Abstract
OBJECTIVE:
To determine the cost-effectiveness of nasal continuous positive pressure (nCPAP) compared with nasal intermittent positive pressure ventilation (NIPPV) in the context of the reported randomized clinical trial.
STUDY DESIGN:
Using patient-level data from the clinical trial, we undertook a prospectively planned economic evaluation. We measured costs, from a third-party payer perspective in all patients, and from a societal perspective in a subgroup with a time horizon through the earlier of discharge, death or 44 weeks post-menstrual age.
RESULTS:
From the third-party payer perspective, the mean cost of hospitalization per infant was statistically similar, $143 745 in the NIPPV group compared to $140 403 in the nCPAP group. Cost-effectiveness evaluation revealed a 61% probability that NIPPV is more expensive and less effective than nCPAP. Similar results were found in subgroup analysis from a societal perspective.
CONCLUSION:
In addition to being clinically equivalent, economic evaluation confirms that NIPPV, as employed in this trial, is also not economically favorable.
INTRODUCTION
Respiratory compromise is among the most common morbidities faced by premature infants.1 For the smallest and most premature infants, the mainstay of treatment has been intubation and mechanical ventilation.2 However, the use of invasive ventilation increases lung injury, and in some cases, causes bronchopulmonary dysplasia bronchopulmonary dysplasia (BPD) or even death.2,3
Nasal continuous positive pressure (nCPAP) has been shown to decrease the need for, and risks of, invasive ventilation.4–6 However, nCPAP either as a first-line therapy or as a bridge to unsupported breathing after invasive support, is not always successful. Nasal intermittent positive pressure ventilation (NIPPV), which provides the same continuous positive pressure as nCPAP with the addition of an intermittent peak inspiratory pressure, has been proposed as a more-effective alternative.
Recently, Kirpalani, et al.7 reported an international, multi-center, randomized controlled trial that compared NIPPV with nCPAP to prevent BPD or death in infants with birth weights < 1000 grams. In the study, the composite outcome of BPD or death was not significantly different between groups (38.4% vs 36.7% in the nCPAP group; P = 0.56).
Although the two strategies of non-invasive ventilation may be equally efficacious, their use may have differing costs. Consequently, we report a prospectively planned economic evaluation alongside the NIPPV trial to determine the economic implications of adopting one therapy over the other with respect to improving survival without BPD.
MATERIALS AND METHODS
The NIPPV study enrolled infants < 30 weeks gestation and < 1000g at birth who required non-invasive ventilation. Patients were recruited from 36 sites in ten countries including the United States, Canada, the United Kingdom, Ireland, Netherlands, Sweden, Belgium, Austria, Singapore, and Qatar. In total, 1009 patients were randomized to receive either NIPPV or nCPAP at the time they needed non-invasive respiratory support within the first 28 days of life. Of these, 987 were available for analysis. The primary outcome was reported as a composite of death or BPD, as defined by a supplemental oxygen requirement at 36 weeks post-menstrual age (PMA) or a positive oxygen-reduction test. Infants were followed until 44 weeks PMA, discharge or death, whichever occurred first. The study had 80% power to determine a 20% relative risk reduction in the primary outcome. With the exception of male sex (P = 0.04), the baseline characteristics of the NIPPV group and nCPAP group were not different. No significant difference was observed for the primary composite outcome of death or BPD between the two groups (38.4% in the NIPPV group vs 36.7% in the nCPAP group; P = 0.56) or in secondary morbidity outcomes. The details of the trial have been previously reported.7 The research ethics boards at both McMaster University and the participating centers approved the original trial.
