Abstract
Colorectal cancer (CRC) is one of the most common cancers in the world. About two third of patients with CRC will develop distant recurrence at some point in time. Liver is the most common site where distant metastasis takes place. While the overall survival (OS) of patients with metastatic CRC was poor about 3 decades ago, there has been tremendous improvement in this area in the recent years. With the advent of effective systemic chemotherapy and biologic agents and better understanding of the biological behaviour of the tumour, aggressive treatment strategies such as metastatectomy of the liver metastases (or lung metastases) are now acceptable. More importantly, it has transformed the way how stage IV CRCs are being managed. From predominantly palliative as the primary aim, a comprehensive multidisciplinary approach is now the mainstay of treatment with very successful outcomes. Combination of systemic therapies with liver resection has been shown to be effective in providing promising survival benefits. In addition, other adjunctive modalities in targeting the liver metastases such as ablation, combining resection and ablation, transarterial chemoembolization, stereotactic body radiotherapy (SBRT), hepatic artery perfusion, etc. have also been demonstrated variable outcome in treating colorectal liver metastasis (CRLM). Very recently, transplant oncologists have also explored using liver transplantation as a treatment modality for unresectable CRLM, which has demonstrated very good long-term survival in well selected cases. The new paradigm in the treatment of metastatic CRC has dawned.
Keywords: Colorectal liver metastasis (CRLM), systemic chemotherapy, liver resection, liver transplantation, liver targeted therapy
Introduction
Colorectal cancer (CRC) is one of the most common cancers in the world, ranking third in terms of incidence (10.2% of all cancer cases worldwide) and second most common cause of cancer mortality (9.2% of all cancer mortality) in the world. Over 1.8 million new CRC cases and 881,000 deaths are estimated to occur in 2018, accounting for about 1 in 10 cancer cases and deaths (1).
There has been a significant improvement in the survival of patients with CRC in the recent decades. Concurrently, the survival of patients with metastatic CRC has improved tremendously as well. In the 1990s, reported 2-year overall survival (OS) for stage IV CRC was only 21% (2). But, in the past 2 decades, the 5-year OS increased to 35–40% in the 2010s (3). Advancements in systemic chemotherapy with greater efficacy, improvements in surgical techniques as well as enhancement in peri-operative care leading to increase in the number of patients undergoing surgical treatment for CRC metastasis have significantly contributed to better outcomes in treating this condition (2,4).
Liver metastasis is the most common site of distant spread, accounting for approximately 15–25% of CRC patients will have distant metastases at the time of primary diagnosis (5). Other 18–25% patients will develop distant metastases within 5 years from the first diagnosis (6). The multidisciplinary approach to managing metastatic CRC has been pivotal in contributing to the improvement in OS of this disease, which previously was rendered to palliative care mainly. A multidisciplinary evaluation is crucial for patients with metastatic CRC as the choice and order of treatments differ depending on presentation, number of sites and location of metastases, and potential for surgical resection (7). Conventionally, neoadjuvant chemotherapy, surgical resection followed by adjuvant chemotherapy is the standard curative treatment for colorectal liver metastases (CRLM), commonly known as the “Sandwich therapy” (8,9). But there are more permutations to the treatment strategies for this disease now. In fact, there is a great likelihood that in the future, molecular signatures may be used to offer the most effective therapies based on tumour genetic subtype (7).
Several studies have shown 5-year OS rates of 47–60% after hepatectomy for colorectal metastases (10-12). However, recurrence occurs in 40–75% of patients after liver resection (13-15). Of these recurrences, 50% occur in the liver (14-16). Repeated hepatectomy after hepatic recurrence has proven to be feasible and improves survival (17). In contrast, the prognosis after recurrence of nonresectable CRC is dismal and the 5-year survival is less than 10% with palliative chemotherapy (18). Clearly, there are those with metastatic disease who can have reasonably good long-term survival with aggressive multidisciplinary approach. This has led to the revision of The American Joint Committee on Cancer seventh edition in 2010 which saw the amendment of Stage IV disease to subcategories of IVA (metastatic to one site) and IVB metastasis to more than one site) (19). Pushing this limit further, well-selected patients with CRLM who underwent liver transplantation seem to provide a potential to change the OS in this group of patients as shown in the SECA trial in Norway (20).
Definition of synchronous and metachronous liver metastasis
There are variable definitions of synchronous and metachronous CRLM reported in the literature. Most of the definitions of synchronous CRLM include liver metastasis detected at or before diagnosis or surgery of the primary tumour, but there are some others who include metastases detected up to 3 months, 4 months or 6 months following diagnosis (21-26).
In order to address this issue as well as to review the recommendations for the management of CRLM, the Expert Group on OncoSurgery management of Liver Metastases group (EGOSLIM) met to debate on this issue (27). The results of the meeting yielded the international consensus statements as follows:
❖ Synchronous CRLM have less favourable cancer biology and expected survival than metachronous, particularly late metachronous metastases.
❖ Synchronous CRLM should be termed “synchronously detected liver metastases”. This is defined as LM detected at or before diagnosis of the primary tumour.
❖ Early metachronous metastases are considered to be those detected within 12 months after diagnosis or surgery of the primary.
❖ Late metachronous metastases are considered to be those detected more than 12 months after diagnosis or surgery of the primary.
It is important to have clear definitions for these conditions as the tumour biology of both are now understood to be different and the natural history of the disease and response to treatment will also be different.
Biology of CRLM
Liver is the most common site for metastatic disease to occur in CRC. When the cancer cells from the primary sites in the colon escape into the bloodstream, the most likely location where they are lodged is the liver. Kelly et al. suggested that micrometastasis occurred when cancer cells from the primary CRC escape from the primary location into the portal circulation. Cancer cells from gastrointestinal malignancies, especially from CRC, hematogenously spread via the portal circulation, often making the liver the first metastasis site. Furthermore, when hepatic metastases grow beyond 2 mm, deriving additional blood supply is crucial for the cancer cells to survive. These metastatic tumours secrete angiogenic factors to induce neovascularisation to derive blood supply from the hepatic artery, while normal hepatocytes are perfused mostly from the portal circulation (28).
Recently, the concept of liver metastasis microenvironment (LME) has emerged as we understand more about the interactions of cancer cells with microenvironment in the liver parenchyma. How the metastatic colon cancer cells engraft in the liver microenvironment and subsequently grow and proliferate within the liver parenchyma, involves an intricate communication process between the cancer cells, the inflammatory and immune cells in the liver as well as the hepatocytes and nonparenchymal cells in the liver. As a result, strategies that harness the engagement of immune system to target both cells and molecules within the LME have shown to be successful approaches which yield highly effective and durable therapeutic modality (29).
We can classify the process of CRLM into 2 specific niches, which can be divided into premetastatic niche formation and the post-tumour invasion niche (29). The latter has 4 distinct phases of the tumour metastasis process, namely the microvascular phase, preangiogenic phase, angiogenic phase and growth phase. It appears that during the premetastatic niche, primary tumour cells secrete factors to recruit nonparenchymal cells, including Kupffer cells (KC), hepatic stellate cells (HepSC), myeloid-derived suppressor cells (MDSC) and neutrophils to aid their invasions. Some recent evidence supporting this postulation demonstrated that tumour-derived factors could activate the cells at the LME to render permissive to metastatic outgrowth in advanced of tumour cell entry (30).
Once the cancer cells enter into the liver microvasculature in the microvascular phase, they need to escape the elimination by the immune cells present locally, including the KC and hepatic natural killer (NK) cells. They can escape the destructive process from the proinflammatory cells by attaching to cytokine-induced endothelial CAM and transmigrating into the space of Disse if they express the corresponding counter receptors (29). After successfully escaping from the proinflammatory cytokines, the tumour cells invade into and expand within the liver parenchyma with facilitation by the quiescent HepSCs in the proangiogenic phase. The HepSCs deposit collagen and fibronectin that provide scaffolding for endothelial migration, angiogenesis and the establishment of extravascular micrometastases, mainly driven by TNFαβ and TGFβ (29,31,32). This set the stage for the angiogenic phase where metastatic cancer cells within the liver parenchyma start co-opting surrounding vessels to draw blood supply in order to prepare for their growth. Classically, the vascular endothelial growth factors (VEGF) and basic FGF (bFGF) are the factors triggering the angiogenic process. Many cells in the LME secrete these factors in response to the cytokine release, including KCs, the newly recruited polarized tumour-associated macrophages (TAM) to M2 phenotype, tumour-associated neutrophils (TAN) and HepSCs (33-36). Now that the tumour cells have gain access to the blood supply, they will proliferate and expand in the growth phase. However, this is not a ‘free-for-all’ situation for the cancer cells. The T-cell mediated response [CD4+ T helper cell and CB8+ cytotoxic T lymphocyte (CTL)] within the liver can curtail the metastatic expansion by activating different cytolytic mechanisms. The tumour cells have been shown to evade the cytolytic process via coinhibitory molecules such as death protein 1 (PD-1) that binds to ligands PD-L1 or PD-L2 on the cancer cell and the CTL-associated protein 4 (CTLA-4), resulting in inhibition of T effector cell functions (29). In the TGFβ-rich tumour microenvironment (TME), TAMs and ATNs can acquire immunosuppressive (M2 and N2 respectively) phenotypes (30). The immunotolerance by the cancer cells are further enhanced by recruitment of immunosuppressive lymphoid and myeloid subsets, including MDSC and regulatory T cells (Treg). Other protumorigenic growth factors in this LME include the type I insulin-like growth factors, EGF, HGF produced by hepatocytes, M2 TAMS and HepSCs respectively.
Understanding the TME is extremely crucial to find the appropriate targets in preventing and treating metastatic disease in the case of CRLM. As illustrated above, the cancer cells depend on the TME to support their survival and growth. In this microenvironment, the cells are genetically stable and their properties and responses are more predictable. Moreover, targeting the microenvironment may be beneficial across tumour types, in particular tumours that metastasize to the same secondary sites such as the liver (29).
Biological markers for CRLM
Based on current standard of care, KRAS and BRAF mutations are probably the most well studied in the context of CRC. KRAS mutant status has been associated with lower likelihood of having resectable CRLM. There is also higher risk of extrahepatic disease, adverse response to targeted anti-EGFR therapy as well as to oxaliplatin or irinotecan-based peri-operative chemotherapy (37-39). RAS mutation status has also been found to confer poorer survival for patients who underwent CRLM metastatectomy (37). Therefore, RAS mutation status is important in guiding decision-making before embarking on aggressive surgical therapies, e.g., 2-stage liver resections (40) and those who are planning for liver resection after second-line chemotherapy (41). On the same note, BRAF mutation in CRC has been found to confer poorer survival and poorer response to biological therapies (42,43). The outcomes of patients with BRAF mutation status who underwent CRLM metastatectomy has also been shown to be poor (44).
In addition to KRAS and BRAF mutations, caudal-type homeobox transcription factor 2 (CXD2), a critical regulator of intestinal development and oncogenesis has been shown to be a good marker for prognostication in CRC (45), and recently has been shown to correlated well with the behaviours of CRLM. Patients with metastatic colorectal disease that has CDX2-negative status was found to have poorer OS and it was correlated with higher likelihood of right-sided primary tumors, poorly differentiated cancers, distant lymphatic metastasis and be women (46,47).
In a systematic review of the tumour biology of synchronous and metachronous CRLM by Slesser et al., they found that the majority of studies demonstrated differences in molecular marker expression between CRLM and their respective primary tumours in both the synchronous and metachronous groups. Studies investigating the genetic aberrations demonstrated that the majority of changes in the primary tumour were ‘maintained’ in the CRLM. In addition, with some conflicting results, they found that the CRLM in synchronous and metachronous groups demonstrated some differences suggesting the more aggressive tumor subtype in the synchronous group (48). One example was the p27 marker which was found to correlate with advanced stages of CRC. In the metachronous group, there was reduction in the expression in the liver metastasis possibly due to ‘post-translational’ degradation of the protein in the liver metastases (49,50). Similarly, the cyclin E expression was also found to be reduced in the synchronous group but its significance and role are unclear at the moment (51). Expression of cyclooxygenase-2 (COX-2) gene was found to be elevated in synchronous CRLM by Pantaleo et al. while contradicting results were found on expression of EGFR in metachronous CRLM, with some showing overexpression while others demonstrated no difference in EGFR expression between synchronous and metachronous CRLM (52-55).
In 2009, Camus et al. described the correlation between relapse of CRC with tumour escape and immune coordination, it has sparked off a major discovery into the role of immune responses in treating cancers (56). Stratification of immune environment and responses has been used to create immunescoring, which is shown to be a better prognostic tool for patients with CRC than MSI (57), which is currently tested to predict the response of these patients to anti-programmed cell death protein 1 (PD-1) therapy (58). Further extrapolation of this idea resulted in the use of ‘liquid biopsy’ to identify certain expressed genomic materials to guide prognostication and therapeutic decision in the treatment of cancer (59). Attempts to rationalize and set instrumental guidelines for personalized therapies have been made. Blank et al. introduced a dynamic model (the ‘cancer immunogram’), which required the assessment of a combination of biomarkers as a tool to guide treatment options for individual patients (60). An initial framework of seven parameters has been established: tumour foreignness; general immune status; immune cell infiltration; absence of checkpoints; absence of soluble inhibitors; absence of inhibitory tumour metabolism; and tumour sensitivity to immune effectors. The evaluation of these factors can be achieved by a combination of tumour genomics, immunoscore assay, immunohistochemistry, standard blood assays and immune gene signature, both pre-therapy and post-therapy, and could be helpful in designing possibly the most efficient therapeutic intervention for individual patients (60). The role of liquid biopsy is likely to become the standard diagnostic modality in the near future.
