Abstract
Introduction:
Currently, the most available treatment for acute ischemic stroke (AIS) is thrombolytic therapy with recombinant tissue plasminogen activator (r-TPA). A challenge in r-TPA therapy is the prediction of recovery in each case.
Objective:
The aim was to find a possible relationship between the cerebral oximetry indexes and the clinical outcome of r-TPA therapy to assess the cerebral oximetry as a non-invasive monitoring agent for therapy.
Methods:
The inclusion criteria were all patients with AIS who received r-TPA. The neurologic status was evaluated based on the national institutes of health stroke scale (NIHSS) score at arrival, and after a period of 24 hours. In addition, the levels of brain oxygenation in both hemispheres were measured before and continuously over the first 24 hours after r-TPA injection, using an oximetric sensor in the frontal lobes. The clinical success was defined as a 4-point improvement from the baseline NIHSS.
Results:
Total 44 patients with the mean age of 58.2 ± 2.18 years were enrolled, of whom 68.18% were male. Twenty-eight patients remained clinically unimproved and 16 patients were improved. A significant difference was found in the mean surface area under the brain oximetric curve in the 24 hour, in the affected hemisphere in the improved group, compared to the unimproved group (P = 0.007). There was a significant difference between the mean increase in brain oxygenation within 24 hours in the improved and unimproved groups (P = 0.002).
Conclusion:
It is likely that, The cerebral oximetry could contribute to predict the likelihood of r-TPA prognosis in patients with AIS.
Keywords: Spectroscopy, Near-Infrared; Stroke; Oximetry; Tissue Plasminogen Activator; Outcome
Introduction
Cerebrovascular disease is the second cause of death and the sixth cause of severe morbidity worldwide, which will rise to 4th place by 2020 (1). About 85% of cerebrovascular disease is acute infarct, classifying to ischemic (85%) and hemorrhagic (15%) (2). Regarding the increasing rate of stroke-related deaths and disability-adjusted life years (DALYs), compared to all diseases, the early diagnosis and consequent appropriate treatment are of paramount of importance. Currently, the treatment of acute ischemic stroke (AIS) is based on the early reperfusion and secondary stroke prevention (3–6). The most available treatment in the acute phase after 3–4.5 hours of the ictus is thrombolytic therapy with recombinant tissue plasminogen activator (r-TPA), which seems to contribute to 30% improvement over a three-month period (3–11). A challenge in reperfusion therapy by r-TPA is the prediction of recovery for each patient. General prognostic factors are patients’ age, female gender, prestrike functional state, infarct volume, diabetes mellitus, heart failure and fever. In recent years, new prognostic factors like hemostasis biomarkers, serum hepatocytes growth factor (HGF) and lesional and contra-lesional brain activity, measured by conventional electroencephalography (EEG), have been of interest, which needed more comprehensive studies (12–16). So far, no distinct measurement has been performed on patients who received r-TPA in order to predict the prognosis. Near-infrared spectroscopy (NIRS) -guided cerebral oximetry, which is associated with regional cerebral perfusion and/or oxygenation, provides non-invasive access to the brain to determine regional cerebral oxygen saturation (rSO2). Despite the use of NIRS-guided oximetry in brain trauma, cardiac surgery and neonatal intensive care unit (ICU), this method has not gained any attention in stroke (17–23). In 2015, Hametneret al. recommended cerebral NIRS index rSO2 as a predictive factor of death in patients with AIS who had been under endovascular intervention (20).Herein, we aimed to show the relationship between the cerebral oximetry measure and the clinical outcome of patients with AIS who had been under intravenous r-TPA in ICU to determine the NIRS guided cerebral oximetry as a tool and non-invasive factor to monitor cerebral function in r-TPA therapy.
Methods
Study design
This correlation study was conducted during 2017 and 2018 in the Poursina hospital, Rasht, Iran. The study was approved by the ethics committee of Guillan University of Medical Sciences (Code: IR.GUMS.REC.1396.351).In addition, written informed consents were obtained from all patients prior to their participation in this study.
Study population
As this is the first study, focusing on cerebral oximetry as a prognostic factor in patients with AIS, who underwent acute reperfusion by r-TPA, and more importantly, due to the limit in the sample size, we had to include all the patients with AIS, referred to our center by the neurologist who fulfilled the criteria of r-TPA (24). Early infarct signs > 1/3 territory of middle cerebral artery was considered as exclusion criteria.