The prospectively planned economic evaluation was completed alongside the clinical trial using individual patient-level data. In the primary analysis, a third-party payer perspective was used; a secondary analysis from a societal perspective was performed for the subgroup of sites at which parental questionnaires, detailing lost productivity and out of pocket costs, were administered. Although the clinical trial reported outcome data at 36 weeks PMA, resource utilization was collected until 44 weeks PMA. The time horizon for the economic evaluation was thus through the latest of discharge, death or 44 weeks PMA. This end point is inclusive of the trial end point of 36 weeks but optimizes the assessment of downstream costs. For the primary analysis, due to unavailability of full costing data in several countries, which together contributed ~ 35% of enrolled patients, unit costs from one of three main contributing countries—United States, United Kingdom or Canada—were applied to the remaining sites. The choice of which set of costs to apply was based on the similarity between the countries’ health expenditures as a percentage of their gross domestic product.8 Conversion to 2013 currency was completed for all direct and indirect medical costs using country-specific health consumer price indices.9–11 Country-specific currency was converted to US dollars using purchasing power parity.12
Resource utilization data were gathered alongside the trial in a detailed daily report of all resources used by the infant. From these forms, the total number of hospital days at each of several levels of acuity, based on respiratory support requirement, was determined. A per diem rate inclusive of all hospital13–15 and physician services16,17 at that acuity was assigned as described previously in economic evaluations alongside neonatal randomized trials.14,18–20 Hospital per diem costs included nursing and other support staff time, diagnostic procedures, nutrition (both enteral and parenteral), respiratory support, hospital overhead and capital equipment. Physician per diem costs covered physician time and services. Additional resources related to the intervention or the primary outcome were costed separately from the per diems in order to improve the accuracy or because of their frequency or magnitude. These included certain medications (antibiotics, antifungals, caffeine, diuretics, erythropoietin, indomethacin, ibuprofen, inhaled corticosteroids, inhaled bronch-odilators, inhaled nitric oxide, surfactant and vitamin A), surgeries (surgery for necrotizing enterocolitis, laser surgery for retinopathy of prematurity and patent ductus arteriosus ligation) and other procedures (chest X ray, abdominal X ray, echocardiogram and packed red blood cell transfusion).14–17,21 A total cost for hospitalization was determined for each patient as well as an average per patient for each arm of the study (NIPPV vs nCPAP). In order to optimize external validity to the main participating countries in the clinical trial, a post hoc secondary analysis was completed using patient and cost data from the United States and Canada exclusively. These countries were chosen as they represented the majority of the patients in the study.
Finally, a subgroup analysis from a societal prospective was completed. To obtain parent out of pocket costs and lost wages, parents were asked to complete a survey alongside the clinical trial, which queried potential sources of cost related to their child’s hospitalization, including as time missed from work, meals, transportation, childcare and lodging. This was used with modification from one previously reported in the literature to reflect an inpatient vs outpatient experience and to include items such as lodging, transportation and meals.19 Costs appropriate to the country of residence were applied to each resource and a total out of pocket cost and wage loss was derived.22–29 This was then added to the third-party payer costs to obtain a total societal cost. Countries in which >50% of patients completed parental surveys (the United and States and Canada) were included for the subgroup analysis. Only patients with completed parental surveys were included in the subgroup. If a survey was completed but contained missing values for costs, imputation based on patients from the same country was used for missing values. Missing cost values occurred for only six cost categories leading to a total of 5 to 24% of values requiring imputation.
First, we completed a direct cost analysis, in which we compared the mean costs for each treatment arm, without consideration of effectiveness. Because of the skewed nature of cost data, we modeled the logarithm of the mean costs using a generalized linear model with a logarithmic link function and gamma distribution.30
Next, we calculated the incremental cost-effectiveness ratio (iCER) as the difference in mean costs between the two study arms, divided by the difference in mean effect between the two study arms. We expressed effectiveness as the inverse of the published outcome, survival without BPD at 36 weeks corrected gestational age.
We assessed uncertainty using nonparametric boot-strapping.31 To accomplish this, we simulated repetitions of the study by obtaining 1000 samples, with replacement, of the 987 patients from the original data set. For each sample the mean costs, mean effects and the iCER were calculated.30,32,33 Additional deterministic sensitivity analysis was accomplished by recalculating the iCER with costs for all per diems and resources varying from 50 to 400% of the original cost, in order to account for broad plausible differences in local cost structure.
Institutions administering the questionnaire received approval from their respective ethics review boards along with the clinical trial approval. The economic evaluation analysis itself was considered exempt by the Beth Israel Deaconess Medical Center Committee on Clinical Investigations and the University of Florida Institutional Review Board.
