Abstract
Prescription opioids, such as oxycodone, are potent analgesics that are used to treat and manage pain. However, oxycodone is one of the most commonly abused prescription drugs. Finding an effective strategy to prevent prescription opioid use disorder is urgent. Orexin receptors (OrxR1 and OrxR2) have been implicated in the regulation of motivation, arousal, and stress, making them possible targets for the treatment of substance use disorder. To study the significance of environmental stimuli in maintaining the vulnerability to relapse to oxycodone use, resistance to the extinction of oxycodone-seeking behavior that was elicited by an oxycodone-related stimulus was examined. Rats were trained to self-administer oxycodone in the presence of a contextual/discriminative stimulus (Sd). Using this procedure, the rats readily acquired oxycodone self-administration and exhibited increases in physical signs of opioid withdrawal. Following extinction, response-reinstating effects of re-exposure to the Sd perseverated. We then tested whether OrxR blockade prevents oxycodone intake and relapse. The effects of the OrxR1 antagonist SB334867 and OrxR2 antagonist TCSOX229 on oxycodone self-administration were tested. SB334867 significantly decreased oxycodone self-administration, whereas TCSOX229 did not produce any effect. To investigate whether OrxR1 and OrxR2 blockade prevents oxycodone seeking, the rats were tested for the ability of SB334867 and TCSOX229 to prevent the Sd-induced conditioned reinstatement of oxycodone-seeking behavior. SB334867 decreased oxycodone-seeking behavior, whereas TCSOX229 was ineffective. These results suggest that OrxR1 antagonism prevents excessive prescription opioid use and relapse and might be beneficial for the treatment of prescription opioid use disorder.
Keywords: opioids, oxycodone, self-administration, relapse, orexin, SB334867
INTRODUCTION
Opioid use disorder (OUD) and overdose have risen to epidemic proportions in the United States (U.S.) in recent years. Prescription opioids are potent analgesics that are used for clinical pain management, but the overprescription of opioids for pain relief has led to a dramatic increase in the prevalence of the nonmedical use of these medications (Jones, Muhuri, & Lurie, 2017; Muhuri, 2013). For example, from 1999 to 2011, hydrocodone consumption more than doubled, and oxycodone consumption increased five-fold (Kolodny et al., 2015). From 1999 to 2016, overdoses in the U.S. resulted in 632,331 deaths, 55.6% (351,630) of which were attributable to opioids. In 2016 alone, 63,632 overdose deaths occurred in the U.S., and opioids accounted for 66.4% (42,249) of these overdose deaths. Currently, an estimated 130 people die every day in the U.S. from opioid-related overdose (National Institute on Drug Abuse, 2019). Rates of overdose deaths that involved prescription opioids, such as oxycodone (OxyContin®), increased by 10.6%. OxyContin® is one of the most commonly abused prescription drugs (Kolodny et al., 2015; Seth, Scholl, Rudd, & Bacon, 2018). The rise in prescription opioid, particularly oxycodone, misuse and abuse has also been associated with a higher rate of the transition to heroin abuse (Compton, Jones, & Baldwin, 2016). Only few medications are available to treat OUD today. For example and importantly for the current study, opioid receptor agonists (e.g., methadone, buprenorphine) are used as substitution therapy and need to be administered in a maintenance regimen to prevent opioid craving and relapse (National Institute on Drug Abuse, 2018). Alternative strategies are urgently needed to prevent and treat prescription OUD.
Orexin (Orx) neurons play a role in modulating reward function, particularly drug-directed behavior (Harris, Wimmer, & Aston-Jones, 2005). Orexin neurons are activated by stimuli that are predictive of food, morphine, cocaine, and alcohol (Dayas, McGranahan, Martin-Fardon, & Weiss, 2008; Harris et al., 2005; Jupp, Krstew, Dezsi, & Lawrence, 2011a; Martin-Fardon, Cauvi, Kerr, & Weiss, 2018; Martin-Fardon, Zorrilla, Ciccocioppo, & Weiss, 2010). Orexin has been implicated in drug-motivated behavior under high demand conditions to obtain the drug or when the motivation for drug seeking is augmented by the presentation of stimuli that were previously associated with the drug (Hollander, Pham, Fowler, & Kenny, 2012; Mahler, Moorman, Smith, James, & Aston-Jones, 2014). In rodents, approximately 50% of Orx neurons express μ-opioid receptors (MORs), the neuropharmacological target of oxycodone (Lalovic et al., 2006; Peckham & Traynor, 2006).
Orexin A (OrxA or hypocretin-1 [Hcrt1]) and orexin B (OrxB or hypocretin-2 [Hcrt2]) are secondary products that are obtained by the proteolytic cleavage of a common precursor, prepro-Orx (de Lecea et al., 1998; Gatfield, Brisbare-Roch, Jenck, & Boss, 2010; Tsujino & Sakurai, 2009), and posttranslational products of the Orx neuropeptide precursor gene. Two Orx receptors have been identified: OrxR1 (Hcrt-r1) and OrxR2 (Hcrt-r2; Ammoun et al., 2003; Sakurai et al., 1998; Scammell & Winrow, 2011). Orexin receptors are distributed throughout the brain, with some overlap between OrxR1 and OrxR2 mRNA expression (Marcus et al., 2001; Trivedi, Yu, MacNeil, Van der Ploeg, & Guan, 1998). For example, the prefrontal cortex (PFC) predominantly expresses OrxR1 mRNA, whereas the nucleus accumbens (NAc) mainly expresses OrxR2 mRNA (Marcus et al., 2001), suggesting different physiological roles for each receptor subtype. A functional difference between OrxR1 and OrxR2 has been suggested (Aston-Jones et al., 2010), with OrxR1 signaling mainly involved in reward seeking and OrxR2 signaling mainly involved in sleep/wake cycle regulation and arousal. These two receptors have been shown to be involved in regulating motivation, especially highly motivated behavior, arousal, and stress (Aston-Jones, Smith, Moorman, & Richardson, 2009; Aston-Jones et al., 2010; Berridge, Espana, & Vittoz, 2010; Cason et al., 2010; Giardino & de Lecea, 2014; James, Campbell, & Dayas, 2017; Koob, 2009; Kuwaki & Zhang, 2012; Tsujino & Sakurai, 2013), making them ideal targets for addiction treatment. For example, the blockade of OrxR1 by peripheral SB334867 administration reduced cocaine self-administration under high demand conditions to obtain the drug (Hollander et al., 2012; James et al., 2018) and reversed conditioned reinstatement that was induced by cocaine-and alcohol-related stimuli (Martin-Fardon & Weiss, 2014a, 2014b). Some studies have shown that morphine seeking and withdrawal depend on Orx signaling (Georgescu et al., 2003; Harris et al., 2005). However, unknown is whether OrxR1 and OrxR2 signaling are required for the taking and seeking of morphine and other opioids. Further studies are required to elucidate the wide-ranging roles of OrxR1 and OrxR2 in prescription opioid-taking and-seeking behaviors.
Using a model of extended access to oxycodone self-administration in rats, the present study investigated the efficacy of two OrxR antagonists (OrxR1 antagonist SB334867 and OrxR2 antagonist TCSOX229) in reducing excessive oxycodone intake. The schedule of oxycodone self-administration and the oxycodone dose that were used in the present study have been previously shown to induce the significant escalation of oxycodone intake over the course of self-administration training (Nguyen, Hwang, Grant, Janda, & Taffe, 2018; Wade, Vendruscolo, Schlosburg, Hernandez, & Koob, 2015). A central problem in treating OUD is the prevalence of relapse to opioid use even after protracted periods of forced or self-imposed abstinence (Gostin, Hodge, & Noe, 2017; Rudd, Seth, David, & Scholl, 2016; Volkow & Collins, 2017). A major factor that contributes to the chronic relapsing and compulsive nature of drug addiction is the process of associative learning, by which environmental stimuli that are repeatedly paired with drug consumption acquire incentive-motivational value, evoking the expectation of drug availability (Miller & Gold, 1994; O’Brien, Childress, Ehrman, & Robbins, 1998; Tiffany & Carter, 1998; Tiffany & Conklin, 2000; van de Laar, Licht, Franken, & Hendriks, 2004). A secondary objective of the present study was to test whether behavior that is guided by associations between environmental stimuli and oxycodone is characterized by extinction-resistant reward seeking similarly to other drugs of abuse (Ciccocioppo, Angeletti, & Weiss, 2001; Gracy, Dankiewicz, Weiss, & Koob, 2000; Martin-Fardon & Weiss, 2017). We also tested whether the blockade of OrxRs prevents oxycodone-seeking behavior in a conditioned reinstatement model.
MATERIALS AND METHODS
Drugs
N-(2-methyl-6-benzoxazolyl)-N’-1,5-naphthyridin-4-yl urea (SB334867, Tocris Bioscience, Bristol, United Kingdom) was dissolved in 10% dimethylsulfoxide (v/v) and 1% hydroxypropyl-β-cyclodextrin (w/v) in sterile water and administered intraperitoneally (i.p.) in a volume of 5 ml/kg, 30 min before behavioral testing. (2S)-1-(3,4-dihydro-6,7-dimethoxy-2(1H)-isoquinolinyl)-3,3-dimethyl-2-[(4-pyridinylmethyl)amino]-1-butanone hydrochloride (TCSOX229, Tocris Bioscience, Bristol, United Kingdom) was dissolved in distilled water and administered i.p. in a volume of 1 ml/kg, 30 min before behavioral testing. Oxycodone hydrochloride (Spectrum Chemicals, Gardena, CA, USA) was dissolved in 0.9% sodium chloride (Hospira, Lake Forest, IL, USA) and administered intravenously (i.v.) at a dose of 0.15 mg/kg/0.1 ml.
Animals
Male Wistar rats (n = 47; Charles River, Wilmington, MA, USA), weighing 200–225 g upon arrival, were housed two per cage at all times in a temperature-and humidity-controlled vivarium on a reverse 12 h/12 h light/dark cycle with ad libitum access to food and water. All of the procedures were conducted in strict adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute. Five separate groups of rats were prepared.
Group 1. Self-administration, extinction training, and oxycodone conditioned reinstatement (Fig. 1A)
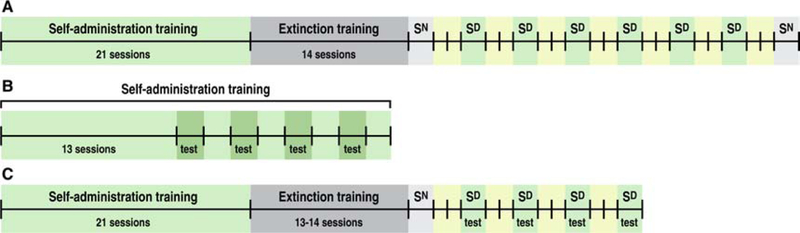
Figure 1.