Intervention and data gathering
Following the hospitalization of patient in the ICU, r-TPA (0.9 mg/ kg up to a maximum dosage of 90 mg) was injected. The severity of the clinical symptoms was evaluated based on the national institutes of health stroke scale (NIHSS) score at arrival, after a period of 24 hours (every 15 minutes) and a week. The clinical success was defined as a 4-point improvement from the baseline NIHSS score (25).
Furthermore, the levels of oxygen of the brain tissue in both hemispheres were measured before r-TPA injection, and continuously over the first 24 hours after reperfusion therapy, by a non-invasive oximetric sensor in the frontal, left and right lobes. The device used in this process was cerebral and somatic oximetry, which is manufactured by the Nonin Medical Company (USA).
Eventually, the data collection was performed by a checklist, prepared by referring to the patients’ chart, including the patients’ characteristics such as age, sex, height, weight, past medical history, habitual patient file, blood pressure, SPO2, brain rSO2 and complication of the r-TPA.
Statistical analysis
After data collection, the statistical analysis was performed, using SPSS software version 22.0. Normal data distribution was performed by the Kolmogorov-Smirnov test. For comparison between two groups, independent t-test and Mann–Whitney test were applied. The odds ratio was estimated by logistic regression test. The mean area under the curve (AUC) of brain oximetric was measured in both clinically improved and unimproved groups.
Results
Totally, 44 patients with the mean age of 58.2±2.18 years were enrolled, of whom 68.18% were males. The summary of patients’ characteristics is presented in table 1. Among them, 28 patients were categorized into the unimproved group and 16 patients in the improved group. Among the unimproved group, three patients died after 24 hours. There was not a statistical difference between age (p=0.82) and sex (p=0.52) in unimproved and improved groups. In addition, no statistical difference was found between primary NIHSS, in two groups (14.75±2.8 and 13.7±2.8 in improved and in unimproved group, respectively) (p=0.085).
Table 1:
Patients’ characteristics at arrival
| Variable | Improved group (n=16) | Unimproved group (n=28) | P value |
|---|---|---|---|
| N (%) | |||
| Age (year) | |||
| <65 | 8 (50.0) | 15 (53.6) | 0.82 |
| ≥65 | 8 (50.0) | 13 (46.4) | 0.82 |
| Sex | |||
| Female | 4 (25.0) | 10 (35.7) | 0.52 |
| Male | 12 (75.0) | 18 (64.3) | 0.52 |
| Involved hemisphere | |||
| Right | 7 (43.8) | 6 (21.4) | 0.118 |
| Left | 9 (56.2) | 22 (78.6) | 0.118 |
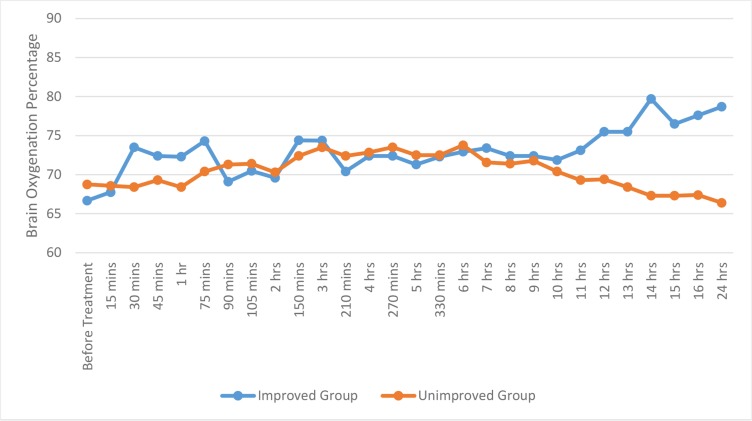
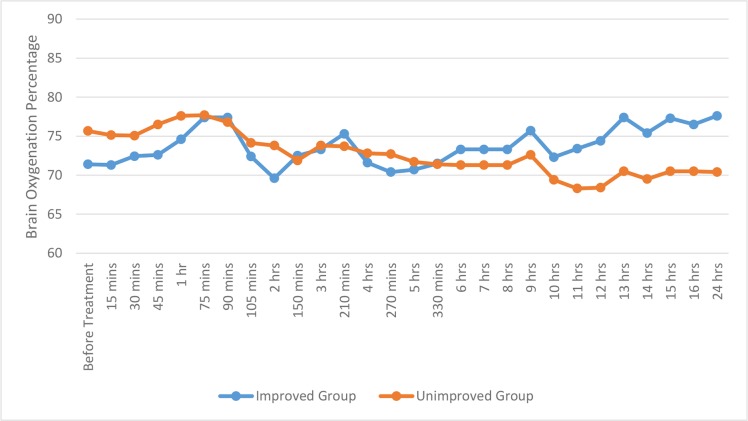
Additionally, the mean rSO2 in both affected and unaffected hemispheres was measured in both groups. Our findings revealed a statistical difference in brain oxygenation of the affected hemisphere, in both improved (p=0.002) and unimproved (p=0.0001) groups over different periods within 24 hours. By using two independent sample t-test, a statistical significant difference in the affected hemisphere oxygenation was observed between improved and unimproved groups, at different times before treatment, such as 15, 45, 60 minutes (p=0.011, 0.026, 0.041 and 0.014, respectively) and 12, 13, 14, 15, 16, 24 hours (p=0.001, 0.0001, 0.0001, 0.0001, 0.0001and 0.0001, respectively). In addition, there was a statistical difference in brain oxygenation of the unaffected hemisphere in both improved (p=0.001) and unimproved (p=0.003) groups over different periods within 24 hours. By using two independent sample t-test, a statistical difference in the unaffected hemisphere oxygenation was observed between improved and unimproved groups at different times such as 30, 45, 60, 75 minutes (p=0.008, 0.044, 0.002 and 0.001, respectively) and 8, 11, 12, 13, 14, 15, 16, 24 hours (p= 0.019, 0.001, 0.001, 0.0001, 0.0001 and 0.0001, respectively). The summary is presented in figures 1 and 2.
Figure 1:
The mean area under the curve of brain oximetric in the affected hemisphere in improved and unimproved groups over different periods within 24 hours
Figure 2:
The mean area under the curve of brain oximetric, in the unaffected hemisphere in improved and unimproved groups over different periods within 24 hours
The mean AUC of brain oximetric in the affected hemisphere was also assessed, showing a considerable difference between improved and unimproved groups (73.02± 3.28 vs. 70.41 ± 2.70; p=0.007). Furthermore, there was not a statistical difference of the mean AUC of the unaffected hemisphere oximetric between improved and unimproved groups (73.72± 2.63 vs. 72.6 ± 4.49; p=0.272).
Moreover, a statistical significant difference was observed between the mean increase in brain oxygenation of the both affected and unaffected hemispheres between the improved and unimproved groups within 24 hours (p=0.0001). In addition, a considerable statistical difference was observed between the mean increase in brain oxygenation between affected and unaffected hemispheres (5.02± 2.22 vs. –0.88 ± 11.1; p=0.002).
Eventually, using Binary Logistic Regression, the intervening factors of neurological improvement, consisting of the mean increase of brain oxygenation, age and sex were evaluated, suggesting that the mean increase of brain oxygenation is the only modifying factor, which potentially can contribute to a clinical improvement. Such as increasing the brain oxygenation of the affected hemisphere leads to 34% clinical improvement (Table 2).
Table 2:
The intervening factor of clinical improvement
| Variables in the Equation | B | SE | Wald | Df | Sig. | Exp (B) | 95.0% CI for EXP (B) | |
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | |||||||
| Affected hemisphere oxygenation increase | 0.298 | 0.101 | 8.813 | 1 | 0.003 | 1.348 | 1.107 | 1.641 |
| Sex | −0.315 | 1.086 | 0.084 | 1 | 0.772 | 0.730 | 0.087 | 6.136 |
| Age | 0.771 | 1.015 | 0.577 | 1 | 0.448 | 2.162 | 0.295 | 15.824 |
| Constant | −1.669 | 0.804 | 4.311 | 1 | 0.038 | 0.188 | - | - |
Discussion
In this study, we aimed to focus on the cerebral perfusion change after 24 hours following r-TPA, as a reperfusion therapy in patients with AIS, who hospitalized in the ICU with continuous NIRS-guided cerebral oximetry.
Given the increasing frequency of stroke in recent years, the early treatment of acute ischemic cerebral infarct is of paramount of importance. The most available treatment in the acute phase of AIS is r-TPA therapy (3–11). A considerable challenge in acute reperfusion therapy is to determine which patient benefit more from the treatment.