Treatment of CRLM
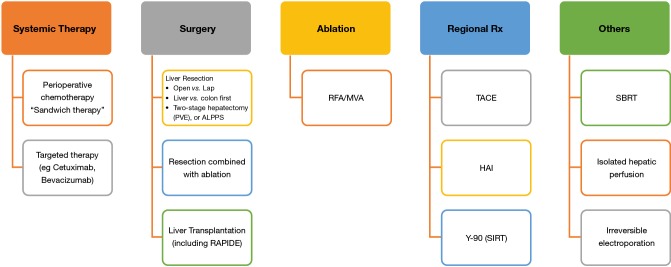
Two decades ago, options to treatment metastatic CRCs were very limited. Fast forward to today, many treatment modalities are effective in providing a reasonably good long term survival as mentioned above. The key to successful treatment of CRC with liver metastases and other extrahepatic metastases is the multidisciplinary approach which involves the medical oncologists, radiation oncologists, colorectal surgeons, hepatobiliary surgeons and others. The wide variety of options for treating CRLM is shown in Figure 1.
Figure 1.
Current treatment options for colorectal liver metastasis.
Systemic therapy for treatment of CRLM
Advances in systemic chemotherapy as well as biologic agents have significantly improved the OS of patients with CRLM and other metastatic disease in CRC treatment. Based on current evidence, possible first line chemotherapy for treating CRC with CRLM include: fluorouracil, leucovorin and oxaliplatin (FOLFOX), fluorouracil, leucovorin, irinotecan (FOLFIRI), capecitabine plus oxaliplatin (XELOX) and fluorouracil, leucovorin, oxaliplatin and irinotecan (FOLFOXFIRI) (61). They can also be complimented with biological agents such as bevacizumab or cetuximab in the course of treatments. The RAS and BRAF status are crucial in deciding if these biologic agents will be useful in the treatment strategies as discussed above. Fakih summarised very nicely the recommendations based on the RAS and BRAF mutation status. FOLFOXFIRI with or without bevacizumab, FOLFOX or FOLFIRI with anti-EGFR are favoured for downstaging for resection. However, bevacizumab must be avoided in patients with high risk of bowel perforation or thrombotic events. These regimens have been shown to have good response rate of >50% with improvement of OS by around 30 months (62-65).
In event that response is not observed after administration of first line therapy, there is still hope to use second for CRLM with FOLFIRI and panitumumab to elicit some treatment response (66). By this time, the chance of resection of the CRLM will significantly decreased, although it may still be possible in selected cases as shown by Adam et al. (67) and Brouquet et al. (68). Bearing in mind the potential toxicity of perioperative chemotherapy on the liver which may result in chemotherapy-associated liver injury (CALI), careful discussion at the multidisciplinary tumour board will help to select appropriate patients for CRLM resection. There are generally 3 distinct histological patterns in CALI, namely steatosis, steatohepatitis and sinusoidal obstruction syndrome. Oxaliplatin is typically described as the culprit of the ‘blue liver’ (sinusoidal obstruction syndrome) while irinotecan is the cause of steatohepatitis (69,70). It has been reported that 2 key factors, including the interval of stopping the systemic chemotherapy as well as the total number of cycles of chemotherapy are both associated with significant increase in post-operative complications after CRLM metastatectomy. Welsh et al. demonstrated the inversely proportional relationship between complication rate post-hepatectomy and length of time between cessation of chemotherapy and surgery (71). In addition, Kishi et al. reported that >9 cycles of systemic chemotherapy were associated with increased risk of sinusoidal injury and hepatic insufficiency without significant improvement in the pathological response rates (72).
It is clear that combination treatment strategies including systemic chemotherapy with or without biologic agents and CRLM resection will improve survival for patients, whom previously would be rendered to palliative treatment due to its stage IV disease status. However, careful selection remains the key. Limiting the duration of preoperative chemotherapy to ≤6 cycles and ensuring adequate FLR volume and function before surgery will be vital to ensure successful CRLM resection.
Resection of CRLM
Two decades ago, patients presented with metastatic CRC were rendered to palliation with limited treatment options as the median life expectancy was around 9 months with an extremely dismal 5-year survival of 3% (73). With the advent of effective systemic therapy such as chemotherapy and/or biologic agents, surgical resection of CRLM has been shown to provide a potential ‘curative’ treatment with good long-term survival. The combination of systemic chemotherapy with resection of CRLM has been shown to provide a 5-year survival of 50% by Norlinger et al. in the EORTC 40983 trial (74). While such combination therapy has yielded a significant OS for 5 years, the chance of recurrence of CRC remains high, reported in up to 70% of cases (75). Nonetheless, this remains a significantly big improvement as compared to the historical scenario that we saw previously. Further research and developments in this area will certainly shed new lights into the optimal treatment strategies for CRLM in the near future.
Selection of patients who will benefit from CRLM liver resection has always been a challenging task. In 1999, Fong et al. created the Clinical Risk Score which was an algorithm containing variables including a positive margin, presence of extrahepatic disease, node-positive primary, disease-free interval from primary to metastases, number of hepatic metastasis >1, largest hepatic lesion >5 cm and CEA level >200 ng/mL (76). Based on the prognostic scoring system, low risk patients demonstrated a 5-year survival of 47% as compared to high risk patients with only 24% of 5-year survival. Following the introduction of the Clinical Risk Score, there were many more iterations of similar scoring systems including the Basingstoke predictive index by Rees et al., Japanese Classification System by Yamaguchi et al. and others (77-83). While they may be able to correlate with prognosis and long-term survival, unfortunately, they remain a rather crude model for selecting patients who will benefit from resection of CRLM with the combination of systemic therapy.
The decision for surgery in patients with CRLM is a complex task as many factors must be taken into considerations, including when to give the systemic chemotherapy with or without anti-EGFR agents before or after liver resection, how to assess response before liver resection is considered safe with adequate FLR, sequence of liver surgery (colon resection first or liver resection first or combined simultaneous surgery) etc. Perhaps in the near future, precision surgery guided by liquid biopsy will be able to accurately identify the exact genetic mutations that manifest as specific biomarkers that will determine the appropriateness of offering surgery to patients with CRLM with clear survival benefit.
Nonetheless, before we reach that final destination, there are currently some recommendations by the international consensus from the EGOSLIM (Expert Group on OncoSurgery management of LIver Metastases) group with regard to the timing and roles of liver resection in the context of synchronous and metachronous CRLM (27).
In general, potentially curative treatments are goals for patients with one site of surgically resectable metastatic disease, such as in CRLM, especially in metachronous setting. For those with more than one site of metastatic disease, the general goal is cancer control.
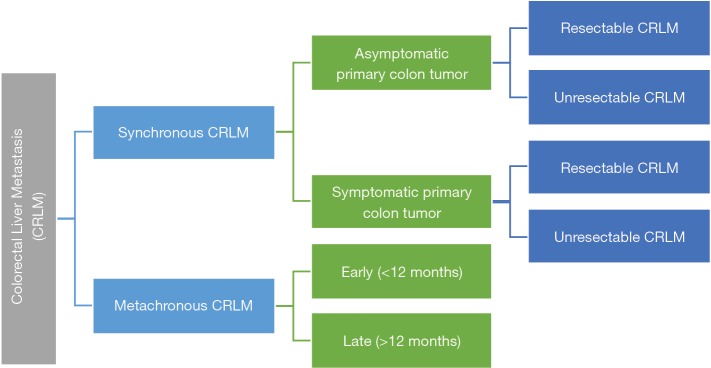
The potential scenarios in which CRLM can present to the liver surgeons for consideration of surgical treatment can be categorised as shown in Figure 2.
Figure 2.
Potential scenarios of colorectal liver metastasis.
Based on the scenario in Figure 2, we can largely summarise the clinical approach to synchronous and metachronous CRLM as follows (27):
-
Synchronous CRLM (patients with resectable CRLM and asymptomatic CRC).
❖ Chemotherapy should be given preoperatively unless surgery of the primary and LM is considered early;
❖ For rectal tumours, preoperative radiotherapy is a standard of care, but not for high rectal tumour or T2 tumours; and one-stage surgery should not be performed;
❖ For colonic primary tumours, one-stage surgery is not advocated for tumours needing complex surgery, in high-risk patients or when hepatectomy would be major;
❖ A total of 6 months of chemotherapy is recommended, independent of whether given pre- or post-operatively;
❖ Postoperative chemotherapy may be different from the pre-op chemotherapy and may be less intense.
-
Synchronous CRLM (patients with non-resectable CRLM and asymptomatic CRC).
❖ Chemotherapy should be administered initially with the aim of achieving resectability of CRLM metastatectomy;
❖ If the CRLM become resectable, a reverse strategy should be advocated;
❖ For rectal cancer, radiotherapy may be given before chemotherapy, or after resection of the CRLM.
-
Synchronous CRLM (patients with resectable CRLM and symptomatic CRC).
❖ For bleeding CRC, following transfusions, pre-operative chemotherapy should be advocated;
❖ For perforations, resection of the primary to remove the tumour (right colon) or suture or creating a stoma (left colon) is advocated;
❖ For proven occlusion with distended evidence of obstruction, resection of the primary should be performed first;
❖ For occlusions, stents are an option but results have been poor;
❖ Liver resection will be done after the crisis for the primary colon tumour is taken care of.
-
Synchronous CRLM (patients with non-resectable CRLM and symptomatic CRC).
❖ The aim of this scenario is to make the CRLM resectable; patients would be managed like the scenario above;
❖ Stents are not recommended unless there is a chance for cure.
-
Metachronous CRLM (patients with resectable CRLM).
❖ Liver resection can be done safely.
-
Metachronous CRLM (patients with unresectable CRLM).
❖ Chemotherapy should be administered initially with the aim of achieving resectability of CRLM metastatectomy.
Liver surgeons are often asked during the multidisciplinary tumour board to review the scans of patients with CRC with liver lesions whether those metastatic lesion(s) are resectable or not. Resectability of the CRLM is determined by many factors such as:
❖ Size and number of nodules;
❖ Relationship of the lesion(s) with major hepatic vessels and segmental localisation of the lesions in the liver;
❖ Response after neoadjuvant chemotherapy;
❖ Non-tumoral liver quality-cirrhotic or not-mandatory to check hep b/c before chemo, NASH or CASH liver;
❖ Anticipated remnant liver volume.
Combining all these factors, there are 4 main areas to consider before deciding on resectability of the CRLM (Figure 3).
Figure 3.
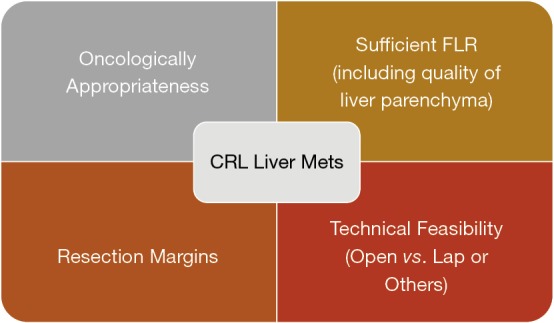
Key considerations in resectability of CRLM. CRLM, colorectal liver metastasis.
Oncologically appropriateness
While previously thought that the greater the number and size of the lesions, the worse prognosis of the CRLM. However, if the resection of CRLM could render R0, the survival is the same regardless of the number of lesions. The main challenge is to be able to undergo liver resection.
As mentioned above, various criteria have been proposed to guide the selection of patients for CRLM going for liver resection, in order to exchange for survival benefits. While the Clinical Risk Score by Fong et al., which was published in 1999, was the first to attempt to provide some form of guidance in the selection of appropriate cases to undergo liver resection, there have been more prognostic scoring system since then (76-83). Many of these scoring systems have demonstrated good correlation with prognosis and ling-term survival, but they remain a crude model for selecting patients who will benefit from resection of CRLM. In the advent of many systemic therapies and possibly immunotherapy options, the future of selection of cases for CRLM liver metastatectomy will likely lie in serum biomarkers and genomic profiling of the tumours as discussed before.
Resection margins
Adequacy of resection margins in CRLM liver resection has long been debated. The “1 cm margin rule” has been disputed as some studies showed that less than 1mm resection margin in CRLM metastatectomy did not influenced the prognosis, provided the patient receive good systemic therapy on time. Although the ‘‘1 cm margin rule’’ is no longer the criteria of resectability, some studies suggest that it may be associated with superior prognosis compared with narrower margins (84-87). On the other hand, other studies demonstrated non-inferior prognosis in resection margin width less than 1 mm (88,89).
In order to address this issue, the EGOSLIM (Expert Group on OncoSurgery management of Liver Metastases) group convened a meeting in 2015. The result of that meeting stated clearly that “safe resection margins are still a goal of therapy and a minimal surgical clearance margin of 1 mm has been suggested as sufficient” (27). Nonetheless, the optimal surgical margin for CLRM remains a debate. Most recently, Margonis et al. published a systematic review and meta-analysis that included 34 studies representing 11,147 hepatic resections and found that wider resection margin (>1 vs. <1 cm) was significantly associated with improved OS at 3 years, 5 years and 10 years. Similarly, DFS was positively associated with >1 cm resection margin at 3, 5, and 10 years. Meta-regression analyses did not reveal any significant modifying role of the study features under investigation, such as the administration of neoadjuvant/adjuvant therapy. As such, they concluded that, while a >1 mm margin is associated with better prognosis than a submillimeter margin, achieving a margin >1 cm may result in even better oncologic outcomes and should be considered if possible (90).
Sufficient future liver remnant (FLR) & quality of the liver parenchyma
It is imperative for the liver surgeon to study the images the liver scan(s) to determine the location and size of the lesion with crucial surrounding structures. The relationship of the lesion(s) to critical inflow pedicular structures such as bile duct, portal vein and hepatic artery as well as outflow structures such as hepatic veins has significant influence on how the surgery will be conducted.
