Abstract
Protein interacting with C kinase-1 (PICK1) regulates intra-cellular trafficking of GluA2-containing AMPA receptors, a process known to play a critical role in cocaine-seeking behavior. This suggests that PICK1 may represent a molecular target for developing novel pharmacotherapies to treat cocaine craving-induced relapse. Emerging evidence indicates that inhibition of PICK1 attenuates the reinstatement of cocaine-seeking behavior, an animal model of relapse. Here, we show that systemic administration of TAT-P4-(DATC5)2, a novel high-affinity peptide inhibitor of the PICK1 PDZ domain, dose-dependently attenuated the reinstatement of cocaine seeking in rats at doses that did not produce operant learning deficits or suppress locomotor activity. We also show that systemic TAT-P4-(DATC5)2 penetrated the brain where it was visualized in the nucleus accumbens shell. Consistent with these effects, infusions of TAT-P4-(DATC5)2 directly into the accumbens shell reduced cocaine, but not sucrose, seeking. The effects of TAT-P4-(DATC5)2 on cocaine seeking are likely due, in part, to inhibition of PICK1 in medium spiny neurons (MSNs) of the accumbens shell as TAT-P4-(DATC5)2 was shown to accumulate in striatal neurons and bind PICK1. Taken together, these findings highlight a novel role for PICK1 in the reinstatement of cocaine seeking and support further studies examining the efficacy of peptide inhibitors of PICK1 in animal and human models of cocaine relapse.
Keywords: relapse, addiction, nucleus accumbens, self-administration, reinstatement, medium spiny neurons
1. Introduction
Protein interacting with C kinase-1 (PICK1) is highly conserved across species and expressed abundantly in the central nervous system (Xia et al., 1999; Xu and Xia, 2006). In neurons, PICK1 is localized at both presynaptic and postsynaptic sites (Haglerod et al., 2017; Haglerod et al., 2009; Perez et al., 2001). PICK1 possesses two important domains, a PDZ (PSD-95/Dlg/ZO1) domain that binds to PDZ motifs in other proteins and a membrane-binding N-BAR (Bin/amphiphysin/Rvs) domain (Herlo et al., 2018; Li et al., 2016). Through its PDZ domain, PICK1 interacts with a wide range of neurotransmitter receptors, transporters and enzymes (Erlendsson et al., 2014; Xu and Xia, 2006). PICK1 has been shown to influence the trafficking and subcellular localization of target proteins through its BAR domain (Jin et al., 2006; Lu and Ziff, 2005; Madsen et al., 2008; Steinberg et al., 2006). Based on its ability to regulate the function of proteins known to be associated with the pathophysiology of neurological and neuropsychiatric disorders, PICK1 may represent a putative molecular target for novel pharmacotherapies (Thorsen et al., 2010).
Emerging preclinical evidence indicates that PICK1 plays an important role in cocaine-induced synaptic plasticity and behavior. Disrupting PICK1 interactions with the AMPA receptor subunit GluA2 in vivo blocks synaptic plasticity and redistribution of GluA2-containing AMPA receptors in VTA dopamine neurons after a single dose of cocaine (Bellone and Luscher, 2006). Moreover, blocking PICK1-GluA2 interactions in the nucleus accumbens shell is sufficient to attenuate the reinstatement of cocaine seeking, an animal model of relapse (Famous et al., 2008). Consistent with these studies, we showed recently that administration of the small molecule PICK1 inhibitor FSC231 directly into the nucleus accumbens shell was sufficient to attenuate the reinstatement of cocaine seeking (Schmidt et al., 2013). Finally, we found that cocaine-induced hyperlocomotion and cocaine self-administration were attenuated in mutant mice lacking PICK1(Jensen et al., 2018). Together, these findings indicate that neuronal PICK1 coordinates cocaine-induced molecular and behavioral plasticity.
FSC231 selectively binds the PDZ domain of PICK1 with a Ki=10.1 μM (Thorsen et al., 2010). Recently, high affinity bivalent peptide inhibitors have been developed. For example, Tat-NPEG4(IEDTV)(2) is a high affinity, dimeric peptide inhibitor of PSD95 tandem PDZ domains with a Ki=4.6 nM (Bach et al., 2012). In addition to being a more potent inhibitor of PSD95 than the monomeric clinical candidate peptide NA-1 (Ballarin and Tymianski, 2018), Tat-NPEG4(IEDTV)(2) is also >100-fold more stable in human plasma indicating that it is not quickly degraded by proteases (Bach et al., 2012). Moreover, TAT11-N-NR2B has improved penetrance of the blood-brain barrier and cell membranes, a property imparted by the HIV-1 TAT portion of the peptide (Bach et al., 2012; Schwarze et al., 1999). Taking a similar approach, we recently developed a high affinity PICK1 PDZ domain inhibitor, TAT-P4-(DATC5)2 (Ki=1.7 nM), which incorporates the best-known PICK PDZ domain ligand, dopamine transporter C-terminus (DATC5), in an analogous bivalent configuration (Erlendsson et al., 2014). Based on its increased affinity and favorable pharmacokinetic profile, TAT-P4-(DATC5)2 may have significantly greater translational potential as a therapeutic compared to FSC231.
The present study had four main goals: 1) to assess the efficacy of systemic injections of TAT-P4-(DATC5)2 to attenuate the reinstatement of cocaine seeking; 2) to determine if systemic TAT-P4-(DATC5)2 crosses the blood brain barrier and localizes in the ventral striatum; 3) to investigate whether TAT-P4-(DATC5)2 accumulates in striatal neurons and binds PICK1; and 4) to characterize the effects of intra-accumbens shell infusions of TAT-P4-(DATC5)2 on cocaine-seeking behavior. Our findings support the hypothesis that inhibition of PICK1 is sufficient to attenuate cocaine priming-induced reinstatement of drug-seeking behavior. Moreover, we identify systemic doses of the PICK1 inhibitor TAT-P4-(DATC5)2 that significantly reduce drug seeking and do not produce operant learning deficits or suppress locomotor activity. These results highlight an important role for PICK1 in cocaine-seeking behavior and support further studies of PICK1 inhibitors in reducing drug-mediated behaviors including cocaine craving-induced relapse.
2. Materials and Methods
2.1. Drugs
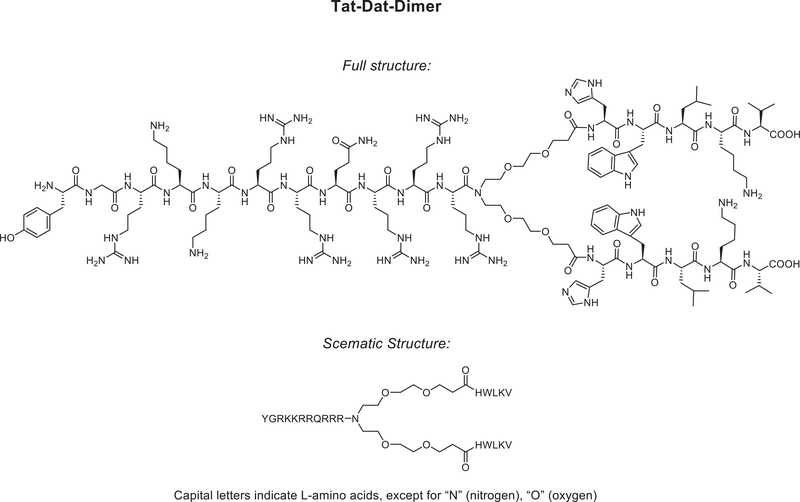
Cocaine was obtained from the National Institute on Drug Abuse (Rockville, MD) and dissolved in bacteriostatic 0.9% saline. The novel PICK1 peptide inhibitor TAT-P4-(DATC5)2 (Figure 1) and the fluorescently labeled peptide inhibitor (carboxytetramethylrhodamine (TAMRA)-conjugated TAT-P4-(DATC5)2) were purchased from Wuxi AppTec Co., Ltd China. The control peptide TAMRA-C5 corresponds to the conjugation of the TAMRA fluorophore to the distal C5 residues (HWLKV) of the dopamine transporter (DAT) and was purchased from TAG-Copenhagen, Denmark. TAT-P4-(DATC5)2, TAMRA-conjugated TAT-P4-(DATC5)2 and TAMRA-C5 were dissolved in bacteriostatic 0.9% saline. The doses and time course of PICK1 peptide inhibitor administration were based on previous in vivo rodent studies of analogous compounds showing that systemically administered TAT11-N-DATC5 and TAMRA-conjugated TAT11-N-DATC5 readily cross the blood-brain barrier, accumulate in neurons and produce behavioral responses (Bach et al., 2012; Kucharz et al., 2017).
Figure 1.
Structure of the dimeric PICK1 peptide inhibitor TAT-P4-(DATC5)2.
