktail of protease inhibitors (Sigma). EV lysates were added to Laemmli sample buffer and rocked at room temperature for 15 minutes and boiled for 5 minutes. Samples were separated by electrophoresis on a 4-20 % Criterion™ TGX™ Precast Gels (Bio-Rad Laboratories, Inc), and proteins transferred to a PVDF membrane. Membranes were incubated with the primary antibody against annexin-A2 (1:1000; ab41803, Abcam) or fibronectin (1:1000; ab610077, BD Bioscience) over night. Proteins were visualized with horseradish peroxidase-conjugated secondary antibodies (anti-mouse IgG and IgM; Santa Cruz Biotechnology) followed by incubation with Clarity™ Western ECL Blotting Substrate (Bio-Rad Laboratories) and chemiluminescent detection. Protein bands were scanned and densities quantified using ImageJ software.
ELISA
The glypican 1 ELISA was carried out according to manufacturer instructions (ELH-GPC1-1, RayBioTech). EVs pellets were dissolved in ice cold PBS buffer by passing the sample through a syringe tip (20x). Equal numbers of EVs (1.38 × 1011) were added to each well of a 96 well plate and incubated overnight to enhance binding. Measurements were obtained at 450 nm using the microplate reader.
Neuraminidase (NEU) activity assay
Neuraminidase (NEU) activity in isolated, intact EVs was measured in a black 96 well clear-bottom plate (CoStar 3603) using a SpectraMax i3c fluorometer and SoftMax Pro 6 software (Molecular Devices, San Hose, CA). EVs (4.5 × 109 per well) in 37 °C warmed sterile-filtered Tris Buffered Saline (TBS) at pH 7.4 were incubated with 15 μM 2’ (4-Methylumbelliferyl) α-D-N-acetylneuraminic acid (4MU-NANA; Sigma Aldrich) substrate suspended in 200 μl TBS). The fluorescence intensity (excitation/emission [ex/em] in nm: 365/460) was measured at baseline before substrate addition and in the presence of substrate between 2 and 35 min at 37°C.
Imaging
Two systems were used for live cell imaging; the WiScan Hermes High Content Imaging System (IDEA Bio-Medical; Rehovot, Israel) and confocal microscopy. For the Hermes, cells were plated in glass bottom 96-well plates (Mattek; Ashland, MA). Recipient cells were labeled with CellTrackerTM Red (1:1000; ThermoFisher, Waltham, MA) and Hoechst 33342 (Thermofisher), EVs were labeled with ExoGlow according to the manufacturer’s instructions (Systems Biosciences, EXOC300A-1). Uptake was imaged using the Wiscan® Hermes Imaging system with a 10x objective; using the following LED light sources: ex/em in nm, 390/440 for Hoechst 33342, 485/525 for ExoGreen and 560/607 for CellTracker Red. Images were acquired every 20 minutes for 2 hours after adding EVs (1.95 × 108) and stored as tif files. For confocal microscopy, recipient cells were plated on glass bottom 96-well plates (Mattek) with cells and EVs labeled as above. Image acquisition was performed 120 minutes after adding EVs (5.2 × 108) using the UltraViewVoX spinning disk confocal microscope equipped for spinning disk confocal microscopy (Eclipse Ti, Nikon, Tokyo, Japan), running Volocity software (Perkin Elmer, Wokingham, UK) on a Windows 64- bit system as previously published [1, 3]. Confocal images were converted into tif files. All tif files were processed using ImageJ software (NIH). The EV (green) - CellTracker (red) colocalized particles were detected with the Colocalization plugin (yellow) and quantified with the Particle analyzer module in ImageJ.
X-stamp mediated targeting of EVs
The Systems Biosciences surface display system was used to place annexin A2 on the surface of EVs. In short, a lentiviral vector was designed and generated by Systems Biosciences, to place Annexin A2 (Addgene; [13]) into the multicloning site of their proprietary XSTP710PA-1 plasmid, flanked by a built-in signal sequence and the downstream C1C2 EV-targeting domain sequence [14]. ARPE-19 cells were grown to 75% confluency in the presence of 10% FBS without antibiotics, followed by transfection in the presence of polybrene (8 μg/mL; Sigma) using 1×105 to 1×107 infectious units [ifus]/mL. Puromycin (GenDEPOT) was added 72 hrs after transduction at 100 μg/ml, and dropped to 50 μg/ml after 1 week. Once 100% confluent, the transduced ARPE-19 cells were trypsinized and seeded onto 6-well transwell filters for the generation of exosomes (see section on Cell culture and Isolation of EVs). Based on cell survival in the presence of puromycin, a final concentration of 2.5×106 ifus/mL was chosen for the final experiment.
Statistics
Data are presented as mean ± SEM. Each TER experiment reflects the average of 3 independent experiments with 3 biological replicates of transwells with 3 measurements taken from different points on the transwell, unless otherwise indicated. TER percentage reflects the change in the TER measurements performed prior to the transfer at 0 hours compared to the measurements performed 4 hours after the exposure to EVs. Western blots and ELISA reflect 3 independent experiments, and neuraminidase activity assay reflects 3 independent experiments with 3 biological replicates. Single comparisons were analyzed using unpaired T-tests; experiments containing multiple parameters were analyzed using ANOVA with Bonferroni correction (Statview; SAS Institute, Cary NC). For imaging, data analysis was performed using Prism GraphPad software (San Diego, CA) using a Student’s T-test.
RESULTS
Bystander effect elicited by EVs requires ligand-dependent EV uptake by ARPE-19 recipient cells.
EVs released apically from either control or H2O2-stimulated monolayers were isolated, equal numbers were transferred to stimulate naïve monolayers, and TER measurements between 0 (baseline) and 4 hours (post treatment) were obtained. As demonstrated previously [3], EVs from stressed cells (exposed to 0.5 mM H2O2, “stress EVs”) elicited a drop in TER (bystander effect) in recipient cells, whereas EVs released from control cells (“control EVs”) had no effect on TER (Fig. 1A).
Figure 1. Transfer assays to study ligands required for cell-cell communication by EVs.
