Abstract
Objective:
Endocan, chemerin, and galectin-3 are discrete biomarkers associated with cardiovascular diseases and acting through different pathophysiological pathways. The aim of this study is to investigate and compare the effects of high doses of atorvastatin and rosuvastatin on serum endocan, chemerin, and galectin-3 levels in patients with acute myocardial infarction (AMI).
Methods:
Sixty-three patients with AMI were randomized to receive atorvastatin (80 mg/day) or rosuvastatin (40 mg/day) after percutaneous revascularization. Serum levels of endocan, chemerin, and galectin-3 were evaluated at baseline and after 4-week therapy.
Results:
Endocan levels were not decreased statistically significantly with atorvastatin 80 mg, but rosuvastatin 40 mg markedly decreased the levels of endocan according to baseline [from 110.27 (86.03–143.69) pg/mL to 99.22 (78.30–122.87) pg/mL with atorvastatin 80 mg and from 110.73 (77.28–165.22) pg/mL to 93.40 (70.48–115.13) pg/mL with rosuvastatin 40 mg, p=0.242 for atorvastatin 80 mg and p=0.014 for rosuvastatin 40 mg]. Chemerin levels significantly decreased in both groups according to baseline [from 264.90 (196.00–525.95) ng/mL to 135.00 (105.95–225.65) ng/mL with atorvastatin 80 mg and from 309.95 (168.87–701.27) ng/mL to 121.25 (86.60–212.65) ng/mL with rosuvastatin 40 mg, p<0.001, respectively, for both groups]. Galectin-3 levels did not change markedly with atorvastatin 80 mg, but they decreased with rosuvastatin 40 mg [from 17.00 (13.10–22.25) ng/mL to 19.30 (15.25–23.45) ng/mL with atorvastatin 80 mg, p=0.721, and from 18.25 (12.82–23.82) ng/mL to 16.60 (10.60–20.15) ng/mL with rosuvastatin 40 mg, p=0.074]. There were no significant between-group differences in terms of absolute and percentage changes of endocan, chemerin, and galectin-3 at 4 weeks.
Conclusion:
We reported that both statins similarly decreased the endocan levels, whereas rosuvastatin seems to have more prominent effects on the reduction of the chemerin and galectin-3 levels in patients with AMI.
Keywords: atorvastatin, chemerin, endocan, galectin-3, myocardial infarction, rosuvastatin
Introduction
Myocardial infarction remains the leading cause of morbidity and mortality worldwide. In 2015, more than 8.75 million people lost their lives due to coronary artery disease (CAD), accounting for 15.5% of all deaths (1). Endothelial dysfunction, inflammation, and disruption-rupture of atherosclerotic plaques play a critical role in the pathogenesis of atherosclerosis and acute myocardial infarction (AMI) (2-5).
Endothelial specific molecule-1 (ESM-1) or endocan, is a soluble dermatan sulfate proteoglycan, secreted and expressed by human vascular endothelial cells (6). The elevated levels of the endocan in patients with tumor progression or in patients with sepsis suggest that endocan may be a probable biomarker for endothelial dysfunction or endothelial activation (7, 8). A previous study showed that endocan levels have been significantly increased in patients with acute coronary syndrome (ACS) (9). In another study, admission endocan levels were found to be associated with in-hospital mortality and the CAD severity index in patients with ST segment elevation myocardial infarction (STEMI) (10).
Adipose tissue is an active endocrine organ and regulates energy homeostasis and metabolism by communicating with liver, skeletal muscle, and brain via secreted soluble protein hormones (also called as adipokines) (11, 12). Chemerin is a novel adipokine that regulates adipogenesis and adipocyte metabolism (13). Chemerin has been shown to be associated with obesity, metabolic syndrome, hypertension, CAD, CAD severity, and ACS (12, 14-17).