2.2. Animals and Housing
Male Sprague-Dawley rats (Rattus norvegicus) weighing 250–300 g were obtained from Taconic Laboratories (Germantown, NY, USA). Rats were housed individually with food and water available ad libitum in their home cage, except as indicated. A 12:12 h light/dark cycle was used with the lights on at 0700 h. All experimental procedures were performed during the light cycle. The experimental protocols were consistent with the guidelines issued by the US National Institutes of Health and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
2.3. Materials
All self-administration experiments were conducted in ventilated, sound attenuating operant conditioning chambers purchased from Med-Associates Inc. (East Fairfield, VT, USA). Each operant chamber was equipped with both active and inactive response levers, a sucrose pellet dispenser, cue lights, tone generator, as well as an automated injection pump for administering drug or vehicle solutions intravenously. Open field locomotor activity experiments were performed with Photobeam Activity Systems (PAS)-Open Field systems from San Diego Instruments (San Diego, CA, USA). Locomotor activity monitoring chambers (17” l × 17” w × 15” h) were surrounded by a photobeam grid with each beam separated by 1”. All beam interruptions were timed stamped per x, y coordinates and sent to a computer for further analysis.
2.4. Surgery
Rats were handled daily and allowed one week to acclimate to their home cages upon arrival. Rats were anesthetized using a mixture of 80 mg/kg ketamine (Midwest Veterinary Supply, Valley Forge, PA, USA) and 12 mg/kg xylazine (Sigma Aldrich/RBI, St. Louis, MO, USA). An indwelling catheter (SAI Infusion Technologies, Lake Villa, IL, USA) was inserted into the right jugular vein and sutured in place. The catheter was routed to a mesh backmount platform that was implanted subcutaneously dorsal to the shoulder blades. To prevent infection and maintain patency, catheters were flushed daily with 0.2 ml of the antibiotic Timentin (0.93 mg/ml; Fisher, Pittsburgh, PA) dissolved in heparinized 0.9% saline (Butler Schein, Dublin, OH). When not in use, catheters were sealed with plastic obturators.
After catheter insertion, some rats were immediately mounted in a stereotaxic apparatus (Kopf Instruments, CA) and implanted with cannulae for intra-cranial microinjections. Bilateral stainless-steel guide cannulas (14 mm, 26 gauge; Plastics One, Roanoke, VA) were implanted 2 mm dorsal to the nucleus accumbens shell and cemented in place by affixing dental acrylic to stainless steel screws secured in the skull. The coordinates for the ventral ends of the guide cannulae, relative to bregma, according to the atlas of Paxinos and Watson (1997), were as follows: +1.0 mm A/P, ±1.0 mm M/L, −5.0 mm D/V. An obturator (14 mm, 33 gauge; Plastics One) was inserted into each guide cannula to prevent occlusion.
2.5. Cocaine self-administration, extinction and reinstatement of cocaine seeking
Rats were allowed 7 days to recover from surgery before behavioral testing commenced. Initially, rats were placed in operant conditioning chambers and allowed to lever-press for intravenous infusions of cocaine (0.25 mg cocaine/59 μl saline, infused over a 5 s period) on a fixed-ratio 1 (FR1) schedule of reinforcement. Rats were allowed to self-administer a maximum of 30 injections per 120 min operant session. Once a rat achieved at least 20 infusions of cocaine in a single daily operant session under the FR1 schedule, the subject was switched to a fixed-ratio 5 (FR5) schedule of reinforcement. The maximum number of injections was again limited to 30 per daily self-administration session under the FR5 schedule. For both FR1 and FR5 schedules, a 20 s time-out period followed each cocaine infusion, during which time active lever responses were tabulated but had no scheduled consequences. Responses made on the inactive lever, which had no scheduled consequences, were also recorded during both the FR1 and FR5 training sessions. Following 21 days of daily cocaine self-administration sessions, drug-taking behavior was extinguished by replacing the cocaine solution with 0.9% saline. Daily extinction sessions continued until responding on the active lever was <15% of the total active lever responses completed on the last day of cocaine self-administration. Typically, it took 5–7 days for rats to meet this criterion. Once cocaine self-administration was extinguished, rats entered the reinstatement phase of the experiment. During reinstatement test sessions, satisfaction of the response requirement (i.e., five presses on the active lever) resulted in an infusion of saline rather than cocaine. Using a between-sessions reinstatement paradigm, each reinstatement test session was followed by extinction sessions until responding was again <15% of the total active lever responses completed on the last day of cocaine self-administration. Generally, 1–2 days of extinction were necessary to reach extinction criterion between reinstatement test sessions. Total active lever responses (mean±SEM) completed on the last day of cocaine self-administration and the last day of extinction prior to subsequent reinstatement tests in which rats were pretreated with systemic TAT-P4-(DATC5)2 were 128.35±6.07 and 13.91±1.11, respectively. Total active lever responses (mean±SEM) completed on the last day of cocaine self-administration and the last day of extinction prior to subsequent reinstatement tests in which rats were pretreated with intra-accumbens shell TAT-P4-(DATC5)2 were 118.0±8.01 and 9.67±0.75, respectively.
During the reinstatement phase, rats were infused intravenously through the jugular catheter with vehicle and TAT-P4-(DATC5)2 (0.3 and 3.0 nmol/g) 45 min prior to an acute injection of cocaine (10 mg/kg, i.p.). Rats were then placed immediately into the operant conditioning chambers and a two-hour reinstatement session commenced. The effects of intra-accumbens shell infusions of TAT-P4-(DATC5)2 on cocaine priming-induced reinstatement of drug-seeking behavior were studied in separate cohorts of rats. TAT-P4-(DATC5)2 (0.3 and 3.0 pmol) and vehicle were microinjected directly into the nucleus accumbens shell 10 min prior to a priming injection of cocaine (10 mg/kg, i.p.). Bilateral infusions into the accumbens shell were performed in a total volume of 500 nl over 2 minutes. Following infusion, microinjectors were left in place for one additional minute to allow for diffusion of the drug solution away from the tips of the microinjectors. A within-subjects design, in which each rat served as its own control, was used for all experiments. To control for potential rank order effects of drug and vehicle administrations, all treatments were counterbalanced across reinstatement test sessions.
Cannula placements were verified according to previously described protocols (Schmidt et al., 2009; Schmidt et al., 2016). Briefly, after completion of all intra-cranial microinjection experiments, rats were given an overdose of pentobarbital (100 mg/kg i.p.). Brains were removed and drop fixed in 10% formalin. Coronal sections (100 μm) were taken at the level of the striatum with a vibratome and mounted on gelatin-coated slides. An individual blinded to behavioral responses verified microinjection sites using light microscopy. Rats with cannula placements outside of the accumbens shell and/or excessive mechanical damage were excluded from subsequent data analyses.
2.6. Sucrose self-administration, extinction and reinstatement of sucrose seeking
Potential nonspecific rate-suppressing effects and operant learning deficits of intra-accumbens shell TAT-P4-(DATC5)2 were evaluated by assessing the influence of TAT-P4-(DATC5)2 on the reinstatement of sucrose-seeking behavior. Separate cohorts of rats were trained initially to self-administer 45 mg sucrose pellets (Research Diets, New Brunswick, NJ) on a FR1 schedule of reinforcement during daily one-hour operant sessions. Once rats achieved stable responding for sucrose (defined as <20% variation in responding over 3 consecutive days) on the FR1 schedule of reinforcement, the response requirement was increased to an FR5 schedule of reinforcement. Rats were limited to 30 sucrose pellets within each daily operant session and were restricted to ~25 g of lab chow (Harlan Teklad, Wilmington, DE) daily in their home cages for the duration of the experiment. Water was available ad libitum in the home cage.
After two weeks of sucrose-maintained responding on a FR5 schedule of reinforcement, rats underwent an extinction phase where active lever pressing no longer resulted in sucrose delivery. Once active lever responding decreased to <15% of the maximum number of responses completed on the last day of sucrose self-administration, rats proceeded to reinstatement testing. Total active lever responses (mean±SEM) completed on the last day of sucrose self-administration and the last day of extinction prior to subsequent reinstatement tests in which rats were pretreated with systemic TAT-P4-(DATC5)2 were 168.1±17.93 and 20.71±3.21, respectively. Total active lever responses (mean±SEM) completed on the last day of sucrose self-administration and the last day of extinction prior to subsequent reinstatement tests in which rats were pretreated with intra-accumbens shell TAT-P4-(DATC5)2 were 159.4±14.17 and 16.86±3.32, respectively. To determine the effects of systemic TAT-P4-(DATC5)2 on sucrose seeking, rats were pretreated with vehicle and 3.0 nmol/g TAT-P4-(DATC5)2 (i.v.) 45 min prior to sucrose reinstatement test sessions. Separate groups of rats were used to study the effects of intra-accumbens shell infusions of TAT-P4-(DATC5)2 on sucrose seeking. Vehicle and TAT-P4-(DATC5)2 (0.3 and 3.0 pmol) were microinjected bilaterally into the accumbens shell 10 min prior to the beginning of the reinstatement test sessions. A within-subjects design was used for all sucrose studies with each rat serving as its own control. Doses were counterbalanced across test sessions. The experimenter remotely administered one sucrose pellet every two min for the first 10 min of each reinstatement test session. A between-session paradigm was used so that each daily reinstatement session was followed by an extinction session the following day until responding was again <15% of the total active lever responses maintained by sucrose.