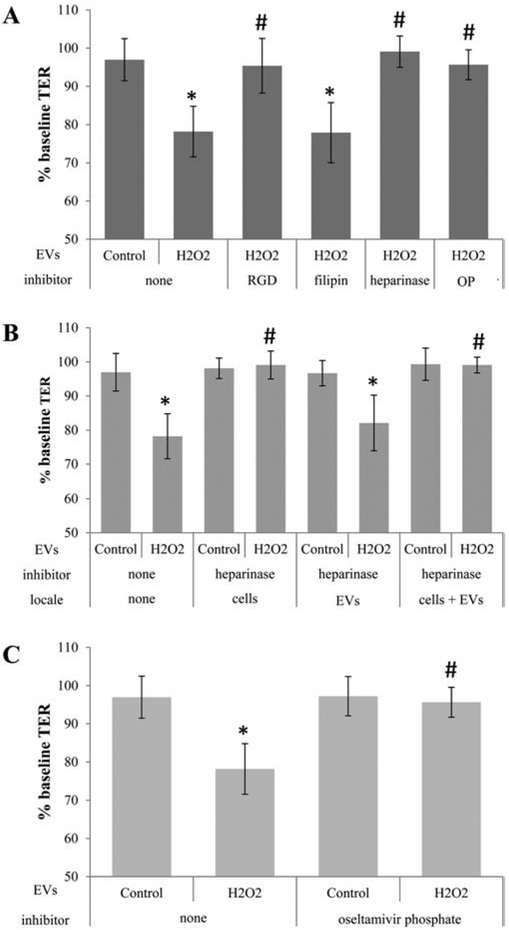
Transepithelial resistance (TER) assays are used to study bystander effects elicited by EV transfer as published, comparing TER measurements between 0 (baseline) and 4 hour time point (post treatment). A reduction in TER is taken as successful uptake of EVs by recipient cells [3]. Tables are arranged as follows indicating the type of EV (stress/H2O2 or control), the inhibitor used, and the location to which the inhibitor is added (recipient cell or EV), if location varies within a graph. (A) EVs from ARPE-19 cell monolayers (donor cells) of control or stressed (H2O2) were transferred directly to the apical side of naïve monolayers (recipient cells). EVs from stressed cells reduced TER. Pretreatment of recipient cells with RGD peptide (integrin receptor inhibitor), heparinase (removes surface proteoglycans) or oseltamivir phosphate (OP; inhibits neuraminidase activity) prevented TER reduction, whereas filipin (inhibits lipid-based interaction) did not. (B) Surface proteoglycans might play a role in binding of EVs to the recipient cell surface. Proteoglycans can be on the EV or the cell membrane of the recipient cell. Incubation of either cells, EVs or both with heparinase confirmed the importance of proteoglycans on the cell membranes of the recipient cells. (C) Transfer assays were repeated on EVs isolated from control or stressed donor ARPE-19 cells and preincubated with OP, which revealed the lack of an effect of OP added to EVs on TER. Bar graphs represent mean ±SEM. Significance was obtained using a posthoc ANOVA with Bonferroni correction. * indicates difference of indicated TER values with those elicited by control EVs; # indicates difference of indicated TER values with those elicited by stress (H2O2) EVs.
To study the role of ligands potentially involved in EV uptake, known ligand-receptor and lipid-based interactions were probed. Previously, we implicated clathrin-dependent endocytosis, a mechanism involving the GTPase dynamin, as well as annexin-A2, a known mediator of endocytic entry into cells, in this process [3]. However, a clathrin-independent mechanism, such as lipid raft-mediated internalization was not investigated. Thus, stress EVs were pretreated with filipin to block lipid interactions and prevent EV uptake [15]. Both filipin-treated and untreated stress EVs resulted in a 20-25% drop in TER after 4 hours (Fig. 1A), suggesting lipid interactions do not play a role in TER reduction of recipient cells by stress EVs. Hence, we next tested protein-based interactions.
Proteins can be stripped from the surface of EVs using proteinase K to reduce their uptake [2], and there is a growing list of protein-protein interactions identified that mediate EV uptake. These include immunoglobulin-integrin interaction or integrin-matrix macromolecule interaction, proteogylcans and their modifications, and annexin-A2 [2][3]. In this study, we focused on the role of integrin and proteoglycans. Pretreatment of the recipient cells with RGD peptide, which blocks the interaction between integrin and its ligands, prevented the drop in TER after exposure to stress EVs compared to untreated cells exposed to stress EVs (Fig. 1A). Proteoglycans, in particular heparin sulphate proteoglycans (HSPG), are required for EVs as well as other complexes such as viruses to enter cells [2]. These HSPGs might be required on the surface of the EVs or recipient cells [16]. Therefore, either recipient cells or EVs were pretreated with heparinase to remove surface proteoglycans prior to the EV transfer assay. Heparinase treatment of cells (Fig. 1A, B), but not EVs (Fig. 1B), completely prevented TER reduction by stress EVs. This suggests that the presence of HSPGs on the cell surface is more important than on the EV surface for the effect of stress EVs on TER. Finally, many glycoproteins are enriched in specific sialic acid residues, and removal of sialic acids from cell surfaces can facilitate virus entry into cells [17]. Pretreatment of cells with oseltamivir phosphate, which inhibits the enzyme neuraminidase and prevents the removal of sialic acids, prevented the drop in TER normally elicited by stress EVs (Fig. 1A, C). Taken together, protein- rather than lipid-based interactions appear to mediate the uptake of stress EVs.
Analysis of potential ligands and enzymes in EVs derived from ARPE-19 cells
In light of the functional assays implicating annexin-A2 [3], integrin, and proteoglycans in EV uptake (Fig. 1A), a preliminary mass spectrometry-based proteomic analysis was performed on control and stress EVs for potential candidates enriched in stress EVs (data not shown). A number of ligands, including annexin-A2, integrin receptor ligands vitronectin and fibronectin, and proteoglycans glypican 1 and HSPG2 (basement membrane-specific heparan sulfate proteoglycan core protein or endorepellin) were identified. In addition, the level of the enzyme neuraminidase (NEU1) was also elevated. To confirm the preliminary proteomic results, EVs from control and H2O2-stressed cells were analyzed by Western blotting (fibronectin, annexin-A2), ELISA (glypican 1), or activity assay (NEU), using equal numbers of EVs per sample. Stress EVs contained ~5 fold more fibronectin than control EVs (Fig. 2B), and annexin-A2, which is present in control EVs at very low levels, was also significantly increased in stress EVs (Fig. 2A). Likewise, EVs released from stressed cells contained ~3 fold more glypican 1 than EVs from control cells (Fig. 2C). Finally, NEU activity was significantly elevated on intact stress EVs compared to control EVs (Fig. 2D). Thus, ligands required for EV uptake, or an enzyme known to aid in uptake, were elevated in stress EVs.
Figure 2. EVs contain ligands/enzymes required for uptake by recipient cells.
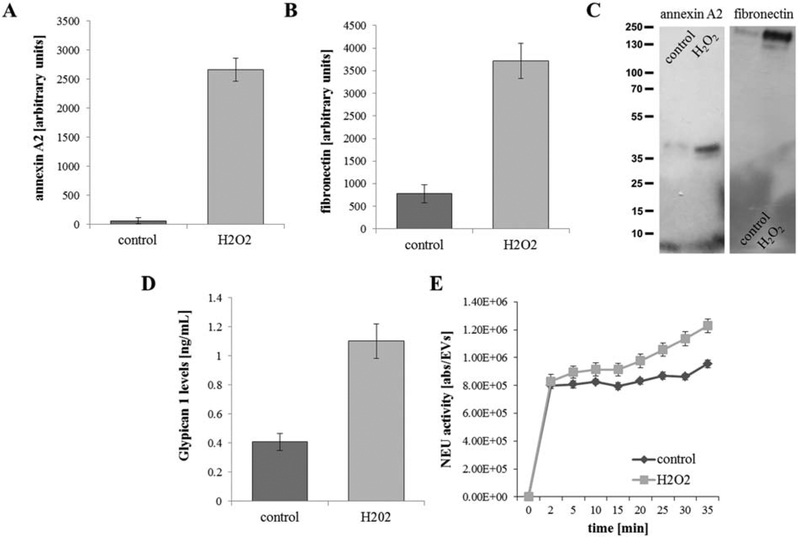
(A) Annexin-A2 and (B) fibronectin levels were obtained by Western blotting, (C) glypican 1 by ELISA and (D) neuraminidase (NEU) activity was measured with a homogenous fluorescence release assay. In summary, all four readouts were significantly elevated in EVs isolated with stressed cells (H2O2) when compared to controls. Bar graphs represent mean ±SEM (n=3 independent experiments for Western, n=3 independent experiments with 3 biological repeats each for ELISA and NEU assay). Significance was obtained using a T-test (Western, ELISA) or posthoc ANOVA with Bonferroni correction (NEU assay). All four comparisons were significant (P<0.01 or less).