RESULTS
Resources used by each group from the time of randomization to the primary end point (death, discharge or 44 weeks PMA) are summarized in Table 1. Resource utilization for medications and procedures between the two groups did not differ significantly. Hospitalization in the NIPPV group was 89 days compared with 87 days in the nCPAP group (P = 0.28). Similarly, infants in the NIPPV group spent 33 days receiving either non-invasive ventilatory support (defined as nasal CPAP or NIPPV), whereas those in the nCPAP arm received 30 days of this level of support (P = 0.07).
Table 1.
Resource utilization
| NIPPV (n = 497) | nCPAP (n = 490) | P-value | |
|---|---|---|---|
| Length of staya | 89.4 (29.8) | 87.3 (29.4) | 0.28 |
| Days in room aira | 25.2 (20.7) | 25.8 (20.2) | 0.67 |
| Days with nasal cannulaa | 14.9 (16.4) | 14.3 (15.6) | 0.53 |
| Days with non-invasive ventilationa | 32.7 (19.3) | 30.4 (19.7) | 0.07 |
| Days with ventiatora | 13.5 (18.8) | 13.6 (18.3) | 0.91 |
| Antibioticsa | 20.3 (17.8) | 19.7 (17.5) | 0.62 |
| Antifungalsa | 16.5 (17.3) | 14.9 (14.6) | 0.34 |
| Surfactanta | 1.1 (0.4) | 1.1 (0.4) | 0.80 |
| Indomethacina | 2.9 (1.5) | 2.9 (1.7) | 0.86 |
| Ibuprofena | 3.4 (1.6) | 3.4 (2) | 0.83 |
| Caffeinea | 48.8 (22.4) | 48.7 (22.6) | 0.99 |
| Furosemidea | 6.6 (9) | 7.6 (11.7) | 0.25 |
| Thiazidea | 25.3 (23.4) | 26 (22.5) | 0.84 |
| Corticosteroidsa | 10.1 (13.2) | 10.1 (12.5) | 0.98 |
| Inhaled corticosteroidsa | 20.3 (23.4) | 18 (22.9) | 0.62 |
| Inhaled brochodilatorsa | 18.1 (21.7) | 19.6 (24.4) | 0.64 |
| Vitamin Aa | 10.8 (6.3) | 11.8 (6.9) | 0.39 |
| Parenteral nutritiona | 26.5 (22.2) | 25.4 (21.3) | 0.97 |
| Packed red blood cell transfusionb | 4.5 (4) | 4.5 (4) | 0.97 |
| Chest X rayb | 9.5 (10) | 9.6 (9.7) | 0.91 |
| Abdominal X rayb | 5.2 (5.3) | 5.4 (5.7) | 0.63 |
| Echocardiogramb | 2.7 (2.1) | 2.8 (2.4) | 0.58 |
| Inhaled nitric oxidec | 4%, 7.5 (7.5) | 5% 9.7 (18.1) | 0.60 |
| Surgery for necrotizing enterocolitisd | 38 (8) | 43 (9) | 0.52e |
| Patent ductus arteriosus ligationd | 43 (9) | 38 (8) | 0.23e |
| Laser surgery-right eyed | 35 (7) | 31 (6) | 0.64e |
| Laser Surgery-left eyed | 34 (7) | 31 (6) | 0.73e |
Abbreviations: nCPAP, nasal continuous positive pressure; NIPPV, nasal intermittent positive pressure ventilation.
Mean number of days of therapy per patient (s.d.).
Mean number of therapy/procedure (s.d.).
Percentage of patients receiving therapy, mean number of days of therapy (s.d.).
Number of patients with procedure (percent).
x2.
Table 2 shows mean costs for hospital fees, physician fees, medications and procedures as well as parent costs in those applicable patients. The NIPPV group had higher absolute mean total costs for each category except medications, as well as for total hospitalization ($143 745) compared with the nCPAP group ($140 404), for a difference in mean costs of $3341, but these were not statistically significant. From the clinical trial, the mean difference in the proportion of survivors without BPD was −0.017. As shown in Table 3, calculation of the iCER resulted in a negative value, confirming that NIPPV was dominated; that is, more costly and less effective (Table 3).
