Abstract
Background:
Previous research has detailed the hemisphere dependence and specific kinematic deficits observed for the less affected arm of patients with unilateral stroke.
Objective:
We now examine whether functional motor deficits in the less affected arm, measured by standardized clinical measures of motor function, also depend on the hemisphere that was damaged and on the severity of contralesional impairment.
Methods:
We recruited 48 left hemisphere damaged (LHD) participants, 62 right hemisphere damaged (RHD) participants, and 54 age-matched control participants. Measures of motor function included: 1) Jebsen-Taylor Hand Function Test (JHFT), 2) Grooved Pegboard Test (GPT) and 3) grip strength. We measured the extent of contralesional arm impairment with the upper extremity component of the Fugl-Meyer (UEFM) assessment of motor impairment.
Results:
Ipsilesional limb functional performance deficits (JHFT) varied with both the damaged hemisphere and severity of contralesional arm impairment with the most severe deficits expressed in LHD participants with severe contralesional impairment (UEFM). GPT and grip strength varied with severity of contralesional impairment, but not with hemisphere.
Conclusions:
Stroke survivors with the most severe paretic arm impairment, who must rely on their ipsilesional arm for performing daily activities, have the greatest motor deficit in the less affected arm. We recommend remediation of this arm to improve functional independence in this group of stroke patients.
Keywords: Stroke, Ipsilesional Deficits, Motor Lateralization, Hemisphere Specific Deficits, Functional Deficits
Introduction
Stroke is a major health problem in the United States that leaves many survivors with chronic motor impairments, including hemiparesis in the limbs that are on the opposite side of the body to the damaged brain hemisphere. Although motor deficits in the less affected arm in unilaterally lesioned stroke patients have been documented as early as 1967 1 more recent research has shown that these deficits are often functionally limiting and persist throughout the chronic phase of stroke 2-10. Studies of motor function in the less affected arm of chronic stroke patients have reported performance deficiencies on a number of clinical tests, including the Purdue Pegboard Test 11, the Jebsen-Taylor Hand Function Test (JHFT) 2, and a variety of tests that directly assess or simulate activities of daily living 12,13. Furthermore, studies of chronic stroke patients that quantify arm and joint kinematics have shown significant deficits in movement coordination and accuracy 2,7-10,14-22.
Previous studies have examined the hemisphere specificity of ipsilesional motor deficits by quantifying detailed kinematic measures during reaching movements 7-10,15,16,18. These studies have proposed that motor deficits observed for the less affected arm result from a loss of contributions to motor control by the ipsilateral hemisphere, and have confirmed that, as a result of brain lateralization, right- and left-hemisphere damage produces different hemisphere-specific deficits in motor control 7,15,18,23. However, the hemisphere-specific nature of ipsilesional motor deficits is controversial because some studies have shown that when measured by clinical tests, such deficits do not appear to vary with hemisphere. Based on our previous studies of hemispheric lateralization for motor control in typical adults and stroke survivors, we now propose that this apparent discrepancy between studies is due to two interacting factors: the extent of contralesional paresis and the hemisphere that is damaged. If this 2-factor dependence exists, then studies that do not stratify participants using both factors can report erroneous dependence on a single factor. It is also plausible that some functional tests and/or components are more sensitive to hemisphere-dependent processes, whereas other components are not. In this study, we will test both of these hypotheses with three functional clinical tests in a large sample of participants with unilateral middle cerebral artery stroke, stratified by severity of contralesional motor impairment and hand, indicating the hemisphere lesioned in stroke participants. We hypothesize that 1) ipsilesional motor deficits are due to the unique contributions to motor control from the ipsilateral hemisphere of the less affected arm, and 2) that impairment in the less affected arm results from a reduced contribution from the ipsilesional hemisphere to the control of both arms. We thus predict that ipsilesional deficits will vary with both the hemisphere that is damaged and the extent of contralesional motor impairment such that ipsilesional motor deficits will be greatest in the less affected arm for those patients with greater contralesional motor deficits. Additionally, we assess the presence or absence of apraxia because it too may have a role in ipsilesional (less affected) arm performance.
Methods
Participants
Sixty-two right hemisphere damaged (RHD), 48 left hemisphere damaged (LHD) chronic unilateral stroke survivors, and 54 age- and education- matched control participants were evaluated at the Penn State Milton S. Hershey Medical Center. Participants were screened and excluded based on a history of (1) hospitalization for substance abuse and/or psychiatric diagnosis; (2) non-stroke neurological diseases or multiple strokes for stroke participants and all neurological diagnoses for the control participants; (3) brain stem or bilateral lesions; and (4) peripheral movement disorders. Stroke participants were right-handed prior to stroke, and control participants were right-handed according to the Edinburgh inventory used to assess handedness 24. Control group participants were randomly assigned to use either their left or right arm for the unilateral clinical tests. Stroke participants always used their less affected, ipsilesional arm. The Pennsylvania State College of Medicine Institutional Review Board approved the study protocol, and written informed consent was obtained from all participants.
Edinburgh Handedness Inventory
We determined handedness using the Edinburgh Handedness Inventory 24. We recruited only right-handed participants because left-handers do not represent a behaviorally- 24 nor a neurologically- 25 homogenous population. In contrast, right-handers are a fairly homogenous population, with respect to both behavioral and neurophysiological measures 26.
Visual Neglect and Aphasia
Visual neglect and aphasia are reported in table 1. Information was obtained based on medical records. Visual neglect information was unavailable for 1 LHD and 2 RHD participants.