Galectin-3 is a member of soluble ß-galactoside-binding lectins, encoded on a single gene, found on chromosome 14, LGALS3 (lectin, galactose-binding soluble 3), secreted by macrophages, monocytes, and epithelial cells, and it has regulatory effects on inflammation, fibrogenesis, immunity, tissue repair, and cell proliferation (18-20). Elevated levels of soluble galectin-3 have been shown to be associated with increased risk of mortality, cardiovascular mortality, and heart failure (21).
In AMI, high-dose potent statin therapy is associated with reduced morbidity and mortality, and current guidelines recommend high-dose potent statin therapy in patients with AMI (22). To the best of our knowledge, there are no data in literature evaluating and comparing the effects of high-dose statins, atorvastatin 80 mg and rosuvastatin 40 mg, on the levels of endocan, chemerin, and galectin-3. In this study, we aimed to compare the effects of atorvastatin 80 mg and rosuvastatin 40 mg on the lipid profiles and the levels of endocan, chemerin, and galectin-3 in patients with AMI.
Methods
Patient population
We designed and conducted a study to investigate and compare the effects of atorvastatin 80 mg and rosuvastatin 40 mg on the lipid profiles and the levels of endocan, chemerin, and galectin-3 in patients with AMI who underwent revascularization as a substudy of a previous article investigating the effects of atorvastatin 80 mg and rosuvastatin 40 mg on the plasma PCSK-9 levels in patients with AMI who underwent revascularization (23). A total of 106 patients hospitalized in the coronary intensive care unit of Selçuk University Faculty of Medicine Department of Cardiology between January 2015 and December 2016 with STEMI and Non-ST-elevation myocardial infarction (NSTEMI) and eligible for our study were enrolled (23). All patients provided written informed consent. Protocol of this study was approved by the Local Institutional Ethics Committee. In the protocol of this study, the measurements of endocan, chemerin, and galectin-3 were not pre-specified. However, it was pre-specified to include measurements of newer markers of inflammation, endothelial dysfunction, and atherosclerosis that were not well known when the protocol was finished. On this basis, we initiated the present substudy of our main article that compares the effects of atorvastatin 80 mg and rosuvastatin 40 mg on the levels of plasma PCSK-9. The present substudy comprised 63 consecutively included patients to examine and compare the effects of atorvastatin 80 mg and rosuvastatin 40 mg on plasma levels of endocan, chemerin, and galectin-3. Of the 106 patients enrolled in this study, 63 (59%) subjects had a baseline plasma specimen available for the measurement of endocan, chemerin, and galectin-3.
STEMI is a clinical syndrome defined by typical symptoms of myocardial ischemia lasting at least 30 minutes or more with persistent electrocardiographic ST elevation and subsequent release of myocardial necrosis biomarkers. The ST elevation was defined as a new ST elevation at the J point in at least 2 contiguous leads ≥2 mm (0.2 mV) in men or ≥1.5 mm (0.15 mV) in women in leads V2–V3 and/or ≥1 mm (0.1 mV) in other contiguous chest leads or the limb leads (24). NSTEMI was defined according to the current guideline for the management of patients with non-ST-elevation ACS (25).
Eligibility criteria were as follows: age >18 years and low-density lipoprotein-cholesterol (LDL-C) >100 mg/dl, and myocardial infarction prior 12 h. Patients with cardiogenic shock; serum creatinine >2.5 mg per deciliter; current statin, fibrate, or other antilipid drug users; body mass index (BMI) >30; chronic muscle disease; contraindication to statin therapy or an unexplained creatine kinase elevation to 2.5-fold to upper normal limits; active infection or sepsis; blood transfusion within 3 months; chronic inflammatory and rheumatic diseases; malignancy; and the presence of obstructive hepatobiliary disease and cirrhosis were excluded from the study.
After revascularization therapy, patients were randomly assigned to receive atorvastatin (80 mg/day) or rosuvastatin (40 mg/day). In addition to statin therapy, acetyl salicylic acid, clopidogrel, or ticagrelor/prasugrel were prescribed in all patients. Same lifestyle changes and exercise recommendations were given for all patients before discharge.