Table 2.
Costs by category
| NIPPV (n = 497) mean (CI) | nCPAP (n = 490) mean (CI) | Cost difference mean (CI) | P-value | |
|---|---|---|---|---|
| Hospital costs | $128 542 (123 431, 133 653) | $124 588 (119 586, 129 589) | 3954.2 (−3188.9, 11 097.2) | 0.28 |
| Physician costs | $11 777 (10 682.2, 12 872.3) | $11 567 (10 497.6, 12 636.0) | 210.4 (−1318.5, 1739.4) | 0.79 |
| Medication costs | $1501 (876.5, 2124.9) | $1970 (532.2, 3407.0) | − 468.9 (−2034.9, 1097.1) | 0.56 |
| Procedure costs | $2962 (2588.4, 3335.7) | $2788 (2417.7, 3158.7) | 173.8 (−351.8, 699.4) | 0.52 |
| Total costs from 3rd party payer perspective | $143 745 (137 323, 150 167) | $140 404 (133 906, 146 902) | 3341.2 (−5783.1, 12 465.6) | 0.47 |
| Parent costsa | $3610.6 (2942.2, 4279.0) | $3577.1 (2986.8, 4167.4) | 33.5259 (−857.1, 924.2) | 0.94 |
| Total costs from societal perspectivea | $147 356 (140 768, 153 943) | $143981 (137 337, 150 625) | 3374.8 (−5969.8, 12 719.3) | 0.48 |
Abbreviations: nCPAP, nasal continuous positive pressure; NIPPV, nasal intermittent positive pressure ventilation.
n=413.
Table 3.
Calculation of point estimate of incremental cost-effectiveness ratio (iCER)
| Parameter | Cost ($) | Incremental cost (Δ cost) | Effectiveness (%) | Incremental effectiveness (Δ effectiveness) | iCER (Δcost/Δeffectiveness) |
|---|---|---|---|---|---|
| nCPAP | 140 404 | — | 0.633 | — | — |
| NIPPV | 143 745 | 3341 | 0.616 | − 0.017 | Dominateda |
Abbreviations: nCPAP, nasal continuous positive pressure; NIPPV, nasal intermittent positive pressure ventilation.
iCER: −197,020.
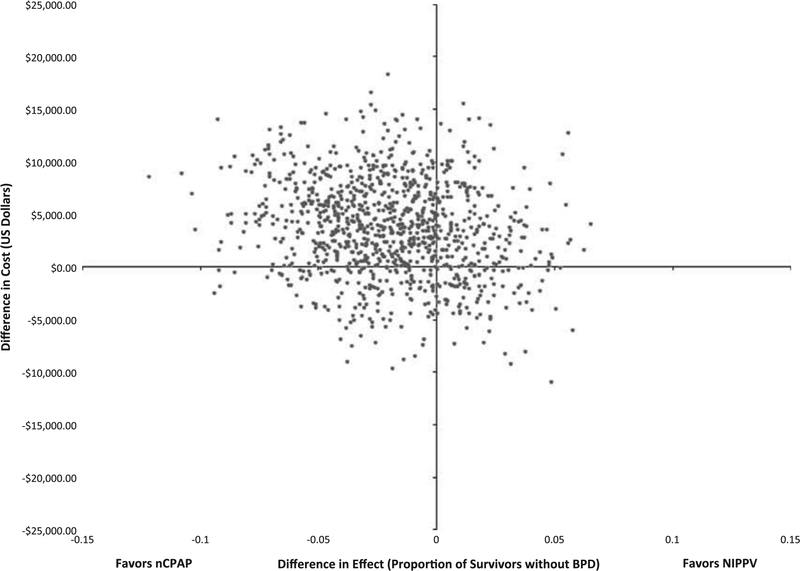
The iCER plot (Figure 1) shows the precision of this estimate. Differences in mean cost and mean effectiveness between treatment and control arms, for each of 1000 bootstrap replications, are shown. The upper left quadrant contains 61% of these replications corresponding to the probability that costs were higher and efficacy lower in the NIPPV arm. Moreover, only 8.6% of the replications lie in the lower right quadrant, which indicates it is unlikely that NIPPV is dominant—that is, both less costly and more effective than the use of nCPAP.
