Abstract
BACKGROUND AND PURPOSE
Exercise plays an important role in supporting overall brain health. However, the mechanisms by which exercise supports brain health are imprecisely defined. Further, brain hemodynamic changes during exercise are not clearly understood, especially in older adults. The primary aim of this study was to compare cerebral blood flow velocity and pulsatility index (PI) during moderate-intensity exercise between older adults with normal pulsatile flow (normal PI) and older adults with elevated pulsatile flow (elevated PI). Secondary aims were to compare cardiovascular disease risk and cognitive function between individuals with elevated and non-elevated PI.
METHODS
Using transcranial Doppler ultrasound (TCD), middle cerebral artery blood velocity (MCAv) and PI were recorded during the rest and moderate intensity exercise. End tidal carbon dioxide (PETCO2) and beat-to-beat mean arterial blood pressure (MAP) were also recorded.
RESULTS
We enrolled 104 older adults into the study. The change in PI was greater in normal PI group (35.5% vs. 21.3%, P=0.005). The change in MCAv was similar in both groups (11.6% for normal PI vs. 10.6% for elevated PI; P=0.22). There was no significant difference in cardiovascular disease risk between the two groups (P=0.77). Individuals with elevated PI performed significantly worse in WAIS-R Digit Symbol and Trail Making Test A (P=0.04 and P=0.01, respectively).
CONCLUSIONS
The percent increase in PI from rest to moderate intensity exercise was attenuated in the older adults with elevated resting PI. Higher resting PI may negatively affect brain health as evidenced by the slower processing speed scores.
Keywords: Transcranial Doppler ultrasound, aging, hemodynamic, pulsatility index
Introduction
The brain comprises only about 2% of the human body mass, but it consumes about one-fifth of the cardiac output making the brain the most energetic organ in the body.1 Unlike muscle cells or other organs, the brain has limited ability to store energy, requiring instead a constant supply of oxygen and energy substance. Because cerebral blood flow is necessary to deliver this continuous supply of oxygen and nutrition,2 brain health depends both on healthy blood vessels and a healthy cardiovascular system.3
Compared with other organs, the brain’s blood supply is high flow and low resistance.4 During the aging process, the cerebrovascular network is subject to adaptive and perhaps maladaptive changes, including the narrowing and stiffening of a small cerebral vessel.4 Pulsatility index (PI) is a measure of the downstream resistance in the cerebral arteries, and it is a measure of cerebrovascular health in the normal elderly population.5,6 Transcranial Doppler (TCD) ultrasound is a non-invasive and cost-effective sensing modality that measures cerebral blood velocities (CBV) with high temporal resolution.7 PI is calculated from the CBV envelope and characterizes the morphology of the pulsatile CBV waveform,8 and has the potential to provide information regarding cerebrovascular health.9
Regular exercise is important for moderating age-related diseases,10,11 and is considered an essential component for optimal brain health.12 According to one study, a morning bout of moderate-intensity exercise led to an improvement in cerebral blood velocity for more than eight subsequent hours in older overweight adults.13 Another study showed no significant change in PI in older adults (n = 10) after a 12-week aerobic exercise training program.4 However, a significant decrease in PI was observed 30 minutes after an acute bout of exercise following the 12-week intervention, which may suggest a decrease in downstream resistance in the cerebral circulation and higher middle cerebral artery velocity (MCAv). It is important to note that these older adults all had normal PI values. Future directions should focus on whether PI decreases following an exercise intervention in those with elevated PI.
Numerous studies showed moderate-intensity exercise has a positive effect on cardiovascular health,14 cognitive function,15 and quality of life.16 However, there is a lack of understanding of the fundamental mechanisms that regulate cerebral blood flow during an acute bout of exercise in older adults. Various TCD studies have shown an increase in MCAv and PI during moderate-intensity exercise in young healthy adults.17-22 However, less is known about the change in MCAv and PI during moderate-intensity exercise in older adults – especially older adults with high pulsatile blood flow velocity (elevated PI). Since elevated PI is related to cardiovascular disease,23 dementia,24 white matter injury,5 traumatic brain injury,25 and intracranial pathology,26 increasing our understanding about MCAv and PI and the potential influencing factors (cardiovascular disease) may help guide optimal therapeutic interventions aimed at brain health. Therefore, the main aim of the present study is to compare MCAv and PI during moderate-intensity exercise between older adults with normal PI and older adults with elevated PI. A secondary aim of this study is to compare cardiovascular disease (CVD) and cognitive function of the same groups: older adults with normal pulsatile blood flow velocity (normal PI) and older adults with high pulsatile blood flow (elevated PI).