Table 1:
Summary of participant information
| Handa | Contra Severity |
N | M | F | Ageb | EDUb | Chronicityc, d | Neglectd. | Aphasiae | Apraxiae |
|---|---|---|---|---|---|---|---|---|---|---|
| Left | Control | 27 | 15 | 12 | 57.4 ± 11.3 | 15.3 ± 2.7 | NA | 0 | 0 | 0 |
| Left | Mild | 32 | 23 | 9 | 61.9 ± 10.5 | 14.5 ± 2.5 | 2.0 ± 1.5 | 2 | 16 | 6 |
| Left | Moderate | 6 | 4 | 2 | 52.4 ± 9.8 | 13.5 ± 2.0 | 5.4 ± 4.1 | 1 | 3 | 1 |
| Left | Severe | 10 | 8 | 2 | 59.8 ± 12.2 | 14.6 ± 2.8 | 2.2 ± 1.6 | 0 | 6 | 5 |
| Right | Control | 27 | 17 | 10 | 58.2 ± 10.9 | 14.9 ± 2.9 | NA | 0 | 0 | 0 |
| Right | Mild | 26 | 20 | 6 | 59.5 ± 10.9 | 14.2 ± 2.7 | 3.1 ± 4.6 | 0 | 0 | 0 |
| Right | Moderate | 8 | 4 | 4 | 64.6 ± 11.7 | 12.0 ± 0.5 | 3.5 ± 3.2 | 2 | 0 | 3 |
| Right | Severe | 28 | 23 | 5 | 57.2 ± 11.4 | 13.5 ± 2.7 | 3.2 ± 2.9 | 2 | 0 | 5 |
Notes: Contra Severity Contralesional impairment severity group; N number in group; M male; F female; EDU education;
Hand represents hemisphere damaged in stroke participants
Statistics give mean ± standard deviation in years
Years post stroke
Number of participants with visual neglect
Number of participants with aphasia
Paretic Arm Evaluation
Upper Extremity Fugl-Meyer Assessment
The Upper Extremity Fugl-Meyer Assessment (UEFM) was used to quantify motor impairment in the contralesional upper limb. Based on the UEFM score, we used the scores suggested by Woytowicz et al. (2017) to stratify participants into 3 impairment groups: Mild (UEFM ≥43), Moderate (UEFM = 29-42) and Severe (UEFM = 0-28). In the severe group, 95-100% were unable to perform wrist movements, mass finger extension, nor the prehension items of the UEFM. These groups are referred to as contralesional impairment groups. The rationale for clustering participants into 3 contralesional impairment groups, based on Fugl-Meyer score is that the Fugl-Meyer assessment does not provide a continuous linear index of impairment, but instead provides a valid and reliable categorical measurement of impairment that can identify 3 groups of clinically identifiable levels of impairment27, 28.
Less affected Arm Evaluations
Primary Measure:
Jebsen-Taylor Hand Function Test
The Jebsen-Taylor Hand Function Test (JHFT) is a clinical assessment of unilateral arm function 29 and consists of a range of tasks that simulate the coordination requirements of functional daily activities 30,31. It includes 7 timed tasks that require dexterity and arm coordination: stacking checkers, feeding, manipulating and lifting objects, writing, and page turning 31. The JHFT represented our primary measure of hand function, because it is a comprehensive assessment of unilateral functional performance.
Secondary Measures:
Grooved Pegboard Test
The Grooved Pegboard Test (GPT) is a timed distal dexterity and perceptual orientation test that consists of placing 25 small pegs in holes of different orientations as quickly as possible (Lafayette instrument). We used the Halstead-Reitan Battery revised comprehensive norms (2004) to calculate standardized T-scores which account for age, gender, education, and ethnic differences32. Greater scores indicate faster performance.
Grip strength
Grip strength was measured using a hand dynamometer (Lafayette instrument), and maximum force was averaged across 3 trials, separated by short rest periods. Standardized T-scores were obtained using the Halstead-Reitan Battery revised comprehensive norms (2004)32.
Ideomotor Limb Apraxia
Ideomotor limb apraxia was measured using a standardized test in which participants were videotaped for scoring 33. Using the ipsilesional arm, stroke participants were asked to imitate five nonrepresentative (e.g., hand behind head), five representative (e.g., salute), and five pantomimed object use (e.g., brush teeth) movements. Each of the 15 items was scored as 1 (correct movement) or 0 (incorrect movement), with scores of 11 or less indicative of the presence of apraxia 34.. It should be stressed that the use of this battery provides a valid cut-off for identification of apraxia. In addition, we identified participants as apraxic, based on medical records in two cases, when apraxia battery scores could not be obtained. We thus categorized participants as either apraxic or non-apraxic, based on these criterion.
Statistics
To confirm our LHD and RHD groups had similar UEFM scores within each severity level, we performed a two-way ANOVA on the stroke participants with severity group (Mild, Moderate, Severe) and hemisphere of damage (Left, Right) as between-subject factors. These severity groups were then treated as a categorical factor for analysis of our dependent variables (Jebsen-Taylor Hand Function Test, Grip Strength, and Pegboard Test).
For each of our ipsilesional deficit measures, the JHFT, grip strength, and GPT, we used a two-way ANOVA analyses with contralesional impairment severity (Control, Mild, Moderate, Severe) and hand (Left, Right) as between-subject factors. Note that our Control group represents the ‘no impairment’ group in this stratification scheme. In stroke participants, ‘hand group’ represented the hemisphere that was damaged, since all stroke participants used their ipsilesional hand to perform the JHFT, grip strength, and GPT. When warranted, we used Bonferroni-adjusted Student t-tests to perform a post-hoc analysis with a p < .05 indicating significant differences between groups.
To examine the effect of apraxia and whether hemisphere differences could be explained by apraxia, we ran the same ANOVAS, but removed apraxic participants from analysis. We then compared performance between all participants with the performance of participants when apraxic participants were removed. As mentioned above, our determination of apraxia did not provide a continuous measure to assess apraxia severity.
Results
Upper-Extremity Fugl-Meyer (UEFM)
Two-way ANOVA indicated no statistically significant main effect for hemisphere (F(1,104) = 1.49, p = .22), nor statistically significant interaction (F(2,104) = .92, p = .4), indicating that our LHD and RHD groups were comparable with respect to contralesional impairment level within each impairment group.
Jebsen-Taylor Hand Function Test (JHFT)
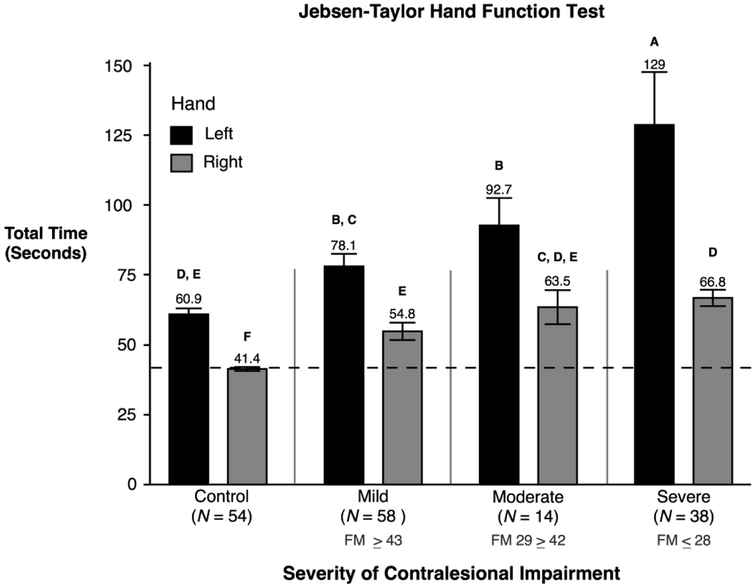
Two-way ANOVA on our primary measure of unilateral arm function, the Jebsen-Taylor Hand Function Test (JHFT), revealed a statistically significant interaction between contralesional impairment severity and hand (F(3,156) = 6.86, p = 0.0002). Post hoc analysis showed several significant differences between groups. Performance time for each stroke group (LHD: Mild, Moderate, Severe and RHD: Mild, Moderate, Severe) was significantly greater than the performance time of their arm-matched control group. The LHD severe group (M = 128.7, SE = 18.93; p < .05) was significantly slower than all other groups. The LHD moderate group (M = 92.72, SE = 9.79; p < .05) was significantly slower than both control groups and all RHD groups, but faster than the LHD severe group. The score was not significantly different than the LHD mild group (M = 78.09, SE = 4.45; p < .05). Thus, regardless of contralesional impairment level, stroke participants with LHD performed slower than participants with RHD. Though the RHD severe group (M = 66.79, SE = 2.91; p < .05) was significantly faster than all LHD groups, it was not significantly different from the RHD moderate group or the control left group. The RHD mild group (M = 54.82, SE = 3.1; p < .05), RHD moderate group (M = 63.47, SE =6.10, p < .05), and control left group (M = 60.92, SE = 2.1; p < .05) scores were not significantly different from each other indicating when RHD participants had only mild or moderate contralesional impairment they performed similar to that of a healthy control that was using their left hand. As expected, control participants using their right hand were significantly faster than all other groups (M = 41.37, SE = .73; p < .05).