Fasting blood samples were taken before the randomization within 24 hours and at the end of the 4-week period of the therapy by a cubital venipuncture avoiding venous stasis to an evacuated serum separator tube. The samples were centrifuged at 1500× g for 15 minutes within 1 hour after collection. After centrifugation, serum samples were transferred to Eppendorf tubes and stored at −80 °C until the assay. The levels of total cholesterol (TC), trygliyceride (TG), and high-density lipoprotein cholesterol (HDL-C) were measured by chemistry autoanalyzer (ARCHITECT c16000, Abbott Diagnostics, USA) via enzymatic colorimetric methods. Levels of LDL-C were calculated by using the Friedewald formula. Oxidized low-density lipoprotein (Oxidized-LDL) (BIOMEDICA, Cat. No: BI-20022), Chemerin (BioVendor, Cat. No: RD191136200R), and ESM1/Endocan PicoKine (MyBiosource, Cat. No: MBS177114) were determined with the enzyme-linked immunosorbent assay technique. Galectin-3 serum concentrations were measured with a chemiluminescent microparticle immunoassay on an ARCHITECT i1000SR auto analyzer (Abbott Diagnostics).
Statistical analysis
Statistical analyses were performed using the SPSS for Mac version 20.0 (SPSS Inc., Chicago, Illinois, USA). Distribution of continuous variables was tested using the Kolmogorov–Smirnov test. Continuous variables with normal distribution were compared using Student’s t-test, and those without normal distribution were compared using the Mann–Whitney’s U test. The chi-squared test was used for comparing categorical variables. Continuous variables were defined as means±standard deviation or median (interquartile range), and categorical variables were given as percentages. Baseline characteristics, as well as post-treatment changes, were compared within groups by using a paired-sample t-test or Wilcoxon signed-ranks test. Also, baseline characteristics, as well as post-treatment changes, were compared between groups by using repeated measures analysis of variance (ANOVA). Because of changes between the baseline and post-treatment in nonparametric variables cannot be studied between groups using the Wilcoxon signed-ranks test, nonparametric variables were analyzed by repeated measures ANOVA after log10 transformation. A p-value <0.05 was considered to be statistically significant for all tests.
Results
The baseline clinical characteristics of the groups are presented in Table 1, and there were no significant differences between the two groups except TC, TG, and blood urea nitrogen (BUN) levels. The groups were also comparable regarding baseline lipid profiles, oxidized-LDL, and hematological parameters. There were no significant differences between the two groups among the baseline levels of endocan, chemerin, and galectin-3 (Table 1). Types of stents (bare metal stent or drug eluting stent) and the number of revascularized vessels were also comparable among the two treatment groups (Table 1).
Table 1.
Comparison of baseline clinical characteristics and laboratory parameters of the patients
| Variable | Atorvastatin | Rosuvastatin n=33 | P-value n=30 |