Figure 1.
Figure 1 displays the incremental cost-effectiveness plot for the primary analysis. The difference in effects is seen on the x axis, whereas the difference in costs is seen on the y axis. Each dot represents one boot-strapping replication.
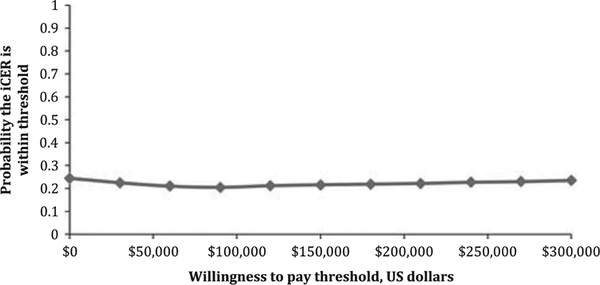
Figure 2 shows the bootstrap results plotted on a ‘cost-effectiveness acceptability curve’.34 This shows whether NIPPV would be economically acceptable to a decision maker at any given threshold of the iCER. Notably, even a high willingness-to-pay threshold of $300 000 per survivor without BPD the probability that NIPPV would be economically desirable is only 23.5%. The $300 000 willingness-to-pay threshold is felt to be high, and in line with other studies, given the majority of these infants will be asymptomatic at 1 year of age.14
Figure 2.
Figure 2 displays the cost-effectiveness acceptability curve. The x axis shows the willingness-to pay in US dollars, whereas the y axis shows the probability that the iCER lies within the threshold.
We also completed deterministic sensitivity analysis in which we varied the costs from 50 to 400% for each of the cost variables: hospital per diem, physician per diem and medication and procedure costs (Table 4). For each scenario, the iCER was recalculated and found to be similar despite the variation in costs.
Table 4.
Sensitivity analyses (% of baseline)
| NIPPV | nCPAP | % Dominated | % Dominant | |
|---|---|---|---|---|
| Hospital | ||||
| 50% | $79 994 (44 419) | $78 364 (46 656) | 58% | 10% |
| 200% | $271 255 (130 933) | $264 483 (128 623) | 63% | 8% |
| 400% | $526 269 (247 907) | $512 642 (241 217) | 63% | 7% |
| Physician | ||||
| 50% | $137 589 (67 911) | $134 620 (68 441) | 62% | 9% |
| 200% | $155 525 (83 280) | $151 971 (83 236) | 60% | 9% |
| 400% | $179 079 (105 425) | $175 104 (104 576) | 58% | 9% |
| Medication costs | ||||
| 50% | $142 997 | $139 419 | 62% | 8% |
| 200% | $145 248 | $142 373 | 58% | 10% |
| 400% | $148 249 | $146 313 | 52% | 12% |
| Procedure costs | ||||
| 50% | $142 266 | $139 010 | 61% | 9% |
| 200% | $146 709 | $143 192 | 62% | 8% |
| 400% | $152 633 | $148 768 | 62% | 8% |
Abbreviations: nCPAP, nasal continuous positive pressure; NIPPV, nasal intermittent positive pressure ventilation.
Because most of the patients were from the United States or Canada, a post hoc subgroup analysis using patients and their appropriate costs from only the United States and Canada was completed (n = 723). The results again were similar although overall the mean costs were higher, at $162 603 in the NIPPV arm and $159 335 in the nCPAP arm. The iCER plot also remained similar, with 60.5% of replications falling in the (dominated) upper left quadrant, and 10.7% of the replications in the (dominant) lower right quadrant.
Finally, to obtain a societal perspective, parent costs were included in a subgroup analysis for patients from countries in which >50% of families participated in the parental survey. This included the United States and Canada (n = 413). Overall parent costs were comparable between the two arms of the study and did not change the results seen in the primary analysis. The parent costs in the NIPPV arm were $3610 while in the nCPAP arm were $3577. In addition, the iCER plot again showed the majority (54%) of the replications in the dominated upper left quadrant.