Peripherally located tumours can be easily resected if the quality of the liver parenchyma allows so. In most circumstances, the liver parenchyma of patients with CRLM should be able to withstand liver resection, provided it is not exposed to excessive amount of systemic chemotherapy which may cause CALI liver as discussed above. Small wedge resection should be reasonably safe in most patients. If the tumours are located deep within the parenchyma of the liver and near to major hepatic veins, portal veins or biliary pedicles, major liver resection will be necessary in order to achieve R0 resection. In this circumstance, careful consideration must be given to the size of the FLR and the adequacy of liver function post resection. In most circumstances, up to 70% to 75% of non-cirrhotic liver could be resected as long as the remnant liver volume contributing to 25% to 30% of the total liver volume (91,92). The safety margin increases significantly in these patients with non-cirrhotic liver if a smaller resection is required.
Further evaluation of the quality and function of the hepatocytes can be achieved by performing the indocyanine green (ICG) clearance test (93). The ICG dye is exclusively cleared by the hepatocytes and excreted into the biliary system, the amount of ICG retained in the blood at a certain duration after injection can be used to stratify the risk of major liver resection. Imamura et al. proposed the use of Makuuchi decisional algorithm using ICG retention at 15 minutes as follows (94):
❖ <10% at 15 min for trisectionectomy or bisectorectomy of liver;
❖ 10% to 19% for hemihepatectomy, right sided sectorectomy;
❖ 20% to 29% for segmentectomy;
❖ 30% to 39% for limited resection (e.g., wedge resection);
❖ >40% for enucleation.
Hepatobiliary surgeons may face the occasional challenging situations where the resection of liver is technically possible and oncologically feasible but the remnant liver volume is deemed inadequate (i.e., <25% of the total liver volume). In such situations, methods to grow the FLR may need to be considered. Options to grow the FLR can be divided into:
❖ Portal vein embolization (PVE) and staged hepatectomy;
❖ Portal vein ligation (PVL) and staged hepatectomy;
❖ Association of Liver Partition with Portal vein ligation for Staged hepatectomy (ALPPS).
To harness the regenerative potential of the liver to grow the FLR, the concepts of PVL was explored by the Japanese in the 1975. Honjo et al. introduced the technique of PVL (95). However, the concepts of inducing liver hypertrophy by manipulating the portal blood flow was first emphasized by Cantlie in 1897 and later by Rous in 1920 (96). PVL is used routinely in two-stage procedures, where sometimes a ‘cleansing’ of the FLR from tumour is performed along with PVL. After reaching adequate hypertrophy of the FLR, resection of the diseased liver part is undertaken during a second stage (97).
Kinoshita et al. (98) and Makuuchi and co-workers (99) in the late 1980s introduced the techniques of PVE by injecting embolizing agents into one of the portal branches. Over the past decades, this approach has gained wide acceptance in the field of liver surgery. Direct comparisons between PVL and PVE regarding the hypertrophy of the FLR were reported with controversial results (97,100-102). While these techniques are popular, it is plagued with a high drop-out rate. The drop-out rate was reported to be up to 35 per cent of patients due to either insufficient liver hypertrophy of the FLR or tumour progression (103,104).
Recently, a new technique of ALPPS has been introduced. Several reports suggested that by combining PVL and partitioning the liver parenchymal in the same setting, greater hypertrophy of FLR could be achieved as compared to PVE or PVL alone, with almost 96–99% of patients undergoing definitive hepatectomy (97,105,106). However, the issues of higher mortality and morbidity (as high as 12% and 40% respectively) associated with ALPPS has dampened the initial enthusiasm with this promising technique (107).
In a recent study done by our center, ALPPS induced a superior volumetric response when compared to PVE/PVL (108). Our study showed that the FLR in ALPPS patients grew by 163.0±90.5 mL representing a 48% increase in size over a median duration of 7 days between both stages. In contrast, the FLR in conventional staged hepatectomy (CSH) (PVL or PVE) patients grew by 57.0±80.8 mL or 12% over a median interval of 20 days. This finding was consistent with a recent meta-analysis by Eshmuminov et al., showing that ALPPS induced 81% FLR increase compared to 35–38% in the PVE/PVL group (97). However, further study demonstrated that, if the underlying disease was HCC requiring ALPPS, the FLR grew significantly less after ALPPS-stage 1 compared to non-HCC patients. We have found that the presence of hepatic fibrosis on the final histopathology was associated with negative impact on the FLR growth. When considering suitability for ALPPS, patients with HCC may benefit from additional pre-operative assessment of fibrosis (109).
It is clear based on current evidence that volume growth in ALPPS is not directly reflective of the functional status of the liver parenchyma. Even when evidence clearly showed that ALPPS rapidly increases FLR volume, Matsuo et al. demonstrated that the hepatocytes seen on light and electron microscopy were immature after ALPPS when compared to PVE (110). In addition, Sparrelid et al. showed that found that using scintigraphy and single-photon emission computed tomography (SPECT), the magnitude of increase in FLR function was 50% of the magnitude of increase in FLR volume (111). As such, there is a new direction recently to better conduct cross-sectional assessment of FLR function after ALPPS-stage 1 with the deployment of liver specific tracers (e.g., 99m Tc-galactosyl, 99m Tc-mebrofenin) and magnetic resonance imaging contrast agents (e.g., gadolinium ethoxybenzyl, gadobenate dimeglumine). These modalities may reflect FLR function more accurately compared to volumetry alone (93,111,112).
While ALPPS may be able to provide the surgeons with a better chance of securing an R0 resection for HCC, the selection of patients remains the key consideration. In the first international expert meeting on ALPPS, HCC was listed as one of the pathologies where ALPPS procedure should be used with caution due to higher morbidity and mortality rate (113). Laparoscopic ALPPS can potentially confer additional benefit as it harnesses the benefits of minimally invasive surgery (MIS) with smaller scars, lesser post-operative pain, faster recovery and shorter hospitalisation (Figure 4A,B,C,D,E,F).
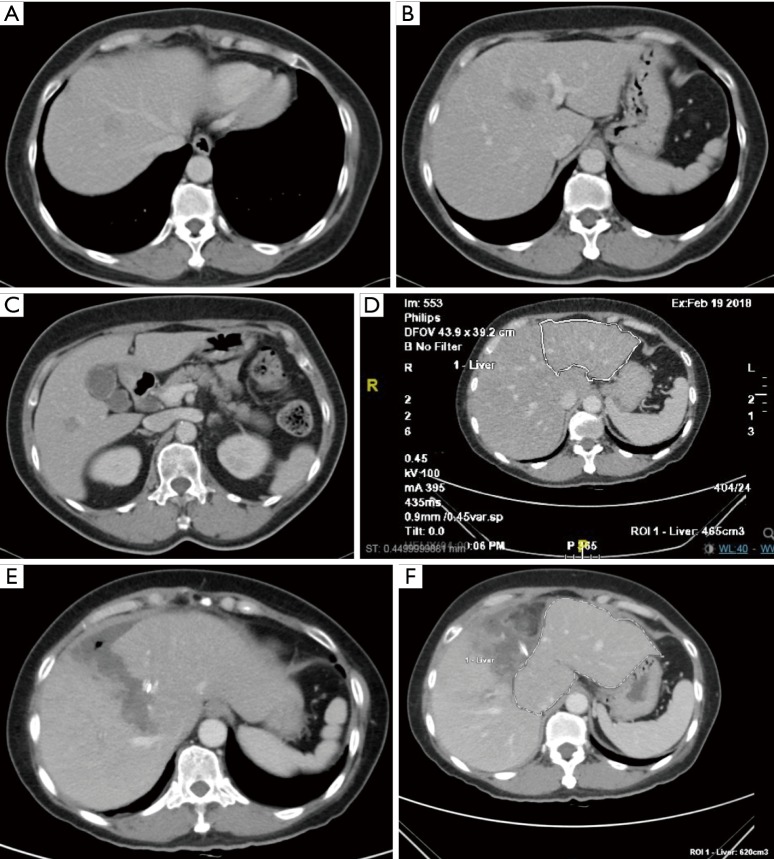
Figure 4.
Resection of colorectal liver metastasis and attempt to grow the future liver remnant (FLR). (A,B,C) Metastatic lesions appearing as hypodense lesion in the liver parenchyma in segment VIII (A), segment IVb (B) and segment VI of the liver (C). In order to help the patient gained a status of “No evidence of disease”, an extended right hepatectomy was required. Based on the CT volumetry, the remnant left lateral section was too small. (D) Based on the CT volumetry, the remnant left lateral section was too small. The FLR of this liver was on 21% of the total volume. As a result, an ALPPS procedure was proposed. As the lesions were all within the liver parenchyma in the right trisection of the liver and not involving the inflow pedicle to the left lobe, it was technically suitable to perform the ALPPS procedure using full laparoscopic method. (E,F) The FLR of this liver had grown from 21% to 35% within 14 days of the ALPPS procedure. In between, the patient was discharged to rest at home on POD 4 following the first surgery and returned to hospital for a scan to assess the volumetry and then proceeded to the second staged of ALPPS later on. The second stage operation took place on the 14th POD after the first stage and the patient recovered uneventfully.
Technical feasibility
Where possible, MIS method of resection of the CRLM [laparoscopic liver resection (LLR)] can be considered. In centres where the MIS technique is mature, patients with CRLM may be able to enjoy the benefits of MIS liver resection. Open liver resection (OLR) for CRLM used to be the standard of care and many opponents of MIS liver surgery argued that the MIS technique was not safe and it may potentially compromise the oncological outcomes of the CRLM metastatectomy (114). However, as MIS quickly becoming the preferred technique to perform this surgery, mainstream evidence has shown that MIS liver resection is as good as open surgery from the oncological perspective, with additional benefits of the MIS surgery as discussed above. A meta-analysis involving 610 patients comparing laparoscopic versus OLR for metastatic CRC performed by Schiffman et al. showed that, compared to the OLR group, the LLR group had lower estimated blood loss, blood transfusion rate, length of stay, and lower overall complication rate. There was no difference in operative time, margins positivity, liver-specific complications, or 30-day mortality. Oncologically, there was no difference in 1-, 2-, and 5-year disease-free survival or OS between the groups (115). Subsequently, the Oslo-CoMet study in Norway reported that, in patients undergoing parenchyma-sparing liver resection for colorectal metastases, laparoscopic surgery was associated with significantly less postoperative complications compared to open surgery. Laparoscopic resection was cost-effective compared to open resection with a 67% probability. The rate of free resection margins was the same in both groups (116). While the initial ORANGE II trial was halted due to very slow recruitment of trial candidates (117), ORANGE II Plus trial is currently still ongoing and hopefully will have results to show by end of this year.
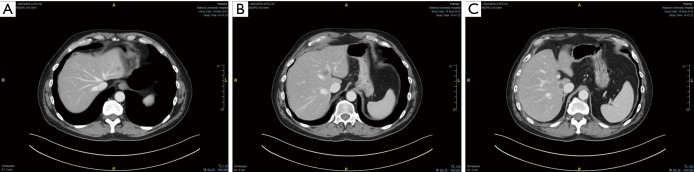
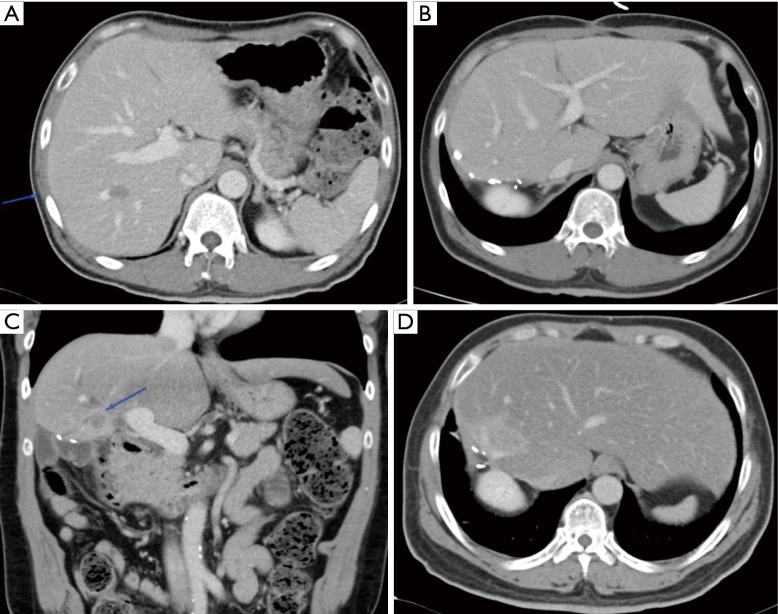
The feasibility of MIS liver resection depends very much on the location of the lesions and their relationship with major structures such as the portal vein, hepatic artery, bile ducts and hepatic veins. In many cases, the lesions could be present in multiple locations and laparoscopic HPB surgeons need to have a strategy tailored to individual patients to achieve R0 for all the lesions, at the same time, leaving behind sufficient FLR for survival of the patients in the immediate post-operative period. Even when the lesions are located at very difficult locations (e.g., at caudate lobe in segment I of liver, Figure 5A,B,C), advances in surgical instruments have made it possible to perform the surgery laparoscopically with very high success rate.
Figure 5.
Resection of liver metastasis from colorectal cancer at difficult location. (A,B,C) The patient has liver metastasis to segment II of the liver. Concurrently, there are two more lesions in segment IVA (B) and caudate lobe of the liver (C). (C) Tumour located at segment I (Caudate lobe) is technically very difficult to resect either open or laparoscopically due to its anatomically location, being situated between the IVC posteriorly and all the inflow structures to the left and right anteriorly. There are plenty of direct branches of PV and bile ducts connected to the caudate lobe e.g., the Spigelian branch of the portal vein.