2.7. Locomotor Testing
The effects of systemic TAT-P4-(DATC5)2 on locomotor activity were evaluated in cocaine-experienced rats whose drug-taking behavior had been extinguished. Rats were habituated to the locomotor testing chambers for 1 hr daily over three consecutive days. On subsequent testing days, rats received intravenous infusions of vehicle and 3.0 nmol/g TAT-P4-(DATC5)2 min prior to an acute injection of cocaine (10 mg/kg, i.p.). Using a within-subjects design, each animal served as its own control and doses were counterbalanced across test sessions. Spontaneous activity in the x-y plane was recorded for 1 hr post injection. Photobeam interruptions/breaks were quantified over 10 min intervals and used as a measurement of locomotor activity (San Diego Instruments Photobeam Activity System).
2.8. Immunohistochemistry and Blood Brain Barrier Permeability
Immunohistochemical analyses were performed to determine if systemic TAT-P4-(DATC5)2 penetrated the brain. Using a between-subjects design, rats were treated acutely with 3.0 nmol/g TAMRA-conjugated TAT-P4-(DATC5)2 (i.v.) once cocaine self-administration had been extinguished. Rats were then deeply anesthetized and transcardially perfused with 0.1 M PBS (pH 7.4) followed by 4% formalin in 0.1 M PBS 15, 45 or 90 minutes post infusion of TAMRA-conjugated TAT-P4-(DATC5)2. Brains were then removed, postfixed overnight in 4% formalin and then cryoprotected in 20% sucrose in 0.1 M PBS at 4°C for three days. Coronal sections (30 μm) were taken at the level of the striatum using a cryostat (Leica 3050S; Leica Corp., Deerfield, IL). Brain sections were stored in 0.1 M PBS at 4°C until processed.
Immunohistochemistry was performed on free-floating coronal sections containing the nucleus accumbens according to modified procedures from previously published studies (Hernandez et al., 2018; Schmidt et al., 2016). Briefly, sections were washed with 1% sodium borohydride followed by 0.1 M PBS. Sections were then blocked in 0.1 M PBS containing 5% normal donkey serum and 0.2% Triton-X for 1 hour at room temperature. Sections were incubated in primary antibodies overnight, and then, following a PBS rinse, they were incubated in secondary antibodies for 2 hours. The primary antibodies used were rabbit anti-NeuN (1:1000; ab177487, Abcam, Cambridge, UK) and goat anti-GFAP (1:1000; ab53554, Abcam). The secondary antibodies used were donkey anti-rabbit Alexa Fluor 488 (1:500) and donkey anti-goat Alexa Fluor 647 (1:500) from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA, USA). Sections were washed and mounted onto glass slides and coverslipped using Vectashield with DAPI (Vector Laboratories, Burlingame, CA, USA). Sections were visualized with a Leica SP5 X confocal microscope using the 20× and 63× oil immersion objectives. Image z-stacks with the 63× oil immersion were collected with a step size of 1 μm, while 2–3x optical zoom z-stack images using the same objective were collected with a step size of 0.5 μm. No evidence of astrogliosis was noted between rats treated with TAT-P4-(DATC5)2 and vehicle-treated controls.
2.9. Neonatal rat striatal culture preparation and transduction
Brains from prenatal E19 rats were dissected and placed in ice-cold dissection media (HBSS, Gibco) supplemented with 30 mM glucose, 10 mM HEPES (pH 7.4, Gibco), 1 mM sodium pyruvate (Gibco), 100 U/ml penicillin and 10 mg/ml streptomycin (Sigma-Aldrich) and cut in half by sagittal incision. Using a dissection microscope, the striatal compartment was punched out using a Pasteur pipet followed by removal of the cortex using a scalpel. The striatum was treated with papain (Worthington) at 37°C for 20 minutes, triturated 5 times with two different diameter fire-polished Pasteur pipettes and filtered through a 70 μm cell strainer to remove cell debris. The cells were seeded on acid treated poly-L-lysine coated 15 mm coverslips at a density of 130,000 cells/well in Neurobasal media (Gibco 21103–049) supplemented with 5% FBS (Gibco 26140079), 2% B27 supplement (Gibco 17504–044), 1:1000 Glutamax (Gibco 35050–038), 25 μM glutamate, and 100 U/mL penicillin streptomycin. After 24 h from the seeding, the growth media was replaced with Neurobasal media supplemented only with 2% B27, 1:1000 Glutamax and 100 U/ml penicillin streptomycin for up to 12–14 days in vitro (DIV). The media was changed every 4 days by replenishing one half of the media with fresh growth media. Nine days after the transfection procedure 5-fluor-2′-deoxyuridine (1:4000 FdU, Sigma) was added to the serum and glutamate free media.
For knockdown and replacement studies of PICK1, striatal neurons were transduced at 14 DIV with three different lentiviruses: FUGWH1sh18GFPPICK1 (GFP-PICK1WT), FUGWH1sh18eGFP (GFP-sh18) or FUGWH1sh18deleGFP (GFP). The replacement plasmid GFP-PICK1WT was kindly provided by Dr. Robert C. Malenka, Stanford University School of Medicine (Citri et al., 2010). Vectors were driven by the dual promoter FUGW with the H1 promoter driving the expression of the small hairpin (ShPICK1) targeting the PICK1 sequence (CTATGAGTACCGCCTTATCCT) and the ubiquitin promoter driving the expression of GFP (PICK1 KD)(Citri et al., 2010). In the control vector (GFP), the sequence of the ShPICK1 was removed before the PCR product of the deleted ShPICK1 fragment and the PICK1 KD vector was digested and ligated together using AfeI and BamHI. Removal of ShPICK1 was achieved by synthesis of a 500 bp PCR fragment using the primers AGTAACGGATCCTTTTTCTAGCCCCAAGGGCG and TCGCCGAGAAGGGACTACTTTTCCTCGCCTG and the original PICK1 KD construct as template. Consequently, the control GFP vector only drives the expression of GFP under the ubiquitin promoter (Holst et al., 2013). This plasmid expresses a short hairpin (sh18) that targets endogenous PICK1 and a resistant eGFP-tagged PICK1. The GFP control vector (GFP) was created by removing the short hairpin (Holst et al., 2013). Lentiviruses were produced as described previously (Jensen et al., 2018).
2.10. Immunocytochemistry
At 20–22 DIV striatal neurons were incubated with 5 nM of TAMRA-conjugated TAT-P4-(DATC5)2 for 1 hr at 37°C, rinsed 3 times in PBS and fixed in 4% PFA + 4% sucrose for 20 minutes (10 min on ice and 10 min at room temperature). Neurons were rinsed in PBS and blocked in 0.05% Triton-X100 with 5% goat serum for 20 min at room temperature. Subsequently, neurons were labelled with rabbit anti-DARRP32 (1:800, Cell Signalling #2306) and chicken anti-GFP (1:2000, Abcam ab13970) for 1 hr at room temperature before incubation with goat anti-rabbit Alexa-647 (1:500, ab32245, Abcam) and goat anti-chicken Alexa-488 secondary antibodies (1:500, ab150173, Abcam). After three final washes with PBS the coverslips were mounted by using Prolong Gold Antifade™ mounting medium (Life Science Technologies).
For cell penetration studies, 14 DIV striatal neurons were treated with 5 μM of TAMRA-conjugated TAT-P4-(DATC5)2 for 1 hr at 37°C, rinsed 3 times in PBS and incubated with 5 μM of the membrane dye DiO (Thermo Fisher, D275) for 10 min at room temperature. After 3 additional washes in PBS, the striatal neurons were fixed in 4% PFA + 4% sucrose for 10 min at room temperature. Following a brief wash in PBS, the coverslips were mounted by using Prolong Gold Antifade™ mounting medium (Life Science Technologies).
2.11. Confocal microscopy
Permeability images were acquired using a Zeiss LSM 510 confocal laser-scanning microscopy, equipped with a 63x oil immersion objective, numerical aperture 1.4, (Zeiss). A 488 nm laser was used to excite the DiO dye, and emission was detected using a 505–550nm bandpass filter. The TAMRA moiety was excited using a 543nm helium-neon laser and emission was detected using a 560–615nm bandpass filter. Images were acquired at 8-bit 1024 × 1024 pixels, with approximately 10 images z-stack with 8 line averaging scans. Images were processed using ImageJ software.
Images for quantification of peptide were acquired with a Zeiss LSM 710 laser-scanning microscopy equipped with oil immersion objective (numerical aperture 1.4, magnification 63x from Zeiss). For excitation of green dyes a 488nm Argon laser was used (emission/detection wavelength 493–551nm), red dyes were excited using a 561nm Solid State laser (emission/detection wavelength 563–660nm) and far-red fluorophores were excited using a 633nm HeNe laser (emission/detection wavelength 638–755nm). Furthermore, the main beam splitter used was MBS 488/561/633. Images were acquired at 16-bit 1584 × 1584 pixels with 4 line averaging scans. All images were processed using ImageJ software. Quantification of the TAMRA-conjugated TAT-P4-(DATC5)2 intensity was performed by defining each neuron as a region of interest. The mean intensity values obtained from each neuron were subsequently normalized to the average of the intensity values of the GFP-induced neurons.