NIRS-guided cerebral oximetry, associated with regional cerebral perfusion and/or oxygenation, provides non-invasive access to the brain to determine rSO2(17–22). Since1997, various studies have been done to yield the effectiveness of regional oximetry in different clinical settings as the study of Shuler et al. who showed tissue oxygenation increase measured by infrared spectroscopy while tibial fracture(23–33). In the study of Orihashi et al. on 59 patients with aortic surgery, after cerebral perfusion monitoring in post-surgery, they showed that a drop in rSO2 over cardiac surgery was highly related to neurologic deficit (24). Interestingly, Robert et al. evaluated six patients with diabetic ketoacidosis after 24 hours with NIRS-guided cerebral oximetry, serial brain CT, trans-cranial Doppler (TCD), and serial measurement of S100; their findings revealed a normal to increased cerebral blood flow, elevated regional cerebral oxygenation and impaired auto-regulation in the patients without a significant change in S100. Due to availability of cerebral oximetry, they recommended cerebral oximetry as a monitoring factor in clinically ill patients with diabetic ketoacidosis (25). In the field of pediatric emergency, Fortune et al. worked on the patients with acute abdominal pain. They showed by using NIRS-guided oximetry, the cerebrosplanchnic oxygenation ratio could be assessed, which could help to detect early splanchnic ischemia in neonates (26).
Moreover, some studies have been done on the influence of NIRS-guided regional oximetry on the management of patients with trauma, which highlighted the importance of regional oximetry to screen neurologic deficit due to hypoxia or to detect compartment syndrome. Furthermore, few studies suggested the cerebral oximetry as a predictive factor of transfusion in hemorrhagic shock (27–30).
In the field of Neuroradiology, NIRS-guided cerebral oximetry has been taken into consideration. In a case report, Luer et al. evaluated a patient with subarachnoid hemorrhage due to ruptured middle cerebral artery aneurysm, and showed NIRS-guided cerebral oximetry was more sensitive than TCD to screen vasospasm (31). Additionally, there are reports of NIRS-guided cerebral in neurovascular interventions as in balloon test occlusion, aneurysm embolization or dural arteriovenous fistula embolization (31–34).
Recently, much attention has been paid to cerebral oximetry over reperfusion therapy during acute ischemic events. In a case series, Hiramatsu demonstrated that despite complete recanalization in all three patients, the NIRS revealed a considerable rSO2 response in large vessel involvement, compared to no change of rSO2 in patients with distal middle cerebral artery occlusion (35). Similarly, in the study of Hametner et al. on 43 patients with acute stroke who underwent thrombectomy with continuous cerebral oximetry, they revealed that before recanalization, 10 distinct rSO2 decreases occurred in 11 patients. During recanalization, two patterns of rSO2 increase occurred in the affected hemisphere. Lower AUC, 10% below baseline was associated with better reperfusion status. Eventually, at the end of the treatment, lower interhemispheric rSO2 difference predicted death within 90 days and higher rSO2 variability predicted poor outcome (20).
Our findings showed that there was a statistical difference of the mean rSO2 in both affected and unaffected hemispheres, in both improved and unimproved groups over different periods within 24 hours (p=0.002and 0.0001, respectively). The changes in the first hours could be explained by the onset, peak and half-life of the r-TPA, and the change in the last hours could be explained by the fact that the cerebral rSO2 is closely related to the systemic changes as cardiac output, pulse rate and vascular resistance, which themselves are influenced by the intracranial pressure, autoregulation and cerebrovascular response to CO2, which are all impaired in AIS (36).
Furthermore, we showed a statistical significant difference between the mean increase in brain oxygenation of the both affected and unaffected hemispheres between the improved and unimproved groups within 24 hours (p=0.001), which was more in the affected hemisphere. We concluded that a ≥10% increase of brain oxygenation, compared to the baseline was related to a clinical improvement. In contrast, in the unimproved group, despite the primary rise of rSO2, the eventual brain oxygenation decreased after 24 h, in comparison to the baseline. Among the three patients who died after 24 hours, we found the baseline brain oxygen saturation was ≤ 50 and during the r-TPA therapy, the brain oxygenation level had been decreasing to more than 20%.
It should be noted that the mean regional oxygenation of the affected hemisphere increased both in the improved and unimproved groups, explaining the impairment of autoregulation of the affected hemisphere, which subsequently results in an increased blood flow due to sympathetic system malfunction and shift of blood flow from the unaffected site toward the affected site. Besides, it mentions the risk of hyperemia in the affected hemisphere (37).