Repeated liver resection for liver metastasis
While resection of the metastatic CRC in the liver has been shown to yield a reasonably 5-year survival at 47% to 60% (10-12), 50% to 70% of patients eventually developed recurrence after hepatectomy and about one in three of them has isolated liver recurrence (76,118-120). Vaillant et al. demonstrated that repeated hepatectomies in selected patients with recurrent CRLM could yield an overall 5-year survival of 30% (121). Following that report, there were other publications showing that repeated liver resection of CRLM could result in favourable survival outcomes in these patients, who would otherwise be considered as palliative candidates (122,123). A meta-analysis done by Wang et al., which pooled the data of 3,039 patients from 34 studies, demonstrated that the OS of repeat hepatectomy for recurrent CRLM was 42%. While the median overall morbidity was reported to be 23% in this cohort, there median mortality rate was 0% (range, 0% to 6%). They have also identified negative prognostic factors related to repeat hepatectomy including high primary tumour stage (T3/T4), multiple tumours, largest tumour size ≥5 cm, positive surgical margin at initial hepatectomy. At repeat hepatectomy, high serum CEA levels, short disease-free interval of ≤12 months, multiple tumours and bilobar disease at recurrence, largest liver lesion ≥5 cm, positive resection margins at repeat hepatectomy and presence of extrahepatic metastases were significantly associated with poorer OS (124).
Repeat hepatectomy is considered challenging due to a few reasons. Exposure for a repeat hepatectomy may be difficult due to post-operative adhesions following previous liver resection. Hypertrophy of the residual liver as well as alterations of the hepatic anatomy from previous hepatectomy may post significant challenging in the vascular and biliary ductal planes (Figure 6A,B,C,D). Such challenges may potentially increase the risk of morbidity and mortality in repeat hepatectomy. Yet, most of the studies showed that it is feasible to perform repeat hepatectomy in CRLM with reasonably low morbidity and mortality (124,125). It is a fine balance between parenchymal preserving and anatomical resection of the liver metastatic lesions. The most important consideration should be the ability to achieve R0 resection. Sparing more liver parenchyma is crucial to prepare for possible future need of another liver resection. In event that repeat liver resection is no longer possible due to limitations in liver vascular and biliary anatomy or inadequate FLR, could there be a role for liver transplantation in the future? There is very little evidence to support this strategy at this point in time but the body of evidence is growing at this juncture (see below on liver transplant in CRLM).
Figure 6.
Repeat resection of CRLM that can still confer survival benefits but each time, making it more difficult to resect the tumours. (A) Patient presented with a solitary CRLM in segment VI/VII of the liver with the primary tumour in situ. After initial 3 cycles of systemic chemotherapy, he underwent posterior sectionectomy and anterior resection of the colon simultaneously. (B) He received another 3 rounds of chemotherapy after the surgery. At 2 years after the initial simultaneous colon and liver resection, he remained free of disease without any chemotherapy. (C) Unfortunately, he was found to have a solitary recurrence in segment V/VIII of the liver and he underwent completion right hepatectomy 2 and a half years after the initial resection of the liver. He received a few more rounds of chemotherapy after that. (D) He remained disease for another 2 years and was found to have another new liver metastasis in segment IVA of the liver at 4 and half years after the initial simultaneous surgery. He opted to have the 3rd liver resection of wedge out the segment IVA lesion. Following that, he has survived for 6 years since the initial diagnosis of stage IV colorectal cancer with liver metastasis. CRLM, colorectal liver metastasis.
In an attempt to achieve curative treatment in CRLM, the role of systemic chemotherapy combining with surgery is pivotal in this strategy. While Norlinger et al. had demonstrated in the EORTC 40983 trial the benefits of this combination therapy for initial hepatectomy, the roles of systemic chemotherapy in repeat hepatectomy should hold true using this same finding (74).
Liver transplantation in CRL liver metastasis
While hepatocellular carcinoma (HCC) has become the standard indication for liver transplantation in the recent decades, there is more evidence in the past few years showing acceptable survival benefits of liver transplantation in unresectable CRLM as demonstrated in the SECA I trial by the Norwegian group in Oslo (20). The key considerations in using liver transplantation as the treatment for CRLM are as follows:
❖ Oncologically sound-survival outcomes comparable to other standard indications of liver transplantation;
❖ Interaction between immunosuppressants, systemic chemotherapy and tumour recurrence;
❖ Availability of organs for liver transplantation-competing with existing indications (HCC and other non-malignant cases);
❖ Technically sound-LDLT vs. DDLT.
One of the earliest reported experience of using liver transplantation for patients with unresectable CRLM came out from the Medical University of Vienna. They published the initial experience of 25 patients transplanted from 1982 to 1994 (126,127). The initial 3- and 5-year survival rate was 32% and 12% respectively (128) but the 30-day mortality rate was high at 30% (126). From this study, they have learnt that patients with negative lymph nodes for metastasis had better long-term survival, triggering a follow up study showing that 15 of 21 patients that initially were classified as lymph node negative had in fact micrometastases. The median survival of patients without lymph node micrometastases that underwent a LT was significantly better at 118 months (compared to 28 months in patients with lymph node micrometastases, P=0.01) (129). Similarly, data from the European Liver Transplant Registry with 58 cases of LT for NRCLM performed between 1977 and 1995 showed poor survival results with the 5-year survival rate only at 18% The reported 1-, 3-, and 5-year survival was 73%, 36%, and 18%, respectively (130).
The initial enthusiasm of transplanting patients with unresectable CRLM died down rapidly due to the unsatisfactory initial results. The key reasons were attributed to poor patient selection with no standardised protocol, learning curve of surgical expertise in LT and the absence of standardized immunosuppression protocols. Indeed, in many initial experiences, the postoperative mortality after LT was high (126-130). Furthermore, the systemic options of chemotherapy for CRC towards the end of the last century were not associated with good outcomes (131). Following that, liver transplant community accepted that unresectable CRLM should not be treated with liver transplantation as it is associated with poor 5-year survival (<20%) and a high recurrence rate.
However, the results of SECA I trial conducted by the group from the Oslo University Hospital, Norway managed to demonstrate excellent results for patients with unresectable CRLM treated with liver transplantation (20). In this study, 21 out of the 25 patients who were recruited in the study underwent deceased donor liver transplantation. Four patients dropped out because they have developed extrahepatic disease. The median follow-up time was 27 [8–60] months and the 1-, 3-, and 5-year OS was 95%, 68%, and 60%, respectively. Recurrence occurred in 19 (90%) patients and 6 (29%) patients died of disseminated CRC after a median of 26 [6–41] months. The strict selection criteria in SECA I trial was the main reason how this result was achievable. The inclusion criteria for this trial were R0 primary colorectal resection; at least 6 weeks of one or more chemotherapy agents received for metastatic disease; nonresectable liver metastases; no extrahepatic disease; and ECOG performance status 0–1. All patients that qualified underwent an intraoperative staging laparotomy to examine the hepatic ligament lymph nodes before the transplant. If there was no disease in the frozen sections then the transplant was performed.
When the results of SECA I trial was retrospectively compared with systemic chemotherapy alone treatment for patients in NORDIC VII trial, it was shown that the 5-year survival of the SECA I trial cohort was 56% and the 5-year survival of the 21 patients with the longest survival in the NORDIC VII Trial cohort was 19% (P=0.01) (132). The NORDIC VII Trial was a multicenter randomized 3-arm trial to assess the OS between fluorouracil/folinic acid and oxaliplatin (FLOX) when administered in bolus (Nordic FLOX) and FLOX combined to cetuximab and intermittent FLOX associated with cetuximab in patients with advanced CCR (133). The 47 patients with liver-only metastases who did not undergo liver resection in the NORDIC VII Trial (and therefore were treated with chemotherapy only) were compared to the 21 patients that underwent LT in the SECA trial (132). The two groups were comparable except for the CEA levels (the SECA trial cohort had a median of 15 µg/L compared with 42 µg/L in the NORDIC VI trial). All patients in the NORDIC VII received first-line chemotherapy while 57% patients on the SECA trial received second and third lines of chemotherapy (132). Given that there are no randomized controlled trials comparing LT to standard chemotherapy in patients with liver-only NRCLM, this is the best available evidence comparing LT with standard of care chemotherapy. However, the evidence of this cohort study is still weak with several bias, and further comparisons are needed.
There are more data showing that well selected patients with CRLM may have good survival benefits with liver transplantation. A European consortium which published a series of 12 patients that underwent LT for unresectable CRLM, showed that the OS of the cohort was 83%, 62% and 50% at 1-, 3-, and 5-year respectively. While 6 patients had recurrence, mainly to the lungs, there were 4 patients were alive without cancer recurrence after 48 months (134). These patients underwent LT after a median of 41 months following the primary CRC resection and 11 patients received chemotherapy before LT. Irinotecan and oxaliplatin were the most common protocols. While this study was a retrospective study with very small sample size, it showed that long-term cure can be achieved with LT in these patients and therefore the results are very encouraging.
The tremendous improvement of the 5-year survival rate of patients with unresectable CRLM treated with LT to around 50% could be credited to better selection criteria coupled with discovery of effective systemic chemotherapy and certainly, the great improvements in the perioperative care of LT recipients (135). However, we must acknowledge that this treatment strategy remains controversial as the cancer recurrence rate remains high. The Oslo group reported in a follow-up study from SECA I trial that the median time to recurrence in their study cohort was 6 months. All the patients who were followed up longer than 11 months experienced recurrence, with lung metastasis being the most common site. With aggressive surgical therapy including resection of the lung lesions, they were able to achieve a 5-year survival rate of 72% after the recurrence was diagnosed in patients with pulmonary first-site metastases (136). Multiple metastatic disease sites can be resected and adjuvant chemotherapy can control tumor progression (137). While tumor recurrence certainly has an impact on patient survival, many would consider metastatic CRC as a chronic disease, just like metastatic breast cancer, due to the effective systemic chemotherapy. CRC patients with metastatic disease can definitively have longer life expectancy (138).
While studies have shown that selected patients with unresectable CRLM may benefit from liver transplantation, one of the key dilemmas in this treatment is availability of liver graft for transplantation. In countries where liver organs are short, it is difficult to justify using deceased donor liver grafts for this treatment at the moment. Norway has a fortunate donor situation, with more donors than potential recipients, and the median waiting time for LT is less than 1 month (20). As such, SECA I trial was successfully conducted, paving the way for this new innovation to demonstrate the benefits of LT in unresectable CRLM. Many liver transplant centres welcome this result with enthusiasm. In order not to encroach onto the already limited liver organ source in the deceased donor pool, some have started offering living donor liver transplantation as the alternative option. The Toronto group is currently conducting a single-arm, prospective study to explore the utility of living donor LT for unresectable CRLM (NCT02864485) (139). It remains to be seen how the long-term results will be but, suffice to say, the key for successful treatment using this strategy will very much rely on extremely careful case selection, standardised chemotherapy protocols as well as great surgical expertise in performing liver transplant surgery with excellent outcomes.
The other studies currently ongoing to evaluate the efficacy of LT for CRLM include the SECA (SEcondary CAncer)-II study (NCT01479608) which is a phase 3 trial, comparing deceased donor LT with liver resection in selected patients with 6 or more liver-only metastases from CRC deemed technically resectable. The results from SECA II Trial will be published soon and we eagerly look forward to its findings. Another randomized control trial currently being conducted in France, called the TRANSMET study aims to compare chemotherapy alone with LT after standard chemotherapy for unresectable CRLM (NCT02597348). Other innovative method combining concepts of ALPPS and living donor liver transplantation to optimise the availability of liver grafts known as RAPID was introduced by the Norwegian group as well. The RAPID concept (Resection And Partial Liver Segment 2/3 Transplantation With Delayed Total Hepatectomy) combines the ALPPS procedure with living donor LT of segments 2 and 3 (left lateral section of the liver graft from living donor), followed by total hepatectomy (140). It is challenging for surgical oncologist to accept the concept that, by resection the left lateral section of the liver with CRLM and concurrently implanting a segment II/III graft in the form of LDLT that the new graft will not be at risk of tumour metastasis during the ‘growth’ period of the graft. Although the diseased liver will eventually be removed with a delayed total hepatectomy, the worry of tumour metastasis to the new graft while patient is on immunosuppressants remains a concern.
Currently, we are limited by imaging technologies and molecular diagnostic tools to accurately predict which patients will be the best candidates for LT in CRLM. With the development of precision (individualized) therapy, exact genetic expressions and mutations of the cancers could potentially be detected via non-invasive method and this can be used to guide patients in selection of appropriate treatment regime. LT for unresectable CRLM will certainly play a pivotal role in providing a potential curative outcome for patients, which previously would be rendered palliative due to its Stage IV disease status (141). The era of transplant oncology has dawned.
Other therapeutic options for CRL liver metastasis
Ablation of CRLM [including radiofrequency ablation (RFA) or microwave ablation (MVA)]
Ablation of liver lesions detected in CRCs is an acceptable option considered as part of the liver-targeted therapy. Selection of patient for ablative therapy as compared to offering liver resection is challenging as ablation of the lesion might not be able to confer similar survival benefits as compared to resection. However, in the situation where tumour has recurred after previous liver resection and re-resection is not an option, ablation of the tumor may be a feasible option to palliate the growth of the tumour, particularly when patients are tired of having prolonged systemic chemotherapy.
While the long-term results of hepatic resection for CRLM is established as shown above, the benefits of RFA as treatment for CRLM is scanty at the moment. Unlike hepatic resection where long-term efficacy is relatively established, evidence related to the benefit of RFA for treatment of hepatic colorectal metastases has been limited. In 2009, a Clinical Evidence Review by the American Society of Clinical Oncology on RFA of CRLM reported that the quality of evidence is limited. It emphasized the need for more and good quality clinical trials to determine the efficacy and utility of RFA for these patients (142).
Depending on the location and number of lesions, RFA can be performed via the percutaneous technique under radiological guidance (either ultrasound-guided or CT scan guided), laparoscopically or open. The use of laparoscopic and open surgical procedures to guide therapy allows for better evaluation of previously undetected intrahepatic disease with IOUS. In addition, the use of IOUS permits better localization of lesions and a method to monitor ablation progress (143). The reported operative morbidity and mortality rates at 30 days are low (3.9% and 0.4%, respectively) (144). Patients with poorer long-term prognoses include those with more than three lesions (median survival, 17 months) and a CEA level greater than 200 ng/mL prior to RFA (16 months median survival vs. 26 months for those with CEA >200). Factors most associated with local recurrence following RFA include tumor size and location. Tumors 3 cm or less and those not immediately adjacent to major vascular structures have considerably less risk of local recurrence in long-term follow-up (145). While some literature reported a high local recurrence rate after RFA (40% to 50%) for CRLM lesions, some studies have reported a 5-year survival of up to 40% (146-151).