Experimental procedure. A. Group 1: Rats underwent 21 sessions of oxycodone self-administration (12 h/day, 5 days/week) in the presence of a discriminative stimulus (Sd), followed by 14 extinction sessions (2 h/day, 7 days/week). After extinction, the rats were tested repeatedly for the cue-induced reinstatement of oxycodone seeking (2 h/session). Sn, reinstatement test with neutral stimuli; Sd, reinstatement test with discriminative stimuli. B. Groups 2 and 3: Rats were tested for the ability of SB334867 and TCSOX229 to reduce oxycodone intake during 12-h self-administration sessions. The tests were performed every other day starting on day 14 of self-administration. C. Groups 4 and 5: Following self-administration and extinction training, the rats were tested for the ability of SB334867 and TCSOX229 to decrease oxycodone seeking during 2 h cue-induced reinstatement tests (Sd). Sn, reinstatement test with neutral stimuli; Sd, reinstatement test with discriminative stimuli.
Rats in Group 1 (n = 10) were surgically prepared with jugular catheters 7–10 days before oxycodone self-administration training in daily 12 h (extended access) sessions. Each session was initiated by the extension of two retractable levers into the operant chamber (29 cm × 24 cm × 19.5 cm, Med Associates, St. Albans, VT, USA). Responses on the right active lever were reinforced on a fixed-ratio 1 (FR1) schedule by intravenous (i.v.) oxycodone (0.15 mg/0.1 ml/infusion) that was infused over 4 s, followed by a 20-s timeout (TO20 s) period that was signaled by the illumination of a cue light above the active lever in the presence of a contextual/discriminative stimulus (Sd; constant 70 dB white noise). Responses on the left inactive lever were recorded but had no scheduled consequences. Body weight was monitored every day, and catheter patency was confirmed weekly (unless otherwise needed) with an injection of 0.1–0.2 ml of Brevital sodium solution (10 mg/ml; Par Pharmaceutical, Woodcliff Lake, NJ, USA). Catheter patency was assumed if the immediate loss of reflexes was observed. The dose of oxycodone was carefully chosen based on earlier published findings from colleagues in our department (Nguyen et al., 2018; Wade et al., 2015). The rats were scored for spontaneous physical withdrawal signs (i.e., jumps, paw tremors, teeth chattering, wet dog shakes, piloerection and ptosis) twice weekly (Tuesdays and Fridays), 11 h after the last self-administration session, by a laboratory assistant who was blind to the experimental conditions. Each withdrawal measure was assigned a score of 0–2, based on the following severity scale: 0 = no sign, 1 = moderate, 2 = severe. The sum of the six observation scores was used as a quantitative measure of withdrawal severity and to confirm dependence. After the training procedure (21 sessions of self-administration, 5 days/week), the rats’ behavior was extinguished in daily 2-h extinction sessions, in which responses on the previously active lever had no programmed consequences (i.e., no oxycodone and no cue presentation) for ~2 weeks (when operant responding typically reached ≤ 10 responses/session). One day after the last extinction session, all of the rats were presented with a neutral stimulus (Sn) in a 2-h session to control for the specificity of the Sd to reinstate extinguished oxycodone seeking. During the Sn session, the illumination of a 2.8 W house light at the top of the chamber’s front panel served as the Sd. Responding on the right active lever was followed by an intermittent tone, during which the lever remained inactive for 20 s. Two days later, the rats were presented with the Sd in a 2-h conditioned reinstatement test and tested repeatedly every third day (with 2 days off between reinstatement tests) for a total of six reinstatement tests. Finally, following the sixth reinstatement test, the rats were tested with an additional Sn (identical to the first one) to control for the specificity of the Sd to reinstate behavior.
Group 2. Effect of SB334867 on oxycodone self-administration (Fig. 1B)
Group 2 (n = 10) was surgically prepared and trained for oxycodone self-administration as described for Group 1. Once oxycodone intake stabilized after escalation, the effects of three doses of SB334867 (5, 10, and 30 mg/kg, i.p.) and its vehicle on oxycodone self-administration were evaluated. The dose selection was based on previous studies (Flores et al., 2014; James, Stopper, et al., 2019; Martin-Fardon & Weiss, 2014a; Smith, See, & Aston-Jones, 2009; Smith, Tahsili-Fahadan, & Aston-Jones, 2010). SB334867 was administered 30 min before beginning the 12-h self-administration sessions. To control for possible order effects of SB33487 dosing on oxycodone self-administration, each animal was tested with all doses of SB334867 in random order using a Latin-square design every other day (sessions 14, 16, 18, and 20). On alternate days (sessions 15, 17, 19, and 21), the rats underwent regular self-administration sessions to confirm that they returned to their pretest baseline levels of self-administration.
Group 3. Effect of TCSOX229 on oxycodone self-administration (Fig. 1B)
Group 3 (n = 10) was surgically prepared and trained for oxycodone self-administration as described for Group 1. The effects of three doses of TCSOX229 (5, 10, and 30 mg/kg, i.p.) and its vehicle on oxycodone self-administration were determined according to the same schedule of injections and intervening sessions as described for Group 2. The dose selection was based on previous studies (Tabaeizadeh et al., 2013; Wang, Li, & Kirouac, 2017).
Group 4. Effect of SB334867 on oxycodone conditioned reinstatement (Fig. 1C)
Group 4 (n = 10) was surgically prepared and trained for oxycodone self-administration and extinction as described for Group 1. One day after the last extinction session, all of the rats were presented with a neutral stimulus (Sn) in a 2-h session. Two days later, the effect of SB338467 on conditioned reinstatement in the presence of the Sd was evaluated. SB338467 (0, 5, 10, and 30 mg/kg, i.p.) was administered 30 min before the reinstatement sessions. To control for possible order effects, the rats were tested with the three doses of SB334867 (and vehicle) in a random sequence according to a Latin-square design. Test sessions were separated by a 2-day period during which the rats remained in their home cages in the vivarium.
Group 5. Effect of TCSOX229 on oxycodone conditioned reinstatement (Fig. 1C)
Group 5 (n = 7) was prepared and trained as described for Group 4. The effects of three doses of TCSOX229 (5, 10, and 30 mg/kg, i.p.) and its vehicle on oxycodone conditioned reinstatement were determined according to the same schedule of injections and intervening sessions as described for Group 4.
Statistical analysis
The acquisition of oxycodone self-administration, extinction training, and conditioned reinstatement were analyzed using two-way repeated-measures analysis of variance (ANOVA), with time (days) and lever (active vs. inactive) as variables. The effects of SB334867 and TCSOX229 on oxycodone self-administration and conditioned reinstatement were analyzed using two-way ANOVA, with dose and lever (active vs. inactive) as variables. Dose-dependent differences in cumulative intake over the 12-h session following SB334867 and TCSOX229 administration were analyzed using two-way ANOVA, with dose and time as variables. Significant main effects and interactions in the ANOVA were followed by the Sidak post hoc test. Withdrawal scores were analyzed using the nonparametric Friedman test. Pearson’s r correlation coefficients were determined to establish the linear dependence between withdrawal scores and the number of oxycodone infusions that were earned on the last day of self-administration training. The results are expressed as mean ± SEM. Values of p < 0.05 were considered statistically significant. The statistical analysis was performed using GraphPad Prism 7 software.
RESULTS
Eight rats were lost (four because of health complications and four because of catheter failure), thus reducing the total number of animals to 39 (Group 1, n = 8; Group 2, n = 9; Group 3, n = 10; Group 4, n = 7; Group 5, n = 5).
Group 1. Acquisition of oxycodone self-administration and conditioned reinstatement
Self-administration, somatic withdrawal signs, and extinction.
Over the 21 days of self-administration training (12 h/day), the rats acquired oxycodone self-administration (two-way ANOVA: day, F20,280 = 14.37, p < 0.001; lever, F1,14 = 27.09, p < 0.001; day × lever interaction, F20,280 = 7.97, p < 0.001; Fig. 2A). The Sidak post hoc test confirmed that the rats emitted more responses of the active lever starting on day 13 vs. the inactive lever and vs. day 1 (Sidak post hoc test following ANOVA: p < 0.05).
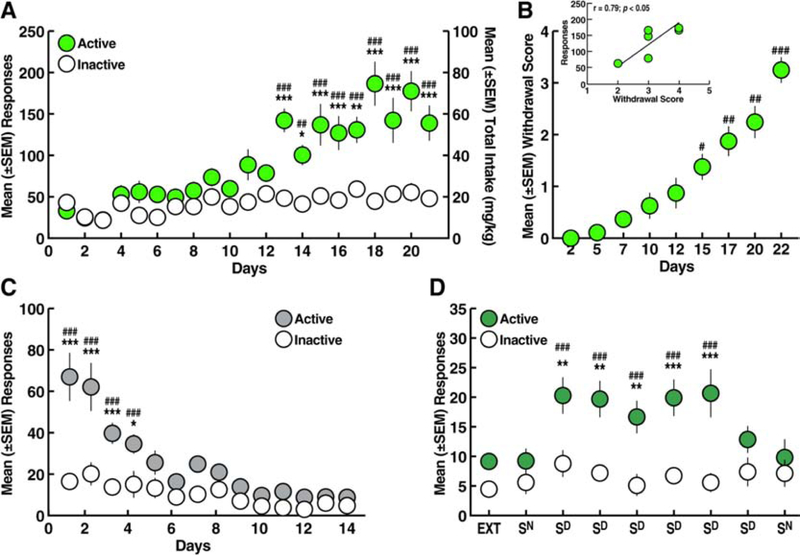
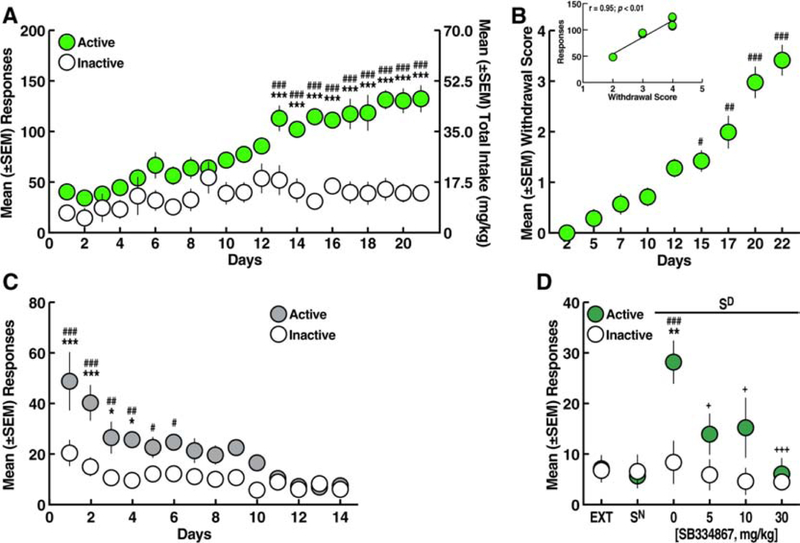
Figure 2.