2.12. Experimental Design and Statistical Analyses
For behavioral experiments, rats underwent cocaine or sucrose self-administration/extinction/reinstatement as described above. For all cocaine and sucrose reinstatement experiments, the total mean active and inactive lever responses were analyzed with two-way repeated measures (RM) ANOVAs. Locomotor data were analyzed using a separate mixed-design ANOVA test to account for the within-subjects design of the experiment while testing for between-subjects effects of drug treatment(s). Pairwise analyses were made using Bonferroni post hoc tests (p<0.05). For in vitro assessment of target engagement, the intensity of fluorescently labeled peptide retained in medium spiny neurons was assessed by one-way ANOVA with Tukey’s multiple comparisons test.
3. Results
3.1. Systemic administration of the novel PICK1 inhibitor TAT-P4-(DATC5)2 dose-dependently attenuates cocaine, but not sucrose, seeking in rats
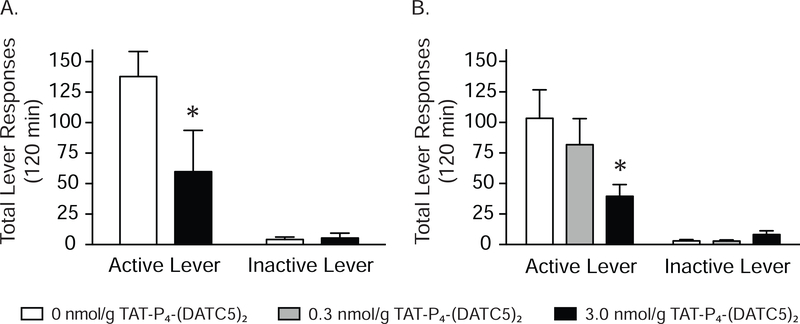
Total lever responses (mean ± S.E.M.) for rats (n=5/treatment) pretreated intravenously with vehicle and 3.0 nmol/g TAT-P4-(DATC5)2 (Figure 1) prior to a cocaine priming-induced reinstatement test session are shown in Figure 2A. These data were analyzed with a two-way RM ANOVA, which revealed significant main effects of treatment [F(1,8)=6.119, p<0.05] and lever [F(1,8)=20.46, p<0.001] as well as a significant interaction between lever and treatment [F(1,8)=6.488, p<0.05]. Subsequent post-hoc analyses identified significant differences in responding on the active lever between rats pretreated with vehicle and 3.0 nmol/g TAT-P4-(DATC5)2 (Bonferroni, p<0.05). The dose-dependent effects of TAT-P4-(DATC5)2 were tested in a separate cohort of rats (n=6/treatment). Total lever responses for rats pretreated with TAT-P4-(DATC5)2 (0, 0.3 and 3.0 nmol/g, i.v.) prior to a cocaine priming-induced reinstatement test session are shown in Figure 2B. A two-way RM ANOVA revealed significant main effects of treatment [F(1,10)=20.80, p<0.01] and lever [F(2,10)=4.456, p<0.05] as well as a significant interaction between lever and treatment [F(2,20)=6.319, p<0.01]. Subsequent post-hoc analyses indicated that active lever responses were significantly different only between vehicle and 3.0 nmol/g TAT-P4-(DATC5)2 (Bonferroni, p<0.05). No significant effects of drug treatment were found on inactive lever responding in either experiment.
Figure 2.
Systemic administration of TAT-P4-(DATC5)2 dose-dependently attenuates cocaine priming-induced reinstatement of drug-seeking behavior. (A) Cocaine seeking was significantly decreased in rats pretreated with 3.0 nmol/g TAT-P4-(DATC5)2 (i.v.) compared to vehicle-treated controls (n=5/treatment). (B) Dose-dependent effects of TAT-P4-(DATC5)2 were tested in a separate cohort of rats (n=6/treatment). Cocaine seeking was significantly reduced in rats pretreated with 3.0 nmol/g TAT-P4-(DATC5)2 (i.v.) compared to vehicle-treated controls and rats pretreated with 0.3 nmol/g TAT-P4-(DATC5)2. No effects of TAT-P4-(DATC5)2 on inactive lever responding were found in either experiment. *p<0.05, Bonferroni
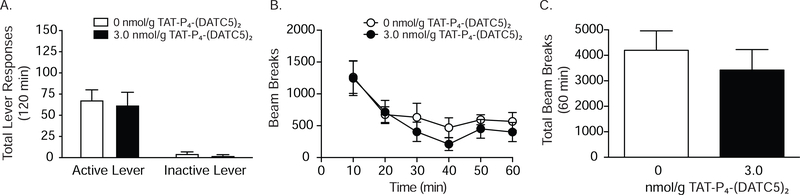
While systemic administration of TAT-P4-(DATC5)2 did not affect inactive lever responding, one could argue that responses on the inactive lever were too low to assess the potential rate-suppressing effects of systemic TAT-P4-(DATC5)2 administration. Therefore, reinstatement of sucrose seeking was assessed in a separate cohort of rats (n=7/treatment) pretreated with the behaviorally relevant dose of TAT-P4-(DATC5)2 (3.0 nmol/g) shown in Figure 2 to attenuate cocaine seeking. No effects of TAT-P4-(DATC5)2 were found on sucrose seeking (Figure 3A) indicating that TAT-P4-(DATC5)2 attenuates cocaine seeking and that these effects are not due to deficits in operant responding.
Figure 3.
Systemic administration of TAT-P4-(DATC5)2 does not affect sucrose seeking or locomotor activity in rats. (A) There were no effects of 3.0 nmol/g TAT-P4-(DATC5)2 (i.v.) on the reinstatement of sucrose-seeking behavior (n=7/treatment). Moreover, systemic TAT-P4-(DATC5)2 did not affect locomotor activity in cocaine-experienced rats (B & C) (n=5/treatment).
3.2. Systemic administration of the novel PICK1 inhibitor TAT-P4-(DATC5)2 does not affect locomotor activity in cocaine-experienced rats
To verify that systemic infusions of TAT-P4-(DATC5)2 did not produce general motor impairments, we assessed the locomotor activity of cocaine-experienced rats treated with the PICK1 inhibitor. Total beam breaks (mean ± SEM) for each 10 min interval of the 60 min test session and the entire test session are shown in Figures 3B and 3C, respectively, for rats (n=5/treatment) pretreated with systemic TAT-P4-(DATC5)2 or vehicle. There were no significant effects of drug treatment on locomotor activity. Taken together, these studies identify a systemic dose of TAT-P4-(DATC5)2 (i.e., 3.0 nmol/g) that reduces cocaine seeking and does not produce motor suppressant effects or operant learning deficits.
3.3. TAMRA-conjugated TAT-P4-(DATC5)2 administered peripherally penetrates the brain and is visualized in the nucleus accumbens shell
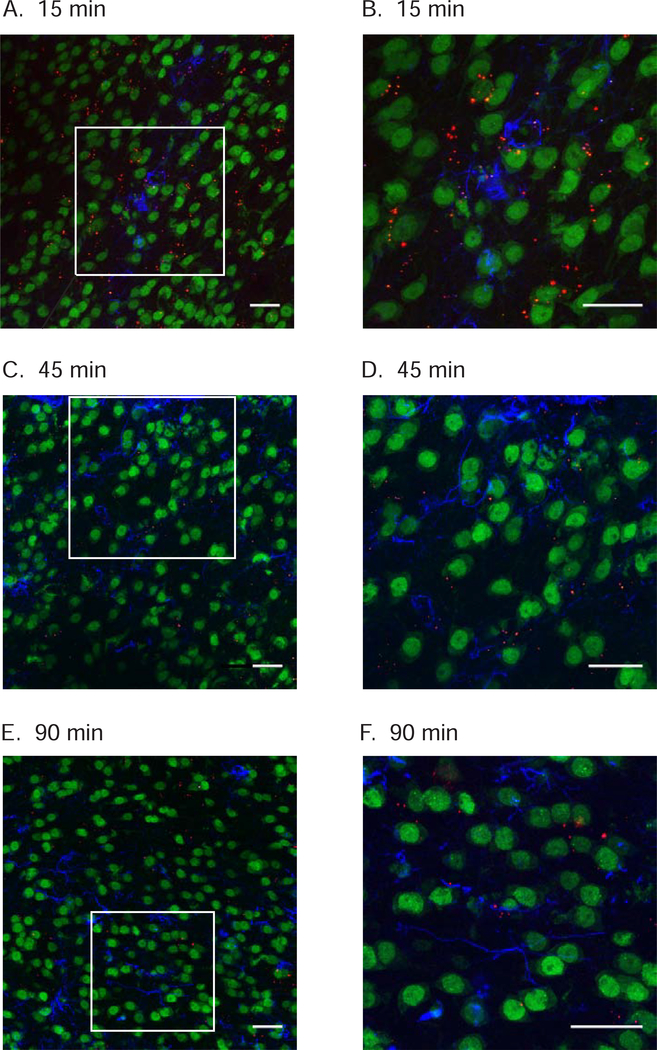
To determine if systemically administered TAT-P4-(DATC5)2 distributes to the brain, rats were pretreated with systemic TAMRA-conjugated TAT-P4-(DATC5)2 and sacrificed 15, 45 or 90 minutes post infusion. Confocal microscopy revealed TAMRA-conjugated TAT-P4-(DATC5)2 located in proximity to GFAP-positive astrocytes and NeuN-positive neurons in the nucleus accumbens shell at all time points shown in Figure 4. Overall, these data revealed that systemically administered TAMRA-conjugated TAT-P4-(DATC5)2 crosses the blood brain barrier and is visualized in the accumbens shell at time points that coincide with reduced cocaine-seeking behavior shown in Figure 2. These results suggest that the suppressive effects of systemic TAT-P4-(DATC5)2 on the reinstatement of cocaine seeking may be due, in part, to inhibition of PICK1 in the nucleus accumbens shell.