The alternative method of ablating CRLM lesion is MVA. MVA employs the electromagnetic field to generate direct heat destruction of the tumours and the microwave field is able to directly heat a larger volume of tissue more rapidly. Moreover, there is less regional heat sink effect from adjacent vessels. This can allow for larger, more predictable tissue destruction, and with greater speed (152). However, reports on the efficacy of MVA remain limited. In a small randomized study about 2 decades ago, it was demonstrated that 30 patients who were treated with either resection or MVA for tumors were found to have similar survival rates also similar complication rates. The median survival rates were also comparable (median survival rates for MVA: 27 months vs. resection: 25 months) (153). A more recent series that included 50 patients with CRLM who underwent MVA for tumors up to 6 cm in size (median, 3 cm) and one to 12 tumors treated per patient reported a median OS of 36 months with a disease-free survival of 12 months after treatment (154).
Combining resection and ablation
In the situation where there are bilobar diseases in CRLM, some centers may render these situations unresectable. However, as illustrated above, innovative methods of growing FLR such as ALPPS and PVE have been shown to be effective in facilitating resection of the liver, whom otherwise will be deemed unresectable. Clearly, the aim of liver resection is to achieve a state of “no evidence of disease (NED)”. However, sometimes, the FLR may have a small lesion and may pose a roadblock for resection. Combining RFA/MVA for small lesion in the FLR while resecting the part of liver with majority of the lesions can increase the chance of liver resection while sparing as much liver parenchyma on the FLR as possible.
The early experience of the combined treatment strategy was shown in a study by Abdalla et al. to be inferior in terms of survival as compared to liver resection alone. In their series, the OS rate was highest after resection (58% at 5 years); 4-year survival after resection, RFA + resection and RFA only were 65%, 36%, and 22%, respectively (P<0.0001). Survival for “unresectable” patients treated with RFA + resection or RFA only was greater than chemotherapy only (P=0.0017) (155). However, in recent years, this combination strategy has been shown to yield similar survival benefits as major resection of the liver for CRLM. Imai et al. reported that hepatectomy combined with RFA can achieve outcomes comparable to hepatectomy alone. They compared 553 patients who received hepatectomy combined with RFA (37 patients) with patients who received hepatectomy alone (516 patients). In this matched cohort, overall and disease-free survival in the hepatectomy + RFA group were no different from those among patients who had hepatectomy alone (5-year OS rate 57 versus 61 per cent, P=0.649; 5-year disease-free survival rate 19 versus 17 per cent, P=0.865) (156).
Transarterial chemoembolisation (TACE)
While TACE is certainly more established as a therapy to slow down the progression of disease in HCC, its role in the treatment of CRLM is limited. The challenges of employing TACE as treatment in CRLM are two folds. Firstly, CRLM lesions are typically hypovascular, presumably due to the portal blood supply that feeds the tumours instead of arterial blood supply in HCC. Therefore, take up of the embolic material is less effective. Secondly, the chemotherapeutic agents to be injected in the TACE therapy can be quite variable. The common chemotherapeutic agents used in TACE for the treatment of CRLM are doxorubicin, cisplatin, and mitomycin C. However, it is important to point out that these agents are distinctly different from the mainstream systemic chemotherapeutic agents used to treat CRLM as stated above. The drugs have been combined with a variety of microspheres and embolic agents used alone or in combination, including lipiodol oil, collagen particles, polyvinyl alcohol particles, or trisacryl gelatin microspheres, in order to occlude tumor vasculature (143).
Most of the data available for the use of TACE in CRLM come from prospective studies with published 2-year survival rates as high as 66% and complete responses of 10% (157). An older study with 40 patients showed a median survival of 10 months with a median duration of response of 7 months (158). A median survival of 8.6 months was reported in a small phase II trial with 30 patients (159). In terms of patient selection, it has been suggested that patients with large tumor burdens (75% of the liver volume) may not benefit from this procedure (157).
Yttrium-90 (Y-90) for liver metastasis
While the deployment of Y-90 as selective internal radiation therapy (SIRT) has demonstrated comparable survival outcomes with palliative systemic therapy, e.g., Sorafenib in the treatment of HCC, its roles in the treatment of CRLM has only been explored recently. With this modality, microspheres incorporated with radioactive Y-90 are selectively delivered to the involved regions of the liver, similar to TACE. There are currently two commercially available vehicles for Y-90 delivery: resin microspheres (SirSpheres, SIRTEX Medical Ltd., Sydney, New South Wales, Australia) and glass microspheres (TheraSpheres, MDS Nordion, Inc., Kanata, Ontario, Canada), each with different performance and delivery characteristics. Limited results have been published in treating CRLM, with the initial studies done in highly selected patients (160). In these studies, improvement in time-to-progression was seen using SIR-Spheres in combination with HAI. In a second small study, Kennedy et al. reported improved survival in patients who responded to 90-Y therapy compared to those who failed to respond (161). Currently, the data on the efficacy of 90-Y radiotherapy are limited, and should be used highly selectively outside of a clinical trial.
Stereotactic body radiotherapy (SBRT)
The use of external-beam radiotherapy to the liver in the treatment of CRLM is uncommon as there is significant concern about the risk of radiation induced hepatitis. The data on its efficacy and survival benefits are limited and it was used previously as one of the palliative options in advanced metastatic disease to the liver (143). More recently, the development of stereotactic radiation strategies has allowed a more precise delivery of greater radiation and yet more focused to the metastatic deposits, has been applied as a treatment option for unresectable colorectal liver metastatic diseases. Therefore, in patients who are symptomatic from the metastatic liver lesions, stereotactic radiation can be considered (162).
In a systematic review on the role of SBRT by Petrelli et al., a total of 18 studies which included 656 patients were analysed. The pooled one- and two-year OS rates were 67.18% and 56.5% respectively. Median PFS and OS were 11.5 and 31.5 months. Mild-moderate and severe liver toxicity were 30.7% and 8.7% respectively. They concluded that SBRT for liver oligometastases is an effective option for patients with advanced CRC, with encouraging local control and survival. However, a definitive validation in large randomised studies is required, due to the retrospective or non-randomised nature of the included studies and the limitations of series with different doses/schedules of treatment (163).
Hepatic artery infusion (HAI)
The technique of HAI in delivering chemotherapy selectively to treat intrahepatic tumours has been tested for over 3 decades now. It has been tested in both the adjuvant and the palliative settings but the results had been mixed. The aim of HAI is to deliver therapeutic agents directly into the liver and to obtain a high drug concentration in the tumor. Technical difficulties for catheter placement have limited the implementation of this method in routine practice (164).
HAI is more often used in palliative setting where the CRLM is not amenable for surgical resection. It has been shown that an increased tumor response rates, up to 80%, could be achieved with a strategy associating HAI-FUDR with IV drugs (irinotecan/5-FU/oxaliplatin or oxaliplatin/irinotecan) (165,166). In selected patients, these combinations have led to 80% response rates as a first-line treatment and 50% as a second-line treatment. In the USA, a systemic combination of FUDR-irinotecan with HAI-oxaliplatin has shown a 90% response rate, with a 50% secondary resection rate (165). A recent French multicentric, prospective, phase II trial (OPTILIV) investigated a triplet chemotherapy by HAI (oxaliplatin/5-FU/irinotecan) combined with systemic cetuximab in patients with unresectable RAS-wild-type LM of CRC, after a first-line systemic treatment. Tolerance was acceptable and tumor responses (40.6% response rate) allowed a R0-R1 secondary resection in 29.7% of cases. Median PFS was 9.3 months. OS was increased two-fold in patients who underwent resection compared with those without resection (35.2 vs. 18.7 months, respectively). Forty-five percent of secondary resected patients were still alive after four years, compared to none in the non-resected group (167).
While the above data seem to show promising results in palliation of unresectable CRLM and some even successfully converted to resectable state, the role of HAI in the adjuvant setting following resection remains controversial. The initial randomised controlled trial by Lorenz et al. comparing HAI and systemic chemotherapy was terminated early due to a low probability of detecting a significant survival benefit when used in combination with resection. The reported median survival was almost 6 months shorter than the control group (34.5 versus 40.8 months) (168). On the other hand, other studies have demonstrated improved OS at 2 years for patients that undergo HAI after resection combined with systemic chemotherapy compared to systemic chemotherapy alone (169,170).
While having some promise, the complexity of drug delivery, high incidence of complications, including biliary sclerosis, and lack of generalizability to more than selected centers have generally dampened enthusiasm for this approach.
Isolated hepatic perfusion (IHP)
IHP is a technique which requires perfusion of treatment agents via the hepatic artery and aspiration of the chemosaturated blood from the inferior vena cava. It is typically performed during the open surgery but can also be achieved via percutaneous method by interventional radiology. In the percutaneous technique, catheters are placed in the hepatic artery to perform the perfusion as well as the IVC to aspirate the chemosaturated blood, which is then filtered and returned to the patient. As it requires invasive vascular access as well as providing treatment near crucial blood vessels, this procedure must be performed with haemodynamic monitoring and support (171). The originally described treatment agents used in IHP was tumor necrosis factor and melphalan, which was able to demonstrate overall objective radiographic response rate of 60–76% (172-174). Most recently, oxaliplatin has been trialled as the agent for IHP as well (175). This treatment modality for CRLM remains experimental as it is complicated to perform and its efficacy is still not clear based on current evidence.
Irreversible electroporation (IRE)
The alternative method of performing ablation to the liver metastases is IRE. In the situation where the lesions are too close to vital structures, conventional ablation is often contraindicated. IRE employs the electrical pulses to cause cell death and results in non-thermal tissue ablation (176,177). It can be used via open surgery or performed percutaneously. Heat sink effect is less of a concern as compared to other conventional ablative method. The ability of IRE to successfully ablate CRLM in humans was demonstrated in patients with resectable CRLM in the COLDFIRE-1 ablate and resect trial (178). The procedure in the trial involved ablating the CRLM lesions using IRE and then performed the resection of the lesions one hour later. The specimens resected showed cell death within 1 hour of the IRE but no significant damage to vascular structures located in the ablated zone. While there have been a mixture of case reports, retrospective studies and prospective studies in the literature, the evidence of IRE as a treatment for CRLM is still weak at the moment.
Conclusions
There has been a huge paradigm shift in the treatment of CRLM. This great advancement is supported by the discovery of effective systemic therapy to treat the metastatic disease including chemotherapy and biologic agents. Concurrently, the safety of liver surgery has also further supported the advancement in the multimodality treatment of CRLM. This is further propelled by the successful adoption of MIS technique in liver resection in the past two decades, facilitating smaller scars with lesser pain and faster recovery without compromising oncological outcomes. More of such surgeries are performed concurrently with resection of the colon primary cancers.
Molecular markers hold a great future in the treatment of CRLM. Appropriate selection of patients by stratifying and identifying patients who will benefit from the multidisciplinary treatment strategies in specific orders, be in chemotherapy or biologics first before surgery or vice versa, can be aided by precise markers in the future. Precision medicine will have an important place in the treatment of patients with CRLM (and other metastasis from CRC) in the future. This can potentially assist in selecting patients for liver transplantation in cases of unresectable CRLM.
Acknowledgments
None.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Footnotes
Conflicts of Interest: The author has no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram, et al. Global cancer statistics 2018: GLOBOSCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677-83. 10.1200/JCO.2008.20.5278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol 2016;27:1386-422. 10.1093/annonc/mdw235 [DOI] [PubMed] [Google Scholar]
- 5.van der Geest LGM, Lam-Boer J, Koopman M, et al. Nationwide trends in incidence, treatment and survival of colorectal cancer patients with synchronous metastases. Clin Exp Metastasis 2015;32:457-65. 10.1007/s10585-015-9719-0 [DOI] [PubMed] [Google Scholar]
- 6.van Gestel YRBM, de Hingh IHJT, van HerkSukel MPP, et al. Patterns of metachronous metastases after curative treatment of colorectal cancer. Cancer Epidemiol 2014;38;448-54. 10.1016/j.canep.2014.04.004 [DOI] [PubMed] [Google Scholar]
- 7.Chakedis J, Schmidt CR. Surgical treatment of Metastatic colorectal cancer. Surg Oncol Clin N Am 2018;27:377-99. 10.1016/j.soc.2017.11.010 [DOI] [PubMed] [Google Scholar]
- 8.Benson AB, Bekaii-Saab T, Chan E, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:141-52. 10.6004/jnccn.2013.0022 [DOI] [PubMed] [Google Scholar]
- 9.Wu Y, Liu F, Cai G, et al. Surgical management of colorectal cancer: the Fudan University Shanghai Cancer Center experience. Transl Cancer Res 2017;6:1351-7. 10.21037/tcr.2017.09.30 [DOI] [Google Scholar]
- 10.House MG, Ito H, Gonen M, et al. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg 2010;210:744-52. 10.1016/j.jamcollsurg.2009.12.040 [DOI] [PubMed] [Google Scholar]
- 11.Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006;13:668-676. 10.1245/ASO.2006.05.039 [DOI] [PubMed] [Google Scholar]
- 12.Leal JN, Bressan AK, Vachharajani N, et al. Time-tosurgery and survival outcomes in resectable colorectal livermetastases: a multi-institutional evaluation. J Am Coll Surg 2016;222:766-79. 10.1016/j.jamcollsurg.2016.01.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Viganò L, Capussotti L, Lapointe R, et al. Early recurrence after liver resection for colorectal metastases: Risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 2014;21:1276-86. 10.1245/s10434-013-3421-8 [DOI] [PubMed] [Google Scholar]
- 14.Yamashita YI, Baba H. How can we predict hepatic insufficiency after resection of colorectal liver metastases? Transl Cancer Res 2017;6:S1435-8. 10.21037/tcr.2017.10.44 [DOI] [Google Scholar]
- 15.Devaud N, Kanji ZS, Dhani N, et al. Liver resection after chemotherapy and tumour downsizing in patients with initially unresectable colorectal cancer liver metastases. HPB 2014;16:475-80. 10.1111/hpb.12159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of Surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 2005;241:715-22. 10.1097/01.sla.0000160703.75808.7d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurster EF, Tenckhoff S, Probst P, et al. A systematic review and meta-analysis of the utility of repeated versus single hepatic resection for colorectal cancer liver metastases. HPB 2017;19:491-7. 10.1016/j.hpb.2017.02.440 [DOI] [PubMed] [Google Scholar]
- 18.Sanoff HK, Sargent DJ, Campbell ME, et al. Five-year data and prognostic factor analysis of oxaliplatin and irinotecan combinations for advanced colorectal cancer: N9741. J Clin Oncol 2008;26:5721-7. 10.1200/JCO.2008.17.7147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The American Joint Committee on Cancer 7th Edition: Colon and Rectum Cancer Staging.