Methods
Participants
All subjects were recruited from a registry of individuals at the University of Kansas Alzheimer’s Disease Center (KU ADC). Inclusion criteria for the study were: (1) 65–90 years of age; (2) sedentary or underactive lifestyle; (3) the absence of major orthopedic disability; (4) the ability to perform moderate physical exercise; (5) cognitively normal/nondemented based on neuropsychological testing and a Clinical Dementia Rating (CDR) =0.27 Exclusion criteria included (1) Diagnostic and Statistical Manual of Mental Disorders-IV defined drug or alcohol abuse within the previous 2 years; (2) clinically significant depression or anxiety; (3) insulin-dependent diabetes; (4) myocardial infarction or symptoms of coronary artery disease within the previous 2 years; (5) acute decompensated congestive heart failure or class IV heart failure. All participants were consented according to a protocol approved by the Institutional Review Board at the University of Kansas Medical Center.
Protocol
Before the study visit, participants abstained from caffeine for 12 hours, physical activity for 24 hours, and eating a large meal for two hours. All participants began study procedures between 7:30 and 9:00 AM. After consent, participants underwent a physical exam including height, weight and heart rate. Cardiovascular disease (CVD) risk (defined as the atherosclerotic cardiovascular disease, ASCVD risk score) and was calculated for each participant. The ASCVD algorithm uses sex, age, race, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure (SBP), as well as smoking, diabetes, and hypertension treatment status to calculate 10-year risk of heart disease or stroke.28 For the ASCVD risk score calculation, SBP was assessed after 20 minutes of supine rest.
Participants were well-characterized as having normal cognition (CDR=0) at a clinical evaluation at the KU ADC prior to the TCD evaluation. During the clinic visit, all participants completed a standard battery of neuropsychological tests,29 that included the Mini-Mental State Examination,30 the Wechsler Adult Intelligence Scale-Revised (WAIS-R) Digit Symbol,31 Trail Making Test A, Trail Making Test B,32 and category naming (animals).33
TCD Evaluation
The laboratory room for the experimental session was dimly lit, the temperature was maintained between 22 and 24°C, and external stimuli were kept to a minimum during testing. All participants sat quietly on the recumbent stepper (NuStep, T5XR) and rested for 15 min. During the last 8 min of rest, we measured MCAv, beat-to-beat mean arterial pressure (MAP), end tidal carbon dioxide (PETCO2), and heart rate (HR).
MCAv was assessed using a 2-MHz probe (RobotoC2MD, Multigon Industries) and TCD ultrasound (TOC2MD Neurovision, Multigon Industries). The TCD transducer, equipped with auto-tracking to find the optimal signal was fixed on the left temporal window. If the left acoustic window did not yield a signal, then the right side was used. MAP was collected using (Finometer Pro, Finapres Medical Systems), which uses a finger plethysmography to continuously measure beat to beat MAP. PETCO2 was assessed using a nasal cannula and capnograph (BCI Capnocheck Sleep 9004 Smiths Medical) and ECG waveform was measured using a 5-lead electrocardiogram (Cardiocard, Nasiff Associates).
After 8 minutes of data collection at rest, the participant performed a single bout of moderate intensity exercise on the recumbent stepper. Moderate intensity was defined as 40% to 60% of age-predicted HR reserve.34 All participants began the exercise at 40 W and a step rate of 90 steps per minute. The resistance was increased until the HR reached the desired exercise intensity.35,36 Once participants were in steady-state for one minute, the 8-minute exercise recording for MCAv, MAP, PETCO2 and HR commenced.
Data Processing
Blood flow MCAv, MAP, PETCO2 data, and HR were sampled at 500 Hz (NI-USB-6212, National Instruments) and then exported for further analysis in MATLAB (R2019 b v. 9.6.0, Mathworks). Before analysis, a lowpass-filter with a passband frequency of < 5 Hz was used to remove high-frequency components of the raw TCD data. ECG R-wave was used to identify cardiac cycles. The customized MATLAB program detected the systolic velocity (Vs), diastolic velocity (Vd), systolic blood pressure (SBP) and diastolic blood pressure (DBP) in each cardiac cycle. Numerical integration via the trapezoidal method was used to calculate MCAv, MAP, PETCO2 for each cardiac cycle.