A. Oxycodone self-administration over 21 sessions (12 h/day). *p < 0.05, **p < 0.01, ***p < 0.001, vs. inactive lever; ##p < 0.01, ###p < 0.001, vs. day 1 (Sidak post hoc test). B. Somatic withdrawal signs. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. day 2 (Dunn’s post hoc test). Inset. Correlation plot between the somatic withdrawal score and the number of oxycodone infusions. C. Extinction training over 14 sessions (2 h/day). *p < 0.05, ***p < 0.001, vs. inactive lever; ###p < 0.001, vs. day 14 (Sidak post hoc test). D. Perseveration of reinstatement by stimuli conditioned to oxycodone (Sd). **p < 0.01, ***p < 0.001, vs. inactive lever; ###p < 0.001, vs. EXT and Sn (Sidak post hoc test). EXT, extinction; Sn, reinstatement test with neutral stimulus; Sd, reinstatement test with discriminative stimulus conditioned to oxycodone. n = 8.
Eleven hours after self-administration on days 1, 4, 6, 9, 11, 14, 16, 19, and 21, somatic signs of withdrawal were measured. Compared with somatic withdrawal signs that were measured after the first day of self-administration (day 2, Fig. 2B), somatic signs of withdrawal were significantly higher starting after the 14th day of self-administration (Dunn’s post hoc test following Friedman ANOVA: H = 49.96; p < 0.001, vs. day 2; Fig. 2B) and included teeth chattering, wet dog shakes, piloerection and ptosis, thus confirming oxycodone dependence (Rehni, Jaggi, & Singh, 2013). Correlational analysis revealed a significant relationship between the somatic withdrawal score and the number of oxycodone infusions that were earned on the last day of self-administration training (Fig. 2B, inset).
During the 14 days of extinction training, a gradual decrease in the number of responses at the active lever was observed (two-way ANOVA: day, F13,182 = 19.39, p < 0.001; lever, F1,14 = 21.04, p < 0.001; day × lever interaction, F13,182 = 7.21, p < 0.001; Fig. 2C). The Sidak post hoc test showed that the rats emitted more responses on the active lever vs. inactive lever from day 1 to day 4 of extinction (p < 0.05), and then responses on the active lever were identical to responses on the inactive lever (Fig. 2C). At the end of extinction training, the rats reached a mean (± SEM) of 9.19 ± 1.11 responses on the active lever.
Perseveration of reinstatement by stimuli conditioned to oxycodone.
Following extinction, re-exposure to the Sd significantly reinstated oxycodone-seeking behavior, whereas presentation of the Sn did not induce any reinstatement of behavior after the first or second presentation (two-way ANOVA: day, F8,112 = 7.21, p < 0.001; lever, F1,14 = 11.99, p < 0.01; day × lever interaction, F8,112 = 4.84, p < 0.001; Fig. 2D). Sd-induced oxycodone-seeking behavior remained stable for five subsequent reinstatement tests (Sidak post hoc test: p < 0.05, vs. extinction, Sn, and inactive lever).
Group 2. Effects of SB334867 on oxycodone self-administration
Over the 21 days of self-administration training, the rats acquired oxycodone self-administration, reflected by a significant increase in the number of responses on the active vs. inactive lever. The rats emitted more responses on the active lever starting on day 8 vs. inactive lever and vs. day 1 (two-way ANOVA: day, F16,256 = 7.82, p < 0.001; lever, F1,16 = 25.60, p < 0.001; day × lever interaction, F16,256 = 8.02, p < 0.001; Sidak post hoc test: p < 0.05; Fig. 3A). Somatic withdrawal signs--teeth chattering, wet dog shakes, piloerection and ptosis--were significantly more pronounced starting on day 11 of oxycodone self-administration vs. the first measurement (Dunn’s post hoc test following Friedman ANOVA: H = 51.38, p < 0.001, vs. day 2; Fig. 3B), confirming oxycodone dependence (Rehni et al., 2013). Similar to the observations in Group 1, the correlational analysis revealed a significant relationship between the somatic withdrawal score and the number of oxycodone infusions that were earned on the last day of self-administration training (Fig. 3B, inset).
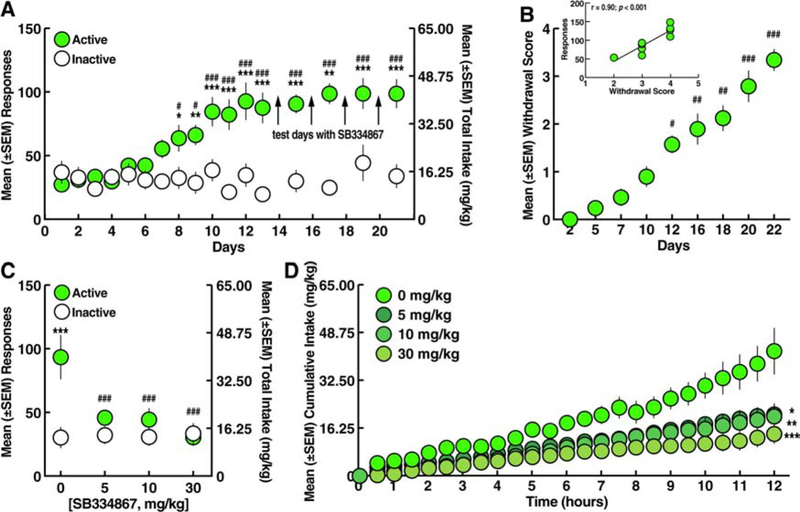
Figure 3.
Effect of SB334867 on oxycodone self-administration. A. Oxycodone self-administration. *p < 0.05, **p < 0.01, ***p < 0.001, vs. inactive lever; #p < 0.05, ###p < 0.001, vs. day 1 (Sidak post hoc test). B. Somatic withdrawal signs. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. day 2 (Dunn’s post hoc test). Inset. Correlation plot between the somatic withdrawal score and number of oxycodone infusions. C. SB334867 (5, 10, and 30 mg/kg) reduced the number of oxycodone infusions. ***p < 0.001, vs. inactive lever; ###p < 0.001, vs. vehicle (Sidak post hoc test). D. SB334867 (5, 10, and 30 mg/kg) reduced the cumulative intake of oxycodone. *p < 0.05, **p < 0.01, ***p < 0.001, vs. vehicle (Sidak post hoc test). n = 9.
The rats were tested for the ability of SB334867 to reduce oxycodone self-administration starting on day 14 (Fig. 3A). Compared with vehicle (0 mg/kg), SB334867 pretreatment decreased oxycodone self-administration (Fig. 3C) at 5, 10, and 30 mg/kg, without affecting responses on the inactive lever (two-way ANOVA: dose, F3,48 = 5.63, p < 0.01; lever, F1,16 = 6.51, p < 0.05; dose × lever interaction, F3,48 = 6.66, p < 0.001; Sidak post hoc test: p < 0.05, vs. 0 mg/kg; Fig. 3C). The two-way ANOVA of the temporal profile of oxycodone self-administration following SB334867 pretreatment showed that SB334867 produced lower cumulative intake across the 12-h self-administration session compared with vehicle (dose, F3,32 = 7.342, p < 0.001; time, F24,768 = 74.93, p < 0.001; dose × time interaction, F72,768 = 4.352, p < 0.001; Fig. 3D), reflected by decreases in the slopes of the cumulative response plots (F3,92 = 229.7, p < 0.001; Fig. 3D). No significant correlation was found between somatic withdrawal scores and the effects of SB334867 (data not shown).
Group 3. Effects of TCSOX229 on oxycodone self-administration
Similar to Groups 1 and 2, Group 3 acquired oxycodone self-administration, reflected by an increase in the number of responses on the active lever starting on day 9 vs. response on the inactive lever. Starting on day 11, a significantly higher number of responses on the active lever vs. day 1 was observed (two-way ANOVA: day, F16,288 = 4.69, p < 0.001; lever, F1,18 = 56.69, p < 0.001; day × lever interaction, F16,288 = 5.84, p < 0.001; Sidak post hoc test: p < 0.05; Fig. 4A). Compared with somatic withdrawal signs that were measured after the first self-administration session, somatic withdrawal signs were significantly higher starting after the 11th oxycodone self-administration session (Dunn’s post hoc test following Friedman ANOVA: H = 62.25, p < 0.001, vs. day 2; Fig. 4B) and were, as described for Group 1 and 2, teeth chattering, wet dog shakes, piloerection and ptosis. The correlational analysis revealed a significant relationship between the somatic withdrawal score and the number of oxycodone infusions that were earned on the last day of self-administration training (Fig. 4B, inset).