Analysis of EV uptake using live-cell imaging in ARPE-19 cells
Previously, we analyzed internalization of EVs by RPE monolayers by live cell imaging [3]. Due to the large number of parameters in this study, imaging was performed using the WiScan Hermes High Content Imaging System, to accommodate analyses in a 96-well plate format. Recipient cells were labeled with CellTracker (red) and DAPI (blue), to visualize cytoplasm and nuclei, respectively. EVs were labeled with ExoGlow (green), which labels proteins present in EVs, prior to addition to recipient cells. Intracellular EVs were identified as co-localized dots between the red and green channel (yellow). As we observed previously [3], stress EVs were rapidly taken up by naïve ARPE-19 cells, whereas EVs derived from control cells showed little uptake over the 2 hour imaging period (Fig. 3A, B). Uptake of stress EVs was inhibited in the presence of RGD peptide, heparinase, or oseltamivir phosphate added to the cells, but not filipin (Fig. 3A, B). Interestingly, fewer EVs overall entered the cell when filipin was added, leading to some aggregation of EVs on the cell surface (Fig. 3A). Hence, the imaging experiments confirmed that interfering with ligand-receptor (i.e., protein-protein) interactions prevented uptake of EVs as predicted by the functional readout.
Figure 3. Live cell imaging investigating ligand-dependent internalization of EVs.
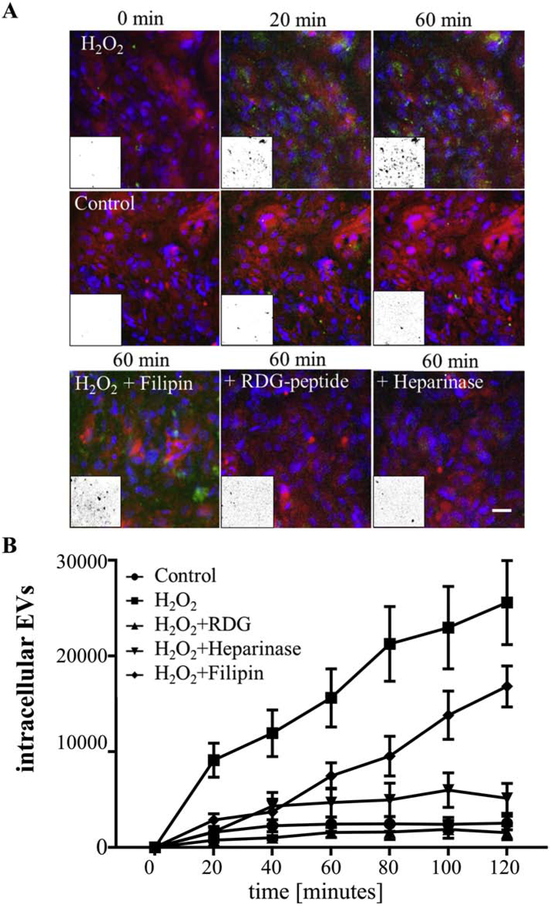
(A) Images of live ARPE-19 cell monolayers exposed to EVs. EVs were labelled with ExoGlow (green), the cytoplasm of recipient cells were labelled with cell tracker (red) and nuclei with Hoechst (blue). Internalization of EVs was imaged using the WiScan Hermes High Content Imaging System at 0-120 minutes. Intracellular EVs were identified as co-localized dots between the red and green channel (= yellow). The yellow channel was binarized (representative examples in insets) and quantified in Image J. (B) Rapid uptake of EVs in naïve ARPE-19 cells was confirmed for those derived from H2O2-treated but not control cells. Uptake of stress-EVs was inhibited in the presence of RGD peptide and heparinase but not filipin. Fewer EVs entered the cell when filipin was added, leading to aggregation of EVs on the cell surface (green clumps). Graphs represent mean ±SD (n=4-8 wells, 6 locations each). Significance was obtained using a posthoc ANOVA with Bonferroni correction. The time course of uptake for control, H2O2 + RGD and H2O2 + heparinase were indistinguishable from each other (P>0.05); whereas H2O2 (P<0.0001) and H2O2 + filipin were significantly increased (P<0.002) when compared to controls.
Control EVs contain sufficient cargo to generate bystander effect if enabled to enter the cells.
The results presented above demonstrated that interfering with ligand-receptor interactions between the surfaces of stress EVs and recipient cells prevented uptake of stress EVs when compared to control EVs. But can control EVs get taken up if a critical ligand-receptor interaction is established? Two separate mechanisms were investigated.
Virus entry into cells is facilitated by removal of sialic acids from cell surfaces by viral neuraminidase [17]; and inhibiting neuraminidase by oseltamivir phosphate to block the removal of sialic acids from the recipient cell surface, prevented a drop in TER elicited by stress EVs (Fig. 1A, C). To determine if removing sialic acids from the recipient cell glycocalyx would aid in uptake of control EVs, recipient cells were pretreated with the native Vibrio cholerae neuraminidase prior to addition of EVs. Pretreatment of the recipient cells with neuraminidase alone had no effect on TER (Fig. 4B), but in the presence of control EVs, resulted in a significant drop in TER (Fig. 4A). Neuraminidase pretreatment did not further increase the TER reduction induced by stress EVs alone (Fig. 4A). In contrast, pretreatment of EVs with neuraminidase had no effect (Fig. 4A). Confocal microscopy at the 120 minute time point confirmed that control EVs were taken up by naïve ARPE-19 cells pretreated with neuraminidase, whereas untreated cells showed little uptake (Fig. 5A, B). Likewise, stress EV uptake by naïve cells was significantly inhibited by the addition of oseltamivir phosphate (Fig. 5A, B). Three conclusions can be drawn from these results. First, control EVs contain sufficient cargo to reduce TER when aided to enter the cells by exogenous neuraminidase added to the recipient cells. Second, exogenous neuraminidase is unable to bind to/interact with control EVs, such that transfer of pretreated EVs had no effect on TER (i.e. the uptake of the EVs) in the recipient cells. And third, this suggests the neuraminidase in stress EVs desialylates proteins on recipient cells facilitating their uptake. Finally, the effect of stress EVs on TER was previously shown to be mediated by HDAC6 packaged within EVs; and while HDAC6 activity was found to be higher in stress EVs when compared to control EVs, both contain the enzyme [3]. To determine if control EVs can affect barrier function in RPE monolayers in an HDAC6-dependent manner, EVs were added to recipient cells pretreated with the HDAC6 inhibitor TSA (pan class I and II HDAC inhibitor) and/or neuraminidase. TSA had no effect on TER following addition of control EVs and prevented TER drop by stress EVs. TSA also reversed the TER drop by control EVs when added to neuraminidase-treated cells (Fig. 4B).
Figure 4. Control EVs exhibit biological effect when provided with critical enzyme required for uptake.