Furthermore, we showed a considerable difference of the mean AUC of the brain oximetric in the affected hemisphere (p=0.007), in comparison to the unaffected hemisphere, which did not show a statistical difference between improved and unimproved groups (p=0.272). This highlights the effect of thrombolytic therapy to preserve the penumbra area, which is susceptible to infarct and irreversible damage.
In addition, our data showed no statistical correlation between sex and the mean increase of brain oxygenation and the mean AUC oximetric, in both improved and unimproved groups, which is similar to the findings of Boehme et al. who evaluated 4925 patients with AIS over 2001–2014. They showed that race and gender were not related to short term outcome (38).
Eventually, we showed no statistical correlation between age and the mean increase of brain oxygenation, and the mean AUC of the oximetric in both improved and unimproved groups, which is not consistent with other studies, such as the study of Berrouschotet al. on the outcome and severe hemorrhagic complications of intravenous r-TPA, showing worse outcome in patients older than 80 years. However, the risk of hemorrhagic transformation was not related to age (39).
Limitation
The main limitation of our study was the small sample size. As one of the most noticeable concerns in AIS is the golden time when the patient is referred to the hospital, we also encountered a gap between the onset of the symptoms and the hospitalization, which consequently resulted in the use of small size of participants.
Conclusions
In summary, we could apply NIRS-guided cerebral oximetry to monitor the cerebral oxygenation over acute reperfusion therapy, which could be used as a predictive factor. As we showed ≥ 10% increase in the brain oxygenation of the affected hemisphere increases the likelihood of clinical improvement up to 34%, over 2h hours of hospitalization. In contrast, a low level of brain oxygenation at the baseline is highly related to poor outcome, which led to death in our three patients. However, the increase of brain oxygenation has the risk of hyperemia and the consequent hyperperfusion syndrome. As a result, we suggest more investigations in this field with the simultaneous use of TCD of the affected hemisphere to monitor the autoregulation of the affected hemisphere. In addition, due to limitation of NIRS-guided cerebral oximetry in small vessel occlusion monitoring, the infarcted region in future researches should be evaluated.
Acknowledgements
The authors would like to thank the Deputy of University Research and Technology, Clinical Research Development Unit of Poursina Hospital, Guilan University of Medical Sciences.
Footnotes
Authors’ contribution
All the authors met the standards of authorship based on the recommendations of the International Committee of Medical Journal Editors.
Conflict of interest
None declared.
Funding
The corresponding author has provided all funds for this study, including the procurement of the cerebral oximetry device.
References
- 1. Menken M, Munsat TL, Toole JF . The global burden of disease study: implications for neurology. Arch Neurol. 2000. : 57(3):418–20. [DOI] [PubMed] [Google Scholar]
- 2. Wein T, Lindsay MP, Côté R, Foley N, Berlingieri J, Bhogal S, et al. Canadian stroke best practice recommendations: Secondary prevention of stroke, practice guidelines, update 2017. Int J Stroke. 2018. : 13(4):420–43. [DOI] [PubMed] [Google Scholar]
- 3. Powers W, Rabinstein A, Ackerson T, Adeoye O, Bambakidis N, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke. Stroke. 2018. : 49(3):e46–e110. [DOI] [PubMed] [Google Scholar]
- 4. Baratloo A, Forouzanfar MM, Hashemi B, Safari S, Kasmaei HD, Rouhipour A, et al. Tissue plasminogen activator: A literature review. Arch Neurosci. 2016. : 3(1):e30452. [Google Scholar]