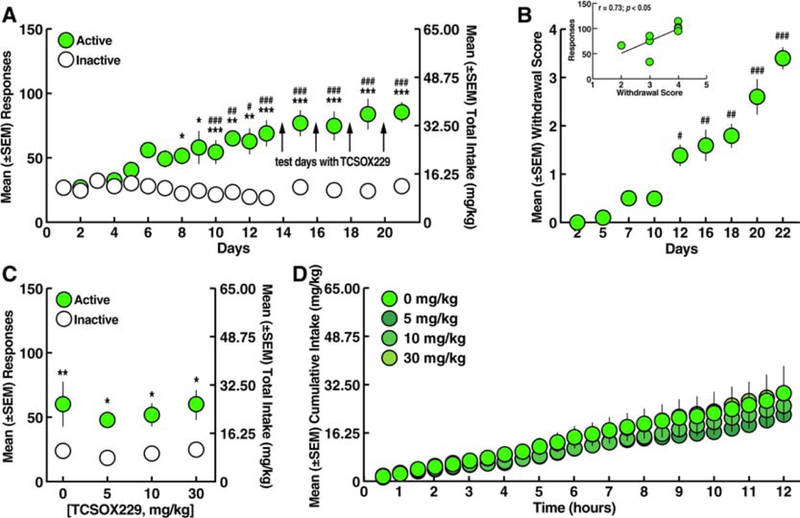
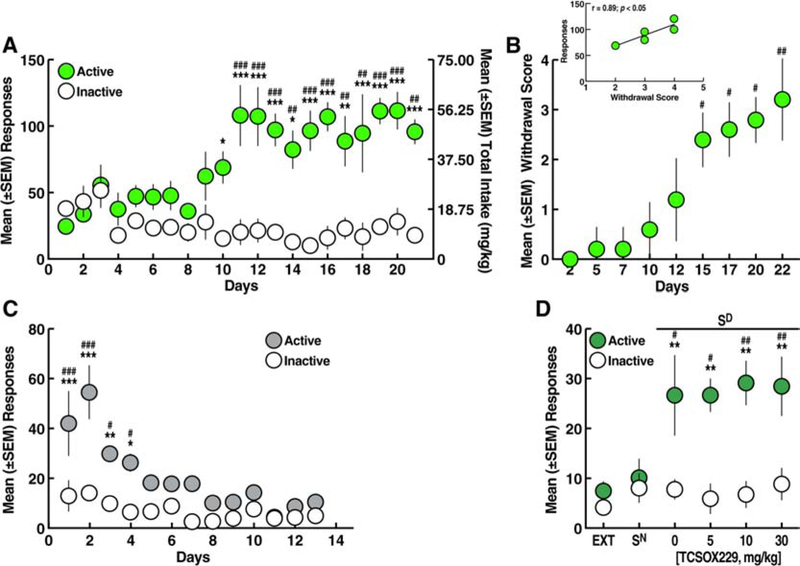
Figure 4.
Effect of TCSOX229 on oxycodone self-administration. A. Oxycodone self-administration. *p < 0.05, **p < 0.01, ***p < 0.001, vs. inactive lever; #p < 0.05, ##p < 0.01, ###p < 0.001, vs. day 1 (Sidak post hoc test). B. Somatic withdrawal signs. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. day 2 (Dunn’s post hoc test). Inset. Correlation plot between the somatic withdrawal score and number of oxycodone infusions. C. TCSOX229 (5, 10, and 30 mg/kg) did not modify oxycodone self-administration. *p < 0.05, **p < 0.01, vs. inactive lever (Sidak post hoc test). D. TCSOX229 (5, 10, and 30 mg/kg) did not alter the cumulative intake of oxycodone. n = 10.
The rats were tested for the ability of TCSOX229 (0, 5, 10, and 30 mg/kg) to reduce oxycodone self-administration starting on day 14 of self-administration (Fig. 4A). Pretreatment with TCSOX229 did not alter oxycodone self-administration or inactive lever responses (two-way ANOVA: dose, F3,54 = 0.63, p > 0.05; lever, F1,18 = 30.03, p < 0.001; dose × lever interaction, F3,54 = 0.11, p > 0.05; Fig. 4C). The two-way ANOVA showed that TCSOX229 did not alter the temporal profile of cumulative oxycodone intake over the 12-h self-administration sessions (dose, F3,36 = 0.26, p > 0.05; time, F23,828 = 69.70, p < 0.001; dose × time interaction, F69,828 = 0.44, p > 0.05; Fig. 4D).
Group 4. Effects of SB334867 on oxycodone conditioned reinstatement
The rats acquired oxycodone self-administration over the 21 days of training and emitted a significantly higher number of responses on the active lever starting on day 13 vs. inactive lever responses and vs. the first day of training (two-way ANOVA: day, F20,240 = 10.73, p < 0.001; lever, F1,12 = 38.55, p < 0.001; day × lever interaction, F20,240 = 5.21, p < 0.001; Sidak post hoc test, p < 0.05; Fig. 5A). Somatic withdrawal signs (teeth chattering, wet dog shakes, piloerection and ptosis) were significantly higher starting after the 14th self-administration session vs. the first measurement (i.e., after day 1 of self-administration; Dunn’s post hoc test following Friedman ANOVA: H = 48.14, p < 0.001, vs. day 2; Fig. 5B), confirming oxycodone dependence. Similar to Groups 1–3, a significant correlation was found between the somatic withdrawal score and the number of oxycodone infusions that were earned on the last day of self-administration training (Fig. 5B, inset).
Figure 5.
Effect of SB334867 on conditioned reinstatement induced by discriminative stimuli associated with oxycodone. A. Oxycodone self-administration. ***p < 0.001, vs. inactive lever; ###p < 0.001, vs. day 1 (Sidak post hoc test). B. Somatic withdrawal signs. #p < 0.05, ##p < 0.01, ###p < 0.001, vs. day 2 (Dunn’s post hoc test). Inset. Correlation plot between the somatic withdrawal score and number of oxycodone infusions. C. Extinction training over 14 sessions (2 h/day). *p < 0.05, ***p < 0.001, vs. inactive lever; #p < 0.05, ##p < 0.01, ###p < 0.001, vs. day 14 (Sidak post hoc test). D. Discriminative stimuli (Sd) that were conditioned to oxycodone induced response-reinstating behavior following the vehicle injection. SB334867 (5, 10, and 30 mg/kg) reduced Sd-induced oxycodone-seeking behavior. **p < 0.01, vs. inactive lever; ###p < 0.001, vs. EXT and Sn; +p < 0.05, +++p < 0.001, vs. vehicle (Sidak post hoc test). n = 7.
During the 14 days of extinction training, the rats gradually reduced their responses on the active lever (two-way ANOVA: days, F13,156 = 12.37, p < 0.001; lever, F1,12 = 12.5, p < 0.001; day × lever interaction, F13,156 = 3.78, p < 0.001; Fig. 5C), reaching a mean (± SEM) of 7.03 ± 2.14 responses at the end of the 2 weeks of extinction training. The Sidak post hoc test showed that the rats emitted more responses on the active lever vs. inactive lever from day 1 to day 6 of extinction (p < 0.05), and then responses on the active lever were statistically identical to responses on the inactive lever. Following extinction training, the effects of SB334867 on oxycodone conditioned reinstatement were tested. SB334867 at doses of 5, 10, and 30 mg/kg decreased oxycodone conditioned reinstatement and completely reversed oxycodone-seeking behavior to both extinction levels and Sn levels (two-way ANOVA: dose, F5,60 = 5.35, p < 0.001; lever, F1,12 = 5.27, p < 0.05; dose × lever interaction, F5,60 = 3.61, p < 0.01; Fig. 5D). No correlation was found between withdrawal scores and the effects of SB334867 on conditioned reinstatement (data not shown).
Group 5. Effects of TCSOX229 on oxycodone conditioned reinstatement
The rats in Group 5 readily acquired oxycodone self-administration over the 21-day training period, confirmed by a significantly higher number of responses on the active vs. inactive lever (starting on day 10) and vs. the first day of training (starting on day 11; two-way ANOVA: day, F20,160 = 3.09, p < 0.001; lever, F1,8 = 53.41, p < 0.001; day × lever interaction, F20,160 = 5.53, p < 0.001; Sidak post hoc test: p < 0.05; Fig. 6A). Starting after the 14th days of oxycodone self-administration, somatic withdrawal signs (i.e., teeth chattering, wet dog shakes, piloerection and ptosis consistent with the measurement in all the previous groups) were significantly higher vs. withdrawal signs that were measured after the first self-administration session (Dunn’s post hoc test following Friedman ANOVA: H = 36.61, p < 0.001, vs. day 2; Fig. 6B), confirming oxycodone dependence. Similar to Groups 1–4, a significant correlation was found between the somatic withdrawal score and the number of oxycodone infusions that were earned on the last day of self-administration training (Fig. 6B, inset).
Figure 6.
Effect of TCSOX229 on conditioned reinstatement induced by discriminative stimuli associated with oxycodone. A. Oxycodone self-administration. *p < 0.05, **p < 0.01, ***p < 0.001, vs. inactive lever; ##p < 0.01, ###p < 0.001, vs. day 1 (Sidak post hoc test). B. Somatic withdrawal signs. #p < 0.05, ##p < 0.01, vs. day 2 (Dunn’s post hoc test). Inset. Correlation plot between the somatic withdrawal score and number of oxycodone infusions. C. Extinction training over 13 sessions (2 h/day). *p < 0.05, **p < 0.01, ***p < 0.001, vs. inactive lever; #p < 0.05, ###p < 0.001, vs. day 13 (Sidak post hoc test). D. Discriminative stimuli (Sd) that were conditioned to oxycodone induced response-reinstating behavior following the vehicle injection. TCSOX229 did not affect Sd-induced oxycodone-seeking behavior. **p < 0.01, vs. inactive lever; #p < 0.05, ##p < 0.01, vs. EXT and Sn (Sidak post hoc test). n = 5.
During extinction training, the rats gradually reduced their responses on the active lever (two-way ANOVA: day, F12,96 = 10.41, p < 0.001; lever, F1,8 = 33.5, p < 0.001; day × lever interaction, F12,96 = 4.13, p < 0.001; Fig. 6C), reaching a mean (± SEM) of 7.53 ± 1.84 responses at the end of extinction training. The Sidak post hoc test showed that the rats emitted more responses on the active lever vs. inactive lever from day 1 to day 4 of extinction (p < 0.05), and then responses on the active lever were statistically identical to responses on the inactive lever. Following extinction training, the effects of TCSOX229 (0, 5, 10, and 30 mg/kg) on oxycodone conditioned reinstatement were tested. TCSOX229 did not modify Sd-induced oxycodone-seeking behavior at any dose tested, reflected by a greater number of responses on the active lever vs. extinction and vs. the Sn (two-way ANOVA: dose, F5,40 = 5.21, p < 0.001; lever, F1,8 = 17.3, p < 0.01; dose × lever interaction, F5,40 = 3.54, p < 0.01; Sidak post hoc test, p < 0.05; Fig. 6D).
DISCUSSION
The present study confirmed earlier findings (Blackwood, Hoerle, et al., 2019; Nguyen et al., 2019; Nguyen et al., 2018; Wade et al., 2015), in which rats readily self-administered oxycodone. With 12-h access, the rats readily acquired oxycodone self-administration, during which they significantly increased their intake and exhibited signs of dependence. The present study sought to characterize persistence of the motivating actions of oxycodone-related environmental stimuli (Sd). Particularly interesting was the finding that the response-reinstating actions of the oxycodone Sd were resistant to extinction, reflected by stability of the ability of the Sd to induce oxycodone-seeking behavior at undiminished levels over five presentations (i.e., 30 days after the last oxycodone self-administration session). The present study also found that OrxR1 blockade decreased oxycodone intake and prevented oxycodone-seeking behavior that was induced by oxycodone-related contextual stimuli, whereas OrxR2 blockade did not produce any effects. The present findings validate the behavioral model for studying prescription OUD and suggest an important role for OrxR1 in mediating the reinforcing action of oxycodone and appetitive behavior that is controlled by oxycodone-related stimuli. These findings identify OrxR1 as a potential treatment target for prescription OUD.