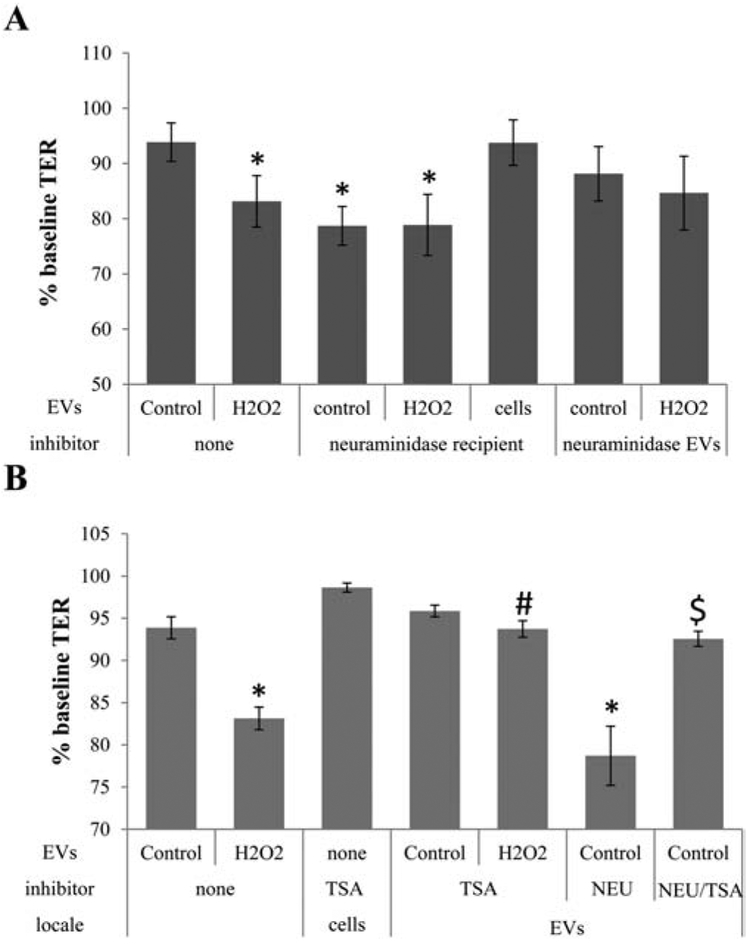
Transepithelial resistance (TER) assays were performed as described in Figure 1, again taking a reduction in TER as successful uptake of EVs by recipient cells [3]. (A) EVs from stressed cells reduce TER. Pretreatment of recipient cells with neuraminidase enabled control EVs to reduce TER to the same level as stress EVs, whereas treatment of recipient cells with neuraminidase (NEU) in the absence of EVs has no effect. Pretreatment of EVs with neuraminidase had no effect on TER. (B) Stress EVs have been shown to contain active HDAC6 that mediate the effect of EVs on tight junctions [3]. Incubation of the recipient cells with the HDAC6 inhibitor TSA (trichostatin A) prevented the effect on TER elicited by neuraminidase (NEU) pretreatment and the addition of control EVs. TSA also inhibited the effect of stress EVs and confirmed our previous results [3]. Bar graphs represent mean ±SEM of n=3 independent experiments with 3 biological replicates. Significance was obtained using a posthoc ANOVA with Bonferroni correction. * indicates difference of indicated TER values with those elicited by control EVs; # indicates difference of indicated TER values with those elicited by stress (H2O2) EVs, $ indicated difference of indicated TER values with those elicited by neuraminidase pretreatment of cells and addition of control EVs.
Figure 5. Live cell imaging confirming neuraminidase-dependent internalization of control EVs.
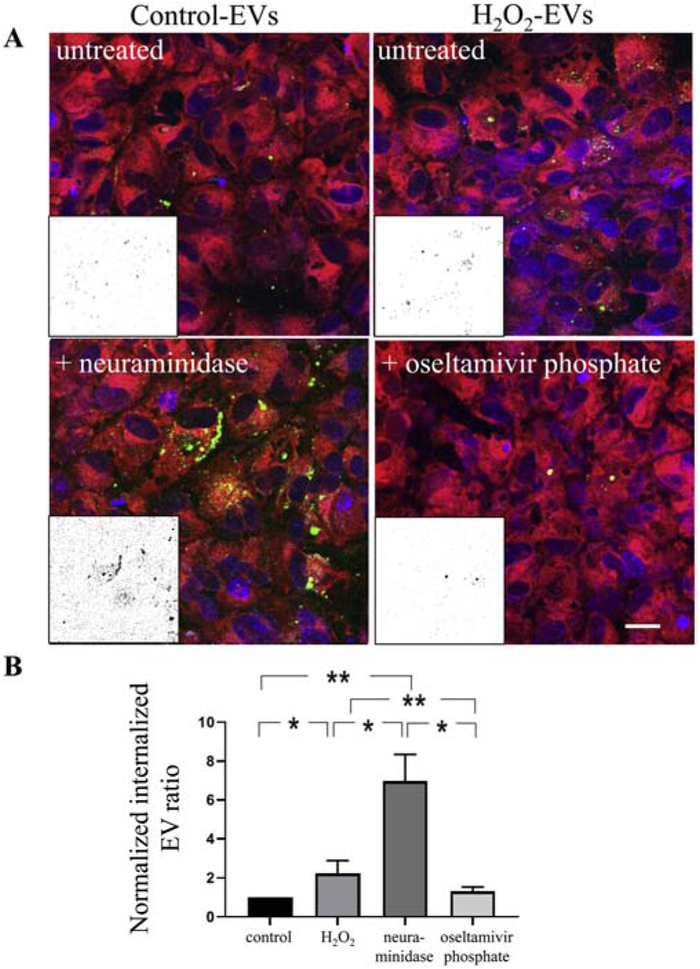
Confocal images of live ARPE-19 cell monolayers exposed to EVs labelled with ExoGlow (green). The cytoplasm of recipient cells was labelled with cell tracker (red) and nuclei with Hoechst (blue). Internalization of EVs was imaged at 120 minutes. Intracellular EVs were identified as co-localized dots between the red and green channel and quantified as described in Figure legend 3. (B) Uptake of EVs in naïve ARPE-19 cells was confirmed for those derived from control cells when recipient cells were treated with neuraminidase. Uptake of stress-EVs (H2O2) was inhibited in the presence of oseltamivir phosphate. Graphs represent mean ±SD (n=4-8 wells, 6 locations each). * indicates P<0.05; ** indicates P<0.01.
Annexin A2 levels are high in stress EVs (Fig. 2A, C) and previously we reported that knockdown of annexin-A2 using siRNA prevented the uptake of stress EVs by naïve ARPE-19 cells [3]. Thus, here we asked if increased delivery of annexin A2 to EVs would enable control EVs to reduce TER in the transfer assays. Lentiviral-mediated delivery of annexin A2 was accomplished using the C1C2 domain known to be involved in targeting ligands to the EV surface [14]. Elevated levels of annexin A2 in control EVs released from transfected cells were confirmed using Western blotting over a 2 log unit range of viral vector load (Fig. 6A). A final dose of 2.5×106 ifus/mL was chosen based on cell survival in the presence of puromycin for selection, resulting in an ~2000 fold increase in annexin A2 associated with EVs (data not shown). Delivery of annexin A2 control EVs to recipient cells resulted in a significant drop in TER (Fig. 6B), an effect that could not be further augmented by H2O2 treatment of the EV donor cells (Fig. 6B). These results further support the conclusion that control EVs contain sufficient cargo to reduce TER when aided to enter the cells by addition of a ligand involved in EV uptake.