|---|---|---|---|
| Age, years | 57.67±9.35 | 58.30±11.98 | 0.815 |
| Male gender, n (%) | 29 (87.9) | 26 (86.7) | 0.885 |
| Body mass index (kg/m2) | 26.33±2.06 | 25.87±1.24 | 0.296 |
| Hypertension, n (%) | 9 (27.3) | 7 (23.3) | 0.720 |
| Diabetes mellitus, n (%) | 4 (12.1) | 7 (23.3) | 0.242 |
| Smoking, n (%) | 11 (33.3) | 8 (26.7) | 0.565 |
| STEMI, n (%) | 13 (39.4) | 16 (53.3) | 0.268 |
| LVEF, % | 48.9±9.5 | 44.4±9.0 | 0.060 |
| SBP, mm Hg | 119.70 ± 9.84 | 118.00±15.40 | 0.609 |
| DBP, mm Hg | 74.55±6.66 | 71.00±15.40 | 0.227 |
| Total cholesterol, mg/dL | 181.64±35.42 | 206.33±36.00 | 0.008 |
| LDL-C, mg/dL | 120.08±27.67 | 131.69±24.61 | 0.085 |
| HDL-C, mg/dL | 36.33±9.76 | 37.60±10.72 | 0.625 |
| Triglyceride, mg/dL* | 116.00 (87.00–182.00) | 154.50 (125.75–215.75) | 0.025 |
| TC/ HDL-C | 5.26±1.56 | 5.80±1.55 | 0.174 |
| Oxidized-LDL, ng/mL | 870.39±239.35 | 862.20±331.87 | 0.910 |
| ALT, IU/L | 27.21±14.51 | 27.30±14.14 | 0.981 |
| AST, IU/L | 33.85±15.80 | 30.57±13.84 | 0.386 |
| CK, IU/L | 118.97±23.82 | 114.77±28.47 | 0.526 |
| Endocan, pg/mL* | 110.27 (86.03–143.69) | 110.73 (77.28–165.22) | 0.934 |
| Chemerin, ng/mL* | 264.90 (196.00–525.95) | 309.95 (168.87–701.27) | 0.804 |
| Galectin-3, ng/mL* | 17.10 (13.10–22.25) | 18.25 (12.82–23.82) | 0.778 |
| Hb, g/dL | 14.41±1.60 | 14.21±1.45 | 0.620 |
| WBC, 103/µL | 10.24±2.62 | 10.59±3.09 | 0.634 |
| Platelet, 103/µL | 245.45±90.61 | 240.27±55.36 | 0.787 |
| Creatinine, mg/dL | 0.84 ±0.16 | 0.83±0.14 | 0.857 |
| BUN, mg/dL | 30.00±8.98 | 35.54±10.18 | 0.025 |
| Na+, mEq/L | 138.79±2.63 | 139.23±1.87 | 0.446 |
| K+, mEq/L | 4.23±0.40 | 4.19±0.39 | 0.660 |
| Coronary intervention | |||
| Drug eluting stent, n (%) | 13 (39.4) | 17 (56.7) | 0.095 |
| Bare metal stents, n (%) | 16 (48.5) | 13 (43.3) | |
| Medical therapy, n (%) | 4 (12.1) | 0 | |
| Number of revascularized vessels | |||
| 0, n (%) | 4 (12.1) | 0 (0) | 0.147 |
| 1, n (%) | 25 (75.8) | 25 (83.3) | |
| 2, n (%) | 3 (9.1) | 5 (16.7) | |
| 3, n (%) | 1 (0.5) | 0 (0) | |
Data given as mean±standard deviation or number (%)
Variables not showing normal distribution given as median (interquartile range)
ALT - alanine aminotransferase; AST - aspartate aminotransferase; BUN - blood urea nitrogen; CK - creatine kinase; DBP - diastolic blood pressure; HDL-C - high-density lipoprotein cholesterol; Hb - hemoglobin; LDL-C - low-density lipoprotein cholesterol; LVEF - left ventricular ejection fraction; TC - total cholesterol; Oxidized-LDL - oxidized low-density lipoprotein cholesterol; SBP - systolic blood pressure; STEMI - ST segment elevation myocardial infarction; WBC - white blood cells
At the end of 1-month therapy, the alanine aminotransferase (ALT), aspartate aminotransferase (AST), and creatin kinase (CK) levels of one patient in the atorvastatin 80 group, increased to three-fold to upper normal limits. Statin treatment of this patient was discontinued for 2 weeks, and after the normalization of liver enzymes and CK levels, statin treatment was continued again with atorvastatin 10 mg.
