Dear Editor,
In critical care, measures that normalize “dose” to physiological response (e.g., PaO2/FiO2 in Acute Respiratory Distress Syndrome) can improve mortality prediction and standardize syndrome definitions [1]. Normalized measures in septic shock - such as blood pressure response to vasopressor dose - are poorly studied, but may improve prognostication and identify patients who benefit from specific treatments. We sought to evaluate the prognostic validity of a novel measure of vasopressor responsiveness in septic shock analogous to PaO2/FiO2 – the blood pressure to vasopressor dose ratio.
We conducted a retrospective study to validate blood pressure:vasopressor dose ratios using two cohorts of patients with septic shock within 24-hours of ICU admission: the MIMIC-III [2] (2008–2012) and eICU Collaborative Research databases [3] (2014–2015). We determined mean arterial pressure (MAP) and norepinephrine equivalent doses (NEQ) [4] (modified with vasopressin 0.02–0.05 units/min = norepinephrine 10 mcg/min) to calculate MAP:NEQ ratios (MAP/NEQ) every 15 minutes, and then identified the lowest MAP/NEQ within 24-hours of ICU admission. We compared the prognostic validity for hospital mortality of MAP/NEQ to NEQ using splines and MAP/NEQ to cardiovascular SOFA (CV-SOFA) and the modified cardiovascular SOFA [5] (mCV-SOFA -includes vasopressors missing from CV-SOFA) scores by generating MAP/NEQ tertiles derived from MIMIC-III data. We used logistic regression models to compare discrimination of hospital mortality using c-statistics with bootstrapping to generate 95% confidence intervals. This study was designated by the Boston University IRB as not Human Subjects Research.
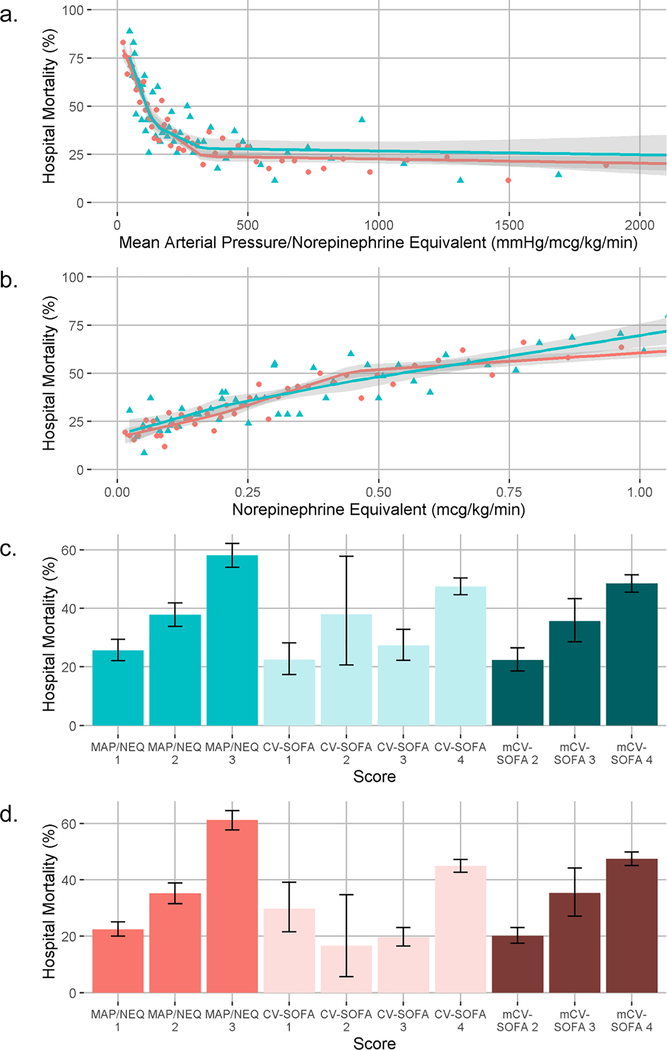
MAP/NEQ (mmHg/mcg/kg/min) ranged 39–8396 and 14–10292 and NEQ (mcg/kg/min) ranged 0.01–1.14 and 0.01–2.75 in MIMIC-III (n=1755) and eICU (n=2564) data, respectively. CV-SOFA ranged 1–4 and mCV-SOFA ranged 2–4 in both cohorts. MAP/NEQ had non-linear relationships with mortality (figure). The tertiles to create MAP/NEQ cutoffs were <136 (3-points), 136–324 (2-points), and ≥325 (1-point). Mortality rates stratified by MAP/NEQ, CV-SOFA, and mCV-SOFA scores are shown in the figure.