In addition to the interaction, two-way ANOVA also revealed a statistically significant main effect due contralesional impairment severity (F(3,156) = 30.6, p < 0.0001). Post hoc comparisons indicated the severe group (M = 83.08, SE = 3.99; p < .05) was significantly slower than all other groups. This indicates that participants with severe contralesional motor deficits also had the most severe ipsilesional deficits. Not surprisingly, the control group (no impairment) was significantly faster than all other groups (M = 51.15, SE = 2.94; p < .05). There was no statistically significant difference in performance between the moderate (M = 76.01, SE = 5.84; p < .05) and mild group (M = 67.67, SE = 2.86; p < .05). These effects are illustrated in Figure 1, which shows the total time that participants took to complete all items on the JHFT separated by contralesional impairment group and hand group. The ordinate axis in Figure 1 represents the cumulative time to complete all 7 JHFT tasks.
Figure 1.
Mean and standard errors of the total time (seconds) to complete the JHFT for control groups and stroke groups are shown, separated by contralesional arm impairment severity. Stroke participants using their left hand (shown in black) had left hemisphere damage. Stroke participants using their right hand (shown in gray) had right hemisphere damage. Groups not connected by same letter are significantly different.
In order to better understand the aspects of ipsilesional performance that were modulated by hand and by severity group, we performed an ANOVA on the individual component scores of the JHFT. We found a significant interaction between severity group and hand (F(3,156) = 13.12, p < 0.0001) for the writing component. Post hoc analysis showed the LHD severe group (M = 82.02, SE = 16.99; p < .05) was significantly slower than all other groups. The LHD moderate group (M = 51.38, SE = 10.87; p < .05) and LHD mild groups (M = 37.38, SE = 2.75; p < .05) were significantly slower than all other groups besides the LHD severe group. Therefore, regardless of the amount of contralesional severity impairment, LHD showed significantly worse performance than controls or participants with RHD. The RHD severe (M = 21.32, SE = 1.53; p < .05) and RHD moderate groups (M = 20.25, SE = 4.35; p < .05) performance were not significantly different from the left control group (M = 26.63, SE = 1.87; p < .05), while the RHD mild group (M = 15.92, SE = 1.18; p < .05) was significantly faster than the left control group on the writing component. This analysis revealed a significant main effect for contralesional impairment severity (severity group) on these tasks: writing (F(3,156) = 25.21, p < 0.0001), lifting small objects (F(3,156) = 10.54, p < 0.0001), placing checkers (F(3,156) = 13.06, p < 0.0001), simulated feeding (F(3,156) = 16.77, p < 0.0001), lifting light objects (F(3,156) = 14.05, p < 0.0001), and lifting heavy objects (F(3,156) = 13.8, p < 0.0001). For each of these, the severe group performed the worst, followed by the moderate group, and the mild group, and the control group always performed the fastest. Some of the between groups analyses were significantly different while others were not but they all showed the same trend: the more severe the contralesional arm impairment level, the worse the ipsilesional arm performance (see table 2).
Table 2:
Time (seconds) to complete Jebsen Taylor Hand Function Test Components
| Hand | Contra Severity |
N | Writing | Page Turn | Small Objectsa |
Feeding | Stacking Checkers |
Light Objectsb |
Heavy Objectsc |
|---|---|---|---|---|---|---|---|---|---|
| Left | Control | 27 | 26.6 ± 1.9 | 4.7 ± 0.2 | 6.9 ± 0.2 | 8.9 ± 0.2 | 5.1 ± 0.2 | 3.8 ± 0.1 | 4.0 ± 0.1 |
| Left | Mild | 32 | 37.4 ± 2.7 | 5.3 ± 0.3 | 8.2 ± 0.4 | 10.3 ± 0.6 | 6.6 ± 0.4 | 5.1 ± 0.4 | 5.1 ± 0.3 |
| Left | Moderate | 6 | 51.4 ± 10.9 | 6.3 ± 1.2 | 8.0 ± 0.6 | 10.2 ± 1.4 | 6.2 ± 0.8 | 5.3 ± 0.6 | 5.3 ± 0.8 |
| Left | Severe | 10 | 82.0 ± 17 | 6.6 ± 0.8 | 8.9 ± 0.5 | 13.6 ± 1.9 | 7.1 ± 0.6 | 5.1 ± 0.5 | 5.3 ± 0.3 |
| Right | Control | 27 | 12.1 ± 0.6 | 4.5 ± 0.2 | 6.4 ± 0.2 | 6.9 ± 0.2 | 4.4 ± 0.1 | 3.5 ± 0.1 | 3.6 ± 0.1 |
| Right | Mild | 26 | 15.9 ± 1.2 | 7.0 ± 1.7 | 7.5 ± 0.3 | 8.3 ± 0.3 | 5.7 ± 0.3 | 4.4 ± 0.2 | 4.5 ± 0.2 |
| Right | Moderate | 8 | 20.3 ± 4.3 | 5.7 ± 0.5 | 9.8 ± 0.6 | 9.7 ± 0.7 | 7.7 ± 0.7 | 5.2 ± 0.6 | 5.2 ± 0.6 |
| Right | Severe | 28 | 21.3 ± 1.5 | 6.7 ± 0.5 | 10.0 ± 0.8 | 10.2 ± 0.4 | 7.2 ± 0.4 | 5.7 ± 0.3 | 5.6 ± 0.3 |
Mean time in seconds ± Standard Error are presented.