DISCUSSION
For this economic evaluation of two interventions with similar clinical efficacy, we used patient-level data from a multi-center, international, randomized controlled trial. Even at willingness-to-pay thresholds well above those typical for interventions in this field, the probability is very low that NIPPV, as used in this clinical trial, is economically desirable. We found neither a clinical nor an economic rationale for adoption in this population.
Exploratory analysis of resource drivers suggests a trend of increased time on non-invasive support (NIPPV and nCPAP) in the NIPPV arm, and similarly a trend toward increased overall length of stay. Although these trends may not affect outcome clinically, they do appear to be the source of higher costs in the NIPPV group. Because nCPAP and NIPPV were recorded similarly, we were unable to determine if the increase in days on non-invasive support was due to transition from NIPPV to nCPAP prior to discontinuation of non-invasive support or a longer need for non-invasive support.
When the societal perspective was incorporated, the cost differences and distribution of the iCER plot remained the same. Most of these parental costs came from time missed from work. To our knowledge this is the first prospective economic evaluation ancillary to a neonatology randomized controlled trial to include measured out of pocket expenses to families of preterm infants. Although there was no impact on the cost difference between trial arms, the absolute family expenses were substantial. This suggests that they might be relevant in another intervention and should be routinely included in economic evaluations ancillary to randomized controlled trials.
One limitation in our study is the inability to distinguish between the daily hospital personnel and ancillary cost to provide respiratory support using NIPPV vs nCPAP, or the daily hospital cost of different types of delivery devices for the same type of support. Current billing schemes do not differentiate between these non-invasive strategies. Of note, although the capital cost may differ between the devices themselves, this cost when amortized over many patients is trivial and was therefore not included. The true cost difference in care of infants on differing levels of support would ideally be studied by a time-motion study, but to date no such literature exists.
Because unit prices for all aspects of neonatal care were unavailable in some countries (corresponding to 35% of enrolled patients), we applied unit costs from one of three fully-costed countries (United States, United Kingdom or Canada) based on the similarity of their healthcare expenditure as a percent of gross domestic product. Importantly, these unit costs were applied to measured resource tallies at the patient-level. Moreover, our study conclusions were unchanged when conducted only for the United States and Canada in the subgroup analysis, suggesting that validity was unlikely to have been adversely affected by this imputation.
CONCLUSION
The majority of formal evaluations of other therapies in the NICU, including caffeine, higher transfusion thresholds, inhaled nitric oxide and earlier treatment for retinopathy of prematurity, have shown economic favorability.14,18,20,35 Formal economic evaluations showing a common NICU therapy to be economically unfavorable or economically equivalent are uncommon. It is unclear whether this is due to negative publication bias or to a hesitation to proceed with retrospective economic analysis when the clinical trial reports a negative outcome. This is arguably a missed opportunity. Decisions regarding adoption of new therapies are typically made based on clinical efficacy, although recent efforts across many specialties, highlighted in the American Board of Internal Medicine Foundation’s Choosing Wisely Campaign, have demonstrated that both effectiveness and economic desirability are now essential factors in such policy decisions.36,37 In this context, the current study calls into question NIPPV as a non-invasive strategy when compared with nCPAP. More generally, our findings support the concept that clinical outcomes are not always in line with resource and economic implications; we suggest that routine concurrent economic evaluation should be considered alongside all significant randomized controlled trials.
ACKNOWLEDGEMENTS
Economic evaluation supported in part by UF CTSI Advanced Postgraduate Program in Clinical Investigation (NIH & NCRR CTSA grant UL1 TR000064); NIPPV trial supported by Canadian Institutes of Health Research (MCT-80246).