Figure 6. Control EVs exhibit biological effect when provided with annexin A2 required for uptake.
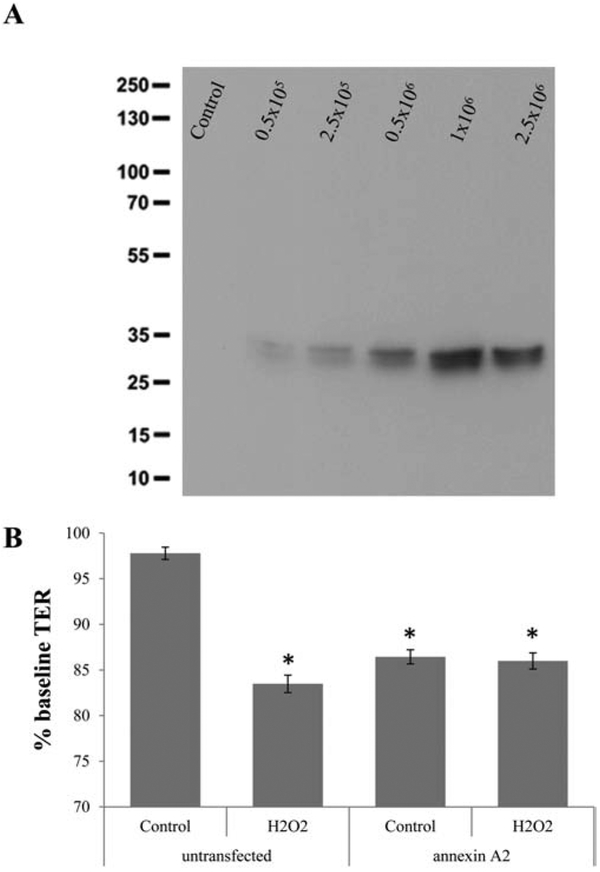
EVs were collected from annexin A2 lentiviral vector transfected cells. The expression vector was designed to target annexin A2 to the surface of EVs. (A) Elevated levels of annexin A2 in control EVs could be confirmed by Western blotting upon transfection with varying concentrations of lentiviral vectors (infectious units/mL). (B) EVs from untransfected stressed cells reduce TER, whereas those from untransfected control cells do not. In contrast, EVs from transfected control cells do reduce TER to the same level as stress EVs; and the addition of stress in transfected cells does not enable further reduction of TER upon EV transfer. Graphs represent mean ±SD (n=3-5 independent Western blots; and n=4-8 wells, 6 locations each for TER). Significance was obtained using a T-test (Western) or posthoc ANOVA with Bonferroni correction (TER assay). Western analysis was significant (P<0.001). * indicates difference of indicated TER values with those elicited by control EVs.
Extracellular vesicle-mediated TER reduction and uptake mechanisms are replicated in primary porcine RPE.
To confirm that ligand-receptor interactions mediating RPE-derived EV uptake by recipient RPE cells is universal, and not an artifact of the ARPE-19 cells, primary porcine RPE cells (pRPE) were examined. As we demonstrated previously, EVs from H2O2-treated donor pRPE reduced TER significantly in recipient pRPE cells, whereas control EVs had no effect (Fig. 7A). The bystander effect elicited by stress EVs from pRPE cells was inhibited in the presence of RGD peptide and heparinase, but not filipin (Fig. 7A), confirming the results obtained from the ARPE-19 cells. Pretreatment of recipient pRPE cells with neuraminidase resulted in a significant drop in TER in response to the addition of control EVs, suggestive of EV uptake (Fig. 7B), an effect that was prevented by pretreatment with TSA (Fig. 7A). Hence, the effect of control EVs is not specific to ARPE-19 cells, but was also verified in neuraminidase treated pRPE cells.
Figure 7. Ligand-dependent uptake of EVs in primary porcine RPE cells.
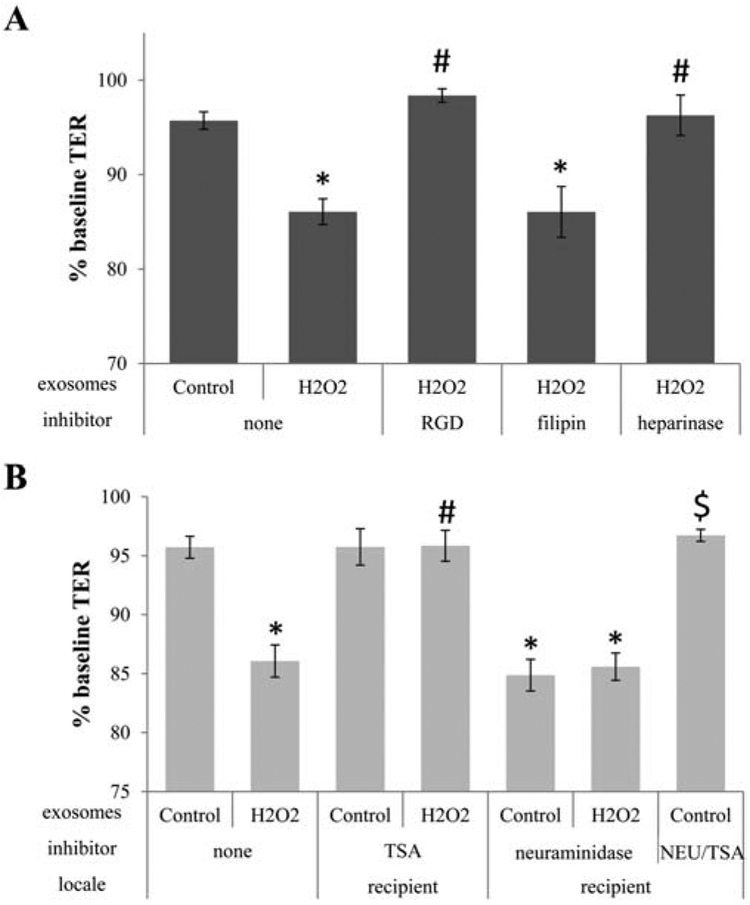
Transepithelial resistance (TER) assays are used to study bystander effects elicited by EV transfer as published, and described in Figure 1. The effect of EVs on TER appears universal for RPE cells as EVs derived from stressed primary porcine RPE (pRPE) cells elicit a bystander effect in naive porcine RPE monolayers [3]. (A) EVs from pRPE cell monolayers (donor cells) of control or stressed (H2O2) cells were transferred directly to the apical side of naïve monolayers (recipient cells). As for ARPE-19 cells, EVs from stressed cells reduce TER, and inhibition of integrin signaling (RGD) or removal of proteoglycans (heparinase) on recipient cells prevented TER reduction, whereas filipin (inhibits lipid-based interaction) does not. (B) To test whether EVs from control cells can be enabled to enter recipient cells, recipient ells were pretreated with neuraminidase. This enables control EVs to reduce TER to the same level as stress EVs, an effect that was inhibited by incubating the recipient cells with the HDAC6 inhibitor TSA. Bar graphs represent mean ±SEM. Significance was obtained using a posthoc ANOVA with Bonferroni correction. * indicates difference of indicated TER values with those elicited by control EVs; # indicates difference of indicated TER values with those elicited by stress (H2O2) EVs, $ indicated difference of indicated TER values with those elicited by neuraminidase pretreatment of cells and addition of control EVs.