Contra Severity Contralesional impairment severity group; N number
Lifting Small Objects
Lifting Light Objects
Lifting Heavy Objects
Since all participants were right-handed, it might be expected that the right hand would perform better for each component of the JHFT, but this was not the case. ANOVA revealed a statistically significant main effect of hand for simulated feeding (F(1,156) = 13.2, p = 0.0004) and writing (copying a sentence) components (F(1,156) = 147.88, p < 0.0001), while none of the other items on the JHFT showed a main effect of hand. The groups using their left hand performed significantly worse for both writing (Left: M = 40.58, SE = 3.37; Right: M = 16.8, SE = .82; p < .05) and feeding (Left: M = 10.24 SE = .41; Right: M = 8.6, SE = .22; p < .05) than the group using their right hand. We expect that this finding is a direct result of premorbid hand dominance.
Ideomotor Limb Apraxia
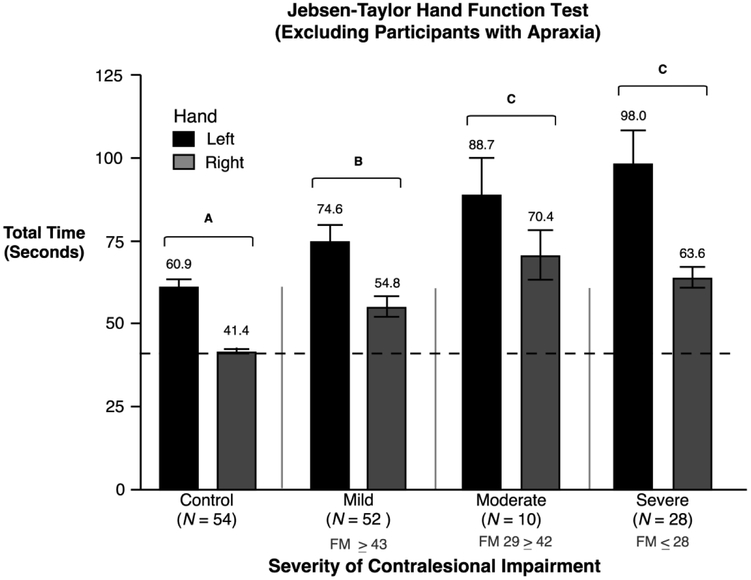
Ideomotor limb apraxia is known to affect skilled, purposeful movements like those assessed by the JHFT, particularly following a left hemisphere stroke 9,35. Twenty of our 110 stroke participants (12 LHD, 8 RHD) were identified as apraxic. In order to assess the effect of apraxia on performance, we removed the 20 apraxic participants from our ANOVA, and found that the main effect of impairment group (F(3,136) = 20.3, p < 0.0001) remained. The severe group (M = 80.82, SE = 3.94) and moderate group (M = 79.51, SE = 6.94; p < .05) performed similar to each other, but significantly slower than the mild (M = 64.72, SE = 3.14; p < .05) and control group (M = 51.15, SE = 1.74; p < .05). ANOVA also indicated a main effect of hand (F(1,136) = 41.62, p < 0.0001) such that participants using their left hand (M =71.72, SE =2.79; p < .05) required significantly more time to complete the JHFT tasks than participants using their right hand (M = 53.79, SE = 1.78; p < .05), similar to when apraxic participants were included in the analysis. This result indicates the presence of a strong effect of impairment group (contralesional impairment severity) and of hand on ipsilesional arm motor performance that are independent of apraxia, since these effects occurred regardless of whether apraxic participants were included in the analysis. However, when the apraxic participants were removed from analysis, the impairment group by hand interaction was no longer significant (F(3,136) = 1.04, p = ns) (Figure 2). It is plausible to speculate that the presence of apraxia might modulate the interaction between hemisphere (hand) and contralesional severity level. However, this interpretation must be viewed cautiously, because removing 20 participants from our analysis also decreased the statistical power of the ANOVA.
Figure 2.
Mean and standard errors of the total time (seconds) to complete the JHFT for control groups and stroke groups are shown, with the exclusion of apraxic participants. Stroke participants using their left hand (shown in black) had left hemisphere damage. Stroke participants using their right hand (shown in gray) had right hemisphere damage. Groups not connected by same letter are significantly different.
Grip Strength and Grooved Pegboard Test
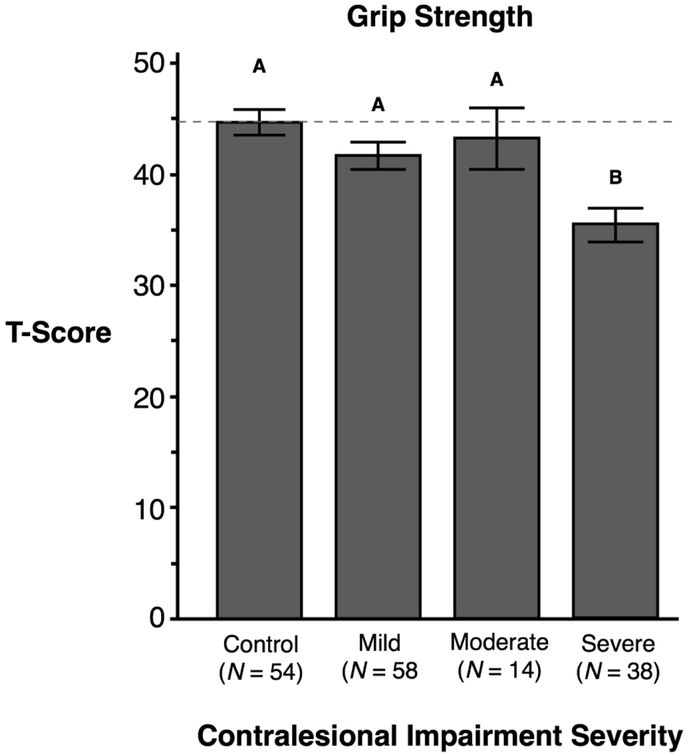
For grip strength, two-way ANOVA revealed a main effect of contralesional impairment severity (F(3,156) = 6.02, p < 0.001), but no effect of hand, nor interaction between factors. Post-hoc analysis showed the severe group (M = 35.38, SE = 1.51; p < .05) was significantly weaker than all other groups. There were no other group differences. Thus, regardless of the hemisphere that was lesioned, severe contralesional motor impairment was associated with a more severe grip strength deficit (see Figure 3).
Figure 3.
The X-axis represents participants’ contralesional arm impairment severity measured by the Fugl-Meyer Assessment in stroke participants. The Y-axis shows the T-score representing grip strength (kg) measured using a hand dynamometer. Mean and standard errors are shown. Groups not connected by same letter are significantly different.