Figure 4.
TAMRA-conjugated TAT-P4-(DATC5)2 administered systemically crosses the blood brain barrier and is visualized in the nucleus accumbens shell. Separate cocaine-experienced rats were injected with TAMRA-conjugated TAT-P4-(DATC5)2 (3.0 nmol/g, i.v.) during extinction. Representative confocal images reveal TAMRA-conjugated TAT-P4-(DATC5)2 (red fluorescence) located in proximity to neurons labeled with NeuN (green fluorescence) and astrocytes labeled with GFAP (blue fluorescence) in the nucleus accumbens shell 15 (A), 30 (C) and 90 (E) minutes post infusion. Images shown in B, D & F are magnified inserts shown in A, C & E, respectively. Images are compressed z-stacks with a 0.5 μm step size (scale bar: 25 μm).
3.4. TAMRA-conjugated TAT-P4-(DATC5)2 penetrates striatal neurons in culture and is retained by PICK1
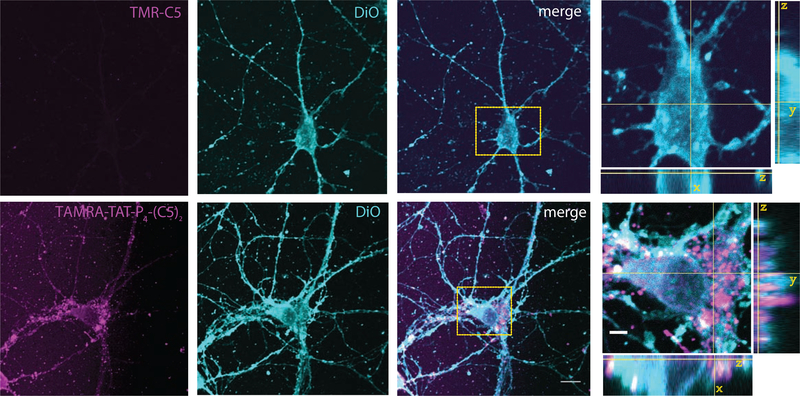
To directly address the cell membrane permeability of TAT-P4-(DATC5)2, we conjugated TAMRA to the N-terminus of the TAT sequence. Striatal neurons were treated for 1 hr with either TAT-P4-(DATC5)2 or the C5 alone (5 μM), both conjugated to TAMRA for visualization, followed by DiO labeling of the plasma membrane. As expected, the TAMRA-C5 control without TAT did not give any fluorescent signal (Figure 5, top panel), whereas TAMRA-conjugated TAT-P4-(DATC5)2 demonstrated a strong fluorescent labeling of the neurons (Figure 5, bottom panel). The peptide overall displayed a punctate localization with prominent localization to the somatic region, but also distinct punctate localization in neurite outgrowths. Most punctate somatic localizations of the peptide (magenta) further appeared enclosed by the DiO signal (cyan), which outline the membrane.
Figure 5.
TAMRA-conjugated TAT-P4-(DATC5)2 penetrates cultured striatal medium spiny neurons. Representative confocal images of live striatal neurons showing the penetration of 5 μM TAMRA-C5 (magenta, top) or TAMRA-conjugated TAT-P4-(DATC5)2 (magenta, bottom) after 1 hr incubation at 37°C in conditioned media. The cell membrane is stained with DiO (light blue). The comparison between the control peptide TAMRA-C5 and TAMRA-conjugated TAT-P4-(DATC5)2 demonstrates the penetration capacity of the second compound over the first one. Scale bar: 10 μm and 3 μm on the orthogonal views zoom.
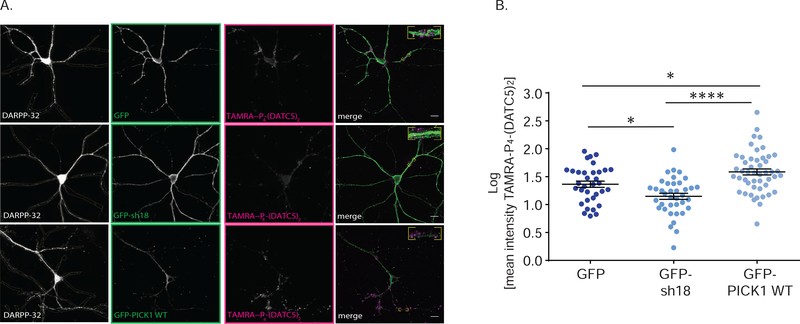
Next, we addressed whether the accumulation of peptide in medium spiny neurons (MSNs) was related to the expression of PICK1. In MSNs with shRNA-mediated knockdown of PICK1, identified by concomitant GFP expression (GFP-sh18) (Citri et al., 2010), the TAMRA-conjugated TAT-P4-(DATC5)2 signal in DARPP-32 positive neurons was significantly reduced compared to neurons expressing GFP without shRNA (GFP) (Figure 6). In contrast, shRNA-mediated knockdown and concomitant overexpression of shRNA insensitive GFP-PICK1 (GFP-PICK1) significantly increased the TAMRA-conjugated TAT-P4-(DATC5)2 signal compared to control (GFP) (Figure 6). Taken together, these experiments suggest that TAT-P4-(DATC5)2 penetrates the plasma membrane and its accumulation in MSNs is related to expression of the target PICK1.
Figure 6.
Altered expression of PICK1 in cultured striatal medium spiny neurons dynamically affects intracellular accumulation of TAMRA-conjugated TAT-P4-(DATC5)2 (5nM). (A) Representative confocal images of striatal medium spiny neurons identified by dopamine and cAMP-regulated neuronal phosphoprotein (DARPP-32) marker. Medium spiny neurons were transduced with the control viral vector encoding GFP (top), the short hairpin 18 silencing PICK1 (GFP-sh18, middle) coupled to GFP expression and the over-expressing vector encoding sh18 as well as shRNA-resistant GFP-PICK1 (WT, bottom). Images show: DARPP-32 in grey, GFP, GFP-sh18, GFP-PICK1WT in green, and TAMRA-conjugated TAT-P4-(DATC5)2 in magenta, as well as the three images merged. Scale bar: 10μm. (B) Quantification of TAMRA-conjugated TAT-P4-(DATC5)2 intensity values of each viral-transduced neuron from (A) normalized to the mean TAMRA-conjugated TAT-P4-(DATC5)2 intensity of the GFP control viral vector. Each dot represents a single neuron, (GFP n =34, GFP-sh18 n=38, GFP-PICK1 WT n=48, all data expressed as means ± S.E.M.). A significant reduction in the TAMRA-conjugated TAT-P4-(DATC5)2 intensity is observed by silencing PICK1 (GFP-sh18) versus both GFP control and GFP-PICK1 WT overexpressing neurons (one way ANOVA with Tukey’s multiple comparisons test * p< 0.05, **** p< 0.0001).