DISCUSSION
The main results of the current study are: 1) EV uptake in RPE cells is mediated by clathrin-dependent endocytosis rather than lipid raft-mediated internalization; 2) Three ligand-receptor interactions, integrins, proteoglycans, and annexin A2 known to be involved in EVs uptake were demonstrated to be necessary for stress EV uptake by naïve RPE cells; 3) Elevated levels of ligands were identified on stress EVs compared to control EVs; 4) Functional readouts, barrier function measurements and live cell imaging, demonstrated the uptake of EVs; 5) A pathway typically used by viruses to enter cells, involving desialylation of cell surface molecules by neuraminidase, was identified as a mechanism involved in RPE uptake of EVs; and 6) Excess targeting of annexin A2 was shown to enable control EVs to reduce TER. In summary, these results suggest that EVs from stressed cells differ from those of control cells predominantly in their ligands on the EV surface for uptake by recipient cells, since control EVs contain sufficient cargo to generate bystander effect if enabled to enter the recipient cells.
Uptake of EVs by recipient cells or cargo transfer from EVs into recipient cells is mediated in two different ways. EVs can fuse with the recipient cell membrane or be taken up via phagocytosis or receptor-/raft-mediated endocytosis to empty/transfer their cargo into recipient cells [18-20]. In RPE cells, we excluded fusion as a mechanism, as membrane patches associated with EV membranes within cell membranes were not observed using live-cell imaging [3]. In contrast, an endocytic mechanism involving clathrin-dependent coated-vesicle formation was suggested based on the inhibitory effect of dynasore, a cell-permeable small molecule that inhibits the GTPase activity of dynamin [3]. However, dynasore does not distinguish between receptor- or raft-mediated endocytosis. Here we addressed the contribution of receptor- or raft-mediated endocytosis to the entry of RPE EVs into recipient RPE cells, and identified potential ligands involved in this process.
Previously, we showed that knockdown of annexin-A2 using siRNA, prevented the uptake of stress EVs by naïve ARPE-19 cells [3], and here we documented that delivering extra annexin A2 to EV surfaces enabled the uptake of control EVs by naïve ARPE-19 cells. In cancer cells, annexin-A2 associates with cholesterol-rich lipid raft microdomains, and filipin disrupts those associations [21]. Thus, if annexin-A2 dependent uptake were mediated via lipid raft-mediated endocytosis, filipin should prevent uptake of stress EVs and prevent the drop in TER. However, both TER measurements and live-cell imaging experiments showed that EV uptake, while diminished, was not driven by this mechanism. Thus, annexin-A2-mediated endocytosis is dependent on an interaction with a yet to be identified endosomal protein receptor, as speculated by others [22]. Our results suggest lipid raft-mediated endocytosis can be excluded as an uptake mechanism based on the lack of inhibition by filipin. While it would be advantageous to test additional raft-disrupting drugs, such as methyl-β-cyclodextrin or statins, those two drugs have direct effects on tight junction integrity and barrier function [23, 24], the readout of our EV transfer assay. Thus, we further investigated receptor-mediated endocytosis. Known ligand-receptor interactions involved in clathrin-dependent endocytosis of EVs include integrins and proteoglycans.
Integrins are transmembrane receptors whose ligands include many extracellular matrix molecules, including fibronectin, vitronectin, collagen and laminin. In RPE cells, apical integrin receptors (alphavbeta5) are essential for photoreceptor outer segment phagocytosis [25]; however the involvement in EV uptake is unknown. Pretreatment with an RGD peptide, which blocks integrin receptor ligand binding sites, was shown to reduce EV uptake in some, but not all cell types [15]. Here we demonstrated RGD peptide treatment of the recipient RPE cells, in both human ARPE-19 cells and pRPE cells, prevented the uptake of stress EVs based on both functional and imaging readouts. Furthermore, the integrin ligand fibronectin was increased in stress EVs compared to control EVs.
Proteoglycans are proteins that have multiple, covalently attached, glycosaminoglycan chains. Proteoglycans can be modified with sulphated glycosaminoglycan polysaccharides [26] or sialic acids [27]. Pretreatment of cells with a heparin sulphate mimetic or heparinase to remove heparin sulphate was shown to reduce EV uptake in different cell types [15, 28]. Pretreatment of naïve recipient cells with heparinase prevented the drop in barrier function normally produced by stress EVs, as well as the rapid uptake seen in live-cell imaging ,and we identified increased levels of the proteoglycan glypican 1 in stress EVs. Interestingly, uptake via heparin sulfate proteoglycans is a mechanism commonly used by viruses [28].
A second mechanism used by viruses for cell entry is sialic acid linked to glycoproteins and gangliosides. Removal of sialic acids from cells by viral neuraminidases facilitate viral entry [17]. Alternatively, sialic acid modified proteins can serve as receptors for cell entry [29]. There are at least four mammalian neuraminidase homologues (NEU1-4) with different cellular distributions, including lysosomal, cytosolic and cellular membrane localization [30]. Here we demonstrated that neuraminidase activity was increased in stress EVs compared to control EVs. Pretreatment of naïve recipient cells with the neuraminidase inhibitor oseltamivir phosphate prevented uptake of stress EVs and prevented the drop in barrier function, further highlighting the similarity between viral particles and EVs [31]. Both are small particles (EVs: 40-150 nm, [5]; viruses: 20 and 300 nm) with a protein coat, carrying cargo (EVs: DNA, RNA, protein; viruses: DNA or RNA). And many viruses not only use the EV pathway for cell entry, but also hijack the EV pathway for cellular protein trafficking, packaging viral components into EVs for delivery to distant cells [31]. Finally, neuraminidase has been identified on the surfaces of exosomes secreted by HeLa cells based on enzyme activity assays and topology analysis, and a proteomic study identified neuraminidase in cancer EVs, suggesting neuraminidase could be involved in differential uptake of these EVs by cells [32].
Taken together, while we provide an example for a potential integrin ligand (fibronectin) and glycan (glypican 1) to be elevated in stress EVs, their role in EV uptake remains to be completely defined. However, our results support membrane proteoglycan and integrin cooperation, and neuraminidase activity in reducing the negative charge and hydrophilicity of the recipient glycocalyx as mechanisms enabling EV uptake/internalization.