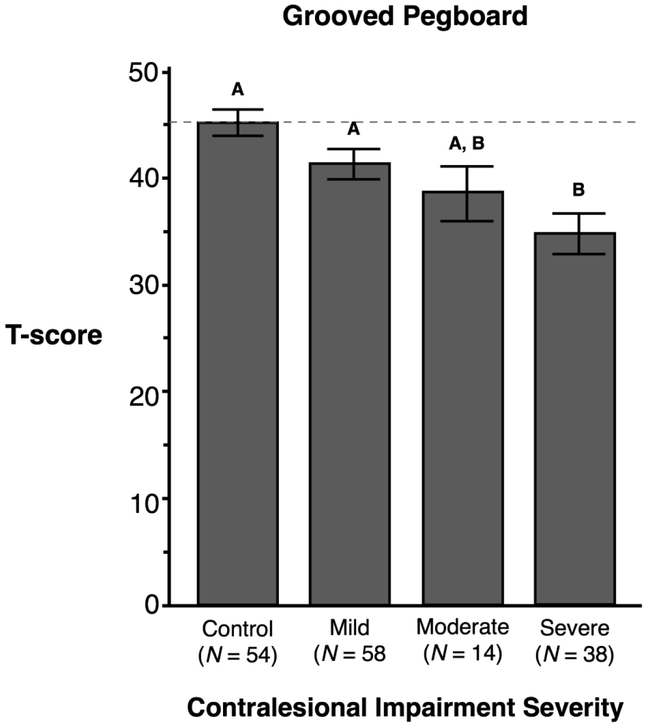
For the GPT, ANOVA revealed a main effect of contralesional impairment severity (F(3,156) = 7.17, p = 0.0002), but no effect of hand, nor interaction between these factors. Post-hoc analysis showed participants with severe contralesional hemiparesis (M = 34.58, SE = 1.89; p < .05) took significantly longer to complete the GPT compared to the Control group (M = 45.07, SE = 1.23; p < .05) and the Mild impairment group (M = 41.14, SE = 1.41; p < .05), but their performance was not significantly different than the Moderate impairment group (M = 38.64, SE = 2.55; p = ns). Thus, regardless of the hemisphere that was lesioned, severe contralesional impairment was associated with less-effective GPT performance. Figure 4 shows the mean and SE for the Grooved Pegboard Test across all 4 severity groups (Control, Mild, Moderate, Severe).
Figure 4.
The X-axis represents participants’ contralesional arm impairment severity measured by the Fugl-Meyer Assessment in stroke participants. The Y-axis shows the participants’ T-score representing the total time to place all pegs. A higher T-score indicates faster performance. Mean and standard errors are shown. Groups not connected by same letter are significantly different.
Discussion
We used three clinical tests of unimanual upper extremity performance to assess functional motor performance of the ipsilesional arm (less affected) of 110 stroke participants and 54 age and sex matched control participants, stratified across the full range of contralesional impairment levels (UEFM) and grouped by hand, which in stroke participants corresponded to the hemisphere that was damaged. Table 1 displays participant characteristics. Data were compared to the same hands of age-matched control participants, representing the zero impairment level groups. Our results revealed three critical findings that have substantial implications for both characterizing the motor deficits in chronic stroke and the development of mechanistic-based rehabilitation interventions. First, all groups showed significant ipsilesional arm motor performance deficits, when compared with age matched control groups. While ipsilesional motor deficits have been identified in stroke survivors as early as 1967 1, this is the first large-scale study to report functional deficits, identified through standardized clinical assessments, stratified by contralesional impairment level and hemisphere of damage. Second, all three ipsilesional arm motor performance measures (grip strength, JHFT, and GPT) varied substantially with contralesional arm impairment. The greater the impairment of the paretic arm, the greater the extent of ipsilesional arm motor deficits in the less affected limb. This is particularly important because those stroke survivors with severe motor impairment, who were unable to use the contralesional arm for grasp and manipulation, had the most substantial deficits in motor control of their ipsilesional arm. The ipsilesional arm, for these patients, is the only means of carrying out object manipulations for activities of daily living. Third, the extent of impairment in the performance of functional activities, as measured by the JHFT, depended on the hemisphere that was affected by the stroke. LHD stroke survivors show greater ipsilesional deficits than RHD stroke survivors. Item analysis of the JHFT revealed that this hemisphere effect is a function of two subtests: writing and simulated feeding.
Previous work from our laboratory with a smaller sample of stroke survivors showed that the score on the JHFT can be similar between RHD and LHD stroke survivors, while deficits in the fundamental motor control processes that gave rise to those similar scores were different 2. In the current study, we did not assess fundamental motor control processes through kinematic analysis, but did find that most of the items of the JHFT had similar total time scores for RHD and LHD participants. This similarity in task performance is likely to be, at least partially, due to deficits in different aspects of motor control. Nevertheless, our findings reveal that JHFT time scores were significantly different between hemisphere damage groups due to differences in component tasks that are most often performed by the dominant arm, writing and feeding. This may be because these subtasks are more extensively dependent on left-hemisphere processes in right-handers.
Bihemispheric Control of Unimanual Arm Movement
Our current findings of ipsilesional motor control deficits in stroke survivors with both right and left hemisphere damage provide strong evidence for a bihemispheric model of limb control 23. While early anatomical and physiological evidence emphasized the role of the contralateral hemisphere in controlling limb movements 36, more recent studies in both animal models of unilateral brain damage 37 and in human stroke patients 1,3,6,12,15,38,39 have demonstrated that the ipsilateral hemisphere plays a significant role in motor control. Based on studies in neurologically intact individuals, we proposed a bihemispheric hybrid model of motor lateralization which attributes specialization of each cerebral hemisphere to distinct but complementary functions: the dominant system for predictive control of limb and task dynamics and the non-dominant system for impedance control mechanisms, which are used to bring the hand to stable positions at the end of a reaching motion 40.
We expect that the lack of hemisphere dependence on most of the JHFT tasks in the current study is related to our previous findings that different underlying deficits can contribute to the same level of functional deficit in stroke survivors with left and right hemisphere damage 19. However, other tasks, such as writing and feeding, may rely so heavily on one hemisphere for performance that the hemisphere dependence of the underlying mechanisms is directly evident in performance. The lack of hemisphere dependence of the grooved pegboard test, however, is surprising as this task tends to show a strong dominant arm preference. In the current study, we did not differentially quantify the phases of this task that resulted in the final score. These include reaction time associated with the cognitive and visual-spatial aspects of the task related to planning the orientation of the peg, and the coordination required to produce this peg orientation. It is likely that RHD survivors might have had greater deficits in the visual spatial analysis and planning phase while LHD patients may have had greater deficits in the manipulation phase 19. This hypothesis, however, requires quantification of the components of performance to assess and cannot be addressed with the current data.