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- 1.Horbar JD, Carpenter JH, Badger GJ, Kenny MJ, Soll RF, Morrow KA et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics 2012; 129(6): 1019–1026. [DOI] [PubMed] [Google Scholar]
- 2.Fischer HS, Buhrer C. Avoiding endotracheal ventilation to prevent bronchopulmonary dysplasia: a meta-analysis. Pediatrics 2013; 132(5): e1351–e1360. [DOI] [PubMed] [Google Scholar]
- 3.Latini G, De Felice HC, Giannuzzi R, Del Vecchio A. Survival rate and prevalence of bronchopulmonary dysplasia in extremely low birth weight infants. Early Hum Dev 2013; 89(Suppl 1): S69–S73. [DOI] [PubMed] [Google Scholar]
- 4.Dunn MS, Kaempf J, de Klerk A, de Klerk R, Reilly M, Howard D et al. Randomized trial comparing 3 approaches to the initial respiratory management of preterm neonates. Pediatrics 2011; 128(5): e1069–e1076. [DOI] [PubMed] [Google Scholar]
- 5.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med 2010; 362(21): 1970–1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morley CJ, Davis PG, Doyle LW, Brion LP, Hascoet JM, Carlin JB. Nasal CPAP or intubation at birth for very preterm infants. N Engl J Med 2008; 358(7): 700–708. [DOI] [PubMed] [Google Scholar]
- 7.Kirpalani H, Millar D, Lemyre B, Yoder BA, Chiu A, Roberts RS. A trial comparing non-invasive ventilation strategies in preterm infants. N Engl J Med 2013; 369(7): 611–620. [DOI] [PubMed] [Google Scholar]
- 8.Bank TW. Health expediture, total (% of GDP). (cited 17 February 2014) Available from http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS.
- 9.Statistics Canada. Consumer Price Index, health and personal care. 2014. (cited 17 February 2014) Available from http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/econ161a-eng.htm.
- 10.UK Statistics Authority. Consumer Price Index for UK. (cited 17 February 2014) Available from http://www.statisticsauthority.gov.uk.
- 11.United States Department of Labor. Consumer Price Index for the United States. 2014. (cited 17 February 2014) Available from http://www.bls.gov/cpi.
- 12.Organisation for Economic Cooperation and Development (OECD). PPPs and Exchange Rates. [cited November 24, 2014]Available from http://stats.oecd.org/index.aspx?DataSetCode=SNA_Table4.
- 13.Lee S, Anderson LBC. Perinatal Services Costing Project: Report on Costs in the Neonatal Intensive Care Unit. Vancouver: BC, Canada, 2004. [Google Scholar]
- 14.Zupancic JA, Hibbs AM, Palermo L, Truog WE, Cnaan A, Black DM et al. Economic evaluation of inhaled nitric oxide in preterm infants undergoing mechanical ventilation. Pediatrics 2009; 124(5): 1325–1332. [DOI] [PubMed] [Google Scholar]
- 15.National Health Services. NHS Reference Costs Collection Guidance for 2013–2014. (cited 23 April 2014) Available from https://www.gov.uk/government/publications/nhs-reference-costs-collection-guidance-for-2013-to-2014.
- 16.Centers for Medicare & Medicaid Services. Physician Fee Schedule Look-Up Tool. (cited 17 February 2014) Available from http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PFSlookup/index.html.
- 17.Ontario Ministry of Health and Long-Term Care. Ontario Health Insurance Schedule of Benefits and Fees. 2013 (cited 17 February 2014) Available from http://www.health.gov.on.ca/english/providers/program/ohip/sob/sob_mn.html.