- 20.Hagness M, Foss A, Line PD, et al. Liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg 2013;257:800-6. 10.1097/SLA.0b013e3182823957 [DOI] [PubMed] [Google Scholar]
- 21.Yin Z, Liu C, Chen Y, et al. Timing of hepatectomy in resectable synchronous colorectal liver metastases (SCRLM): simultaneous or delayed? Hepatology 2013;57:2346-57. 10.1002/hep.26283 [DOI] [PubMed] [Google Scholar]
- 22.Ruers T, Punt C, van Coevorden F, et al. Radiofrequency ablation combined with systemic treatment versus systemic treatment alone in patients with non-resectable colorectal liver metastases: a randomized EORTC Intergroup phase II study (EORTC 40004). Ann Oncol 2012;23:2619-26. 10.1093/annonc/mds053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.LiverMetSurvey. International registry of patients operated for colorectal liver metastasis. Available online: http://www.livermetsurvey.org [accessed 2311.11].
- 24.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 25.Mekenkamp LJ, Koopman M, Teerenstra S, et al. Clinicopathological features and outcome in advanced colorectal cancer patients with synchronous vs metachronous metastases. Br J Cancer 2010;103:159-64. 10.1038/sj.bjc.6605737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Siriwardena AK, Mason JM, Mullamitha S, et al. Management of colorectal cancer presenting with synchronous liver metastases. Nat Rev Clin Oncol 2014;11:446-59. 10.1038/nrclinonc.2014.90 [DOI] [PubMed] [Google Scholar]
- 27.Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: A multidisciplinary international consensus. Cancer Treat Rev 2015;41:729-41. 10.1016/j.ctrv.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 28.Kelly RJ, Kemeny NE, Leonard GD. Current strategies using hepatic arterial infusion chemotherapy for the treatment of colorectal cancer. Clin Colorectal Cancer 2005;5:166-74. 10.3816/CCC.2005.n.027 [DOI] [PubMed] [Google Scholar]
- 29.Milette S, Sicklick JK, Lowy AM, et al. Molecular pathways: Targeting the microenvironment of liver metastases. Clin Cancer Res 2017;23:6390-9. 10.1158/1078-0432.CCR-15-1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brodt P. Role of the microenvironment in liver metastasis: from pre- to prometastatic niches. Clin Cancer Res 2016;22:5971-82. 10.1158/1078-0432.CCR-16-0460 [DOI] [PubMed] [Google Scholar]
- 31.Gressner AM, Bachem MG. Molecular mechanisms of liver fibrogenesis-a homage to the role of activated fat-storing cells. Digestion 1995;56:335-46. 10.1159/000201257 [DOI] [PubMed] [Google Scholar]
- 32.Friedman SL. Hepatic stellate cells: protean, multifunctional, and enigmatic cells of the liver. Physiol Rev 2008;88:125-72. 10.1152/physrev.00013.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fridlender ZG, Sun J, Kim S, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009;16:183-94. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schouppe E, De Baetselier P, Van Ginderachter JA, et al. Instruction of myeloid cells by the tumor microenvironment: Open questions on the dynamics and plasticity of different tumor-associated myeloid cell populations. Oncoimmunology 2012;1:1135-45. 10.4161/onci.21566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taura K, De Minicis S, Seki E, et al. Hepatic stellate cells secrete angiopoietin 1 that induces angiogenesis in liver fibrosis. Gastroenterology 2008;135:1729-38. 10.1053/j.gastro.2008.07.065 [DOI] [PubMed] [Google Scholar]
- 36.Copple BL, Bai S, Burgoon LD, et al. Hypoxia-inducible factor-1alpha regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int 2011;31:230-44. 10.1111/j.1478-3231.2010.02347.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vauthey JN, Zimmitti G, Kopetz SE, et al. RAS mutation status predicts survival and patterns of recurrence in patients undergoing hepatectomy for colorectal liver metastases. Ann Surg 2013;258:619-26. 10.1097/SLA.0b013e3182a5025a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brudvik KW, Kopetz SE, Li L, et al. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg 2015;102:1175-83. 10.1002/bjs.9870 [DOI] [PubMed] [Google Scholar]
- 39.Zimmitti G, Shindoh J, Mise Y, et al. RAS mutations predict radiologic and pathologic response in patients treated with chemotherapy before resection of colorectal liver metastases. Ann Surg Oncol 2015;22:834-42. 10.1245/s10434-014-4042-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Passot G, Chun YS, Kopetz SE, et al. Predictors of safety and efficacy of 2-stage hepatectomy for bilateral colorectal liver metastases. J Am Coll Surg 2016;223:99-108. 10.1016/j.jamcollsurg.2015.12.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passot G, Chun YS, Kopetz SE, et al. Prognostic factors after resection of colorectal liver metastases following preoperative second-line chemotherapy: impact of RAS mutations. Eur J Surg Oncol 2016;42:1378-84. 10.1016/j.ejso.2016.02.249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morris V, Overman MJ, Jiang ZQ, et al. Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer. Clin Colorectal Cancer 2014;13:164-71. 10.1016/j.clcc.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaczirek K, Ciuleanu TE, Vrbanec D, et al. FOLFOX4 plus cetuximab for patients previously untreated metastatic colorectal cancer according to tumour RAS and BRAF mutation status: updated analysis of the CECOG/CORE 1.2.002 study. Clin Colorectal Cancer 2015;14:91-8. 10.1016/j.clcc.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 44.Yaeger R, Cercek A, Chou JF, et al. BRAF mutation predicts for poor outcomes after metastatectomy in patients with metastatic colorectal cancer. Cancer 2014;120:2316-24. 10.1002/cncr.28729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dalerba P, Sahoo D, Paik S, et al. CDX2 as a prognostic biomarker in stage II and stage III colon cancer. N Engl J Med 2016;374:211-22. 10.1056/NEJMoa1506597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang BY, Jones JC, Briggler AM, et al. Lack of caudal-type homeobox transcription factor 2 expression as a prognostic biomarker in metastatic colorectal cancer. Clin Colorectal Cancer 2017;16:124-8. 10.1016/j.clcc.2016.09.003 [DOI] [PubMed] [Google Scholar]
- 47.Kemeny NE, Chou JF, Capanu M, et al. KRAS mutation influences recurrence patterns in patients undergoing hepatic resection of colorectal metastases. Cancer 2014;120:3965-71. 10.1002/cncr.28954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Slesser AAP, Gerogious P, Brown G, et al. The tumor biology of synchronous and metachronous colorectal liver metastases: a systematic review. Clin Exp Metastasis 2013;30:457-70. 10.1007/s10585-012-9551-8 [DOI] [PubMed] [Google Scholar]
- 49.Thomas GV, Szigeti K, Murphy M, et al. Down-regulation of p27 is associated with development of colorectal adenocarcinoma metastases. Am J Pathol 1998;153:681-7. 10.1016/S0002-9440(10)65610-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lloyd RV, Erickson LA, Jin L, et al. p27kip1: a multifunctional cyclindependent kinase inhibitor with prognostic significance in human cancers. Am J Pathol 1999;154:313-23. 10.1016/S0002-9440(10)65277-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li JQ, Miki H, Ohmori M, et al. Expression of cyclin E and cyclin-dependent kinase 2 correlates with metastasis and prognosis in colorectal carcinoma. Hum Pathol 2001;32:945-53. 10.1053/hupa.2001.27116 [DOI] [PubMed] [Google Scholar]
- 52.Pantaleo MA, Astolfi A, Nannini M, et al. Gene expression profiling of liver metastases from colorectal cancer as potential basis for treatment choice. Br J Cancer 2008;99:1729-34. 10.1038/sj.bjc.6604681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Prabhudesai SG, Rekhraj S, Roberts G, et al. Apoptosis and chemo-resistance in colorectal cancer. J Surg Oncol 2007;96:77-88. 10.1002/jso.20785 [DOI] [PubMed] [Google Scholar]
- 54.Yarom N, Jonker DJ. The role of the epidermal growth factor receptor in the mechanism and treatment of colorectal cancer. Discov Med 2011;11:95-105. [PubMed] [Google Scholar]
- 55.De Jong KP, Stellema R, Karrenbeld A, et al. Clinical relevance of transforming growth factor alpha, epidermal growth factor receptor, p53, and Ki67 in colorectal liver metastases and corresponding primary tumors. Hepatology 1998;28:971-9. 10.1002/hep.510280411 [DOI] [PubMed] [Google Scholar]
- 56.Camus M, Tosolini M, Mlecnik B, et al. Coordination of intratumoral immune reaction and human colorectal cancer recurrence. Cancer Res 2009;69:2685-93. 10.1158/0008-5472.CAN-08-2654 [DOI] [PubMed] [Google Scholar]
- 57.Mlecnik B, BIndea G, Angell HK, et al. Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 2016;44:698-711. 10.1016/j.immuni.2016.02.025 [DOI] [PubMed] [Google Scholar]
- 58.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology 2010;138:2073-2087.e3. 10.1053/j.gastro.2009.12.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197-218. 10.1038/s41573-018-0007-y [DOI] [PubMed] [Google Scholar]
- 60.Blank CU, Haanen JB, Ribas A, et al. The “cancer immunogram”. Science 2016;352:658-60. 10.1126/science.aaf2834 [DOI] [PubMed] [Google Scholar]
- 61.Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol 2015;33:1809-24. 10.1200/JCO.2014.59.7633 [DOI] [PubMed] [Google Scholar]
- 62.Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol 2014;15:1065-75. 10.1016/S1470-2045(14)70330-4 [DOI] [PubMed] [Google Scholar]
- 63.Venook A, Niedzwiecki D, Lenz HJ, et al. CALGB/SWOG 80405: phase III trial of irinotecan/5-fu/leucovorin (FOLFIRI) or oxaliplatin/5-fu/leucovorin (mFOLFOX6) with bevacizumab (bv) or cetuximab (cet) for patients (pts) with KRAS wild-type (wt) untreated metastatic adenocarcinoma of the colon or rectum (MCRC). J Clin Oncol 2014;32:No 15 Suppl. [Google Scholar]
- 64.Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med 2014;371:1609-18. 10.1056/NEJMoa1403108 [DOI] [PubMed] [Google Scholar]
- 65.Garufi C, Torsello A, Tumolo S, et al. Cetuximab plus chronomodulated irinotecan, 5-fluorouracil, leucovorin and oxaliplatin as neoadjuvant chemotherapy in colorectal liver metastases: POCHER trial. Br J Cancer 2010;103:1542-7. 10.1038/sj.bjc.6605940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Peeters M, Price TJ, Cervantes A, et al. Randomized phase III study of panitumumab with fluorouracil, leucovorin, and irinotecan (FOLFIRI) compared with FOLFIRI alone as second-line treatment in patients with metastatic colorectal cancer. J Clin Oncol 2010;28:4706-13. 10.1200/JCO.2009.27.6055 [DOI] [PubMed] [Google Scholar]
- 67.Adam R, Aloia T, Levi F, et al. Hepatic resection after rescue cetuximab treatment for colorectal liver metastases previously refractory to conventional systemic therapy. J Clin Oncol 2007;25:4593-602. 10.1200/JCO.2007.10.8126 [DOI] [PubMed] [Google Scholar]
- 68.Brouquet A, Overman MJ, Kopetz S, et al. Is resection of colorectal liver metastases after a second-line chemotherapy regimen justified? Cancer 2011;117:4484-92. 10.1002/cncr.26036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vigano L, Capussotti L, De Rosa G, et al. Liver resection for colorectal metastases after chemotherapy: impact of chemotherapy-related liver injuries, pathological tumour response and micrometastases on long-term survival. Ann Surg 2013;258:731-40. 10.1097/SLA.0b013e3182a6183e [DOI] [PubMed] [Google Scholar]
- 70.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065-72. 10.1200/JCO.2005.05.3074 [DOI] [PubMed] [Google Scholar]
- 71.Welsh FK, Tilney HS, Tekkis PP, et al. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer 2007;96:1037-42. 10.1038/sj.bjc.6603670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kishi Y, Zorzi D, Contreras CM, et al. Extended preoperative chemotherapy does not improve pathologic response and increases postoperative liver insufficiency after hepatic resection for colorectal liver metastases. Ann Surg Oncol 2010;17:2870-6. 10.1245/s10434-010-1166-1 [DOI] [PubMed] [Google Scholar]
- 73.Mohammad WM, Balaa FK. Surgical management of colorectal liver metastasis. Clin Colon Rectal Surg 2009;22:225-32. 10.1055/s-0029-1242462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nordlinger B, Sorbye H, Glimelius B, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled phase 3 trial. Lancet Oncol 2013:14:1208-15. 10.1016/S1470-2045(13)70447-9 [DOI] [PubMed] [Google Scholar]