PI was calculated using Gosling’s pulsatility index equation as PI=(Vs-Vd)/MFV.37 Then two grand averages of MCAv, MAP, PETCO2 and PI were calculated for each participant during the eight minutes of rest and the eight minutes of exercise. Based on the measured PI value, the study population was divided into two groups: 1) individuals with normal PI and 2) individuals with elevated PI. Since PI increases with age (per year 0.01),7,38 the distinction between normal PI and elevated PI was made by a formula (the equation of the linear regression) implemented from a large population study:38
| (1) |
PI (cal) is the calculated age-appropriate expected PI. If the measured PI is higher than the PI (cal) by 1.5 standard deviations, then the participant was classified as elevated PI. This equation is only valid for individuals above 64 years old.
Statistical Analysis
Means and standard deviations were calculated for all quantitative variables. Frequencies and proportions were calculated for all categorical variables. The Lilliefors test was used to check the normality assumption of the data.
Between-group (elevated PI vs normal PI) differences of quantitative normally distributed variables were assessed using Welch's t-test. Wilcoxon rank-sum test was used for non-normally distributed quantitative variables. Large-sample categorical data were evaluated using Chi-squared and small-sample categorical data were evaluated using Fisher’s exact test.
Percentage change between rest and exercise were calculated and between-group percentage change (Δ%) comparisons were made with Welch's t-test. All statistics were computed using the MATLAB Statistics and Machine Learning Toolbox and P < 0.05 was considered statistically significant.
Results
Participant Characteristics
One hundred and four participants enrolled in this study. The demographic data is presented in Table 1. Using the equation to calculate PI, we found 77 participants demonstrated a normal PI and 27 were considered to have an elevated PI. There were no statistical differences between groups in age or the distribution by gender or education (p > 0.05). Additionally, there were no significant differences in resting HR or body mass index (BMI). All participants reached the target HR range using the appropriate estimated HR equations. There were no significant differences in ASCVD risk score between participants with elevated PI and those with normal PI. Moreover, none of the variables used in the ASCVD prediction equation were significantly different between elevated and non-elevated PI. Individuals with elevated PI performed significantly worse in WAIS-R Digit Symbol and Trail Making Test A. No significant differences were found for the MMSE, Animal Naming or Trail Making Test B.
Table 1.
Demographic and neuropsychological data
| Characteristics | Overall N=104 |
Elevated PI N=27 |
Normal PI N=77 |
P Value |
|---|---|---|---|---|
| Age(y) | 70.39 ± 4.78 | 70.22 ± 3.97 | 70.45 ± 5.0 | 0.78 |
| Gender (F:M) | 75:39 | 17:10 | 58:19 | 0.29 |
| Education (y) | 16.78± 2.59 | 16.78 ± 2.9 | 16.83 ± 2.4 | 0.81 |
| BMI (kg/m2) | 26.8± 4.45 | 27.39 ± 5.5 | 26.68 ± 4.01 | 0.62 |
| Resting HR | 64.8± 8.2 | 62.22± 10.13 | 65.66± 7.2 | 0.11 |
| MMSE | 28.01± 1.52 | 28.74± 1.7 | 28.8± 1.50 | 0.95 |
| WAIS-R Digit Symbol | 49.85± 9.5 | 46.33± 8.4 | 51.18± 9.7 | 0.04* |
| Trail Making Test A | 31.44± 10.8 | 35.70± 11.7 | 29.83± 10.2 | 0.01* |
| Trail Making Test B | 79.22± 37.6 | 79.74± 30.6 | 79.32± 39.7 | 0.6 |
| Animal Naming | 21.99± 5.1 | 21.70± 4.6 | 22.05± 5.4 | 0.85 |
| ASCVD score | 14.8± 9.0 | 14.78± 8.2 | 14.78± 9.3 | 0.77 |
| Resting Systolic blood pressure (mm Hg) | 130.54± 14.34 | 134.77± 16.42 | 128.89± 13.33 | 0.09 |
| Total cholesterol (mmol/L) | 186.30± 36.10 | 186.30± 37.30 | 187.30± 37.30 | 0.86 |
| HDL cholesterol (mmol/L) | 60.72± 17.7 | 61.07± 18.06 | 60.38± 17.71 | 0.64 |
| Blood pressure treatment % | 46.48 | 58.82 | 42.6 | 0.47 |
| Diabetes % | 4 | 0 | 5.48 | 0.57 |
| Smoking status % | 1.96 | 3.85 | 1.3 | 0.43 |
N=number of subjects; PI=pulsatility index; F=female; M=male; Y=years; BMI= body mass index; HR=heart rate; MMSE=The Mini-Mental State Exam; ASCVD = atherosclerotic cardiovascular disease; HDL=High-density lipoprotein. Values are presented as mean ± standard deviation (range) or number (percentage).