Previosuly, HDAC6 packaged into EVs was identified as a mediator of the stress signal released from the apical side leading to TER loss [3]. Specifically, we showed that EVs from oxidatively stressed RPE cells contained substantial amounts of active HDAC6, and when transferred to naïve recipient RPE monolayers lead to tubulin deacetylation and impaired the integrity of tight junctions leading to reduced TER. HDAC6 inhibition by either pretreating the recipient cells or incubating the EVs with TSA prevented these effects. HDAC6 was shown to be the effector mediating this bystander effect of stress EVs generated by H2O2 treatment in both ARPE-19 and pRPE monolayer. The mechanism by which HDAC6 mediates its effect on barrier function in RPE cells was discussed previously [3]. In short, α-tubulin is a substrate of HDAC6 [33, 34], its acetylation stabilizes microtubule structure [33, 35], overexpression of HDAC6 reduces α-tubulin acetylation [36], and tight junction stability in various endothelial [37, 38] and epithelial tissues [39, 40] was shown to improve in the presence of the HDAC6 inhibitor TSA. A question that could not be asked in our previous publication was whether the amount of HDAC6 present in control EVs is sufficient to reduce tight junction stability, as the control EVs did not enter the recipient cells [3]. Physiological concentrations of neuraminidase added to the recipient cells enabled the uptake of control EVs as shown by live-cell imaging, followed by a drop in barrier function. This drop in barrier function was inhibited by TSA. Thus, while we originally speculated that stress EVs mediate their effect on barrier function by virtue of the high levels of HDAC6 they transfer to the recipient cells [3], our results here suggest the levels of HDAC6 present in control EVs is sufficient to trigger the partial disassembly of tight junctions when enabled to enter the cells.
One of the goals of this research was to identify EVs that are specific to control or stressed RPE cells as a means to monitor the health of the tissue. The results presented here demonstrates EVs from stressed cells differ from those of control cells specifically in the amount of ligands on their surface. However, the ligands identified here are not unique to RPE cells. Fibronectin, annexin-A2, and glypican 1 are common markers of EVs [15]. Fibronectin and annexin-A2 were identified in pRPE EVs by the Bowes-Rickman lab [41], whereas NEU1 has so far only been identified by proteomic analysis of human parotid gland EVs collected in human saliva [42]. Based on published DNA microarray data, ARPE-19 cells express NEU1 [43], an enzyme required in RPE cells to digest rod outer segments. Analyzing the effects of neuraminidase on lectin binding sites in photoreceptor cells of the rat, monkey and human retina identified terminal sialyl residues on rod outer segments [44-46], suggesting that neuramindase expression by RPE is not species-specific. However, whether NEU1 enrichment is unique to RPE-derived stress EVs requires further investigation.
In conclusion, as demonstrated by the results presented here, we further characterized the uptake mechanism for EVs released from stressed RPE cells by naïve RPE cells. Receptor-mediated uptake mechanisms, in particular those also used for viral entry into cells, were identified as playing a critical role in transferring the information from donor to recipient cells. Surprisingly, control EVs were shown to contain sufficient cargo to generate a bystander effect if enabled to enter the recipient cells. These findings increase our basic understanding of cell-cell communication between RPE cells, and will aid in future biomarker development characterizing the health of the RPE.
SUMMARY.
Extracellular vesicles (EVs) from stressed cells (= stress EVs) but not control cells (= control EVs) are taken up by naïve recipient cells.
Internalization of stress EVs by recipient cells requires integrin signaling and the presence of glycans as shown by imaging and functional assays.
Stress EVs contain elevated levels of an integrin ligand and a proteoglycan both known to be required for EV uptake in other cells.
Elevated levels of neuraminidase activity is associated with intact stress EVs, and pre-treatment of recipient cells with neuraminidase enables control EVs previously unable to enter the cells, to be rapidly taken up.
Elevated levels of annexin A2 is associated with stress EVs, and targeting of annexin A2 to control EVs enables singling based on functional assays
We speculate that membrane proteoglycans and integrins and/or annexin A2 cooperate during EV uptake, and neuraminidase activity on EVs reduces the negative charge and hydrophilicity of the recipient cell glycocalyx to enable EV internalization.
EV-mediated cell-cell communication might contribute to RPE dysfunction in aging and disease.
ACKNOWLEDGEMENTS
Funding for this project was provided in part by the National Institutes of Health (NIH) (R01EY019320) (BR), the Department of Veterans Affairs (RX000444, BX003050) (BR) and the South Carolina SmartState Endowment (BR), and the Office of the Assistant Secretary of Defense for Health Affairs through the Peer-Reviewed Medical Research Program Lupus Topic Area Award W81XWH-16-1-0640 (TN).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Competing Interests: The authors have no financial or non-financial competing interests to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Ishii M, Rohrer B, Bystander effects elicited by single-cell photo-oxidative blue-light stimulation in retinal pigment epithelium cell networks, Cell Death Discov, 3 (2017) 16071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ishii M, Rohrer B, Mechanisms of bystander effects in retinal pigment epithelium cell networks, Cell Death Dis, 8 (2017) e3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Shah N, Ishii M, Brandon C, Ablonczy Z, Cai J, Liu Y, Chou CJ, Rohrer B, Extracellular vesicle-mediated long-range communication in stressed retinal pigment epithelial cell monolayers, Biochim Biophys Acta, 1864 (2018) 2610–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Antwi-Baffour SS, Molecular characterisation of plasma membrane-derived vesicles, J Biomed Sci, 22 (2015) 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhang W, Ma Y, Zhang Y, Yang J, He G, Chen S, Photo-Oxidative Blue-Light Stimulation in Retinal Pigment Epithelium Cells Promotes Exosome Secretion and Increases the Activity of the NLRP3 Inflammasome, Curr Eye Res, 44 (2019) 67–75. [DOI] [PubMed] [Google Scholar]
- [6].Nanbo A, Kawanishi E, Yoshida R, Yoshiyama H, Exosomes derived from Epstein-Barr virus-infected cells are internalized via caveola-dependent endocytosis and promote phenotypic modulation in target cells, J Virol, 87 (2013) 10334–10347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tian T, Zhu YL, Zhou YY, Liang GF, Wang YY, Hu FH, Xiao ZD, Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery, J Biol Chem, 289 (2014) 22258–22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Svensson KJ, Christianson HC, Wittrup A, Bourseau-Guilmain E, Lindqvist E, Svensson LM, Morgelin M, Belting M, Exosome uptake depends on ERK1/2-heat shock protein 27 signaling and lipid Raft-mediated endocytosis negatively regulated by caveolin-1, J Biol Chem, 288 (2013) 17713–17724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feng D, Zhao WL, Ye YY, Bai XC, Liu RQ, Chang LF, Zhou Q, Sui SF, Cellular internalization of exosomes occurs through phagocytosis, Traffic, 11 (2010) 675–687. [DOI] [PubMed] [Google Scholar]