- 75.Shindoh J, Loyer EM, Kopetz S, et al. Optimal morphologic response to preoperative chemotherapy: an alternative outcome end point before resection of hepatic colorectal metastases. J Clin Oncol 2012;30:4566-72. 10.1200/JCO.2012.45.2854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. 10.1097/00000658-199909000-00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. 10.1097/SLA.0b013e31815aa2c2 [DOI] [PubMed] [Google Scholar]
- 78.Yamaguchi T, Mori T, Takahashi K, et al. A new classification system for liver metastases from colorectal cancer in Japanese multicenter analysis. Hepatogastroenterology 2008;55:173-8. [PubMed] [Google Scholar]
- 79.Kattan MW, Gonen M, Jarnagin WR, et al. A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg 2008;247:282-7. 10.1097/SLA.0b013e31815ed67b [DOI] [PubMed] [Google Scholar]
- 80.Damm R, Seidensticker R, Ulrich G, et al. Y90 Radioembolization in chemo-refractory metastastic, liver dominant colorectal cancer patients: outcome assessment applying a predictive scoring system. BMC Cancer 2016;16:509. 10.1186/s12885-016-2549-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hill CR, Chagpar RB, Callender GG, et al. recurrence following hepatectomy for metastatic colorectal cancer: development of a model that predicts patterns of recurrence and survival. Ann Surg Oncol 2012;19:139-44. 10.1245/s10434-011-1921-y [DOI] [PubMed] [Google Scholar]
- 82.Sasaki K, Morioka D, Conci S, et al. The tumor burden score: a new “Metro-ticket” prognostic tool for colorectal liver metastases based on tumor size and number of tumors. Ann Surg Jan 2018;267:132-41. 10.1097/SLA.0000000000002064 [DOI] [PubMed] [Google Scholar]
- 83.Brudvik KW, Jones RP, Giuliante F, et al. RAS mutation clinical risk score to predict survival after resection of colorectal liver metastases. Ann Surg Jan 2019;269:120-6. 10.1097/SLA.0000000000002319 [DOI] [PubMed] [Google Scholar]
- 84.Are C, Gonen M, Zazzali K, et al. The impact of margins on outcome after hepatic resection for colorectal metastasis. Ann Surg 2007;246:295-300. 10.1097/SLA.0b013e31811ea962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cady B, Jenkins RL, Steele GD, Jr, et al. Surgical margin in hepatic resection for colorectal metastasis: a critical and improvable determinant of outcome. Ann Surg 1998;227:566-71. 10.1097/00000658-199804000-00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shirabe K, Takenaka K, Gion T, et al. Analysis of prognostic risk factors in hepatic resection for metastatic colorectal carcinoma with special reference to the surgical margin. Br J Surg 1997;84:1077-80. 10.1002/bjs.1800840810 [DOI] [PubMed] [Google Scholar]
- 87.Nuzzo G, Giuliante F, Ardito F, et al. Influence of surgical margin on type of recurrence after liver resection for colorectal metastases: a single-center experience. Surgery 2008;143:384-93. 10.1016/j.surg.2007.09.038 [DOI] [PubMed] [Google Scholar]
- 88.Inoue Y, Hayashi M, Komeda K, et al. Resection margin with anatomic or nonanatomic hepatectomy for liver metastasis from colorectal cancer. J Gastrointest Surg 2012;16:1171-80. 10.1007/s11605-012-1840-7 [DOI] [PubMed] [Google Scholar]
- 89.Truant S, Sequier C, Leteurtre E, et al. Tumour biology of colorectal liver metastasis is a more important factor in survival than surgical margin clearance in the era of modern chemotherapy regimens. HPB 2015;17:176-84. 10.1111/hpb.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Margonis GA, Sengetanis TN, Ntanassis-Stathopoulos I, et al. Impact of Surgical Margin Width on Recurrence and Overall Survival Following R0 Hepatic Resection of Colorectal Metastases. A Systematic Review and Meta-analysis. Ann Surg 2018;267:1047-55. 10.1097/SLA.0000000000002552 [DOI] [PubMed] [Google Scholar]
- 91.Shoup M, Gonen M, D’Angelica M, et al. Volumetric analysis predicts hepatic dysfunction in patients undergoing major liver resection. J Gastrointest Surg 2003;7:325-30. 10.1016/S1091-255X(02)00370-0 [DOI] [PubMed] [Google Scholar]
- 92.Vauthey JN, Chaoui A, Do KA, et al. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery 2000;127:512-9. 10.1067/msy.2000.105294 [DOI] [PubMed] [Google Scholar]
- 93.Hoekstra LT, de Graaf W, Nibourg GAA, et al. Physiological and biochemical basis of clinical liver function tests: A review. Ann Surg 2013;257:27-36. 10.1097/SLA.0b013e31825d5d47 [DOI] [PubMed] [Google Scholar]
- 94.Imamura H, Sano K, Sugawara Y, et al. Assessment of hepatic reserve for indication of hepatic resection: decision tree incorporating indocyanine green test. J Hepatobiliary Pancreat Surg 2005;12:16-22. 10.1007/s00534-004-0965-9 [DOI] [PubMed] [Google Scholar]
- 95.Honjo I, Suzuki T, Ozawa K, et al. Ligation of a branch of the portal vein for carcinoma of the liver. Am J Surg 1975;130:296-302. 10.1016/0002-9610(75)90389-X [DOI] [PubMed] [Google Scholar]
- 96.van Gulik TM, van den Esschert JW. James Cantlie’s early messages for hepatic surgeons: how the concept of pre-operative portal vein occlusion was defined. HPB (Oxford) 2010;12:81-3. 10.1111/j.1477-2574.2009.00124.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Eshmuminov D, Raptis DA, Linecker M, et al. Meta-analysis of associating liver partition with portal vein ligation and portal vein occlusion for two-stage hepatectomy. Br J Surg 2016;103:1768-82. 10.1002/bjs.10290 [DOI] [PubMed] [Google Scholar]
- 98.Kinoshita H, Sakai K, Hirohashi K, et al. Preoperative portal vein embolization for hepatocellular carcinoma. World J Surg 1986;10:803-8. 10.1007/BF01655244 [DOI] [PubMed] [Google Scholar]
- 99.Makuuchi M, Thai BL, Takayasu K, et al. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery 1990;107:521-7. [PubMed] [Google Scholar]
- 100.Aussilhou B, Lesurtel M, Sauvanet A, et al. Right portal vein ligation is as efficient as portal vein embolization to induce hypertrophy of the left liver remnant. J Gastrointest Surg 2008;12:297-303. 10.1007/s11605-007-0410-x [DOI] [PubMed] [Google Scholar]
- 101.Robles R, Marin C, Lopez-Conesa A, et al. Comparative study of right portal vein ligation versus embolisation for induction of hypertrophy in two-stage hepatectomy for multiple bilateral colorectal liver metastases. Eur J Surg Oncol 2012;38:586-93. 10.1016/j.ejso.2012.03.007 [DOI] [PubMed] [Google Scholar]
- 102.van Lienden KP, Hoekstra LT, Bennink RJ, et al. Intrahepatic left to right portoportal venous collateral vascular formation in patients undergoing right portal vein ligation. Cardiovasc Intervent Radiol 2013;36:1572-9. 10.1007/s00270-013-0591-5 [DOI] [PubMed] [Google Scholar]
- 103.Broering DC, Hillert C, Krupski G, et al. Portal vein embolization versus portal vein ligation for induction of hypertrophy of the future liver remnant. J Gastrointest Surg 2002;6:905-13. 10.1016/S1091-255X(02)00122-1 [DOI] [PubMed] [Google Scholar]
- 104.Capussotti L, Muratore A, Baracchi F, et al. Portal vein ligation as an efficient method of increasing the future liver remnant volume in the surgical treatment of colorectal metastases. Arch Surg 2008;143:978-82. 10.1001/archsurg.143.10.978 [DOI] [PubMed] [Google Scholar]
- 105.Schnitzbauer AA, Lang SA, Goessmann H, et al. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 2012;255:405-14. 10.1097/SLA.0b013e31824856f5 [DOI] [PubMed] [Google Scholar]
- 106.Schadde E, Ardiles V, Slankamenac K, et al. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 2014;38:1510-9. 10.1007/s00268-014-2513-3 [DOI] [PubMed] [Google Scholar]
- 107.Alvarez FA, Ardiles V, de Santibanes M, et al. Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 2015;261:723-32. 10.1097/SLA.0000000000001046 [DOI] [PubMed] [Google Scholar]
- 108.Chia DKA, Yeo Z, Loh S, et al. Greater hypertrophy can be achieved with Associating Liver Partition with Portal vein ligation for Staged hepatectomy compared to Conventional Staged Hepatectomy, but with a higher price to pay? Am J Surg 2018;215:131-7. 10.1016/j.amjsurg.2017.08.013 [DOI] [PubMed] [Google Scholar]
- 109.Chia DKA, Yeo Z, Loh SEK, et al. ALPPS for hepatocellular carcinoma is associated with decreased liver remnant growth. J Gastrointest Surg 2018;22:973-80. 10.1007/s11605-018-3697-x [DOI] [PubMed] [Google Scholar]
- 110.Matsuo K, Murakami T, Kawaguchi D, et al. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery 2016;159:1289-98. 10.1016/j.surg.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 111.Sparrelid E, Jonas E, Tzortzakakis A, et al. Dynamic Evaluation of Liver Volume and Function in Associating Liver Partition and Portal Vein Ligation for Staged Hepatectomy. J Gastrointest Surg 2017;21:967-74. 10.1007/s11605-017-3389-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Geisel D, Ludemann L, Hamm B, et al. Imaging-Based Liver Function Tests—Past, Present and Future. Rofo 2015;187:863-71. 10.1055/s-0035-1553306 [DOI] [PubMed] [Google Scholar]
- 113.Oldhafer KJ, Stavrou GA, van Gulik TM, et al. ALPPS-Where Do We Stand, Where Do We Go? Eight Recommendations From the First International Expert Meeting. Ann Surg 2016;263:839-41. 10.1097/SLA.0000000000001633 [DOI] [PubMed] [Google Scholar]
- 114.Ito K, Ito H, Are C, et al. Laparoscopic versus open liver resection: a matched-pair case control study. J Gastrointest Surg 2009;13:2276-83. 10.1007/s11605-009-0993-5 [DOI] [PubMed] [Google Scholar]
- 115.Schiffman SC, Kim KH, Tsung A, et al. Laparoscopic versus open liver resection for metastatic colorectal cancer: a meta-analysis of 610 patients. Surgery 2015;157:211-22. 10.1016/j.surg.2014.08.036 [DOI] [PubMed] [Google Scholar]
- 116.Fretland ÅA, Dagenborg VJ, Bjornelv GMV, et al. Laparoscopic Versus Open Resection for Colorectal Liver Metastases. The OSLO-COMET Randomized Controlled Trial. Ann Surg 2018;267:199-207. 10.1097/SLA.0000000000002353 [DOI] [PubMed] [Google Scholar]
- 117.Wong-Lun-Hing EM, van Dam RM, van Breukelen GJ, et al. Randomized clinical trial of open versus laparoscopic left lateral hepatic sectionectomy within an enhanced recovery after surgery programme (ORANGE II study). Br J Surg 2017;104:525-35. 10.1002/bjs.10438 [DOI] [PubMed] [Google Scholar]
- 118.Yan TD, Sim J, Black D, et al. Systematic review on safety and efficacy of repeat hepatectomy for recurrent liver metastases from colorectal. Ann Surg Oncol 2007;14:2069-77. 10.1245/s10434-007-9388-6 [DOI] [PubMed] [Google Scholar]
- 119.Chu QD, Vezeridis MP, Avradopoulos KA, et al. Repeat hepatic resection for recurrent colorectal cancer. World J Surg 1997;21:292-6. 10.1007/s002689900231 [DOI] [PubMed] [Google Scholar]
- 120.Chiappa A, Zbar AP, Biella F, et al. Survival after repeat hepatic resection for recurrent colorectal metastases. Hepatogastroenterology 1999;46:1065-70. [PubMed] [Google Scholar]
- 121.Vaillant JC, Balladur P, Nordlinger B, et al. Repeat liver resection for recurrent colorectal metastases. Br J Surg 1993;80:340-4. 10.1002/bjs.1800800324 [DOI] [PubMed] [Google Scholar]
- 122.Wicherts DA, de Haas RJ, Salloum C, et al. Repeat hepatectomy for recurrent colorectal metastases. Br J Surg 2013;100:808-18. 10.1002/bjs.9088 [DOI] [PubMed] [Google Scholar]
- 123.Jönsson K, Grondahl G, Salo M, et al. Repeated liver resection for colorectal liver metastases: a comparison with primary liver resections concerning perioperative and long-term outcome. Gastroenterol Res Pract 2012;2012:568214. 10.1155/2012/568214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang SJ, Si XY, Cai ZB, et al. Survival after repeat hepatectomy for recurrent colorectal liver metastasis: A review and meta-analysis of prognostic factors. Hepatobiliary Pancreat Dis Int 2019;18:313-20. 10.1016/j.hbpd.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 125.Lopez P, Marzano E, Piardi T, et al. Repeat hepatectomy for liver metastases from colorectal primary cancer: A review of the literature. J Visc Surg 2012;149:e97-103. 10.1016/j.jviscsurg.2012.01.001 [DOI] [PubMed] [Google Scholar]
- 126.Mühlbacher F, Huk IU, Steininger R, et al. Is orthotopic liver transplantation a feasible treatment for secondary cancer of the liver? Transplant Proc 1991;23:1567-8. [PubMed] [Google Scholar]
- 127.Mühlbacher F, Piza F. Orthotopic liver transplantation for secondary malignancies of the liver. Transplant Proc 1987;19:2396-8. [PubMed] [Google Scholar]
- 128.Moris D, Tsilimigras DI, Chakedis J, et al. Liver transplantation for unresectable colorectal liver metastases: A systematic review. J Surg Oncol 2017;116:288-97. 10.1002/jso.24671 [DOI] [PubMed] [Google Scholar]