P < 0.05 for Welch's t-test or Wilcoxon rank sum test or Chi-square test or Fisher’s exact test
Absolute TCD Parameters and Blood Pressure
TCD parameters during rest and exercise are summarized in Table 2. In addition to the inherent significant differences in PI at rest, PI remained significantly higher during exercise in subjects with elevated PI. Vs was significantly higher at rest and during exercise in participants with elevated PI. However, no significant group differences were found for MCAv, Vd, MAP, SBP or PETCO2 at rest and during exercise. MAP and SBP were not significantly different between elevated and non-elevated PI at rest and during exercise. Participants with elevated PI showed a significantly lower DBP at rest but not during exercise.
Table 2.
TCD parameters of subjects with high resting PI and subjects with low resting PI at rest.
| Overall N=104 |
Elevated PI N=27 |
Normal PI N=77 |
P Value | |
|---|---|---|---|---|
| Rest PI | 1.02 ± 0.2 | 1.26 ± 0.1 | 0.95 ± 0.1 | <0.001* |
| Exercise PI | 1.33 ± 0.2 | 1.52 ± 0.3 | 1.26 ± 0.2 | <0.001* |
| Rest MCAv (cm/s) | 47.01± 11.15 | 47.90± 11.9 | 46.62± 11.0 | 0.62 |
| Exercise MCAv (cm/s) | 51.93± 12.6 | 52.30± 12.4 | 51.81± 12.9 | 0.86 |
| Rest Vs (cm/s) | 80.08± 19.8 | 90.45± 22.4 | 76.43± 17.5 | 0.005* |
| Exercise Vs (cm/s) | 94.32± 22.5 | 103.10± 23.6 | 91.4± 21.5 | 0.03* |
| Rest Vd (cm/s) | 31.96 ± 7.0 | 30.13 ± 6.6 | 32.61 ± 7.07 | 0.11 |
| Exercise Vd (cm/s) | 31.18 ± 7.4 | 29.5 ± 6.7 | 31.7 ± 7.6 | 0.2 |
| Rest PETCO2 (mmHg) | 33.97± 4.7 | 33.9± 4.01 | 33.5± 5.2 | 0.96 |
| Exercise PETCO2 (mmHg) | 38.33± 4.2 | 38.1± 4.1 | 38.4± 3.9 | 0.66 |
| Rest MAP (mmHg) | 81.33± 12.1 | 79.37± 13.0 | 83.06± 13.0 | 0.212 |
| Exercise MAP (mmHg) | 112.54± 19.4 | 113.59± 21.5 | 112.0± 21.9 | 0.75 |
| Rest SBP (mmHg) | 131.76± 20.7 | 136.33± 22.8 | 130.13± 19.8 | 0.22 |
| Exercise SBP (mmHg) | 187.62± 34.9 | 192.70± 39.7 | 185.82± 33.2 | 0.44 |
| Rest DBP (mmHg) | 70.80± 11.2 | 66.58± 11.8 | 72.25± 10.61 | 0.03* |
| Exercise DBP (mmHg) | 91.14± 18.03 | 89.2± 17.2 | 91.8± 18.44 | 0.71 |
N=number of subjects; PI= pulsatility index; MCAv=mean middle cerebral artery velocity; Vs= systolic velocity; Vd= diastolic velocity; PETCO2 = end-tidal carbon dioxide; SBP=systolic blood pressure; DBP= diastolic blood pressure. Values are presented as mean ± standard deviation.
P < 0.05 for Welch's t-test or Wilcoxon rank sum test between elevated and normal PI percentage absolute values. *P< 0.05 Welch's t-test or Wilcoxon rank sum test between elevated and normal PI percentage change from rest to exercise.