The model of extended access to oxycodone self-administration in the present study was shown to be reliable for studying prescription OUD, characterized by an increase in oxycodone intake (escalation) over time, the presence of somatic withdrawal signs, and reliable perseveration of the reinstatement of oxycodone-seeking behavior, similar to other drugs of abuse (Ciccocioppo, Martin-Fardon, & Weiss, 2004; Gracy et al., 2000; Martin-Fardon & Weiss, 2017; Weiss et al., 2001). Notably, however, the rats in the present study underwent oxycodone self-administration training by being directly subjected to a 12 h/day self-administration session. Therefore, the “escalation” of oxycodone intake that was observed in the present study may also reflect a normal pattern of the acquisition of an operant task. Although this a possibility, the escalation of opioid self-administration has been demonstrated to occur earlier for heroin, fentanyl, and oxycodone when rats are trained under extended-access conditions compared with short access (Blackwood, Hoerle, et al., 2019; Blackwood, Leary, Salisbury, McCoy, & Cadet, 2019; Wade et al., 2015), strongly suggesting that it also occurred in the present study. One hypothesis is that the transition from stable/low to escalated levels of drug intake depends on the amount of drug exposure, which has been demonstrated for cocaine and heroin self-administration (Ahmed, Kenny, Koob, & Markou, 2002; Ahmed, Walker, & Koob, 2000; Lenoir, Guillem, Koob, & Ahmed, 2012), suggesting that the escalation of intake is a common process for both opioids and psychostimulants. The transition to an increase in drug intake might result from gradual changes (i.e., tolerance) in the hedonic set point for the regulation of drug self-administration (Ahmed & Koob, 1998, 1999). Importantly, however, a recent study reported that intermittent access to cocaine was sufficient to induce signs of addiction-like behavior in rats, and the increase in cocaine consumption was not necessarily correlated with changes in motivation toward the drug or tolerance of the Orx system (James, Stopper, et al., 2019). This suggests that the pattern or schedule of drug self-administration may be as important as the amount of drug that is consumed to develop addiction-like phenotypes. This issue requires further investigation for opioids in general and oxycodone in particular. The phenomenon of the escalation of oxycodone intake might also be a hallmark of the resistance to extinction of oxycodone-seeking behavior that was observed herein. The persistent reinstatement of oxycodone-seeking behavior by oxycodone-related stimuli shows that, similar to other drugs of abuse, the perseveration of reinstatement that results from reward-environment associations is a phenomenon that is preferentially linked to drugs of abuse (e.g., Martin-Fardon et al., 2018; Martin-Fardon & Weiss, 2017). However, repeated conditioned reinstatement testing could be argued to simply result from a nonspecific behavioral response that occurs through general arousal. Notwithstanding this possibility, the behavioral effects of the oxycodone Sd are not likely attributable to nonspecific arousal or spontaneous recovery because responding on the inactive lever remained negligible and, more importantly, responding in the presence of the Sn remained at extinction levels. These observations indicate that the animals’ behavior was controlled selectively by the Sd. Moreover, the nearly identical behavioral results that were obtained during vehicle tests in the drug (SB338467 and TCSOX229) treatment groups (Groups 4 and 5) and the repeated reinstatement tests (Group 1) confirmed the generality of resistance to the extinction of oxycodone-cue effects.
The interest in evaluating the role of OrxR antagonists to treat prescription OUD derived from previous studies that indicated that the Orx system plays a role in modulating the high motivation to consume and seek drugs of abuse (Borgland et al., 2009; Boutrel, Steiner, & Halfon, 2013; Calipari & Espana, 2012; Mahler, Smith, Moorman, Sartor, & Aston-Jones, 2012; Marchant, Millan, & McNally, 2012; Yeoh, Campbell, James, Graham, & Dayas, 2014). The administration of SB334867 (5, 10, and 30 mg/kg) significantly reduced oxycodone self-administration, which is consistent with studies of heroin, fentanyl, and remifentanil self-administration (Fragale, Pantazis, James, & Aston-Jones, 2019; Mohammadkhani, James, Pantazis, & Aston-Jones, 2019; Smith & Aston-Jones, 2012). However, our findings contrast with some reports of cocaine self-administration, in which the OrxR1 antagonist SB334867 did not affect responding for cocaine (Borgland et al., 2009; Espana et al., 2010) unless the contingency to obtain cocaine was maintained under a higher-effort schedule of reinforcement (Bentzley & Aston-Jones, 2015; James et al., 2018). This suggests the drug-dependent involvement of OrxR1 during low-effort self-administration conditions (i.e., different across drug classes), supporting the hypothesis that addiction to opioids and psychostimulants involves distinct behavioral and neurobiological processes (Badiani, Belin, Epstein, Calu, & Shaham, 2011). The mechanisms that are responsible for changes in oxycodone intake that were observed in the present study following SB334867 administration are difficult to explain. SB334867 might decrease the motivation for oxycodone, reflected by a reduction of intake (self-administration; Fig. 3C, D) and seeking (reinstatement; Fig. 5C). Another possibility is that the reduction of oxycodone intake resulted from SB334867 enhancing the reinforcing action of oxycodone, such that less oxycodone was required to produce the desired reinforcing effect. This interpretation, however, is inconsistent with the observation that SB334867 significantly reduced the motivation to seek oxycodone (Fig. 5C).
In the present study, SB334867 also prevented oxycodone conditioned reinstatement, strongly suggesting that OrxR1 is involved in both the reinforcing effects of oxycodone and appetitive behavior that is controlled by oxycodone-related stimuli. This is consistent with several studies that showed that the Orx system, particularly Orx transmission that is mediated by OrxR1, is involved in reward seeking that is elicited by conditioned stimuli. SB334867 reduced cue-and context-induced reward-seeking behavior for fentanyl, heroin, cocaine, alcohol, and sucrose (Cason et al., 2010; Fragale et al., 2019; Jupp, Krstew, Dezsi, & Lawrence, 2011b; Lawrence, Cowen, Yang, Chen, & Oldfield, 2006; Martin-Fardon & Weiss, 2014a, 2014b; Smith & Aston-Jones, 2012; Smith et al., 2009; Smith et al., 2010). Additionally, SB334867 reduced the preference for contextual cues that were associated with morphine, cocaine, and amphetamine in the conditioned place preference (CPP) paradigm (Harris et al., 2005; Hutcheson et al., 2011). Notably, Orx appears to be involved specifically in reward seeking that is triggered by external stimuli when the motivation for drug seeking is enhanced by the presentation of stimuli that are previously associated with the drug (Mahler et al., 2014). One possibility is that the SB334867-induced decreases in both oxycodone self-administration and oxycodone conditioned reinstatement could be attributable to nonspecific effects, such as generalized motor impairment. However, this possibility is unlikely when considering that no alterations of the number of responses on the inactive lever were observed following SB334867 treatment. Moreover, SB334867 was previously reported to not affect locomotion (Richards et al., 2008; Smith et al., 2009; Voorhees & Cunningham, 2011) and was ineffective in reducing prime-induced heroin and cocaine seeking, cue-induced food seeking, and cocaine self-administration under low motivation/effort conditions within the dose range that was tested herein (Espana et al., 2010; James et al., 2018; Mahler, Smith, & Aston-Jones, 2013; Martin-Fardon & Weiss, 2014a, 2014b; Smith & Aston-Jones, 2012; Smith et al., 2009). Overall, these findings strongly suggest a specific effect of SB334867 in the present study.
Previous studies have shown that SB334867 exerts more pronounced effects in animals with higher motivation to obtain cocaine or alcohol (e.g., Moorman, James, Kilroy, & Aston-Jones, 2017; James, Stopper, et al., 2019; Pantazis, James, Bentzley, & Aston-Jones, 2019), with measurable effects in the 10–30 mg/kg dose range. For cocaine (James, Stopper, et al., 2019; Pantazis et al., 2019), this may be attributable to a higher number of Orx neurons in the lateral hypothalamus during cocaine abstinence. In the present study, SB334867 decreased oxycodone self-administration and reinstatement at a dose as low as 5 mg/kg, suggesting that low doses of OrxR1 antagonists might be effective selectively in individuals with substance use disorders. We found significant correlations between somatic withdrawal signs and the number of oxycodone infusions, but no correlation was found between withdrawal scores and the effects of SB334867 on oxycodone self-administration or reinstatement. This suggests that the magnitude of effects of SB334867 was not directly related to the severity of oxycodone dependence. Remaining to be determined are whether the number of Orx cells is higher in rats that exhibit higher oxycodone dependence and whether using other behavioral approaches (e.g., intermittent access with behavioral economics procedure) could reveal different outcomes (i.e., correlation between addiction-like phenotypes and SB334867 efficacy).