Lipid parameters
The value of the serum levels of TC (from 181.64±35.42 mg/dL to 138.36±34.08 mg/dL in the atorvastatin group, p<0.001, and from 206.33±36.00 mg/dL to 143.27±39.95 mg/dL in the rosuvastatin group, p<0.001, respectively), LDL-C (from 120.08±27.68 mg/dL to 72.22±25.09 mg/dL in the atorvastatin group, p<0.001, and from 131.69±24.61 mg/dL to 69.06±26.62 mg/dL in the rosuvastatin group, p<0.001, respectively) were significantly reduced within atorvastatin and rosuvastatin groups (Table 2). TG levels decreased within both groups, but this decrease was not statistically significant [from 116.00 (87.00–182.00) mg/dL to 110.00 (89.00–154.00) mg/dL in the atorvastatin group; p=0.532, and from 154.50 (125.75–215.75) mg/dL to 135.00 (91.00–182.50) mg/dL in the rosuvastatin group, p=0.052, respectively] (Table 2). HDL-C levels were slightly elevated in both groups, but this elevation was not statistically significant. Oxidized-LDL levels showed a significant reduction in both groups (p<0.001). The ratio of TC/HDL-C also showed remarkable reduction in both groups (p<0.001), (Table 2).
Table 2.
Effects of atorvastatin 80 mg and rosuvastatin 40 mg on laboratory parameters after 4-week treatment
| Atorvastatin 80 mg, n=33 | Rosuvastatin 40 mg, n=30 | |||||
|---|---|---|---|---|---|---|
| Baseline | 4th week of the therapy | P-value | Baseline | 4th week of the therapy | P-value | |
| TC, mg/dL | 181.64±35.42 | 138.36±34.08 | <0.001 | 206.33±36.00 | 143.27±39.95 | <0.001 |
| LDL-C, mg/dL | 120.08±27.68 | 72.22±25.09 | <0.001 | 131.69±24.61 | 69.06±26.62 | <0.001 |
| HDL-C, mg/dL | 36.33±9.76 | 36.73±9.62 | 0.665 | 37.60±10.72 | 38.84±10.14 | 0.323 |
| TC/ HDL-C | 5.26±1.56 | 3.88±0.95 | <0.001 | 5.80±1.55 | 3.81±1.04 | <0.001 |
| Triglyceride, mg/dL | 116.00 (87.00–182.00) | 110.00 (89.00–154.00) | 0.532 | 154.50 (125.75–215.75) | 135.00 (91.00–182.50) | 0.052 |
| Oxidized-LDL, ng/mL | 870.39±239.35 | 742.61±189.83 | <0.001 | 862.20±331.87 | 703.87±186.00 | <0.001 |
| ALT, IU/L | 27.21±14.51 | 25.70±11.15 | 0.461 | 27.30±14.14 | 28.67±19.87 | 0.710 |
| AST, IU/L | 33.85±15.80 | 30.18±12.24 | 0.118 | 30.57±13.84 | 30.20±21.31 | 0.925 |
| CK, IU/L | 118.97±23.82 | 121.24±38.42 | 0.759 | 114.77±28.47 | 122.63±57.47 | 0.483 |
| Endocan, pg/mL | 110.27 (86.03–143.69) | 99.22 (78.30–122.87) | 0.242 | 110.73 (77.28–165.22) | 93.40 (70.48–115.13) | 0.014 |
| Chemerin, ng/mL | 264.90 (196.00–525.95) | 135.00 (105.95–225.65) | <0.001 | 309.95 (168.87–701.27) | 121.25 (86.60–212.65) | <0.001 |
| Galectin-3, ng/mL | 17.10 (13.10–22.25) | 19.30 (15.25–23.45) | 0.721 | 18.25 (12.82–23.82) | 16.60 (10.60–20.15) | 0.074 |
| WBC, 103/µL | 10.24±2.62 | 8.1.±1.83 | <0.001 | 10.59±3.09 | 7.71±1.46 | <0.001 |
ALT - alanine aminotransferase; AST - aspartate aminotransferase; CK - creatine kinase; HDL-C - high-density lipoprotein cholesterol; LDL-C - low-density lipoprotein cholesterol;
TC - total cholesterol; Oxidized-LDL - oxidized low-density lipoprotein cholesterol; WBC - white blood cells
There were no statistically significant differences between both treatment arms among the results of lipid parameters at the end of 1- month therapy except LDL-C levels. Rosuvastatin 40 mg was more effective than the atorvastatin 80 mg to reduce the LDL-C levels at the end of 1-month therapy (p=0.039, Table 2).