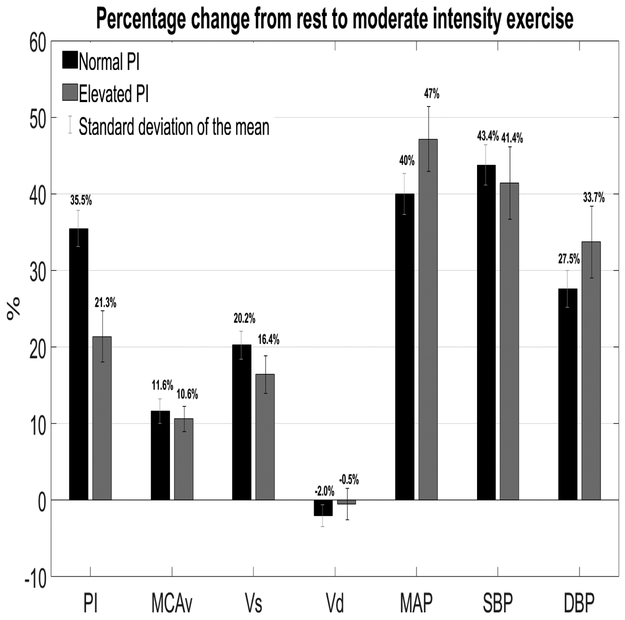
Relative TCD Parameters and Blood Pressure
Between-group percent change from rest to exercise are summarized in Figure 1. In those with elevated PI, the percent change from rest to exercise (ΔPI) was significantly lower than subjects with normal PI (P=0.005). However, there were no significant difference with the other variables of interest.
Figure 1.
PI= pulsatility index; MCAv=middle cerebral artery velocity; Vs= systolic velocity; Vd= diastolic velocity; MAP=mean arterial pressure; SBP=systolic blood pressure; DBP= diastolic blood pressure
Discussion
This experiment provided novel insight into the ΔPI and ΔMCAv in older adults at rest and during exercise. The major findings of the study are twofold. First, during moderate intensity exercise, cerebral perfusion was similar between the groups. Second, the ΔPI% in the group with elevated PI was 14.2% lower than in the group with normal PI (Figure 1).
Pulsatility index
PI has traditionally been interpreted as a descriptor of distal cerebrovascular resistance. The driving force in PI between elevated and normal PI values in our study can be explained by Vs. We suspect that the reason for the high PI value at rest in the elevated PI group is a significant increase in Vs. Also, we observed that the ΔPI from rest to moderate intensity exercise in people with elevated PI is lower than those with normal PI. This result suggests that individuals with elevated cerebral pulsatility may have a protective mechanism to prevent a further increase in PI during exercise. This result is an essential first step to understand the hemodynamic response to moderate intensity exercise in those with elevated PI. This could have significant implications for those with impaired cerebral autoregulation such as AD39 and traumatic brain injury.40
Middle Cerebral Blood Flow Velocity
Both groups increased the MCAv to the brain during exercise by 11.6% for individuals with normal PI and 10.6% for those with elevated PI. In contrast to the breadth of research gathered from healthy young individuals, few studies have examined ΔMCAv% and ΔPI% in older individuals from rest to moderate intensity exercise. Prior work showed an increase of 7% (n=9) and 24% (n=14) during moderate intensity exercise in older adults.41,42 Therefore, to our knowledge, we are the first to present a larger dataset of well characterized older adults and show the ΔMCAv% from rest to moderate exercise in older individuals with normal and elevated PI. Other TCD studies have shown an attenuation in cerebral perfusion at rest with age.7 This age-related reduction in cerebral perfusion is independent of other physiological factors such as BP, MAP, or BMI22 and is postulated to reflect global brain atrophy or reduced neuronal activity.43 In addition to low resting MCAv, our study showed a lower ΔMCAv% from resting levels to moderate intensity exercise levels when compared to young healthy adults (~20% in MCAv).22
Blood Pressure
Figure 1 shows an increase in MAP, SBP and DBP with exercise for both groups (elevated and normal PI). This increase in arterial blood pressure is necessary to supply blood to the vital organs and the working muscle during moderate intensity exercise. However, studies in young healthy individuals showed an increase in MAP and SBP with moderate intensity exercise, while DBP remained unchanged.20,44 Therefore, in healthy young individuals, only MCAv and Vs, and not Vd, increase with moderate intensity exercise. To our surprise, Vd did not increase in our study, while DBP increased by 27.5% in the normal PI group and 33.7% in the elevated PI group. These findings suggest that dynamic cerebral autoregulation in older adults is intact and was able to maintain unchanged Vd despite an increase in DBP.