- 129.Kappel S, Kandioler D, Steininger R, et al. Genetic detection of lymph node micrometastases: a selection criterion for liver transplantation in patients with liver metastases after colorectal cancer. Transplantation 2006;81:64-70. 10.1097/01.tp.0000189711.98971.9c [DOI] [PubMed] [Google Scholar]
- 130.Foss A, Adam R, Dueland S. Liver transplantation for colorectal liver metastases: Revisiting the concept. Transpl Int 2010;23:679-85. 10.1111/j.1432-2277.2010.01097.x [DOI] [PubMed] [Google Scholar]
- 131.Lucas AS, O’Neil BH, Goldberg RM. A decade of advances in cytotoxic chemotherapy for metastatic colorectal cancer. Clin Colorectal Cancer 2011;10:238-44. 10.1016/j.clcc.2011.06.012 [DOI] [PubMed] [Google Scholar]
- 132.Dueland S, Guren T, Hagness M, et al. Chemotherapy or liver transplantation for nonresectable liver metastases from colorectal cancer? Ann Surg 2015;261:956-60. 10.1097/SLA.0000000000000786 [DOI] [PubMed] [Google Scholar]
- 133.Tveit KM, Guren T, Glimelius B, et al. Phase III trial of cetuximab with continuous or intermittent fluorouracil, leucovorin, and oxaliplatin (Nordic FLOX) versus FLOX alone in firstline treatment of metastatic colorectal cancer: the NORDIC-VII study. J Clin Oncol 2012;30:1755-62. 10.1200/JCO.2011.38.0915 [DOI] [PubMed] [Google Scholar]
- 134.Toso C, Marques HP, Andres A, et al. Liver transplantation for colorectal liver metastasis: Survival without recurrence can be achieved. Liver Transpl 2017;23:1073-6. 10.1002/lt.24791 [DOI] [PubMed] [Google Scholar]
- 135.Chua TC, Liauw W, Chu F, et al. Viewing metastatic colorectal cancer as a curable chronic disease. Am J Clin Oncol 2012;35:77-80. 10.1097/COC.0b013e3181fe4444 [DOI] [PubMed] [Google Scholar]
- 136.Hagness M, Foss A, Egge TS, et al. Patterns of recurrence after liver transplantation for nonresectable liver metastases from colorectal cancer. Ann Surg Oncol 2014;21:1323-9. 10.1245/s10434-013-3449-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Leung U, Gonen M, Allen RJ, et al. Colorectal cancer liver metastases and concurrent extrahepatic disease treated with resection. Ann Surg 2017;265:158-65. 10.1097/SLA.0000000000001624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.D’Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol 2011;18:1096-103. 10.1245/s10434-010-1409-1 [DOI] [PubMed] [Google Scholar]
- 139.Hibi T, Sapisochin G. What is transplant oncology? Surgery 2019;165:281-5. 10.1016/j.surg.2018.10.024 [DOI] [PubMed] [Google Scholar]
- 140.Line PD, Hagness M, Berstad AE, et al. A novel concept for partial liver transplantation in nonresectable colorectal liver metastases: The RAPID concept. Ann Surg 2015;262:e5-9. 10.1097/SLA.0000000000001165 [DOI] [PubMed] [Google Scholar]
- 141.Riley JM, Cross AW, Paulos CM, et al. The clinical implications of immunogenomics in colorectal cancer: A path for precision medicine. Cancer 2018;124:1650-9. 10.1002/cncr.31214 [DOI] [PubMed] [Google Scholar]
- 142.Wong SL, Mangu PB, Choti MA, et al. American Society of Clinical Oncology 2009 clinical evidence review on radiofrequency ablation of hepatic metastases from colorectal cancer. J Clin Oncol 2010;28:493-508. 10.1200/JCO.2009.23.4450 [DOI] [PubMed] [Google Scholar]
- 143.Alsina J, Choti MA. Liver-directed therapies in Colorectal cancer. Semin Oncol 2011;38:561-7. 10.1053/j.seminoncol.2011.05.010 [DOI] [PubMed] [Google Scholar]
- 144.Berber E, Siperstein AE. Perioperative outcome after laparoscopic radiofrequency ablation of liver tumors: an analysis of 521 cases. Surg Endosc 2007;21:613-8. 10.1007/s00464-006-9139-y [DOI] [PubMed] [Google Scholar]
- 145.Siperstein AE, Berber E, Ballem N, et al. Survival after radiofrequency ablation of colorectal liver metastases: 10-year experience. Ann Surg 2007;246:559-65. 10.1097/SLA.0b013e318155a7b6 [DOI] [PubMed] [Google Scholar]
- 146.Gillams AR, Lees WR. Five-year survival in 309 patients with colorectal liver metastases treated with radiofrequency ablation. Eur Radiol 2009;19:1206-13. 10.1007/s00330-008-1258-5 [DOI] [PubMed] [Google Scholar]
- 147.Schindera ST, Nelson RC, DeLong DM, et al. Intrahepatic tumor recurrence after partial hepatectomy: value of percutaneous radiofrequency ablation. J Vasc Interv Radiol 2006;17:1631-7. 10.1097/01.RVI.0000239106.98853.B8 [DOI] [PubMed] [Google Scholar]
- 148.Suppiah A, White TJ, Roy-Choudhury SH, et al. Long-term results of percutaneous radiofrequency ablation of unresectable colorectal hepatic metastases: final outcomes. Dig Surg 2007;24:358-60. 10.1159/000107717 [DOI] [PubMed] [Google Scholar]
- 149.Oshowo A, Gillams AR, Lees WR, et al. Radiofrequency ablation extends the scope of surgery in colorectal liver metastases. Eur J Surg Oncol 2003;29:244-7. 10.1053/ejso.2002.1419 [DOI] [PubMed] [Google Scholar]
- 150.Tepel J, Hinz S, Klomp HJ, et al. Intraoperative radiofrequency ablation (RFA) for irresectable liver malignancies. Eur J Surg Oncol 2004;30:551-5. 10.1016/j.ejso.2004.03.010 [DOI] [PubMed] [Google Scholar]
- 151.Solbiati L, Lerace T, Tonolini M, et al. Radiofrequency thermal ablation of hepatic metastases. Eur J Ultrasound 2001;13:149-58. 10.1016/S0929-8266(01)00127-6 [DOI] [PubMed] [Google Scholar]
- 152.Lubner MG, Brace CL, Hinshaw JL, et al. Microwave tumor ablation: mechanism of action, clinical results, and devices. J Vasc Interv Radiol 2010;21:S192-203. 10.1016/j.jvir.2010.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Shibata T, Niinobu T, Ogata N, et al. Microwave coagulation therapy for multiple hepatic metastases from colorectal carcinoma. Cancer 2000;89:276-84. [DOI] [PubMed] [Google Scholar]
- 154.Martin RC, Scoggins C, McMasters K. Safety and efficacy of microwave ablation of hepatic tumors: a prospective review of a 5-year experience. Ann Surg Oncol 2010;17:171-8. 10.1245/s10434-009-0686-z [DOI] [PubMed] [Google Scholar]
- 155.Abdalla EK, Vauthey JN, Ellis LM, et al. Recurrence and outcomes following hepatic resection, radiofrequency ablation, and combined resection/ablation for colorectal liver metastases. Ann Surg 2004;239:818-25; discussion 825-7. 10.1097/01.sla.0000128305.90650.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Imai K, Allard MA, Castro Benitez C, et al. Long-term outcomes of radiofrequency ablation combined with hepatectomy compared with hepatectomy alone for colorectal liver metastases. Br J Surg 2017;104:570-9. 10.1002/bjs.10447 [DOI] [PubMed] [Google Scholar]
- 157.Vogl TJ, Zangos S, Eichler K, et al. Colorectal liver metastases: regional chemotherapy via transarterial chemoembolization (TACE) and hepatic chemoperfusion: an update. Eur Radiol 2007;17:1025-34. 10.1007/s00330-006-0372-5 [DOI] [PubMed] [Google Scholar]
- 158.Sanz-Altamira PM, Spence LD, Huberman MS, et al. Selective chemoembolization in the management of hepatic metastases in refractory colorectal carcinoma: a phase II trial. Dis Colon Rectum 1997;40:770-5. 10.1007/BF02055430 [DOI] [PubMed] [Google Scholar]
- 159.Tellez C, Benson AB, 3rd, Lyster MT, et al. Phase II trial of chemoembolization for the treatment of metastatic colorectal carcinoma to the liver and review of the literature. Cancer 1998;82:1250-9. [DOI] [PubMed] [Google Scholar]
- 160.Garrean S, Muh A, Bui JT, et al. Complete eradication of hepatic metastasis from colorectal cancer by yttrium-90 SIRT. World J Gastroenterol 2007;13:3016-9. 10.3748/wjg.v13.i21.3016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kennedy AS, Salem R. Radioembolization (yttrium-90 microspheres) for primary and metastatic hepatic malignancies. Cancer J 2010;16:163-75. 10.1097/PPO.0b013e3181d7e8cf [DOI] [PubMed] [Google Scholar]
- 162.Stintzing S, Hoffmann RT, Heinemann V, et al. Frameless single-session robotic radiosurgery of liver metastases in colorectal cancer patients. Eur J Cancer 2010;46:1026-32. 10.1016/j.ejca.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 163.Petrelli F, Comito T, Barni S, et al. SBRT for CRC liver metastases. Stereotactic body radiotherapy for colorectal cancer liver metastases: A systematic review. Radiother Oncol 2018;129:427-34. 10.1016/j.radonc.2018.06.035 [DOI] [PubMed] [Google Scholar]
- 164.Chapelle N, Matysiak-Budnik T, Rougier P, et al. Hepatic arterial infusion in the management of colorectal cancer liver metastasis: Current and future perspectives. Review Article. Dig Liver Dis 2018;50:220-5. 10.1016/j.dld.2017.12.004 [DOI] [PubMed] [Google Scholar]
- 165.Kemeny NE, Melendez FDH, Capanu M, et al. Conversion to resectability using hepatic artery infusion plus systemic chemotherapy for the treatment of unresectable liver metastases from colorectal carcinoma. J Clin Oncol 2009;27:3465-71. 10.1200/JCO.2008.20.1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kemeny N, Jarnagin W, Paty P, et al. Phase I trial of systemic oxaliplatin combination chemotherapy with hepatic arterial infusion in patients with unresectable liver metastases from colorectal cancer. J Clin Oncol 2005;23:4888-96. 10.1200/JCO.2005.07.100 [DOI] [PubMed] [Google Scholar]
- 167.Lévi FA, Boige V, Hebbar M, et al. Conversion to resection of liver metastases from colorectal cancer with hepatic artery infusion of combined chemotherapy and systemic cetuximab in multicenter trial OPTILIV. Ann Oncol 2016;27:267-74. 10.1093/annonc/mdv548 [DOI] [PubMed] [Google Scholar]
- 168.Lorenz M, Muller HH, Schramm H, et al. Randomized trial of surgery versus surgery followed by adjuvant hepatic arterial infusion with 5-fluorouracil and folinic acid for liver metastases of colorectal cancer. German Cooperative on Liver Metastases. Ann Surg 1998;228:756-62. 10.1097/00000658-199812000-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Kemeny N, Huang Y, Cohen AM, et al. Hepatic arterial infusion of chemotherapy after resection of hepatic metastases from colorectal cancer. N Engl J Med 1999;341:2039-48. 10.1056/NEJM199912303412702 [DOI] [PubMed] [Google Scholar]
- 170.Lygidakis NJ, Sgourakis G, Vlachos L. Metastatic liver disease of colorectal origin: the value of locoregional immunochemotherapy combined with systemic chemotherapy following liver resection. Results of a prospective randomized study. Hepatogastroenterology 2001;48:1685-91. [PubMed] [Google Scholar]
- 171.de Leede EM, Burgmans MC, Martini CH, et al. Percutaneous hepatic perfusion (PHP) with melphalan as a treatment for unresectable metastases confined to the liver. J Vis Exp 2016. doi: . 10.3791/53795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bartlett DL, Libutti SK, Figg WD, et al. Isolated hepatic perfusion for unresectable hepatic metastases from colorectal cancer. Surgery 2001;129:176-87. 10.1067/msy.2001.110365 [DOI] [PubMed] [Google Scholar]
- 173.Alexander HR, Libutti SK, Bartless DL, et al. Hepatic vascular isolation and perfusion for patients with progressive unresectable liver metastases from colorectal carcinoma refractory to previous systemic and regional chemotherapy. Cancer 2002;95:730-6. 10.1002/cncr.10686 [DOI] [PubMed] [Google Scholar]
- 174.Alexander HR, Libutti SK, Pingpank JF, et al. Isolated hepatic perfusion for the treatment of patients with colorectal cancer liver metastases after irinotecan-based therapy. Ann Surg Oncol 2005;12:138-44. 10.1245/ASO.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 175.Boone BA, Bartless DL, Zureikat AH. Isolated hepatic perfusion for the treatment of liver metastases. Curr Probl Cancer 2012;36:27-76. 10.1016/j.currproblcancer.2011.12.001 [DOI] [PubMed] [Google Scholar]
- 176.Schoellhammer HF, Goldner B, Merchant SJ, et al. Colorectal liver metastases: making the unresectable resectable using irreversible electroporation for microscope positive margins-a case report. BMC Cancer 2015;15;271. 10.1186/s12885-015-1279-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Scheffer HJ, Vroomen LG, Nielsen K, et al. Colorectal liver metastatic disease: efficacy of irreversible electroporation-a single-arm Phase II clinical trial (COLDFIRE-2 trial). BMC Cancer 2015;15:772. 10.1186/s12885-015-1736-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Scheffer HJ, Nielsen K, van Tilborg AA, et al. Ablation of colorectal liver metastases by irreversible electroporation: results of the COLDFIRE-1 ablate-and-resect study. Eur Radiol 2014;24:2467-75. 10.1007/s00330-014-3259-x [DOI] [PubMed] [Google Scholar]