The absolute and percentage changes of lipid parameters after 4-week therapy in both groups are listed in Table 3. Rosuvastatin 40 mg/day provided a statistically significant reduction in the absolute change of LDL-C levels (48 mg/dL vs. 63 mg/dL, p=0.039). On the other hand, when the decrease in LDL-C levels was examined in terms of percentage change, there was no statistically significant difference between the two groups (39% vs. 47%, p=0.091). Absolute and percentage changes of TG, HDL-C, and Oxidized-LDL levels were similar in both groups, and there was no statistically significant difference between groups after 4-week therapy (Table 3).
Table 3.
Comparison of atorvastatin and rosuvastatin by means of absolute and percentage change of laboratory parameters
| Absolute change | Percent change, % | |||||
|---|---|---|---|---|---|---|
| Atorvastatin | Rosuvastatin | P-value | Atorvastatin | Rosuvastatin | P-value | |
| TC, mg/dL | -43±41 | -63±40 | 0.058 | -22±22 | -30±17 | 0.101 |
| LDL-C, mg/dL | -48±26 | -63±29 | 0.039 | -39±20 | -47±20 | 0.091 |
| HDL-C, mg/dL | 0.4±5.2 | 1.2±6.8 | 0.577 | 2.3±14.8 | 5.3±18.9 | 0.470 |
| TC/HDL-C | -1.37±1.21 | -1.99±1.27 | 0.054 | -23.1±18.6 | -32.6±16.5 | 0.038 |
| Triglyceride, mg/dL | 2 (-63.50–23.00) | -23.50 (-56.0 – -12.0) | 0.378 | 1.81 (-35.11–29.19) | -15.05 (-31.68–7.04) | 0.335 |
| Oxidized-LDL, ng/mL | -128±184 | -158±223 | 0.554 | -12.5±15.9 | -14.6±16.2 | 0.607 |
| Endocan, pg/mL | -9.73 (-55.84–18.91) | -28.91 (-57.18 – 12.63) | 0.349 | -7.96 (-43.75 – 27.64) | -26.61 (-46.56–15.65) | 0.349 |
| Chemerin, ng/mL | -134.10 (-323.90–-47.70) | -148.30 (-369.20–-34.40) | 0.815 | -42.79 (-64.72 – -26.68) | -56.08 (-72.06–-23.49) | 0.650 |
| Galectin-3, ng/mL | 0.10 (-4.80–4.25) | -2.25 (-7.97 – 1.25) | 0.141 | 0.88 (-19.96 – 28.57) | -16.23 (-30.70–9.13) | 0.071 |
| WBC, 103/µL | -2.1±2.6 | -2.9±2.6 | 0.255 | -17.3±22.2 | -23.2±19.9 | 0.275 |
HDL-C - high-density lipoprotein cholesterol; LDL-C - low-density lipoprotein cholesterol; Oxidized-LDL - oxidized low-density lipoprotein cholesterol; TC - total cholesterol;
WBC - white blood cells
Endocan, chemerin, and galectin-3
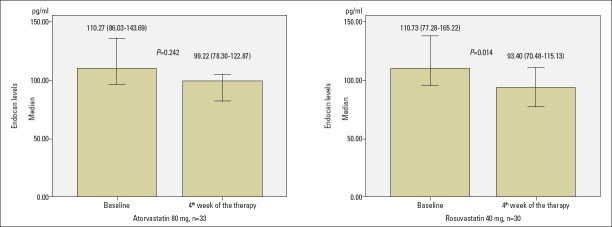
Endocan levels were not decreased statistically significantly with atorvastatin 80 mg, but rosuvastatin 40 mg markedly decreased the levels of endocan according to baseline [from 110.27 (86.03–143.69) pg/mL to 99.22 (78.30–122.87) pg/mL with atorvastatin 80 mg and from 110.73 (77.28–165.22) pg/mL to 93.40 (70.48–115.13) pg/mL with rosuvastatin 40 mg, p=0.242 for atorvastatin 80 mg and p=0.014 for rosuvastatin 40 mg, as shown in Table 2 and Fig. 1]. Absolute change of endocan was −9.73 (−55.84–18.91) pg/mL with atorvastatin 80 mg and −28.91 (−57.18–12.63) pg/mL with rosuvastatin 40 mg, and there was no statistically significant difference between groups (p=0.349, Table 3). The percentage change of endocan was −7.96 (−43.75–27.64) % with atorvastatin 80 mg and −26.61 (−46.56–15.65) % with rosuvastatin 40 mg, and there was no statistically significant difference between groups (p=0.349, Table 3).