- 18.Dukhovny D, Lorch SA, Schmidt B, Doyle LW, Kok JH, Roberts RS et al. Economic evaluation of caffeine for apnea of prematurity. Pediatrics 2011; 127(1): e146–e155. [DOI] [PubMed] [Google Scholar]
- 19.Dukhovny D, Dennis CL, Hodnett E, Weston J, Stewart DE, Mao W et al. Prospective economic evaluation of a peer support intervention for prevention of postpartum depression among high-risk women in Ontario, Canada. Am J Perinatol 2013; 30(8): 631–642. [DOI] [PubMed] [Google Scholar]
- 20.Kamholz KL, Dukhovny D, Kirpalani H, Whyte RK, Roberts RS, Wang N et al. Economic evaluation alongside the Premature Infants in Need of Transfusion randomised controlled trial. Arch Dis Childh Fetal Neonatal Ed 2012; 97(2): F93–F98. [DOI] [PubMed] [Google Scholar]
- 21.Tretiak R, Laupacis A, Riviere M, McKerracher K, Souetre E. Cost of allogeneic and autologous blood transfusion in Canada. Canadian Cost of Transfusion Study Group. CMAJ 1996; 154(10): 1501–1508. [PMC free article] [PubMed] [Google Scholar]
- 22.Agency CR. Automobile Allowance Rates. (cited 2/4/15) Available from http://www.cra-arc.gc.ca/tx/bsnss/tpcs/pyrll/bnfts/tmbl/llwnc/rts-eng.html.
- 23.Canada EaSD. Work - Weekly Hours Worked. (cited 2/4/15) Available from http://www4.hrsdc.gc.ca/3ndic.1t.4r@-eng.jsp?iid=9-M_2.
- 24.Canada S. Average hourly wages of empluees by selected characteristics and occupation. (cited 2/4/15) Available from http://www.statcan.gc.ca/tables-tableaux/sum-som/l01/cst01/labr69a-eng.htm.
- 25.Canada S. National Occupational Classification Statistics. (cited 2/4/15) Available from http://stds.statcan.gc.ca/soc-cnp/2006/cs-rc-eng.asp?cretaria=G811.
- 26.Service IR. Standard Mileage Rates for 2013. 2012. (cited 2/5/14) Available from http://www.irs.gov/uac/Newsroom/2013-Standard-Mileage-Rates-Up-1-Cent-per-Mile-for-Business,-Medical-and-Moving.
- 27.United States Departemnt of Labor BoLS. Occupational Employment Statistics. 2013. (cited 2/4/15) Available from http://www.bls.gov/oes/current/oes372012.htm.
- 28.United States Departemnt of Labor BoLS. American Time Use Survey 2013 Results. 2014. (cited 2/4/15) Available from http://www.bls.gov/news.release/atus.nr0.htm.
- 29.United States Department of Labor BoLS. Household Data Annual Averages. 2013. (cited 2/4/15) Available from http://www.bls.gov/cps/cpsaat39.pdf.
- 30.Glick H, Doshi J, Sonnad S, Polsky D. Economic Evaluation in Clinical Trials. 2nd edn (. Oxford Press: New York, NY, USA, 2014. [Google Scholar]
- 31.Efron B, Tibshirani R. An Introduction to the Booststrap. Chapman and Hall: Boca Raton, FL,1993. [Google Scholar]
- 32.O’Brien BJ, Drummond MF, Labelle RJ, Willan A. In search of power and significance: issues in the design and analysis of stochastic cost-effectiveness studies in health care. Med Care 1994; 32(2): 150–163. [DOI] [PubMed] [Google Scholar]
- 33.Ramsey S, Willke R, Briggs A, Brown R, Buxton M, Chawla A et al. Good research practices for cost-effectiveness analysis alongside clinical trials: the ISPOR RCT-CEA Task Force report. Value Health 2005; 8(5): 521–533. [DOI] [PubMed] [Google Scholar]
- 34.Fenwick E, O’Brien BJ, Briggs A. Cost-effectiveness acceptability curves--facts, fallacies and frequently asked questions. Health Economics 2004; 13(5): 405–415. [DOI] [PubMed] [Google Scholar]
- 35.Kamholz KL, Cole CH, Gray JE, Zupancic JA. Cost-effectiveness of early treatment for retinopathy of prematurity. Pediatrics 2009; 123(1): 262–269. [DOI] [PubMed] [Google Scholar]
- 36.Ho T, Dukhovny D, Zupancic JA, Goldmann DA, Horbar JD, Pursley DM. Choosing Wisely in Newborn Medicine: five Opportunities to Increase Value. Pediatrics 2015; 136(2): e482–e489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing wisely--the politics and economics of labeling low-value services. N Engl J Med 2014; 370(7): 589–592. [DOI] [PMC free article] [PubMed] [Google Scholar]