Cardiovascular Disease Risk and Cognitive Function
Current evidence suggests that high pulsatile blood velocity is associated with CVD.45 However, our results did not support this hypothesis and showed no significant difference in ASCVD score between the elevated and normal PI group in our sample of relatively healthy older adults. However, SBP tended be higher in subjects with elevated PI. This is not surprising since prior studies have shown that increased pulse wave (SBP-DBP) is associated with increased resting PI.46
Our results suggest that elevated PI may be associated with cognitive changes in the brain. Individuals with elevated PI display lower processing speed and psychomotor speed by scoring worse in WAIS-R Digit Symbol and Trail Making Test A. Our results agree with a previously published study showing the association between high PI and slower processing speed.47 Therefore, microvascular damage measured by PI may contributes to the cognitive changes in cognitively normal older individuals.
Experimental considerations
The present study has several strengths including inclusion of both male and female participants. It included a sample size of one hundred and four well characterized, cognitively normal older adults who underwent a cardiovascular risk assessment and cognitive screening. We believe a strength of our study is the addition of other MCA data such as PI, Vs and Vd. Examination of all indices provides more perception to understand the complexity of the overlapping regulatory mechanism of blood velocity during exercise. TCD measures of MCAv as an index of cerebral blood flow are challenged by the assumption of constant MCA diameter,48 and the error in velocity estimate caused by the angle of insonation.49 However, in our study, the increase in PETCO2 in our study was less than +7.5, which is the amount needed to see a change in MCA diameter.50 Therefore, the implications of MCA diameter changes in our study is unlikely.
In conclusion, older adults with elevated PI present with an attenuated ΔPI% between rest to exercise and slower cognitive processing speed. SBP increases during moderate-intensity exercise in older individuals. However, autoregulation appears intact and able to retain (Vs) from increasing in the phase of SBP.
Acknowledgments and Disclosures:
We wish to thank Allegra Morton for her assistance in preparing the manuscript. SA Billinger and ED Vidoni report a patent pending (18KU028M-02). No other authors have conflicts of interest to report. S. A. Billinger was supported in part by K01HD067318 from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, by the American Heart Association Grant 16GRNT30450008 and from the Wohlgemuth Faculty Scholar Award. E. D. Vidoni and J. M. Burns are supported by the University of Kansas Alzheimer's Disease Center (P30AG035982). S. Perdomo is supported by the National Institute on Aging Diversity Supplement to R01 AG058162. REDCap and clinical space at University of Kansas Medical Center is supported by CTSA Award # ULTR000001 from NCRR and NCATS awarded to the University of Kansas Medical. R01AG043962 provided additional support for characterization of the participants. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or NCATS. The Georgia Holland Research in Exercise and Cardiovascular Health (REACH) laboratory space was supported by the Georgia Holland Endowment Fund.
References
- 1.Bundgaard-Nielsen M1, Sørensen H, Dalsgaard M, Rasmussen P, Secher NH. Relationship between stroke volume, cardiac output and filling of the heart during tilt. Acta Anaesthesiol Scand 2009;53:1324–8. [DOI] [PubMed] [Google Scholar]
- 2.Alexandrov AV, Sloan MA, Tegeler CH, et al. Practice standards for transcranial Doppler (TCD) ultrasound. part II: clinical indica- tions and expected outcomes. J Neuroimaging 2012;22:215–24. [DOI] [PubMed] [Google Scholar]
- 3.Attwell D, Buchan AM, Charpak S, et al. Glial and neuronal control of brain blood flow. Nature 2010;468:232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Akazawa N, Tanahashi K, Kosaki K, et al. Aerobic exercise training enhances cerebrovascular pulsatility response to acute aerobic exercise in older adults. Physiol Rep 2018;6:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fleysher R, Lipton ML, Noskin O, et al. White matter structural integrity and transcranial Doppler blood flow pulsatility in normal aging. Magn Reson Imaging 2018;47:97–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mok V, Ding D, Fu J, et al. Transcranial Doppler ultrasound for screening cerebral small vessel disease: A community study. Stroke 2012;43:2791–3. [DOI] [PubMed] [Google Scholar]