3.5. Administration of TAT-P4-(DATC5)2 directly into the nucleus accumbens shell dose-dependently attenuates cocaine, but not sucrose, seeking in rats
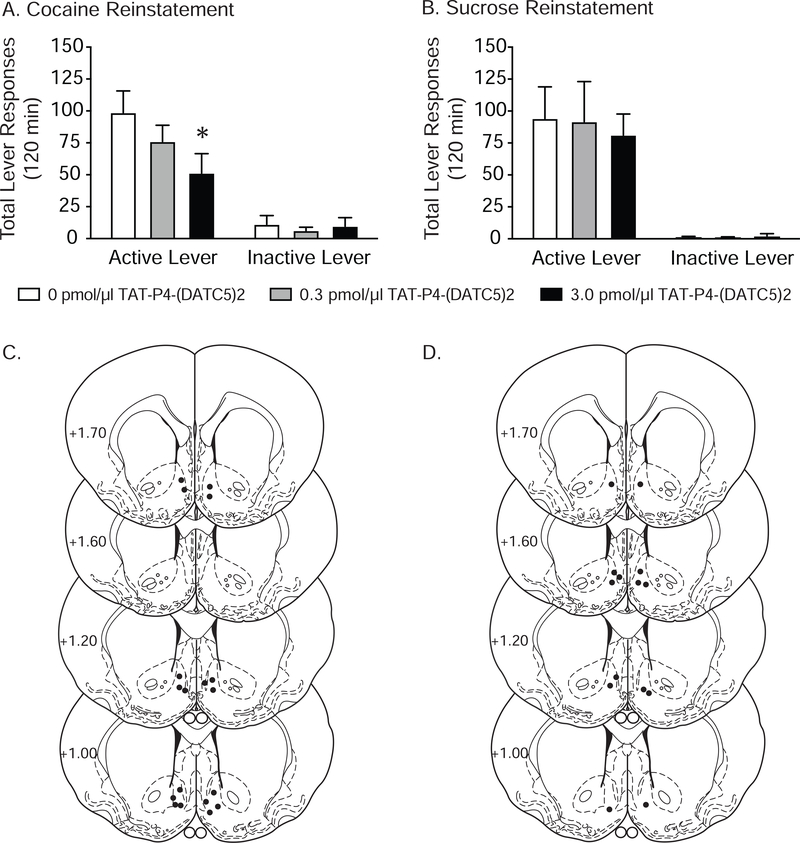
Having shown that systemic TAT-P4-(DATC5)2 penetrates the brain and is visualized in the accumbens shell, we next investigated whether infusing TAT-P4-(DATC5)2 locally into the accumbens shell would be sufficient to block the reinstatement of cocaine seeking. Total lever responses (mean ± S.E.M.) for rats pretreated with vehicle and TAT-P4-(DATC5)2 (0.03 and 3.0 pmol/μl) in the shell prior to a cocaine priming-induced reinstatement test session are shown in Figure 7A (n=9/treatment). These data were analyzed with a two-way RM ANOVA, which revealed significant main effects of treatment [F(2,32)=6.001, p<0.01] and lever [F(1,16)=22.72, p<0.001] as well as a significant interaction between lever and treatment [F(2,32)=5.326, p<0.01]. Subsequent post-hoc analyses revealed significant differences in responding on the active lever between rats pretreated with vehicle and 0.3 pmol/μl TAT-P4-(DATC5)2 (Bonferroni, p<0.05). There were no significant effects of drug treatment on inactive lever responding. To rule out any potential rate-suppressing effects, we assessed the ability of intra-accumbens shell TAT-P4-(DATC5)2 to attenuate sucrose reinstatement in a separate cohort of rats (n=7/treatment). No effects of TAT-P4-(DATC5)2 infusions into the accumbens shell were found on sucrose seeking (Figure 7B). Corresponding microinjection sites in the shell for the cocaine and sucrose experiments are shown in Figures 7C and 7D, respectively. Together, these results identify an important role for PICK1 in the accumbens shell in cocaine seeking.
Figure 7.
Administration of TAT-P4-(DATC5)2 directly into the nucleus accumbens shell dose-dependently attenuated cocaine priming-induced reinstatement of drug-seeking behavior. (A) Infusions of TAT-P4-(DATC5)2 (3.0 pmol/μl) directly into the shell prior to a cocaine priming injection reduced active lever responses during subsequent reinstatement test sessions compared to vehicle-treated controls and rats pretreated with 0.3 pmol/μl TAT-P4-(DATC5)2 (n=9/treatment). (B) Administration of TAT-P4-(DATC5)2 directly into the accumbens shell did not affect the reinstatement of sucrose seeking (n=7/treatment). Intra-shell microinjection sites for the cocaine and sucrose reinstatement studies are shown in C & D, respectively. *p<0.05, Bonferroni
4. Discussion
The present study provides the first evidence that systemically administered PICK1 peptide inhibitors have efficacy in reducing addiction-like behaviors in rats. Specifically, we show that systemic administration of a novel high-affinity inhibitor of the PICK1 PDZ domain, TAT-P4-(DATC5)2, significantly attenuates cocaine priming-induced reinstatement of drug-seeking behavior. Importantly, we identify doses of TAT-P4-(DATC5)2 that reduce cocaine seeking and do not affect sucrose seeking or locomotor activity in cocaine-experienced rats. We also show that systemic TAT-P4-(DATC5)2 crosses the blood-brain barrier and accumulates in the nucleus accumbens shell at time points that coincide with reduced cocaine-seeking behavior. Consistent with these effects, infusions of TAT-P4-(DATC5)2 directly into the nucleus accumbens shell dose-dependently reduce cocaine seeking. Furthermore, we demonstrate membrane permeability in vitro in MSNs as well as target engagement, results that confirm route from site of administration to the intracellular target. The behavioral effects of systemic and intra-cranial infusions of TAT-P4-(DATC5)2 are likely due to inhibition of PICK1 in MSNs based on our in vitro studies showing that TAT-P4-(DATC5)2 accumulates in striatal neurons and binds endogenous PICK1. Collectively, these findings provide strong support for developing peptide inhibitors of PICK1 for the treatment of cocaine craving and addiction.
4.1. PICK1 inhibitors and cocaine seeking
From a translational perspective, our systemic studies showing that TAT-P4-(DATC5)2 penetrates the brain and reduces cocaine seeking highlight the potential clinical significance of PICK1 peptide inhibitors. In contrast to most peptides that are susceptible to enzymatic cleavage in biological fluids and tissues, TAT-P4-(DATC5)2 is likely to be a protease-resistant peptide with a relatively long half-life as it is highly similar to the PSD95 inhibitor Tat-NPEG4(IETDV)(2) (Bach et al., 2012; Kucharz et al., 2017). These unique pharmacokinetic parameters along with a HIV-1 TAT cell-penetrating peptide sequence (Brooks et al., 2005) allow TAT-P4-(DATC5)2 to cross the blood-brain barrier, accumulate in neurons and produce behavioral responses in mice. Our findings expand these studies and show that intravenous infusions of TAT-P4-(DATC5)2 penetrate the brain, localize in the nucleus accumbens shell and reduce cocaine seeking in rats. Importantly, cerebral blood flow and metabolic responses are preserved following intravenous infusions of PICK1 inhibitors indicating that systemic administration of a PICK1 inhibitor does not alter normal brain function (Attwell et al., 2010; Kucharz et al., 2017). Disrupting PICK1 interactions with key binding partners, including GluA2, mGluR7, ASICs, and PKCα, could potentially give rise to adverse effects including absence-like seizures and impaired learning and memory (Bertaso et al., 2008; Volk et al., 2010). However, we did not observe absence-like seizures in our rats following systemic or intra-accumbens administration of TAT-P4-(DATC5)2 or in mice treated with equipotent doses of TAT-P4-(DATC5)2 (data not shown). While TAT-P4-(DATC5)2 did not affect sucrose reinstatement, indicating intact operant learning in our study, the putative effects of TAT-P4-(DATC5)2 on other measures of cognitive function in cocaine-exposed rodents remains to be tested. Taken together, these preclinical studies support further preclinical development ultimately leading to pilot clinical trials of PICK1 peptide inhibitors for the treatment of neurological and neuropsychiatric disorders including cocaine use disorder (Focant and Hermans, 2013).
4.2. Nucleus accumbens PICK1 and cocaine seeking
Extinction following cocaine self-administration is associated with increased PICK1 expression in the nucleus accumbens shell, but not the core (Ghasemzadeh et al., 2009). These results suggest that enhanced PICK1 function in the accumbens shell facilitates cocaine seeking during drug abstinence. This hypothesis is supported by studies showing that pharmacological inhibition of PICK1 in the accumbens shell is sufficient to attenuate cocaine priming-induced reinstatement of cocaine seeking (the present findings and Schmidt et al., 2013). Consistent with these effects, disrupting PICK1 interactions with the GluA2 AMPA receptor subunit in the nucleus accumbens reduces cocaine seeking (Famous et al., 2008). These findings suggest that peptide inhibitors of PICK1 attenuate cocaine seeking by blocking PICK1-mediated molecular mechanisms in the nucleus accumbens shell. Consistent with this hypothesis, systemic and intra-cranial infusions of Tat-GluA2(3Y), a small interference peptide that blocks GluA2/BRAG2 interactions and facilitates internalization of GluA2 (Scholz et al., 2010), prevents expression of psychostimulant-induced behavioral sensitization (Brebner et al., 2005), attenuates cue-induced reinstatement of heroin seeking (Van den Oever et al., 2008) and facilitates extinction of morphine-induced conditioned place preference (CPP) (Dias et al., 2012). Together with the present findings, these studies highlight endocytosis of GluA2-containing AMPA receptors as a target for drug development in the treatment of substance use disorders. In addition to GluA2, PICK1 interacts with at least 40 other target proteins, some of which have been shown to play important roles in drug-seeking behaviors (Xu and Xia, 2006). Thus, it is likely that the effects of TAT-P4-(DATC5)2 on cocaine seeking are due to disrupting PICK1 interactions with multiple proteins. Future studies that comprehensively measure PICK1 binding to target proteins in the accumbens shell of cocaine-experienced rats are required to elucidate the exact molecular mechanisms underlying the effects of TAT-P4-(DATC5)2 on cocaine seeking.