- 5. Boudreau DM, Guzauskas GF, Chen E, Lalla D, Tayama D, Fagan SC, et al. Cost-effectiveness of recombinant tissue-type plasminogen activator within 3 hours of acute ischemic stroke: current evidence. Stroke. 2014. : 45(10):3032–9. [DOI] [PubMed] [Google Scholar]
- 6. De Keyser J, Gdovinová Z, Uyttenboogaart M, Vroomen PC, Luijckx GJ . Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke. 2007. : 38(9):2612–8. [DOI] [PubMed] [Google Scholar]
- 7. Patel RA, White C . Acute ischemic stroke treatment: State of the art. Vasc Med. 2011. : 16(1):19–28. [DOI] [PubMed] [Google Scholar]
- 8. Bernheisel CR, Schlaudecker JD, Leopold K . Subacute management of ischemic stroke . Am Fam Physician . 2011. December 15 ; 84 ( 12 ): 1383 – 8 . [PubMed] [Google Scholar]
- 9. Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, et al. . Solitaire™ with the Intention for Thrombectomy as Primary Endovascular Treatment for Acute Ischemic Stroke (SWIFT PRIME) trial: protocol for a randomized, controlled, multicenter study comparing the Solitaire revascularization device with IV tPA with IV tPA alone in acute ischemic stroke . Int J Stroke . 2015. April ; 10 ( 3 ): 439 – 48 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schellinger PD, Jansen O, Fiebach JB, Heiland S, Steiner T, Schwab S, et al. Monitoring intravenous recombinant tissue plasminogen activator thrombolysis for acute ischemic stroke with diffusion and perfusion MRI. Stroke. 2000. : 31(6):1318–28. [DOI] [PubMed] [Google Scholar]
- 11. Cheng NT, Kim AS . Intravenous thrombolysis for acute ischemic stroke within 3 hours versus between 3 and 4.5 hours of symptom onset. Neurohospitalist. 2015. : 5(3):101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N . Endovascular therapy for ischemic stroke with perfusion imaging selection . N Engl J Med . 2015. ; 372 ( 11 ): 1009 – 1 . [DOI] [PubMed] [Google Scholar]
- 13. van Seeters T, Biessels GJ, Kappelle LJ, van der Schaaf IC, Dankbaar JW, Horsch AD, et al. The prognostic value of CT angiography and Ct perfusion in acute ischemic stroke. Cerebrovasc Dis. 2015. : 40( 5–6 ): 258 – 69 . [DOI] [PubMed] [Google Scholar]
- 14. Kim JS . Stroke in A sia: a global disaster. Int J Stroke. 2014. : 9(7):856–7. [DOI] [PubMed] [Google Scholar]
- 15. Donkel SJ, Benaddi B, Dippel DW, ten Cate H, de Maat MP . Prognostic hemostasis biomarkers in acute ischemic stroke: a systematic review. Arterioscler Thromb Vasc Biol. 2019. : 39(3):360–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Kaam RC, van Putten MJ, Vermeer SE, Hofmeijer J . Contralesional brain activity in acute ischemic stroke. Cerebrovasc Dis. 2018. : 45( 1–2 ): 85 – 92 . [DOI] [PubMed] [Google Scholar]
- 17. Zhu Z, Xu T, Guo D, Huangfu X, Zhong C, Yang J, et al. Serum hepatocyte growth factor is probably associated with 3-month prognosis of acute ischemic stroke. Stroke. 2018. : 49(2):377–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Drayna PC, Abramo TJ, Estrada C . Near-infrared spectroscopy in the critical setting . Pediatr Emerg Care . 2011. May ; 27 ( 5 ): 432 – 9 . [DOI] [PubMed] [Google Scholar]
- 19. Joana M, Carolina MC, Liliane G, Manuel TC . Noninvasive Neurophysiological Monitoring in Acute Ischemic Stroke Treatment. J Neurol Neurosurg. 2017. : 4(1):555 628 . [Google Scholar]
- 20. Kim W, Taw B, Yokosako S, Koyanagi M, Fukuda H, Sinclair D, et al. The future of non-invasive cerebral oximetry in neurosurgical procedures: A systematic review. MNI Open Res. 2018. : 2( 3 ):( 10.12688/mniopenres.12779.1 ). [DOI] [Google Scholar]
- 21. Hametner C, Stanarcevic P, Stampfl S, Rohde S, Veltkamp R, Bösel J, et al. Noninvasive cerebral oximetry during endovascular therapy for acute ischemic stroke: an observational study. J Cereb Blood Flow Metab. 2015. : 35(11):1722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elser HE, Holditch-Davis D, Brandon DH . Cerebral Oxygenation Monitoring: A Strategy to Detect IVH and PVL. Newborn Infant Nurs Rev. 2011. : 11( 3 ): 153 – 9 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shuler MS, Reisman WM, Whitesides TE, Jr, Kinsey TL, Hammerberg EM, Davila MG, et al. Near-infrared spectroscopy in lower extremity trauma. J Bone Joint Surg Am. 2009. : 91(6):1360–8. [DOI] [PubMed] [Google Scholar]