- 7.Tegeler CH, Crutchfield K, Katsnelson M, et al. Transcranial Doppler velocities in a large, healthy population. J Neuroimaging 2013;23:466–7 [DOI] [PubMed] [Google Scholar]
- 8.Altmann M, Thommessen B, Rønning OM, Benth JŠ, Reichenbach AS, Fure B. Middle cerebral artery pulsatility index is associated with cognitive impairment in lacunar stroke. J Neuroimaging 2016;26:431–5. [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, el-Saden S, Livshits Z, Martin N, Glenn TC, Saver JL. Transcranial Doppler pulsatility indices as a measure of diffuse small-vessel disease. J Neuroimaging 2001;11:229–35. [DOI] [PubMed] [Google Scholar]
- 10.Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation 2000;102:1270–5. [DOI] [PubMed] [Google Scholar]
- 11.Larson EB, Bruce RA. Health benefits of exercise in an aging society. Arch Intern Med 1987;147:353–6. [PubMed] [Google Scholar]
- 12.Gallaway PJ, Miyake H, Buchowski MS, et al. Physical activity: A viable way to reduce the risks of mild cognitive impairment, alzheimer’s disease, and vascular dementia in older adults. Brain Sci 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wheeler MJ, Dunstan DW, Smith B, et al. Morning exercise mitigates the impact of prolonged sitting on cerebral blood flow in older adults. J Appl Physiol 2019;126:1049–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Myers J Cardiology patient pages: exercise and cardiovascular health. Circulation 2003;107:e2–5. [DOI] [PubMed] [Google Scholar]
- 15.Cassilhas RC, Viana VA, Grassmann V, et al. The impact of resistance exercise on the cognitive function of the elderly. Med Sci Sports Exerc 2007;39:1401–7. [DOI] [PubMed] [Google Scholar]
- 16.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate exercise training in chronic heart failure: Effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99:1173–82. [DOI] [PubMed] [Google Scholar]
- 17.Larsen TS, Rasmussen P, Overgaard M, et al. Non-selective β-adrenergic blockade prevents reduction of the cerebral metabolic ratio during exhaustive exercise in humans. J Physiol 2008;586:2807–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lefferts WK, Augustine JA, Heffernan KS. Effect of acute resistance exercise on carotid artery stiffness and cerebral blood flow pulsatility. Front Physiol 2014;5:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moraine JJ, Lamotte M, Berré J, et al. Physiology and occupational physiology relationship of middle cerebral artery blood flow velocity to intensity during dynamic exercise in normal subjects. Eur J Appl Physiol Occup Physiol 1993;67:35–8. [DOI] [PubMed] [Google Scholar]
- 20.Jørgensen LG, Perko G, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. J Appl Physio 1992;73:1825–30. [DOI] [PubMed] [Google Scholar]
- 21.Secher NH, Seifert T, Van Lieshout JJ. Cerebral blood flow and metabolism during exercise: implications for fatigue. J Appl Physiol 2008;104:306–14. [DOI] [PubMed] [Google Scholar]
- 22.Smith KJ, Ainslie PN. Regulation of cerebral blood flow and metabolism during exercise. Exp Physiol 2017;102:1356–71. [DOI] [PubMed] [Google Scholar]
- 23.Poels MM, Zaccai K, Verwoert GC, et al. Arterial stiffness and cerebral small vessel disease: The rotterdam scan study. Stroke 2012;43:2637–42. [DOI] [PubMed] [Google Scholar]
- 24.Buratti L, Viticchi G, Falsetti L, et al. Thresholds of impaired cerebral hemodynamics that predict short-term cognitive decline in asymptomatic carotid stenosis. J Cereb Blood Flow Metab 2016;36:1804–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bouzat P, Almeras L, Manhes P, et al. Transcranial Doppler to predict neurologic outcome after mild to moderate traumatic brain injury. Anesthesiology 2016;125:346–54. [DOI] [PubMed] [Google Scholar]
- 26.Bellner J, Romner B, Reinstrup P, et al. Transcranial Doppler sonography pulsatility index (PI) reflects intracranial pressure (ICP). Surg Neuro 2004;62:45–51.. [DOI] [PubMed] [Google Scholar]