4.3. PKC, PICK1, AMPA receptor trafficking and cocaine seeking
An emerging literature indicates that cocaine seeking is mediated, in part, by increased protein kinase C (PKC) signaling in the nucleus accumbens (Ortinski et al., 2015; Schmidt et al., 2015; Schmidt et al., 2013). Indeed, cocaine seeking is associated with increase phosphorylation of PKCγ in the accumbens shell and core (Schmidt et al., 2015; Schmidt et al., 2013). Consistent with these findings, pharmacological inhibition of PKC in the shell and core attenuates cocaine priming-induced reinstatement (Schmidt et al., 2015; Schmidt et al., 2013). The downstream targets that mediate the effects of PKC on cocaine seeking are not known but may include AMPA receptors (Schmidt and Pierce, 2010). Cocaine priming-induced reinstatement of drug seeking is associated with increased phosphorylation of accumbens GluA2 at Ser880, a PKC phosphorylation site (Famous et al., 2008). PICK1 has been shown to interact with GluA2 (Hanley et al., 2002; Thorsen et al., 2010) and regulate its trafficking (Anggono et al., 2011; Hirbec et al., 2003; Malinow and Malenka, 2002; Steinberg et al., 2006; Xia et al., 2000). Specifically, PKC-induced phosphorylation of GluA2 at Ser880 and the subsequent association of GluA2 with PICK1 result in rapid internalization of GluA2-containing AMPA receptors (Chung et al., 2000; Collingridge et al., 2004; Perez et al., 2001; Terashima et al., 2004) (but see, Gardner et al., 2005; Liu and Cull-Candy, 2005). PKCγ, the same PKC isoform identified in our previous studies to play a role in cocaine seeking (Schmidt et al., 2015; Schmidt et al., 2013), phosphorylates GluA2 at Ser880 and together with PICK1 promote internalization of GluA2 subunits (Patten and Ali, 2009). Our results indicate that administration of a PICK1 inhibitor into the shell attenuates cocaine seeking and imply that cocaine reinstatement is associated with endocytosis of GluA2-containing AMPA receptors. This hypothesis is consistent with studies demonstrating that disrupting PICK1-GluA2 interactions is sufficient to attenuate cocaine seeking (Famous et al., 2008) and prevent cocaine-induced synaptic plasticity (Bellone and Luscher, 2006). Thus, endocytosis of GluA2-containing AMPA receptors in the accumbens shell during cocaine seeking may depend on PICK1 interactions with PKC.
4.4. PICK1 and Dopamine Homeostasis
As mentioned above, it is likely that multiple targets mediate the effects of PICK1 inhibition on cocaine-mediated behaviors. Cocaine self-administration and sensitization to the locomotor-activating effects of cocaine are reduced in mutant mice lacking PICK1 (Jensen et al., 2018). These effects are associated with reduced dopamine uptake in striatal synaptosomes, increased expression of tyrosine hydroxylase (TH) from striatal homogenates and increased dopamine release in the striatum (Jensen et al., 2018). Taken together, these results suggest that PICK1 may alter dopamine homeostasis in the striatum through a negative-feedback mechanism involving striatal TH expression. While it is not clear whether these same cellular and neurochemical mechanisms underlie the effects of PICK1 inhibitors on cocaine seeking, these results indicate that the mechanisms underlying PICK1 inhibition on cocaine seeking are likely to be complex.
5. Conclusions
Through its ability to broadly bind numerous protein targets and regulate their intracellular trafficking, PICK1 plays an important role in synaptic plasticity and behavior. Therefore, PICK1 may represent a molecular target for novel pharmacotherapies aimed at treating neurological and neuropsychiatric disorders including substance use disorders. Here, we provide the first evidence supporting the efficacy of systemic administration of a novel peptide inhibitor of PICK1 in an animal model of cocaine craving-induced relapse. While our results clearly identify a role for PICK1 in cocaine seeking through actions in the nucleus accumbens shell, the mechanisms underlying PICK1 function in drug-seeking behavior are unclear. Future studies aimed at identifying PICK1-targeted molecular substrates and how extinction following cocaine self-administration dynamically alters the functional significance of these interactions will be critical towards understanding how PICK1 coordinates cellular function in the accumbens shell to regulate cocaine seeking.
Systemic infusions of the novel PICK1 inhibitor TAT-P4-(DATC5)2 cross the blood brain barrier and attenuate cocaine seeking.
TAT-P4-(DATC5)2 accumulates in medium spiny striatal neurons and binds PICK1.
Administration of TAT-P4-(DATC5)2 directly into the nucleus accumbens shell attenuates cocaine, but not sucrose, seeking.
Acknowledgements
This work was supported by the following grants from the National Institutes of Health: R01 DA037897 (H.D.S.). H.D.S. and J.W. were partially supported by a JumpStart for Juniors Research Grant from the Center for Undergraduate Research & Fellowships at the University of Pennsylvania. N.S.H. is a Howard Hughes Medical Institute Gilliam Fellow. K.L.M. and M.D.L. were supported by the Lundbeck Foundation and the Danish Research Counsel. The authors would also like to thank John Maurer for his technical assistance.
Footnotes
Conflict of Interest
The authors declare no competing financial interests. A patent (P4997EP00) has been filed for TAT-P4-(DATC5)2.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anggono V, Clem RL, Huganir RL, 2011. PICK1 loss of function occludes homeostatic synaptic scaling. J Neurosci 31, 2188–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA, 2010. Glial and neuronal control of brain blood flow. Nature 468, 232–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach A, Clausen BH, Moller M, Vestergaard B, Chi CN, Round A, Sorensen PL, Nissen KB, Kastrup JS, Gajhede M, Jemth P, Kristensen AS, Lundstrom P, Lambertsen KL, Stromgaard K, 2012. A high-affinity, dimeric inhibitor of PSD-95 bivalently interacts with PDZ1–2 and protects against ischemic brain damage. Proc Natl Acad Sci U S A 109, 3317–3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballarin B, Tymianski M, 2018. Discovery and development of NA-1 for the treatment of acute ischemic stroke. Acta Pharmacol Sin 39, 661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Luscher C, 2006. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci 9, 636–641. [DOI] [PubMed] [Google Scholar]
- Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, Lerner-Natoli M, 2008. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nat Neurosci 11, 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brebner K, Wong TP, Liu L, Liu Y, Campsall P, Gray S, Phelps L, Phillips AG, Wang YT, 2005. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 310, 1340–1343. [DOI] [PubMed] [Google Scholar]
- Brooks H, Lebleu B, Vives E, 2005. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev 57, 559–577. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL, 2000. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci 20, 7258–7267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citri A, Bhattacharyya S, Ma C, Morishita W, Fang S, Rizo J, Malenka RC, 2010. Calcium binding to PICK1 is essential for the intracellular retention of AMPA receptors underlying long-term depression. J Neurosci 30, 16437–16452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge GL, Isaac JT, Wang YT, 2004. Receptor trafficking and synaptic plasticity. Nat Rev Neurosci 5, 952–962. [DOI] [PubMed] [Google Scholar]
- Dias C, Wang YT, Phillips AG, 2012. Facilitated extinction of morphine conditioned place preference with Tat-GluA2(3Y) interference peptide. Behav Brain Res 233, 389–397. [DOI] [PubMed] [Google Scholar]
- Erlendsson S, Rathje M, Heidarsson PO, Poulsen FM, Madsen KL, Teilum K, Gether U, 2014. Protein interacting with C-kinase 1 (PICK1) binding promiscuity relies on unconventional PSD-95/discs-large/ZO-1 homology (PDZ) binding modes for nonclass II PDZ ligands. J Biol Chem 289, 25327–25340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famous KR, Kumaresan V, Sadri-Vakili G, Schmidt HD, Mierke DF, Cha JH, Pierce RC, 2008. Phosphorylation-dependent trafficking of GluR2-containing AMPA receptors in the nucleus accumbens plays a critical role in the reinstatement of cocaine seeking. J Neurosci 28, 11061–11070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Focant MC, Hermans E, 2013. Protein interacting with C kinase and neurological disorders. Synapse 67, 532–540. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Suh JG, Johnson R, Yu S, Huganir RL, 2005. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron 45, 903–915. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller CR, Seubert C, Mantsch JR, 2009. Region-specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Res 1267, 89–102. [DOI] [PubMed] [Google Scholar]