Figure 1.
Change of endocan levels with atorvastatin 80 mg and rosuvastatin 40 mg at end of 4-week therapy
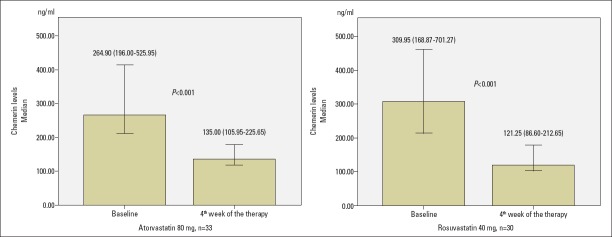
Chemerin levels significantly decreased in both groups according to baseline [from 264.90 (196.00–525.95) ng/mL to 135.00 (105.95–225.65) ng/mL with atorvastatin 80 mg and from 309.95 (168.87–701.27) ng/mL to 121.25 (86.60–212.65) ng/mL with rosuvastatin 40 mg, p<0.001, respectively, for both groups, Table 2 and Fig. 2]. However, when both groups were compared in terms of chemerin decrease according to baseline, there was no statistically significant difference between groups at the end of 4-week therapy. The absolute change of chemerin was −134.10 (−323.90–−47.70) ng/mL with atorvastatin 80 mg and −148.30 (−369.20–−34.40) ng/mL with rosuvastatin 40 mg (p=0.815, Table 3). The percent change of chemerin was −42.79 (−64.72–−26.68) % with atorvastatin 80 mg and −56.08 (−72.06–−23.49) % with rosuvastatin 40 mg (p=0.650, Table 3).
Figure 2.
Change of chemerin levels with atorvastatin 80 mg and rosuvastatin 40 mg at end of 4-week therapy
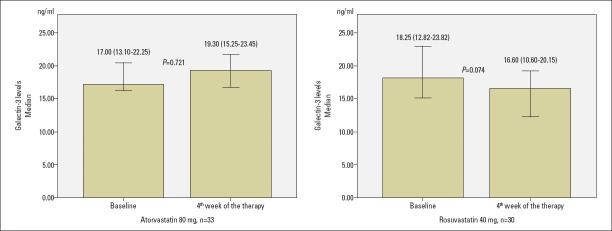
Galectin-3 levels did not changed markedly with atorvastatin 80 mg, but they decreased with rosuvastatin 40 mg [from 17.00 (13.10–22.25) ng/mL to 19.30 (15.25–23.45) ng/mL with atorvastatin 80 mg, p=0.721, and from 18.25 (12.82–23.82) ng/mL to 16.60 (10.60–20.15) ng/mL with rosuvastatin 40 mg, p=0.074, Table 2 and Fig. 3]. Although there was no statistically significant difference between atorvastatin 80 mg and rosuvastatin 40 mg groups in terms of decrease in the galectin-3 levels according to baseline, there was a trend favoring the rosuvastatin arm. The absolute change of galectin-3 was 0.10 (−4.80–4.25) ng/mL with atorvastatin 80 mg and −2.25 (−7.97–1.25) ng/mL with rosuvastatin 40 mg (p=0.141, Table 3). The percentage change of galectin-3 was 0.88 (−19.96–28.57) % with atorvastatin 80 mg and −16.23 (−30.70–9.13) % with rosuvastatin 40 mg (p=0.071, Table 3).