- [10].Horibe S, Tanahashi T, Kawauchi S, Murakami Y, Rikitake Y, Mechanism of recipient cell-dependent differences in exosome uptake, BMC Cancer, 18 (2018) 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Conner SD, Schmid SL, Regulated portals of entry into the cell, Nature, 422 (2003) 37–44. [DOI] [PubMed] [Google Scholar]
- [12].Thurman JM, Renner B, Kunchithapautham K, Ferreira VP, Pangburn MK, Ablonczy Z, Tomlinson S, Holers VM, Rohrer B, Oxidative stress renders retinal pigment epithelial cells susceptible to complement-mediated injury, J Biol Chem, 284 (2009) 16939–16947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rescher U, Zobiack N, Gerke V, Intact Ca(2+)-binding sites are required for targeting of annexin 1 to endosomal membranes in living HeLa cells, J Cell Sci, 113 ( Pt 22) (2000) 3931–3938. [DOI] [PubMed] [Google Scholar]
- [14].Antes TJ, Middleton RC, Luther KM, Ijichi T, Peck KA, Liu WJ, Valle J, Echavez AK, Marban E, Targeting extracellular vesicles to injured tissue using membrane cloaking and surface display, J Nanobiotechnology, 16 (2018) 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mulcahy LA, Pink RC, Carter DR, Routes and mechanisms of extracellular vesicle uptake, J Extracell Vesicles, 3 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Christianson HC, Svensson KJ, van Kuppevelt TH, Li JP, Belting M, Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity, Proc Natl Acad Sci U S A, 110 (2013) 17380–17385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Matrosovich MN, Matrosovich TY, Gray T, Roberts NA, Klenk HD, Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium, J Virol, 78 (2004) 12665–12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].McKelvey KJ, Powell KL, Ashton AW, Morris JM, McCracken SA, Exosomes: Mechanisms of Uptake, J Circ Biomark, 4 (2015) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Camussi G, Deregibus MC, Bruno S, Grange C, Fonsato V, Tetta C, Exosome/microvesicle-mediated epigenetic reprogramming of cells, Am J Cancer Res, 1 (2010) 98–110. [PMC free article] [PubMed] [Google Scholar]
- [20].Raposo G, Stoorvogel W, Extracellular vesicles: exosomes, microvesicles, and friends, J Cell Biol, 200 (2013) 373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Maji S, Chaudhary P, Akopova I, Nguyen PM, Hare RJ, Gryczynski I, Vishwanatha JK, Exosomal Annexin II Promotes Angiogenesis and Breast Cancer Metastasis, Mol Cancer Res, 15 (2017) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Grill D, Matos ALL, de Vries WC, Kudruk S, Heflik M, Dorner W, Mootz HD, Jan Ravoo B, Galla HJ, Gerke V, Bridging of membrane surfaces by annexin A2, Sci Rep, 8 (2018)14662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lambert D, O'Neill CA, Padfield PJ, Methyl-beta-cyclodextrin increases permeability of Caco-2 cell monolayers by displacing specific claudins from cholesterol rich domains associated with tight junctions, Cell Physiol Biochem, 20 (2007) 495–506. [DOI] [PubMed] [Google Scholar]
- [24].Wilkinson EL, Sidaway JE, Cross MJ, Statin regulated ERK5 stimulates tight junction formation and reduces permeability in human cardiac endothelial cells, J Cell Physiol, 233 (2018) 186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nandrot EF, Chang Y, Finnemann SC, Alphavbeta5 integrin receptors at the apical surface of the RPE: one receptor, two functions, Adv Exp Med Biol, 613 (2008) 369–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sarrazin S, Lamanna WC, Esko JD, Heparan sulfate proteoglycans, Cold Spring Harb Perspect Biol, 3 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Varki A, Biological roles of glycans, Glycobiology, 27 (2017) 3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].van Dongen HM, Masoumi N, Witwer KW, Pegtel DM, Extracellular Vesicles Exploit Viral Entry Routes for Cargo Delivery, Microbiol Mol Biol Rev, 80 (2016) 369–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Matrosovich M, Herrler G, Klenk HD, Sialic Acid Receptors of Viruses, Top Curr Chem, 367 (2015) 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Glanz VY, Myasoedova VA, Grechko AV, Orekhov AN, Sialidase activity in human pathologies, Eur J Pharmacol, 842 (2019) 345–350. [DOI] [PubMed] [Google Scholar]
- [31].Crenshaw BJ, Gu L, Sims B, Matthews QL, Exosome Biogenesis and Biological Function in Response to Viral Infections, Open Virol J, 12 (2018) 134–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Paolini L, Orizio F, Busatto S, Radeghieri A, Bresciani R, Bergese P, Monti E, Exosomes Secreted by HeLa Cells Shuttle on Their Surface the Plasma Membrane-Associated Sialidase NEU3, Biochemistry, 56 (2017) 6401–6408. [DOI] [PubMed] [Google Scholar]
- [33].Yu J, Ma Z, Shetty S, Ma M, Fu J, Selective HDAC6 inhibition prevents TNF-alpha-induced lung endothelial cell barrier disruption and endotoxin-induced pulmonary edema, Am J Physiol Lung Cell Mol Physiol, 311 (2016) L39–47. [DOI] [PubMed] [Google Scholar]
- [34].Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P, HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo, EMBO J, 22 (2003) 1168–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Asthana J, Kapoor S, Mohan R, Panda D, Inhibition of HDAC6 deacetylase activity increases its binding with microtubules and suppresses microtubule dynamic instability in MCF-7 cells, J Biol Chem, 288 (2013) 22516–22526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hubbert C, Guardiola A, Shao R, Kawaguchi Y, Ito A, Nixon A, Yoshida M, Wang XF, Yao TP, HDAC6 is a microtubule-associated deacetylase, Nature, 417 (2002) 455–458. [DOI] [PubMed] [Google Scholar]
- [37].Edelman JL, Lutz D, Castro MR, Corticosteroids inhibit VEGF-induced vascular leakage in a rabbit model of blood-retinal and blood-aqueous barrier breakdown, Exp Eye Res, 80 (2005) 249–258. [DOI] [PubMed] [Google Scholar]
- [38].Stewart MW, Anti-VEGF therapy for diabetic macular edema, Curr Diab Rep, 14 (2014) 510. [DOI] [PubMed] [Google Scholar]
- [39].Ablonczy Z, Dahrouj M, Tang PH, Liu Y, Sambamurti K, Marmorstein AD, Crosson CE, Human Retinal Pigment Epithelium Cells as Functional Models for the RPE In Vivo, Invest Ophthalmol Vis Sci, 52 (2011) 8614–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Desjardins D, Liu Y, Crosson CE, Ablonczy Z, Histone Deacetylase Inhibition Restores Retinal Pigment Epithelium Function in Hyperglycemia, PLoS One, 11 (2016) e0162596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Klingeborn M, Dismuke WM, Skiba NP, Kelly U, Stamer WD, Bowes Rickman C, Directional Exosome Proteomes Reflect Polarity-Specific Functions in Retinal Pigmented Epithelium Monolayers, Sci Rep, 7 (2017) 4901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Gonzalez-Begne M, Lu B, Han X, Hagen FK, Hand AR, Melvin JE, Yates JR, Proteomic analysis of human parotid gland exosomes by multidimensional protein identification technology (MudPIT), J Proteome Res, 8 (2009) 1304–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Sharma RK, Orr WE, Schmitt AD, Johnson DA, A functional profile of gene expression in ARPE-19 cells, BMC ophthalmology, 5 (2005) 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Uehara F, Muramatsu T, Sameshima M, Kawano K, Koide H, Ohba N, Effects of neuraminidase on lectin binding sites in photoreceptor cells of monkey retina, Jpn J Ophthalmol, 29 (1985) 54–62. [PubMed] [Google Scholar]
- [45].Kivela T, Characterization of galactose-containing glycoconjugates in the human retina: a lectin histochemical study, Curr Eye Res, 9 (1990) 1195–1209. [DOI] [PubMed] [Google Scholar]
- [46].Cohen D, Nir I, Cytochemical characterization of sialoglycoconjugates on rat photoreceptor cell surface, Invest Ophthalmol Vis Sci, 28 (1987) 640–645. [PubMed] [Google Scholar]