The Effect of Apraxia, Hand Dominance, and Hemisphere on Ipsilesional Arm Performance
We found that the amplitude of functional deficits, measured by JHFT, was significantly different between our hemisphere groups (RHD and LHD). Item analysis of the individual components of the JHFT indicated that only the writing and simulated feeding components varied with the hemisphere of damage. It is plausible that dependence on hemisphere of specific items from the JHFT is a function of apraxia, a clinical condition that is more common in LHD stroke survivors, and is known to be expressed in performance of the ipsilesional arm. In fact, there is evidence indicating that ipsilesional motor coordination deficits processes are potentiated by ideomotor limb apraxia 9 and predict speed deficits on functional tasks 41. Goldmann, Gross and Grossman (2008) showed that praxic disorders impacted both ipsilesional and contralesional motor function. While there are different types of apraxia, ideomotor limb apraxia was the only type assessed in this study. Our findings indicated that although apraxia potentiated the effects of hand (hemisphere damaged in stroke participants) on JHFT performance, removal of participants with apraxia did not remove the effect of hand on the writing and feeding components of the JHFT.
It should be stressed that removal of the 20 apraxic participants did eliminate the hand by severity group interaction. That interaction was characterized by a steeper decrement of JHFT performance (more time) in the left vs the right arm (left or right hemisphere damage in stroke participants), across severity levels. Thus, it seems plausible to conclude that the interaction between hand and severity level in the current study reflects the presence of apraxia. Indeed, previous research has reported that left hemisphere patients with apraxia have substantially worse JHFT performance than those without apraxia, and that apraxia has a negative impact on functional recovery following stroke 43. It is therefore critical to consider the presence or absence of apraxia, as well as the hemisphere of damage, when designing an intervention protocol to address functional deficits in stroke survivors.
Our current findings indicate that patients with severe contralesional paresis who do not have functional hand movement in the paretic arm, have the greatest deficits in their less affected arm. Such non-paretic arm movement deficits will clearly affect the efficiency of functional performance in activities of daily living, as indicated by the JHFT. Thus, we recommend that identification of potential motor deficits in the less affected arm is critical to address the amelioration of these deficits through targeted rehabilitation intervention.
Further, because the main effect of hand across severity groups was restricted to the writing and simulated feeding components of the JHFT, tasks that are commonly performed with the dominant arm, it also appears reasonable to conclude that the main effect of hand in this study was primarily due to differences in control of the dominant and non-dominant arms. As mentioned earlier, we previously reported that similar deficits in JHFT scores between the hands of RHD and LHD patients have been shown to correlate with different aspects of control and coordination, assessed through kinematic analysis of reaching movements2. Specifically, JHFT scores in the less-affected arm (left) of LHD patients were correlated with kinematic errors in linearity of motion, while JHFT scores in the less-affected arm (right) of RHD patients correlated with reaction time deficits during reaching movements2. Thus, damage to the right or the left hemisphere may result in different underlying deficits in motor control, yet appear similar in timed scores of functional activities, such as measured by the JHFT. Nevertheless, the importance of considering limb dominance as a factor in clinical rehabilitation is emphasized by our main effect of hand in this study: RHD patients with severe paresis in the contralesional arm are able to perform activities of daily living with the dominant ipsilesional arm, while LHD patients must use the less-efficient non-dominant arm as the primary manipulator for performance of most activities of daily living. This suggests that dominance retraining in the ipsilesional arm may provide an effective rehabilitation strategy to produce more efficient performance of functional activities in patients with left hemisphere damage.
Contralesional Impairment Severity
The severity of contralesional impairment reflects the extent to which motor-related circuits have been lesioned in the damaged hemisphere. Because we have previously provided evidence that both hemispheres contribute to control of each arm, we hypothesized that unilateral lesions will affect performance in the ipsilesional arm. The current findings confirm that the extent of ipsilesional deficits varies with contralesional impairment severity. Our functional measure, the JHFT showed a main effect of contralesional impairment severity such that the more extensive the contralesional arm impairment, the more extensive the performance deficiencies for the ipsilesional arm.
Our findings are consistent with a number of previous studies that have shown that ipsilesional motor performance impairments vary with contralesional arm impairment severity5. In a recent study, Bustren and colleagues (2017) showed stroke participants with moderate contralesional paresis had more prominent movement deficits than participants with mild contralesional paresis on an ipsilesional drinking task. Therefore, even when stratifying within a small range of impairment, ipsilesional movement deficits varied with contralesional impairment severity. That study, however, did not include participants with severe contralesional impairment, which we found to be the group with the most extensive ipsilesional deficits. Using accelerometers, Lang et al. (2007) showed a positive correlation between the amount of use of the more affected and less affected arm following stroke in patients with mild to moderate hemiparesis. Regardless of hemisphere that was damaged, hemiparetic participants that used their more affected arm for shorter durations also used their less affected arm for shorter durations. Though that study looked at patients in the sub-acute phase of stroke rather than chronic, our data suggests it’s possible that the positive correlation was due to that fact that the more severe the impairment in the more affected arm, the more severe the impairment was in their less affected arm, therefore they used both arms less. Noskin et al. (2008) reported contralesional severity measured by maximum grip strength did not vary with ipsilesional motor deficits, measured by the 9-hole peg test (9HPT). It should be noted that the participants with the most severe deficits could not perform grip strength, and therefore were not included in their correlation analysis. However, at one-year post stroke, the study showed a strong correlation between contralesional 9HPT performance, a test of distal manipulation, and ipsilesional 9HPT.
In our current study, we found that both the JHFT and the GPT varied substantially with contralesional arm impairment level (UEFM). In support of the idea that contralesional impairment severity level is an important factor in the expression of ipsilesional motor deficits, Haaland et al. (2009) demonstrated that stroke participants with paresis, but not those without paresis, showed deficits in performance of a single joint reaching task. Our current results indicate a strong dependence of ipsilesional motor deficits on contralesional impairment severity level, and thus may explain some discrepancies in previous literature. Studies that do not stratify contralesional impairment level, or that only assess participants in the mild range of impairment spectrum may not identify deficits in ipsilesional motor performance.