In contrast to our observations with SB334867, the OrxR2 antagonist TCSOX229 did not alter oxycodone self-administration or oxycodone conditioned reinstatement. These findings were surprising when considering earlier studies of other drugs of abuse that tested other OrxR2 antagonists. For example, JNJ10397049 dose-dependently reduced alcohol self-administration in rats and attenuated the acquisition, expression, and reinstatement of alcohol-induced CPP and alcohol-induced hyperactivity in mice (Shoblock et al., 2011). LSN2424100 reduced breakpoints on a progressive-ratio schedule and reduced alcohol consumption in alcohol-preferring rats (Anderson, Becker, Adams, Jesudason, & Rorick-Kehn, 2014). 2-SORA 18, although ineffective on nicotine self-administration and nicotine prime-induced reinstatement, blocked the cue-induced reinstatement of nicotine-seeking behavior (Uslaner et al., 2014). Intracerebroventricular TCSOX229 administration reduced alcohol self-administration but had no effect on the cue-induced reinstatement of alcohol seeking (Brown, Khoo, & Lawrence, 2013). When injected in the nucleus accumbens core and anterior paraventricular nucleus of the thalamus, TCSOX229 decreased alcohol intake (Barson, Ho, & Leibowitz, 2015; Brown et al., 2013). Intra-nucleus accumbens and intra-hippocampus TCSOX229 administration inhibited the reinstatement of morphine-seeking behavior in a CPP procedure in rats (Edalat, Kavianpour, Zarrabian, & Haghparast, 2018; Farzinpour, Taslimi, Azizbeigi, Karimi-Haghighi, & Haghparast, 2019; Sadeghi, Ezzatpanah, & Haghparast, 2016). An injection of TCSOX229 in the posterior paraventricular nucleus of the thalamus also decreased OrxA prime-induced cocaine seeking (Matzeu, Kerr, Weiss, & Martin-Fardon, 2016). Schmeichel et al. (2015) showed that NBI80713 decreased heroin self-administration in rats that were given extended access to heroin (12 h), suggesting that OrxR2, in addition to OrxR1, may be a potential therapeutic target for the treatment of OUD. Based on this study, the lack of effect of TCSOX229 on oxycodone intake was not expected and is difficult to reconcile. One explanation could be the differential pharmacological profiles of OrxR antagonists that are tested in different studies. Given the moderate selectivity of NBI80713 for OrxR2 vs. OrxR1 (Schmeichel et al., 2015), the attenuation of heroin self-administration may be partially mediated by OrxR1 blockade. Indeed, NBI80713 has nearly 40-fold higher affinity for OrxR2 vs. OrxR1, and TCSOX229 is a potent and selective OrxR2 antagonist with 250-fold higher affinity for OrxR2 vs. OrxR1 (Hirose et al., 2003; Mould, Brown, Marshall, & Langmead, 2014). Nevertheless, OrxR2 signaling might be involved in drug intake and seeking under conditions (i.e., high-demand conditions, prime-or stress-induced reinstatement) that are different from the conditions that were tested in the present study. Finally, one cannot exclude that the lack of effect of TCSOX229 on oxycodone self-administration and seeking could be due to the poor bioavailability of this compound when administered peripherally. Unfortunately to our knowledge, the pharmacokinetic profile of TCSOX229 has not yet been adequately described and will require further investigation in order to completely disregard this possibility.
The present study showed that the OrxR1 antagonist SB334867 effectively reduced oxycodone self-administration and oxycodone-seeking behavior, but the underlying neuronal mechanisms are still unknown. The Orx system has been shown to be recruited in behaviors that involve the stimulation of dopaminergic signaling in the ventral tegmental area (VTA) and NAc (Aston-Jones et al., 2009; Bonci & Borgland, 2009; Borgland, Ungless, & Bonci, 2010; Calipari & Espana, 2012), similar to the cue-induced reinstatement of cocaine seeking (James et al., 2011; Mahler et al., 2013). The involvement of the Orx system in mediating the behavioral effects of opioids, particularly morphine, has emerged in the past decade. For example, Orx neurons respond to opioid receptor antagonist-induced morphine withdrawal with an increase in Fos expression (Georgescu et al., 2003). In the same study, the authors found that Orx gene expression was induced by opioid withdrawal (Georgescu et al., 2003). Moreover, mice self-administered morphine directly in the lateral hypothalamus (Cazala, Darracq, & Saint-Marc, 1987), and the activation of Orx neurons or application of Orx in the VTA reinstated morphine seeking (Harris et al., 2005). Therefore, SB334867 might decrease oxycodone self-administration and reinstatement through actions on the mesolimbic system.
In conclusion, the present findings provide preclinical evidence of the efficacy of OrxR1 blockade in reducing oxycodone self-administration and oxycodone-seeking behavior. Although the neural mechanisms that underlie the involvement of the Orx system in oxycodone reinforcement and relapse remain to be established, the present findings indicate that selective OrxR1 antagonists may be beneficial for the treatment of prescription OUD.
Highlights.
OrxR1 blockade significantly decreased oxycodone self-administration and conditioned reinstatement.
OrxR2 blockade did not affect oxycodone self-administration or conditioned reinstatement.
OrxR1 antagonism might prevent excessive prescription opioid use and relapse.
Targeting OrxR1 might be beneficial for the treatment of prescription opioid use disorder.
ACKOWLEDGEMENTS
This is publication number 29852 from The Scripps Research Institute. The authors thank Hayoung (Haidi) Lee, Juan Jimenez, and Justin Norton for technical assistance and Michael Arends for assistance with manuscript preparation. This work was supported by the National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism (grant no. DA033344, AA024146, AA006420, AA022249, and AA026999 to RM-F).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed SH, Kenny PJ, Koob GF, & Markou A. (2002). Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci, 5(7), 625–626. doi: 10.1038/nn872 [DOI] [PubMed] [Google Scholar]
- Ahmed SH, & Koob GF. (1998). Transition from moderate to excessive drug intake: change in hedonic set point. Science, 282(5387), 298–300. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, & Koob GF. (1999). Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology (Berl), 146(3), 303–312. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Walker JR, & Koob GF. (2000). Persistent increase in the motivation to take heroin in rats with a history of drug escalation. Neuropsychopharmacology, 22(4), 413–421. doi: 10.1016/S0893-133X(99)00133-5 [DOI] [PubMed] [Google Scholar]
- Ammoun S, Holmqvist T, Shariatmadari R, Oonk HB, Detheux M, Parmentier M, … Kukkonen JP. (2003). Distinct recognition of OX1 and OX2 receptors by orexin peptides. J Pharmacol Exp Ther, 305(2), 507–514. doi: 10.1124/jpet.102.048025 jpet.102.048025 [pii] [DOI] [PubMed] [Google Scholar]
- Anderson RI, Becker HC, Adams BL, Jesudason CD, & Rorick-Kehn LM. (2014). Orexin-1 and orexin-2 receptor antagonists reduce ethanol self-administration in high-drinking rodent models. Front Neurosci, 8, 33. doi: 10.3389/fnins.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Moorman DE, & Richardson KA. (2009). Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology, 56 Suppl 1, 112–121. doi: 10.1016/j.neuropharm.2008.06.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, & Richardson KA. (2010). Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res, 1314, 74–90. doi: 10.1016/j.brainres.2009.09.106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badiani A, Belin D, Epstein D, Calu D, & Shaham Y. (2011). Opiate versus psychostimulant addiction: the differences do matter. Nat Rev Neurosci, 12(11), 685–700. doi: 10.1038/nrn3104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Ho HT, & Leibowitz SF. (2015). Anterior thalamic paraventricular nucleus is involved in intermittent access ethanol drinking: role of orexin receptor 2. Addict Biol, 20(3), 469–481. doi: 10.1111/adb.12139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentzley BS, & Aston-Jones G. (2015). Orexin-1 receptor signaling increases motivation for cocaine-associated cues. Eur J Neurosci, 41(9), 1149–1156. doi: 10.1111/ejn.12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Espana RA, & Vittoz NM. (2010). Hypocretin/orexin in arousal and stress. Brain Res, 1314, 91–102. doi: 10.1016/j.brainres.2009.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood CA, Hoerle R, Leary M, Schroeder J, Job MO, McCoy MT, … Cadet JL. (2019). Molecular Adaptations in the Rat Dorsal Striatum and Hippocampus Following Abstinence-Induced Incubation of Drug Seeking After Escalated Oxycodone Self-Administration. Mol Neurobiol, 56(5), 3603–3615. doi: 10.1007/s12035-018-1318-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwood CA, Leary M, Salisbury A, McCoy MT, & Cadet JL. (2019). Escalated Oxycodone Self-Administration Causes Differential Striatal mRNA Expression of FGFs and IEGs Following Abstinence-Associated Incubation of Oxycodone Craving. Neuroscience, 415, 173–183. doi: 10.1016/j.neuroscience.2019.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonci A, & Borgland S. (2009). Role of orexin/hypocretin and CRF in the formation of drug-dependent synaptic plasticity in the mesolimbic system. Neuropharmacology, 56 Suppl 1, 107–111. doi: 10.1016/j.neuropharm.2008.07.024 [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, … Bonci A. (2009). Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci, 29(36), 11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Ungless MA, & Bonci A. (2010). Convergent actions of orexin/hypocretin and CRF on dopamine neurons: Emerging players in addiction. Brain Res, 1314, 139–144. doi: 10.1016/j.brainres.2009.10.068 [DOI] [PubMed] [Google Scholar]
- Boutrel B, Steiner N, & Halfon O. (2013). The hypocretins and the reward function: what have we learned so far? Front Behav Neurosci, 7, 59. doi: 10.3389/fnbeh.2013.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RM, Khoo SY, & Lawrence AJ. (2013). Central orexin (hypocretin) 2 receptor antagonism reduces ethanol self-administration, but not cue-conditioned ethanol-seeking, in ethanol-preferring rats. Int J Neuropsychopharmacol, 16(9), 2067–2079. doi: 10.1017/S1461145713000333 [DOI] [PubMed] [Google Scholar]
- Calipari ES, & Espana RA. (2012). Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Front Behav Neurosci, 6, 54. doi: 10.3389/fnbeh.2012.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cason AM, Smith RJ, Tahsili-Fahadan P, Moorman DE, Sartor GC, & Aston-Jones G. (2010). Role of orexin/hypocretin in reward-seeking and addiction: implications for obesity. Physiol Behav, 100(5), 419–428. doi: 10.1016/j.physbeh.2010.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazala P, Darracq C, & Saint-Marc M. (1987). Self-administration of morphine into the lateral hypothalamus in the mouse. Brain Res, 416(2), 283–288. [DOI] [PubMed] [Google Scholar]
- Cherrier MM, Amory JK, Ersek M, Risler L, & Shen DD. (2009). Comparative cognitive and subjective side effects of immediate-release oxycodone in healthy middle-aged and older adults. J Pain, 10(10), 1038–1050. doi: 10.1016/j.jpain.2009.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccocioppo R, Angeletti S, & Weiss F. (2001). Long-lasting resistance to extinction of response reinstatement induced by ethanol-related stimuli: role of genetic ethanol preference. Alcohol Clin Exp Res, 25(10), 1414–1419. [DOI] [PubMed] [Google Scholar]
- Ciccocioppo R, Martin-Fardon R, & Weiss F. (2004). Stimuli associated with a single cocaine experience elicit long-lasting cocaine-seeking. Nat Neurosci, 7(5), 495–496. doi: 10.1038/nn1219 [DOI] [PubMed] [Google Scholar]