- Haglerod C, Hussain S, Nakamura Y, Xia J, Haug FS, Ottersen OP, Henley JM, Davanger S, 2017. Presynaptic PICK1 facilitates trafficking of AMPA-receptors between active zone and synaptic vesicle pool. Neuroscience 344, 102–112. [DOI] [PubMed] [Google Scholar]
- Haglerod C, Kapic A, Boulland JL, Hussain S, Holen T, Skare O, Laake P, Ottersen OP, Haug FM, Davanger S, 2009. Protein interacting with C kinase 1 (PICK1) and GluR2 are associated with presynaptic plasma membrane and vesicles in hippocampal excitatory synapses. Neuroscience 158, 242–252. [DOI] [PubMed] [Google Scholar]
- Hanley JG, Khatri L, Hanson PI, Ziff EB, 2002. NSF ATPase and alpha-/beta-SNAPs disassemble the AMPA receptor-PICK1 complex. Neuron 34, 53–67. [DOI] [PubMed] [Google Scholar]
- Herlo R, Lund VK, Lycas MD, Jansen AM, Khelashvili G, Andersen RC, Bhatia V, Pedersen TS, Albornoz PBC, Johner N, Ammendrup-Johnsen I, Christensen NR, Erlendsson S, Stoklund M, Larsen JB, Weinstein H, Kjaerulff O, Stamou D, Gether U, Madsen KL, 2018. An Amphipathic Helix Directs Cellular Membrane Curvature Sensing and Function of the BAR Domain Protein PICK1. Cell Rep 23, 2056–2069. [DOI] [PubMed] [Google Scholar]
- Hernandez NS, Ige KY, Mietlicki-Baase EG, Molina-Castro GC, Turner CA, Hayes MR, Schmidt HD, 2018. Glucagon-like peptide-1 receptor activation in the ventral tegmental area attenuates cocaine seeking in rats. Neuropsychopharmacology in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec H, Francis JC, Lauri SE, Braithwaite SP, Coussen F, Mulle C, Dev KK, Coutinho V, Meyer G, Isaac JT, Collingridge GL, Henley JM, 2003. Rapid and differential regulation of AMPA and kainate receptors at hippocampal mossy fibre synapses by PICK1 and GRIP. Neuron 37, 625–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holst B, Madsen KL, Jansen AM, Jin C, Rickhag M, Lund VK, Jensen M, Bhatia V, Sorensen G, Madsen AN, Xue Z, Moller SK, Woldbye D, Qvortrup K, Huganir R, Stamou D, Kjaerulff O, Gether U, 2013. PICK1 deficiency impairs secretory vesicle biogenesis and leads to growth retardation and decreased glucose tolerance. PLoS Biol 11, e1001542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen KL, Sorensen G, Dencker D, Owens WA, Rahbek-Clemmensen T, Brett Lever M, Runegaard AH, Riis Christensen N, Weikop P, Wortwein G, Fink-Jensen A, Madsen KL, Daws L, Gether U, Rickhag M, 2018. PICK1-Deficient Mice Exhibit Impaired Response to Cocaine and Dysregulated Dopamine Homeostasis. eNeuro. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W, Ge WP, Xu J, Cao M, Peng L, Yung W, Liao D, Duan S, Zhang M, Xia J, 2006. Lipid binding regulates synaptic targeting of PICK1, AMPA receptor trafficking, and synaptic plasticity. J Neurosci 26, 2380–2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharz K, Sondergaard Rasmussen I, Bach A, Stromgaard K, Lauritzen M, 2017. PSD-95 uncoupling from NMDA receptors by Tat- N-dimer ameliorates neuronal depolarization in cortical spreading depression. J Cereb Blood Flow Metab 37, 1820–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Zhang N, Wang YN, Shen Y, Wang Y, 2016. Multiple faces of protein interacting with C kinase 1 (PICK1): Structure, function, and diseases. Neurochem Int 98, 115–121. [DOI] [PubMed] [Google Scholar]
- Liu SJ, Cull-Candy SG, 2005. Subunit interaction with PICK and GRIP controls Ca2+ permeability of AMPARs at cerebellar synapses. Nat Neurosci 8, 768–775. [DOI] [PubMed] [Google Scholar]
- Lu W, Ziff EB, 2005. PICK1 interacts with ABP/GRIP to regulate AMPA receptor trafficking. Neuron 47, 407–421. [DOI] [PubMed] [Google Scholar]
- Madsen KL, Eriksen J, Milan-Lobo L, Han DS, Niv MY, Ammendrup-Johnsen I, Henriksen U, Bhatia VK, Stamou D, Sitte HH, McMahon HT, Weinstein H, Gether U, 2008. Membrane localization is critical for activation of the PICK1 BAR domain. Traffic 9, 1327–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Malenka RC, 2002. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci 25, 103–126. [DOI] [PubMed] [Google Scholar]
- Ortinski PI, Briand LA, Pierce RC, Schmidt HD, 2015. Cocaine-seeking is associated with PKC-dependent reduction of excitatory signaling in accumbens shell D2 dopamine receptor-expressing neurons. Neuropharmacology 92, 80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patten SA, Ali DW, 2009. PKCgamma-induced trafficking of AMPA receptors in embryonic zebrafish depends on NSF and PICK1. Proc Natl Acad Sci U S A 106, 6796–6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 1997. The rat brain in stereotaxic coordinates. Academic Press, New York. [DOI] [PubMed] [Google Scholar]
- Perez JL, Khatri L, Chang C, Srivastava S, Osten P, Ziff EB, 2001. PICK1 targets activated protein kinase Calpha to AMPA receptor clusters in spines of hippocampal neurons and reduces surface levels of the AMPA-type glutamate receptor subunit 2. J Neurosci 21, 5417–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Famous KR, Pierce RC, 2009. The limbic circuitry underlying cocaine seeking encompasses the PPTg/LDT. Eur J Neurosci 30, 1358–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Kimmey BA, Arreola AC, Pierce RC, 2015. Group I metabotropic glutamate receptor-mediated activation of PKC gamma in the nucleus accumbens core promotes the reinstatement of cocaine seeking. Addict Biol 20, 285–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Mietlicki-Baase EG, Ige KY, Maurer JJ, Reiner DJ, Zimmer DJ, Van Nest DS, Guercio LA, Wimmer ME, Olivos DR, De Jonghe BC, Hayes MR, 2016. Glucagon-Like Peptide-1 Receptor Activation in the Ventral Tegmental Area Decreases the Reinforcing Efficacy of Cocaine. Neuropsychopharmacology 41, 1917–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Pierce RC, 2010. Cocaine-induced neuroadaptations in glutamate transmission: potential therapeutic targets for craving and addiction. Ann N Y Acad Sci 1187, 35–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt HD, Schassburger RL, Guercio LA, Pierce RC, 2013. Stimulation of mGluR5 in the accumbens shell promotes cocaine seeking by activating PKC gamma. J Neurosci 33, 14160–14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholz R, Berberich S, Rathgeber L, Kolleker A, Kohr G, Kornau HC, 2010. AMPA receptor signaling through BRAG2 and Arf6 critical for long-term synaptic depression. Neuron 66, 768–780. [DOI] [PubMed] [Google Scholar]
- Schwarze SR, Ho A, Vocero-Akbani A, Dowdy SF, 1999. In vivo protein transduction: delivery of a biologically active protein into the mouse. Science 285, 1569–1572. [DOI] [PubMed] [Google Scholar]
- Steinberg JP, Takamiya K, Shen Y, Xia J, Rubio ME, Yu S, Jin W, Thomas GM, Linden DJ, Huganir RL, 2006. Targeted in vivo mutations of the AMPA receptor subunit GluR2 and its interacting protein PICK1 eliminate cerebellar long-term depression. Neuron 49, 845–860. [DOI] [PubMed] [Google Scholar]
- Terashima A, Cotton L, Dev KK, Meyer G, Zaman S, Duprat F, Henley JM, Collingridge GL, Isaac JT, 2004. Regulation of synaptic strength and AMPA receptor subunit composition by PICK1. J Neurosci 24, 5381–5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen TS, Madsen KL, Rebola N, Rathje M, Anggono V, Bach A, Moreira IS, Stuhr-Hansen N, Dyhring T, Peters D, Beuming T, Huganir R, Weinstein H, Mulle C, Stromgaard K, Ronn LC, Gether U, 2010. Identification of a small-molecule inhibitor of the PICK1 PDZ domain that inhibits hippocampal LTP and LTD. Proc Natl Acad Sci U S A 107, 413–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Oever MC, Goriounova NA, Li KW, Van der Schors RC, Binnekade R, Schoffelmeer AN, Mansvelder HD, Smit AB, Spijker S, De Vries TJ, 2008. Prefrontal cortex AMPA receptor plasticity is crucial for cue-induced relapse to heroin-seeking. Nat Neurosci 11, 1053–1058. [DOI] [PubMed] [Google Scholar]
- Volk L, Kim CH, Takamiya K, Yu Y, Huganir RL, 2010. Developmental regulation of protein interacting with C kinase 1 (PICK1) function in hippocampal synaptic plasticity and learning. Proc Natl Acad Sci U S A 107, 21784–21789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia J, Chung HJ, Wihler C, Huganir RL, Linden DJ, 2000. Cerebellar long-term depression requires PKC-regulated interactions between GluR2/3 and PDZ domain-containing proteins. Neuron 28, 499–510. [DOI] [PubMed] [Google Scholar]
- Xia J, Zhang X, Staudinger J, Huganir RL, 1999. Clustering of AMPA receptors by the synaptic PDZ domain-containing protein PICK1. Neuron 22, 179–187. [DOI] [PubMed] [Google Scholar]
- Xu J, Xia J, 2006. Structure and function of PICK1. Neurosignals 15, 190–201. [DOI] [PubMed] [Google Scholar]