Our different measures of ipsilesional motor performance showed different dependencies on severity of contralesional impairment and on hemisphere of damage, suggesting that the nature of the test is also an important factor in the expression of ipsilesional motor deficits. Our functional measure (JHFT), the GPT, and grip strength all showed an effect of contralesional impairment severity, but with different patterns. The JHFT showed differences between the Mild impairment group and the non-disabled control group, whereas grip strength and GPT did not. On these measures, performance of participants with mild contralesional impairment were similar to that of the non-disabled control group. In fact, Noskin et al. (2008) found as the ipsilesional 9HPT improved over the course of one year, ipsilesional grip strength did not, emphasizing that these two measures of ipsilesional deficits do not represent the same aspects of performance. Metrot et al. (2013) reported that clinical scores on the 9HPT were comparable to that of non-disabled controls, yet identified ipsilesional deficits in smoothness during reaching movements when measured with kinematics. We conclude that the discrepancies in the literature regarding the effect of contralesional impairment level on ipsilesional motor deficits are likely related to differences between studies in the inclusion range of contralesional impairment levels, lack of stratification by side of damage and impairment level, and variations in tasks and measures employed to quantify ipsilesional arm motor deficits.
Limitations
This study had some limitations that should be noted. 1) Although all participants demonstrated cognitive understanding of the tasks, we did not quantitatively assess cognitive status. 2) While both aphasia and visual neglect were reported, we did not consider these as covariates in our analysis because we did not have significant representation of these deficits across our impairment groups. 3) Ideomotor limb apraxia was either identified by the 15-item battery proposed by Haaland and Flaherty (1984), or by medical records when this battery was not available. We used the 15-item limb apraxia battery (Haaland and Flaherty, 1984) to provide a cut-off for identification of apraxia, but not to provide a continuous measure of apraxia severity. Therefore, apraxia was treated as a categorical variable in our analysis. Furthermore, only 20 of the 110 participants were identified as apraxic, and we could not include apraxia as a factor in our ANOVA because it was inconsistently represented across our factors and levels. 4) Although our overall sample size was large, we did not have equal numbers of participants across all our groups: The smallest impairment level group (moderate) was comprised of 14 participants, while the largest (mild) was comprised of 58 participants, and the severe group was comprised of 38 participants. Our sample was limited by the availability of participants during recruitment.
Clinical Implications
Deficits in coordination of the less affected arm can produce substantial limitations in the speed and efficacy of functional activities in participants with severe contralesional deficits. In fact, our findings indicate that unilateral tasks with the less affected arm take, on average, twice as long to complete as the comparable arm of age-matched control participants, regardless of which hemisphere is damaged, indicative of labored and inefficient movement that can interfere with an individual’s participation in activities. However, these deficits are generally more pronounced following LHD. Unfortunately, clinical rehabilitation has yet to recognize the need to address less affected arm motor deficits, largely because scientific evidence has not yet been translated into clinical practice, nor has the best practice for this translation been specified through innovative intervention studies. This is particularly important for right-handed LHD patients with severe paresis, because they cannot use the paretic arm for manipulations in functional activities and must rely on their non-dominant arm as their primary controller 47.
Vega-González and Granat (2005) found stroke participants used their less affected arm 3 to 6 times more than their affected arm for ADLs. Despite this increased arm use, our participants, who were all in the chronic phase of stroke, still persisted with significant ipsilesional deficits. To provide a reference, imagine using only your non-dominant arm to carry out all your ADLs, such as preparing food, getting dressed, etc. This would be somewhat frustrating. This would be equivalent to the difference between our control groups using their left or right hand. Now imagine that your non-dominant arm has become more than twice as slow and less coordinated than it was, which is the case for the severely impaired LHD participants enrolled in this study. On the other hand, RHD participants are able to use the previously dominant arm, but with a 66% decrement in function. In either case, these impairments are substantially limiting. Based on these findings, we recommend future research address whether training of the less affected arm can improve functional independence in stroke patients with severe impairment in the paretic arm.
Conclusion
Our results indicate that regardless of contralesional severity level, the stroke groups showed significant deficits in performance with the ipsilesional arm when compared to age-matched control participants using the same arm. In addition, all clinical tests indicated that ipsilesional arm performance deficits increase with contralesional severity. However, only the components of the JHFT that show large disparities between the dominant and nondominant hands in non-disabled individuals consistently varied with hemisphere of damage. In addition, neither grip strength nor GPT performance varied with hemisphere of damage. Most importantly, movement function in the less effected arm of participants with severe contralesional paresis was substantially less-efficient than that of age-matched controls. This is particularly important because this group of stroke survivors with severe motor impairment cannot use the paretic arm for manipulations during functional activities.
Acknowledgements:
This research was supported by the NIH Grant # R01 HD059783 to RLS, and the American Heart Association Grant # 16GRNT31010001.
Footnotes
The authors declare that there is no conflict of interest.
References
- 1.Wyke M. Effect of brain lesions on the rapidity of arm movement. Neurology. 1967;17:1113–1120. [DOI] [PubMed] [Google Scholar]
- 2.Schaefer SY, Haaland KY, Sainburg RL. Hemispheric specialization and functional impact of ipsilesional deficits in movement coordination and accuracy. Neuropsychologia. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arya KN, Pandian S, Kumar D. Task-based mirror therapy enhances ipsilesional motor functions in stroke: A pilot study. J. Bodyw. Mov. Ther 2017. [DOI] [PubMed] [Google Scholar]
- 5.Bustrén EL, Sunnerhagen KS, Alt Murphy M. Movement Kinematics of the Ipsilesional Upper Extremity in Persons with Moderate or Mild Stroke. Neurorehabil. Neural Repair 2017. [DOI] [PubMed] [Google Scholar]
- 6.Semrau JA, Herter TM, Kenzie JM, Findlater SE, Scott SH, Dukelow SP. Robotic Characterization of Ipsilesional Motor Function in Subacute Stroke. Neurorehabil. Neural Repair 2017. [DOI] [PubMed] [Google Scholar]