- 27.Morris JC, Ernesto C, Schafer K, et al. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1997;48:1508–10. [DOI] [PubMed] [Google Scholar]
- 28.Rana JS, Tabada GH, Solomon MD, et al. Accuracy of the atherosclerotic cardiovascular risk equation in a large contemporary, multiethnic population. J Am Coll Cardiol. 2016;67:2118–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weintraub S, Salmon D, Mercaldo N, et al. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS). Alzheimer Dis Assoc Disord 2009;23:91–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” Aprac- tical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- 31.Salthouse T What do adult age differences in the digit symbol substitution test reflect? J Gerontol 1992;47:121–8. [DOI] [PubMed] [Google Scholar]
- 32.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol 2004;19:203–14. [DOI] [PubMed] [Google Scholar]
- 33.Henry JD, Crawford JR, Phillips LH. Verbal fluency perfor- mance in dementia of the Alzheimer’s type: a meta-analysis. Neuropsychologia 2004;42:1212–22. [DOI] [PubMed] [Google Scholar]
- 34.American College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. Philadelphia, PA: Lippincott Williams and Wilkins; 2014. [Google Scholar]
- 35.Sisante J-FV, Vidoni ED, Kirkendoll K, et al. Blunted cerebrovascular response is associated with elevated beta-amyloid. J Cereb Blood Flow Metab 2019;39:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke 2012;43:2803–5 [DOI] [PubMed] [Google Scholar]
- 37.Gosling G, King D. Arterial assessment by Doppler-shift ultrasound. Proc R Soc Med 1974;67:447–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bakker SLM, de Leeuw FE, den Heijer T, Koudstaal PJ, Hofman A, Breteler MMB. Cerebral haemodynamics in the elderly: the Rotterdam study. Neuroepidemiology 2004;23:178–84. [DOI] [PubMed] [Google Scholar]
- 39.Stefani A, Sancesario G, Pierantozzi M, et al. CSF biomarkers, impairment of cerebral hemodynamics and degree of cognitive decline in Alzheimer’s and mixed dementia. J Neurol Sci 2009;283:109–15. [DOI] [PubMed] [Google Scholar]
- 40.Pierre Bouzat, Mauro Oddo, Jean-François Payen. Transcranial Doppler after traumatic brain injury: is there a role? Curr Opin Crit Care 2014;20:153–60. [DOI] [PubMed] [Google Scholar]
- 41.Fisher JP, Hartwich D, Seifert T, et al. Cerebral perfusion, oxygenation and metabolism during exercise in young and elderly individuals. J Physiology 2013;591:1859–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ward JL, Craig JC, Liu Y, et al. Effect of healthy aging and sex on middle cerebral artery blood velocity dynamics during moderate-intensity exercise. Am J Physiol Heart Circ Physiol 2018;315:H492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogoh S, Ainslie PN. Cerebral blood flow during exercise: mechanisms of regulation. J Appl Physiol 2009;107:1370–80. [DOI] [PubMed] [Google Scholar]
- 44.Querido JS, Sheel AW. Regulation of cerebral blood flow during exercise. Sports Med 2007;37:765–82. [DOI] [PubMed] [Google Scholar]
- 45.Pase MP, Grima NA, Stough CK, Scholey A, Pipingas A. Cardiovascular disease risk and cerebral blood flow velocity. Stroke 2012;43:2803–5. [DOI] [PubMed] [Google Scholar]
- 46.Muller M, Van Der Graaf Y, Visseren FL, et al. Hypertension and longitudinal changes in cerebral blood flow: The SMART-MR study. Ann Neurol 2012;71:825–33. [DOI] [PubMed] [Google Scholar]
- 47.Mitchell GF, Van Buchem MA, Sigurdsson S, et al. Arterial stiffness, pressure and flow pulsatility and brain structure and function: The age, gene/environment susceptibility-Reykjavik Study. Brain 2011;134:3398–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ainslie PN, Hoiland RL. Transcranial Doppler ultrasound: valid, invalid, or both? J Appl Physiol 2014;117:1081–3. [DOI] [PubMed] [Google Scholar]
- 49.Eicke BM, Tegeler CH, Dalley G, et al. Angle correction in transcranial Doppler sonography. J Neuroimaging 1994;4:29–33. [DOI] [PubMed] [Google Scholar]
- 50.Verbree J, Bronzwaer ASGT, Ghariq E, et al. Assessment of middle cerebral artery diameter during hypocapnia and hypercapnia in humans using ultra-high-field MRI. J Appl Physio 2014;117:1084–9. [DOI] [PubMed] [Google Scholar]