- 24. Hametner C, Stanarcevic P, Stampfl S, Rohde S, Veltkamp R, Bösel J . Noninvasive cerebral oximetry during endovascular therapy for acute ischemic stroke: an observational study. J Cereb Blood Flow Metab. 2015. : 35(11):1722–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Louis ED, Mayer SA, Rowland LP . Merritt's neurology . 13th edition . Lippincott Williams & Wilkins; ; 2015. . [Google Scholar]
- 26. Balucani C, Levine SR, Khoury JC, Khatri P, Saver JL, Broderick JP . Acute ischemic stroke with very early clinical improvement:a national institute of neurological disorders and stroke recombinant tissue plasminogen activator stroke trials exploratory analysis. J Stroke Cerebrovasc Dis. 2016. : 25(4):894–901. [DOI] [PubMed] [Google Scholar]
- 27. Jobsis FF . Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977. : 198(4323):1264–7. [DOI] [PubMed] [Google Scholar]
- 28. Orihashi K, Sueda T, Okada K, Imai K . Near-infrared spectroscopy for monitoring cerebral ischemia during selective cerebral perfusion. Eur J Cardiothorac Surg. 2004. : 26(5):907–11. [DOI] [PubMed] [Google Scholar]
- 29. Roberts JS, Vavilala MS, Schenkman KA, Shaw D, Martin LD, Lam AM . Cerebral hyperemia and impaired cerebral autoregulation associated with diabetic ketoacidosis in critically ill children. Crit Care Med. 2006. : 34(8):2217–23. [DOI] [PubMed] [Google Scholar]
- 30. Fortune PM, Wagstaff M, Petros AJ . Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia in neonates. Intensive Care Med. 2001. : 27(8):1401–7. [DOI] [PubMed] [Google Scholar]
- 31. Kirkness CJ, Burr RL, Cain KC, Newell DW, Mitchell PH . Effect of continuous display of cerebral perfusion pressure on outcomes in patients with traumatic brain injury. Am J Crit Care. 2006. : 15(6):600–9. [PubMed] [Google Scholar]
- 32. Adelson PD, Nemoto E, Colak A, Painter M . The use of near infrared spectroscopy (NIRS) in children after traumatic brain injury: a preliminary report. Acta Neurochir Suppl. 1998. : 71: 250 – 4 . [DOI] [PubMed] [Google Scholar]
- 33. Giannotti G, Cohn SM, Brown M, Varela JE, McKenney MG, Wiseberg JA . Utility of near-infrared spectroscopy in the diagnosis of lower extremity compartment syndrome. J Trauma. 2000. : 48(3):396–401. [DOI] [PubMed] [Google Scholar]
- 34. Plachky J, Hofer S, Volkmann M, Martin E, Bardenheuer HJ, Weigand MA . Regional cerebral oxygen saturation is a sensitive marker of cerebral hypoperfusion during orthotopic liver transplantation. Anesth Analg. 2004. : 99(2):344–9. [DOI] [PubMed] [Google Scholar]
- 35. Shidoh S, Akiyama T, Ohira T, Yoshida K . Cerebral perfusion change of venous hypertension on near-infrared spectroscopy signals after operation for dural arteriovenous fistula. J Stroke Cerebrovasc Dis. 2014. : 23(5):823–8. [DOI] [PubMed] [Google Scholar]
- 36. Mutoh T, Kobayashi S, Tamakawa N, Ishikawa T . Multichannel near-infrared spectroscopy as a tool for assisting intra-arterial fasudil therapy for diffuse vasospasm after subarachnoid hemorrhage. Surg Neurol Int. 2011. : 2 : 68 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bhatia R, Hampton T, Malde S, Kandala NB, Muammar M, Deasy N, et al. The application of near-infrared oximetry to cerebral monitoring during aneurysm embolization: a comparison with intraprocedural angiography. J Neurosurg Anesthesiol. 2007. : 19(2):97–104. [DOI] [PubMed] [Google Scholar]
- 38. Hiramatsu R, Furuse M, Yagi R, Ohnishi H, Ikeda N, Nonoguchi N, Kawabata S, Miyachi S, Kuroiwa T . Limit of intraoperative near-infrared spectroscopy monitoring during endovascular thrombectomy in acute ischemic stroke. Interv Neuroradiol. 2018. : 24(1):57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Brott T, Bogousslavsky J . Treatment of acute ischemic stroke. N Engl J Med. 2000. : 343(10):710–22. [DOI] [PubMed] [Google Scholar]