Fig.
a. Relationship between hospital mortality and mean arterial pressure/norepinephrine equivalent dose. Each point represents the percentage of patients who died in the hospital grouped from 50 quantiles of the mean arterial pressure/norepinephrine equivalent dose. Green triangles are from the MIMIC-III cohort, pink circles are from the eICU cohort. Overlying green and pink lines are fitted linear splines (knots at terciles) with 95% confidence intervals. b. Relationship between hospital mortality and norepinephrine equivalent dose. Each point represents the percentage of patients who died in the hospital grouped from 50 quantiles of the norepinephrine equivalent dose. Green triangles are from the MIMIC-III cohort, pink circles are from the eICU cohort. Overlying green and pink lines are fitted linear splines (knots at terciles) with 95% confidence intervals. c. Stratified mortality of 3-point mean arterial pressure/norepinephrine equivalent dose, cardiovascular sequential organ failure assessment score, and modified cardiovascular sequential organ failure assessment score in the MIMIC-III cohort. Bars show the percent of patients who died and 95% confidence intervals for each score category. d. Stratified mortality of 3-point mean arterial pressure/norepinephrine equivalent dose, cardiovascular sequential organ failure assessment score, and modified cardiovascular sequential organ failure assessment score in the eICU cohort. Bars show the percent of patients who died and 95% confidence intervals for each score category. MAP/NEQ: mean arterial pressure: norepinephrine equivalent dose; CV-SOFA: cardiovascular sequential organ failure assessment; mCV-SOFA: modified cardiovascular sequential organ failure assessment
In MIMIC-III data, MAP/NEQ had a higher c-statistic (0.65, 95% CI 0.63–0.67) than CV-SOFA (0.60, 95% CI 0.58–0.62, p<0.001)) and mCV-SOFA (0.61, 95% CI 0.59–0.65, p=0.001). In eICU, MAP/NEQ also had a higher c-statistic (0.68, 95% CI 0.66–0.71) than CV-SOFA (0.60, 95% CI 0.58–0.62, p<0.001) and mCV-SOFA (0.63, 95% CI 0.61–0.65, p<0.001). Using splines, MAP/NEQ had a higher c-statistic (0.71, 95% CI 0.69–0.73) than NEQ alone (0.66, 95% CI 0.64–0.69, p<0.001) in eICU data but not in MIMIC-III data (0.68, 95% CI 0.65–0.70 vs 0.67, 95% CI 0.64–0.70, p=0.10).
We evaluated a novel measure of septic shock severity using the ratio of MAP to vasopressor dose (MAP/NEQ). The MAP/NEQ improved prognostic validity compared to commonly used sepsis severity scores and to vasopressor dose alone. A normalized measure such as MAP/NEQ that characterizes vasopressor responsiveness may have prognostication value and standardize comparisons between patients with different MAP targets. Additionally, similar to PaO2/FiO2, MAP/NEQ may identify thresholds at which to initiate additional therapies (i.e. corticosteroids in patients with lower MAP/NEQ values) and improve consensus definitions. Future studies may evaluate how MAP/NEQ compares to measures not included in our models (e.g., lactic acid, SvO2) and test whether MAP/NEQ predicts responsiveness to therapies.
Acknowledgements
Contributions: Nicholas A Bosch takes responsibility for the integrity of the work as a whole, from inception to published article. All authors substantially contributed to the conception and design of this study. Nicholas A Bosch acquired the data. All authors were involved in the interpretation of data. Nicholas A Bosch drafted the manuscript and all authors revised it critically for important intellectual content. All authors read and approved the final manuscript.
Source of Funding. Financial/Non-financial disclosures: The authors have reported the following: AJW is supported by the following NHLBI grants: 5K01HL116768-03, 1R01HL136660-01. NAB is supported by the following NIH grants: T32HL7035-42, 1F32GM133061-01.
Footnotes
Conflicts of Interest and Source of Funding. Financial/Non-financial disclosures: The authors have disclosed that they do not have any conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, et al. (2012) Acute respiratory distress syndrome: the Berlin Definition. JAMA 307:2526–2533 [DOI] [PubMed] [Google Scholar]
- 2.Johnson AEW, Pollard TJ, Shen L, et al. (2016) MIMIC-III, a freely accessible critical care database. Sci Data 3:160035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pollard TJ, Johnson AEW, Raffa JD, et al. (2018) The eICU Collaborative Research Database, a freely available multi-center database for critical care research. Sci Data 5:180178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Russell JA, Walley KR, Singer J, et al. (2008) Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med 358:877–887 [DOI] [PubMed] [Google Scholar]
- 5.Yadav H, Harrison AM, Hanson AC, et al. (2015) Improving the Accuracy of Cardiovascular Component of the Sequential Organ Failure Assessment Score. Crit Care Med 43:1449- [DOI] [PubMed] [Google Scholar]