- 7.Schaefer SY, Mutha PK, Haaland KY, Sainburg RL. Hemispheric specialization for movement control produces dissociable differences in online corrections after stroke. Cereb. Cortex 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mutha PK, Sainburg RL, Haaland KY. Left Parietal Regions Are Critical for Adaptive Visuomotor Control. J. Neurosci 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mutha PK, Sainburg RL, Haaland KY. Coordination deficits in ideomotor apraxia during visually targeted reaching reflect impaired visuomotor transformations. Neuropsychologia. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mutha PK, Sainburg RL, Haaland KY. Critical neural substrates for correcting unexpected trajectory errors and learning from them. In: Brain.; 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapin I, Tourk LM, Costa LD. Evaluation of the Purdue Pegboard as a Screening Test for Brain Damage. Dev. Med. Child Neurol 1966. [DOI] [PubMed] [Google Scholar]
- 12.Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the “unaffected” upper extremity of elderly stroke patients. Stroke. 1996. [DOI] [PubMed] [Google Scholar]
- 13.Haaland KY, Mutha PK, Rinehart JK, Daniels M, Cushnyr B, Adair JC. Relationship between arm usage and instrumental activities of daily living after unilateral stroke. Arch. Phys. Med. Rehabil 2012. [DOI] [PubMed] [Google Scholar]
- 14.Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989. [DOI] [PubMed] [Google Scholar]
- 15.Winstein CJ, Pohl RS, CJ W, PS P. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res. 1995. [DOI] [PubMed] [Google Scholar]
- 16.Mutha PK, Haaland KY, Sainburg RL. The effects of brain lateralization on motor control and adaptation. J. Mot. Behav 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esparza DY, Archambault PS, Winstein CJ, Levin MF. Hemispheric specialization in the co-ordination of arm and trunk movements during pointing in patients with unilateral brain damage. Exp. Brain Res 2003. [DOI] [PubMed] [Google Scholar]
- 18.Mutha PK, Haaland KY, Sainburg RL. Rethinking Motor Lateralization: Specialized but Complementary Mechanisms for Motor Control of Each Arm. PLoS One. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schaefer SY, Haaland KY, Sainburg RL. Dissociation of initial trajectory and final position errors during visuomotor adaptation following unilateral stroke. Brain Res. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haaland KY, Prestopnik JL, Knight RT, Lee RR. Hemispheric asymmetries for kinematic and positional aspects of reaching. Brain. 2004. [DOI] [PubMed] [Google Scholar]
- 21.Haaland KY, Harrington DL. Hemispheric asymmetry of movement. Curr. Opin. Neurobiol 1996. [DOI] [PubMed] [Google Scholar]
- 22.Haaland KY, Harrington D. The role of the hemispheres in closed loop movements. Brain Cogn. 1989. [DOI] [PubMed] [Google Scholar]
- 23.Sainburg RL. Convergent models of handedness and brain lateralization. Front. Psychol 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971. [DOI] [PubMed] [Google Scholar]
- 25.Kim SG, Ashe J, Hendrich K, et al. Functional magnetic resonance imaging of motor cortex: Hemispheric asymmetry and handedness. Science (80-.). 1993. [DOI] [PubMed] [Google Scholar]
- 26.Perelle IB, Ehrman L. An international study of human handedness: The data. Behav. Genet 1994. [DOI] [PubMed] [Google Scholar]
- 27.Woytowicz EJ, Rietschel JC, Goodman RN, et al. Determining Levels of Upper Extremity Movement Impairment by Applying a Cluster Analysis to the Fugl-Meyer Assessment of the Upper Extremity in Chronic Stroke. In: Archives of Physical Medicine and Rehabilitation.; 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Woodbury ML, Velozo CA, Richards LG, Duncan PW. Rasch analysis staging methodology to classify upper extremity movement impairment after stroke. Arch. Phys. Med. Rehabil 2013. [DOI] [PubMed] [Google Scholar]
- 29.Beebe JA, Lang CE. Relationships and responsiveness of six upper extremity function tests during the first six months of recovery after stroke. J. Neurol. Phys. Ther 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jebsen RH, Griffith ER, Long EW, Fowler R. Function of “normal” hand in stroke patients. Arch. Phys. Med. Rehabil 1971;52:170–4 passim. [PubMed] [Google Scholar]
- 31.Jebsen RH, Taylor N, Trieschmann RB, Trotter MJ, Howard LA. An objective and standardized test of hand function. Arch. Phys. Med. Rehabil 1969. [PubMed] [Google Scholar]
- 32.Heaton RK, Miller S, Taylor M, Grant I. Revised comprehensive norms for an expanded Halstead-Reitan Battery: Demographically adjusted neuropsychological norms for African American and Caucasian adults scoring programs.; 2004. [Google Scholar]
- 33.Haaland KY, Flaherty D. The different types of limb apraxia errors made by patients with left vs. right hemisphere damage. Brain Cogn. 1984. [DOI] [PubMed] [Google Scholar]
- 34.Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000. [DOI] [PubMed] [Google Scholar]
- 35.Berti A, Garbarini F, Neppi-Modona M. Disorders of higher cortical function. In: Neurobiology of Brain Disorders. Elsevier; 2015:525–541. [Google Scholar]
- 36.Sperry RW. Cerebral Organization and Behavior: The split brain behaves in many respects like two separate brains, providing new research possibilities. Science (80-.). 1961. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez CLR, Gharbawie OA, Williams PT, Kleim JA, Kolb B, Whishaw IQ. Evidence for bilateral control of skilled movements: Ipsilateral skilled forelimb reaching deficits and functional recovery in rats follow motor cortex and lateral frontal cortex lesions. Eur. J. Neurosci 2004. [DOI] [PubMed] [Google Scholar]
- 38.Yarosh C a, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J. Neurophysiol 2004. [DOI] [PubMed] [Google Scholar]
- 39.Tretriluxana J, Gordon J, Fisher BE, Winstein CJ. Hemisphere specific impairments in reach-to-grasp control after stroke: Effects of object size. Neurorehabil. Neural Repair 2009. [DOI] [PubMed] [Google Scholar]
- 40.Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp. Brain Res 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poole JL, Sadek J, Haaland KY. Ipsilateral Deficits in 1-Handed Shoe Tying After Left or Right Hemisphere Stroke. Arch. Phys. Med. Rehabil 2009. [DOI] [PubMed] [Google Scholar]
- 42.Gross RG, Grossman M. Update on apraxia. Curr. Neurol. Neurosci. Rep 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch. Phys. Med. Rehabil 2005. [DOI] [PubMed] [Google Scholar]
- 44.Noskin O, Krakauer JW, Lazar RM, et al. Ipsilateral motor dysfunction from unilateral stroke: Implications for the functional neuroanatomy of hemiparesis. J. Neurol. Neurosurg. Psychiatry 2008. [DOI] [PubMed] [Google Scholar]
- 45.Haaland KY, Schaefer SY, Knight RT, et al. Ipsilesional trajectory control is related to contralesional arm paralysis after left hemisphere damage. Exp. Brain Res 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Metrot J, Froger J, Hauret I, Mottet D, Van Dokkum L, Laffont I. Motor recovery of the ipsilesional upper limb in subacute stroke. Arch. Phys. Med. Rehabil 2013. [DOI] [PubMed] [Google Scholar]
- 47.Rinehart JK, Singleton RD, Adair JC, Sadek JR, Haaland KY. Arm use after left or right hemiparesis is influenced by hand preference. Stroke. 2009. [DOI] [PubMed] [Google Scholar]
- 48.Vega-González A, Granat MH. Continuous monitoring of upper-limb activity in a free-living environment. Arch. Phys. Med. Rehabil 2005;86:541–548. [DOI] [PubMed] [Google Scholar]