- Compton WM, Jones CM, & Baldwin GT. (2016). Relationship between Nonmedical Prescription-Opioid Use and Heroin Use. N Engl J Med, 374(2), 154–163. doi: 10.1056/NEJMra1508490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayas CV, McGranahan TM, Martin-Fardon R, & Weiss F. (2008). Stimuli linked to ethanol availability activate hypothalamic CART and orexin neurons in a reinstatement model of relapse. Biol Psychiatry, 63(2), 152–157. doi: 10.1016/j.biopsych.2007.02.002 [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, … Sutcliffe JG. (1998). The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A, 95(1), 322–327. doi: 10.1073/pnas.95.1.322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edalat P, Kavianpour M, Zarrabian S, & Haghparast A. (2018). Role of orexin-1 and orexin-2 receptors in the CA1 region of hippocampus in the forced swim stress-and food deprivation-induced reinstatement of morphine seeking behaviors in rats. Brain Res Bull, 142, 25–32. doi: 10.1016/j.brainresbull.2018.06.016 [DOI] [PubMed] [Google Scholar]
- Espana RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, & Jones SR. (2010). The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. Eur J Neurosci, 31(2), 336–348. doi: 10.1111/j.1460-9568.2009.07065.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzinpour Z, Taslimi Z, Azizbeigi R, Karimi-Haghighi S, & Haghparast A. (2019). Involvement of orexinergic receptors in the nucleus accumbens, in the effect of forced swim stress on the reinstatement of morphine seeking behaviors. Behav Brain Res, 356, 279–287. doi: 10.1016/j.bbr.2018.08.021 [DOI] [PubMed] [Google Scholar]
- Flores A, Valls-Comamala V, Costa G, Saravia R, Maldonado R, & Berrendero F. (2014). The hypocretin/orexin system mediates the extinction of fear memories. Neuropsychopharmacology, 39(12), 2732–2741. doi: 10.1038/npp.2014.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragale JE, Pantazis CB, James MH, & Aston-Jones G. (2019). The role of orexin-1 receptor signaling in demand for the opioid fentanyl. Neuropsychopharmacology. doi: 10.1038/s41386-019-0420-x [DOI] [PMC free article] [PubMed]
- Gatfield J, Brisbare-Roch C, Jenck F, & Boss C. (2010). Orexin receptor antagonists: a new concept in CNS disorders? ChemMedChem, 5(8), 1197–1214. doi: 10.1002/cmdc.201000132 [DOI] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, … DiLeone RJ. (2003). Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci, 23(8), 3106–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardino WJ, & de Lecea L. (2014). Hypocretin (orexin) neuromodulation of stress and reward pathways. Curr Opin Neurobiol, 29, 103–108. doi: 10.1016/j.conb.2014.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostin LO, Hodge JG Jr., & Noe SA. (2017). Reframing the Opioid Epidemic as a National Emergency. JAMA, 318(16), 1539–1540. doi: 10.1001/jama.2017.13358 [DOI] [PubMed] [Google Scholar]
- Gracy KN, Dankiewicz LA, Weiss F, & Koob GF. (2000). Heroin-specific stimuli reinstate operant heroin-seeking behavior in rats after prolonged extinction. Pharmacol Biochem Behav, 65(3), 489–494. [DOI] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, & Aston-Jones G. (2005). A role for lateral hypothalamic orexin neurons in reward seeking. Nature, 437(7058), 556–559. doi: 10.1038/nature04071 [DOI] [PubMed] [Google Scholar]
- Hirose M, Egashira S, Goto Y, Hashihayata T, Ohtake N, Iwaasa H, … Yamada K. (2003). N-acyl 6,7-dimethoxy-1,2,3,4-tetrahydroisoquinoline: the first orexin-2 receptor selective non-peptidic antagonist. Bioorg Med Chem Lett, 13(24), 4497–4499. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, & Kenny PJ. (2012). Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Front Behav Neurosci, 6, 47. doi: 10.3389/fnbeh.2012.00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson DM, Quarta D, Halbout B, Rigal A, Valerio E, & Heidbreder C. (2011). Orexin-1 receptor antagonist SB-334867 reduces the acquisition and expression of cocaine-conditioned reinforcement and the expression of amphetamine-conditioned reward. Behav Pharmacol, 22(2), 173–181. doi: 10.1097/FBP.0b013e328343d761 [DOI] [PubMed] [Google Scholar]
- James MH, Bowrey HE, Stopper CM, & Aston-Jones G. (2019). Demand elasticity predicts addiction endophenotypes and the therapeutic efficacy of an orexin/hypocretin-1 receptor antagonist in rats. Eur J Neurosci, 50(3), 2602–2612. doi: 10.1111/ejn.14166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James MH, Campbell EJ, & Dayas CV. (2017). Role of the Orexin/Hypocretin System in Stress-Related Psychiatric Disorders. Curr Top Behav Neurosci, 33, 197–219. doi: 10.1007/7854_2016_56 [DOI] [PubMed] [Google Scholar]
- James MH, Charnley JL, Levi EM, Jones E, Yeoh JW, Smith DW, & Dayas CV. (2011). Orexin-1 receptor signalling within the ventral tegmental area, but not the paraventricular thalamus, is critical to regulating cue-induced reinstatement of cocaine-seeking. Int J Neuropsychopharmacol, 14(5), 684–690. doi: 10.1017/S1461145711000423 [DOI] [PubMed] [Google Scholar]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, & Aston-Jones G. (2018). Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry. doi: 10.1016/j.biopsych.2018.07.022 [DOI] [PMC free article] [PubMed]
- James MH, Stopper CM, Zimmer BA, Koll NE, Bowrey HE, & Aston-Jones G. (2019). Increased Number and Activity of a Lateral Subpopulation of Hypothalamic Orexin/Hypocretin Neurons Underlies the Expression of an Addicted State in Rats. Biol Psychiatry, 85(11), 925–935. doi: 10.1016/j.biopsych.2018.07.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Muhuri PK, & Lurie PG. (2017). Trends in the Nonmedical Use of OxyContin, United States, 2006 to 2013. Clin J Pain, 33(5), 452–461. doi: 10.1097/AJP.0000000000000426 [DOI] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, & Lawrence AJ. (2011a). Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin receptors. Br J Pharmacol, 162(4), 880–889. doi: 10.1111/j.1476-5381.2010.01088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jupp B, Krstew E, Dezsi G, & Lawrence AJ. (2011b). Discrete cue-conditioned alcohol-seeking after protracted abstinence: pattern of neural activation and involvement of orexin(1) receptors. Br J Pharmacol, 162(4), 880–889. doi: 10.1111/j.1476-5381.2010.01088.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodny A, Courtwright DT, Hwang CS, Kreiner P, Eadie JL, Clark TW, & Alexander GC. (2015). The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health, 36, 559–574. doi: 10.1146/annurev-publhealth-031914-122957 [DOI] [PubMed] [Google Scholar]
- Koob GF. (2009). Brain stress systems in the amygdala and addiction. Brain Res, 1293, 61–75. doi: 10.1016/j.brainres.2009.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwaki T, & Zhang W. (2012). Orexin neurons and emotional stress. Vitam Horm, 89, 135–158. doi: 10.1016/B978-0-12-394623-2.00008-1 [DOI] [PubMed] [Google Scholar]
- Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, & Shen DD. (2006). Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther, 79(5), 461–479. doi: 10.1016/j.clpt.2006.01.009 [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, & Oldfield B. (2006). The orexin system regulates alcohol-seeking in rats. Br J Pharmacol, 148(6), 752–759. doi: 10.1038/sj.bjp.0706789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenoir M, Guillem K, Koob GF, & Ahmed SH. (2012). Drug specificity in extended access cocaine and heroin self-administration. Addict Biol, 17(6), 964–976. doi: 10.1111/j.1369-1600.2011.00385.x [DOI] [PubMed] [Google Scholar]
- Li Y, & van den Pol AN. (2008). Mu-opioid receptor-mediated depression of the hypothalamic hypocretin/orexin arousal system. J Neurosci, 28(11), 2814–2819. doi: 10.1523/JNEUROSCI.5447-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Moorman DE, Smith RJ, James MH, & Aston-Jones G. (2014). Motivational activation: a unifying hypothesis of orexin/hypocretin function. Nat Neurosci, 17(10), 1298–1303. doi: 10.1038/nn.3810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, & Aston-Jones G. (2013). Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl), 226(4), 687–698. doi: 10.1007/s00213-012-2681-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Moorman DE, Sartor GC, & Aston-Jones G. (2012). Multiple roles for orexin/hypocretin in addiction. Prog Brain Res, 198, 79–121. doi: 10.1016/B978-0-444-59489-1.00007-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant NJ, Millan EZ, & McNally GP. (2012). The hypothalamus and the neurobiology of drug seeking. Cell Mol Life Sci, 69(4), 581–597. doi: 10.1007/s00018-011-0817-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, & Elmquist JK. (2001). Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol, 435(1), 6–25. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Cauvi G, Kerr TM, & Weiss F. (2018). Differential role of hypothalamic orexin/hypocretin neurons in reward seeking motivated by cocaine versus palatable food. Addict Biol, 23(1), 6–15. doi: 10.1111/adb.12441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, & Weiss F. (2014a). Blockade of hypocretin receptor-1 preferentially prevents cocaine seeking: comparison with natural reward seeking. Neuroreport, 25(7), 485–488. doi: 10.1097/WNR.0000000000000120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, & Weiss F. (2014b). N-(2-methyl-6-benzoxazolyl)-N’-1,5-naphthyridin-4-yl urea (SB334867), a hypocretin receptor-1 antagonist, preferentially prevents ethanol seeking: comparison with natural reward seeking. Addict Biol, 19(2), 233–236. doi: 10.1111/j.1369-1600.2012.00480.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, & Weiss F. (2017). Perseveration of craving: effects of stimuli conditioned to drugs of abuse versus conventional reinforcers differing in demand. Addict Biol, 22(4), 923–932. doi: 10.1111/adb.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Fardon R, Zorrilla EP, Ciccocioppo R, & Weiss F. (2010). Role of innate and drug-induced dysregulation of brain stress and arousal systems in addiction: Focus on corticotropin-releasing factor, nociceptin/orphanin FQ, and orexin/hypocretin. Brain Res, 1314, 145–161. doi:S0006-8993(09)02661-4 [pii] 10.1016/j.brainres.2009.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzeu A, Kerr TM, Weiss F, & Martin-Fardon R. (2016). Orexin-A/Hypocretin-1 Mediates Cocaine-Seeking Behavior in the Posterior Paraventricular Nucleus of the Thalamus via Orexin/Hypocretin Receptor-2. J Pharmacol Exp Ther, 359(2), 273–279. doi: 10.1124/jpet.116.235945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, & Gold MS. (1994). Dissociation of “conscious desire” (craving) from and relapse in alcohol and cocaine dependence. Ann Clin Psychiatry, 6(2), 99–106. [DOI] [PubMed] [Google Scholar]
- Mohammadkhani A, James MH, Pantazis CB, & Aston-Jones G. (2019). Persistent effects of the orexin-1 receptor antagonist SB-334867 on motivation for the fast acting opioid remifentanil. Brain Res, 146461. doi: 10.1016/j.brainres.2019.146461 [DOI] [PMC free article] [PubMed]
- Moorman DE, James MH, Kilroy EA, & Aston-Jones G. (2017). Orexin/hypocretin-1 receptor antagonism reduces ethanol self-administration and reinstatement selectively in highly-motivated rats. Brain Res, 1654(Pt A), 34–42. doi: 10.1016/j.brainres.2016.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould R, Brown J, Marshall FH, & Langmead CJ. (2014). Binding kinetics differentiates functional antagonism of orexin-2 receptor ligands. Br J Pharmacol, 171(2), 351–363. doi: 10.1111/bph.12245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhuri PKG, J.C.; Davies MC. (2013). Associations of Nonmedical Pain Reliever Use and Initiation of Heroin Use in the United States.
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, … Suzuki T. (2006). Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci, 26(2), 398–405. doi: 10.1523/JNEUROSCI.2761-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Drug Abuse (2018). Medications to Treat Opioid Use Disorder. National Institute on Drug Abuse, Advancing Addiction Science. Retrieved from https://www.drugabuse.gov/publications/research-reports/medications-to-treat-opioid-addiction/overview
- National Institute on Drug Abuse (2019). Opioid Overdose Crisis. National Institute of Drug of Abuse; Advancing Addiciton Science. Retrieved from https://www.drugabuse.gov/drugs-abuse/opioids/opioid-overdose-crisis
- Nguyen JD, Grant Y, Creehan KM, Hwang CS, Vandewater SA, Janda KD, … Taffe MA. (2019). Delta(9)-tetrahydrocannabinol attenuates oxycodone self-administration under extended access conditions. Neuropharmacology, 151, 127–135. doi: 10.1016/j.neuropharm.2019.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen JD, Hwang CS, Grant Y, Janda KD, & Taffe MA. (2018). Prophylactic vaccination protects against the development of oxycodone self-administration. Neuropharmacology, 138, 292–303. doi: 10.1016/j.neuropharm.2018.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP, Childress AR, Ehrman R, & Robbins SJ. (1998). Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol, 12(1), 15–22. doi: 10.1177/026988119801200103 [DOI] [PubMed] [Google Scholar]
- Pantazis CB, James MH, Bentzley BS, & Aston-Jones G. (2019). The number of lateral hypothalamus orexin/hypocretin neurons contributes to individual differences in cocaine demand. Addict Biol, e12795. doi: 10.1111/adb.12795 [DOI] [PMC free article] [PubMed]
- Peckham EM, & Traynor JR. (2006). Comparison of the antinociceptive response to morphine and morphine-like compounds in male and female Sprague-Dawley rats. J Pharmacol Exp Ther, 316(3), 1195–1201. doi: 10.1124/jpet.105.094276 [DOI] [PubMed] [Google Scholar]
- Rehni AK, Jaggi AS, & Singh N. (2013). Opioid withdrawal syndrome: emerging concepts and novel therapeutic targets. CNS Neurol Disord Drug Targets, 12(1), 112–125. [DOI] [PubMed] [Google Scholar]
- Richards JK, Simms JA, Steensland P, Taha SA, Borgland SL, Bonci A, & Bartlett SE. (2008). Inhibition of orexin-1/hypocretin-1 receptors inhibits yohimbine-induced reinstatement of ethanol and sucrose seeking in Long-Evans rats. Psychopharmacology (Berl), 199(1), 109–117. doi: 10.1007/s00213-008-1136-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosow CE. (1987). The clinical usefulness of agonist-antagonist analgesics in acute pain. Drug Alcohol Depend, 20(4), 329–337. [DOI] [PubMed] [Google Scholar]
- Rudd RA, Seth P, David F, & Scholl L. (2016). Increases in Drug and Opioid-Involved Overdose Deaths-United States, 2010–2015. MMWR Morb Mortal Wkly Rep, 65(50–51), 1445–1452. doi: 10.15585/mmwr.mm655051e1 [DOI] [PubMed] [Google Scholar]
- Sadeghi B, Ezzatpanah S, & Haghparast A. (2016). Effects of dorsal hippocampal orexin-2 receptor antagonism on the acquisition, expression, and extinction of morphine-induced place preference in rats. Psychopharmacology (Berl), 233(12), 2329–2341. doi: 10.1007/s00213-016-4280-3 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, … Yanagisawa M. (1998). Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell, 92(4), 573–585. [DOI] [PubMed] [Google Scholar]
- Scammell TE, & Winrow CJ. (2011). Orexin receptors: pharmacology and therapeutic opportunities. Annu Rev Pharmacol Toxicol, 51, 243–266. doi: 10.1146/annurev-pharmtox-010510-100528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeichel BE, Barbier E, Misra KK, Contet C, Schlosburg JE, Grigoriadis D, … Vendruscolo LF. (2015). Hypocretin receptor 2 antagonism dose-dependently reduces escalated heroin self-administration in rats. Neuropsychopharmacology, 40(5), 1123–1129. doi: 10.1038/npp.2014.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth P, Scholl L, Rudd RA, & Bacon S. (2018). Overdose Deaths Involving Opioids, Cocaine, and Psychostimulants-United States, 2015–2016. MMWR Morb Mortal Wkly Rep, 67(12), 349–358. doi: 10.15585/mmwr.mm6712a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock JR, Welty N, Aluisio L, Fraser I, Motley ST, Morton K, … Galici R. (2011). Selective blockade of the orexin-2 receptor attenuates ethanol self-administration, place preference, and reinstatement. Psychopharmacology (Berl), 215(1), 191–203. doi: 10.1007/s00213-010-2127-x [DOI] [PubMed] [Google Scholar]
- Smith RJ, & Aston-Jones G. (2012). Orexin / hypocretin 1 receptor antagonist reduces heroin self-administration and cue-induced heroin seeking. Eur J Neurosci, 35(5), 798–804. doi: 10.1111/j.1460-9568.2012.08013.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, & Aston-Jones G. (2009). Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci, 30(3), 493–503. doi: 10.1111/j.1460-9568.2009.06844.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, & Aston-Jones G. (2010). Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology, 58(1), 179–184. doi: 10.1016/j.neuropharm.2009.06.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabaeizadeh M, Motiei-Langroudi R, Mirbaha H, Esmaeili B, Tahsili-Fahadan P, Javadi-Paydar M, … Dehpour AR. (2013). The differential effects of OX1R and OX2R selective antagonists on morphine conditioned place preference in naive versus morphine-dependent mice. Behav Brain Res, 237, 41–48. doi: 10.1016/j.bbr.2012.09.010 [DOI] [PubMed] [Google Scholar]
- Tiffany ST, & Carter BL. (1998). Is craving the source of compulsive drug use? J Psychopharmacol, 12(1), 23–30. doi: 10.1177/026988119801200104 [DOI] [PubMed] [Google Scholar]
- Tiffany ST, & Conklin CA. (2000). A cognitive processing model of alcohol craving and compulsive alcohol use. Addiction, 95(8 Suppl 2), 145–153. [DOI] [PubMed] [Google Scholar]
- Trivedi P, Yu H, MacNeil DJ, Van der Ploeg LH, & Guan XM. (1998). Distribution of orexin receptor mRNA in the rat brain. FEBS Lett, 438(1–2), 71–75. [DOI] [PubMed] [Google Scholar]
- Tsujino N, & Sakurai T. (2009). Orexin/hypocretin: a neuropeptide at the interface of sleep, energy homeostasis, and reward system. Pharmacol Rev, 61(2), 162–176. doi:61/2/162 [pii] 10.1124/pr.109.001321 [DOI] [PubMed] [Google Scholar]
- Tsujino N, & Sakurai T. (2013). Role of orexin in modulating arousal, feeding, and motivation. Front Behav Neurosci, 7, 28. doi: 10.3389/fnbeh.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uslaner JM, Winrow CJ, Gotter AL, Roecker AJ, Coleman PJ, Hutson PH, … Renger JJ. (2014). Selective orexin 2 receptor antagonism blocks cue-induced reinstatement, but not nicotine self-administration or nicotine-induced reinstatement. Behav Brain Res, 269, 61–65. doi: 10.1016/j.bbr.2014.04.012 [DOI] [PubMed] [Google Scholar]
- van de Laar MC, Licht R, Franken IH, & Hendriks VM. (2004). Event-related potentials indicate motivational relevance of cocaine cues in abstinent cocaine addicts. Psychopharmacology (Berl), 177(1–2), 121–129. doi: 10.1007/s00213-004-1928-1 [DOI] [PubMed] [Google Scholar]
- Volkow ND, & Collins FS. (2017). The Role of Science in Addressing the Opioid Crisis. N Engl J Med, 377(4), 391–394. doi: 10.1056/NEJMsr1706626 [DOI] [PubMed] [Google Scholar]
- Voorhees CM, & Cunningham CL. (2011). Involvement of the orexin/hypocretin system in ethanol conditioned place preference. Psychopharmacology (Berl), 214(4), 805–818. doi: 10.1007/s00213-010-2082-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, & Koob GF. (2015). Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology, 40(2), 421–428. doi: 10.1038/npp.2014.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Li S, & Kirouac GJ. (2017). Role of the orexin (hypocretin) system in contextual fear conditioning in rats. Behav Brain Res, 316, 47–53. doi: 10.1016/j.bbr.2016.08.052 [DOI] [PubMed] [Google Scholar]
- Weiss F, Martin-Fardon R, Ciccocioppo R, Kerr TM, Smith DL, & Ben-Shahar O. (2001). Enduring resistance to extinction of cocaine-seeking behavior induced by drug-related cues. Neuropsychopharmacology, 25(3), 361–372. doi: 10.1016/S0893-133X(01)00238-X [DOI] [PubMed] [Google Scholar]
- Yeoh JW, Campbell EJ, James MH, Graham BA, & Dayas CV. (2014). Orexin antagonists for neuropsychiatric disease: progress and potential pitfalls. Front Neurosci, 8, 36. doi: 10.3389/fnins.2014.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, & Kreek MJ. (2006). Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol, 191(1), 137–145. doi: 10.1677/joe.1.06960 [DOI] [PubMed] [Google Scholar]