Figure 3.
Change of galectin-3 levels with atorvastatin 80 mg and rosuvastatin 40 mg at end of 4-week therapy
Discussion
Our study showed that high-dose atorvastatin (80 mg/day) and high-dose rosuvastatin (40 mg/day) had similar effects on lipid parameters in patients with AMI, except LDL-C levels. Rosuvastatin 40 mg significantly reduced LDL-C levels when compared with atorvastatin 80 mg. Rosuvastatin 40 mg seems to have more prominent effects on the levels of chemerin and galectin-3, whereas the two high-dose statin regimens have similar effects on the levels of endocan.
Comparison of effects of atorvastatin (80 mg/day) and rosuvastatin (40 mg/day) on lipid parameters
Current dyslipidemia guidelines recommendation is to “initiate or continue high-dose statins early after admission in all ACS patients without contraindication regardless of initial LDL-C values” (22). We know from previous studies that atorvastatin 80 mg and rosuvastatin 40 mg are the most potent statins (26). One of the landmark articles about the effectiveness of high-dose statins is the TNT-Trial (27). The TNT-Trial showed that atorvastatin 80 mg provided a significant clinical benefit beyond that provided by atorvastatin 10 mg (27). On the other hand, in the ASTEROID trial, rosuvastatin 40 mg resulted in significant regression of the atherosclerotic plaque burden (28). For this reason, we chose high-dose atorvastatin and rosuvastatin for our research.
In our study, rosuvastatin 40 mg resulted in further reductions in LDL-C levels when compared with atorvastatin 80 mg. Atorvastatin 80 mg led to a 39% and rosuvastatin 40 mg led to a 47% reduction of LDL-C levels from baseline, respectively, at the end of 4 weeks. This finding is consistent with previous studies. In the LUNAR study, while the atorvastatin 80 mg provided a 42% reduction in LDL-C levels, rosuvastatin 40 mg provided a 46.8% reduction in LDL-C levels at the end of 6 weeks (29).
The effects of atorvastatin (80 mg/day) and rosuvastatin (40 mg/day) therapy on endocan levels
Endothelial dysfunction, vascular inflammation, atherosclerotic plaque formation, and the rupture of these plaques constitute the basis of the pathophysiology of AMI.
Endothelial specific molecule-1 (ESM-1), named endocan (50 kDa), is a soluble dermatan sulfate proteoglycan, secreted and expressed by human vascular endothelial cells and found to be associated with vascular smooth muscle cell proliferation and migration (6, 30). Menon et al. (6) showed the expression of endocan in atherosclerotic plaques of apolipoprotein E null mice, fed with high-fat diet. Immunohistochemical analysis revealed that endocan is highly expressed in these plaques, and the authors hypothesized that endocan may contribute the neointimal formation process during atherosclerosis (6).
Previous studies have shown the association between the endocan levels and CAD, AMI, newly diagnosed hypertension, and coronary ectasia (9, 10, 31, 32). Kundi et al. (10) reported that the admission of high endocan levels is an independent predictor of in-hospital mortality and an increased SYNTAX score in patients with STEMI. Xiong et al. (33) investigated the relationship between endocan levels and the presence and severity of CAD in patients with hypertension, and they found an independent correlation between endocan levels and the presence and severity of CAD.
Previous studies showed that statins have some beneficial effects independent of the LDL reduction, which are called pleiotropic effects (34). A post-hoc analysis of the WOSCOPS study showed improved outcomes with pravastatin independent of the LDL-C reduction, and this was the first research that proposed the pleiotropic effects of statins (35). Statins show its pleiotropic effects via various ways: anti-inflammatory, antioxidant, anti-neovascularization, and healing effects on endothelial functions (36-39). To the best of our knowledge, the present study is the first study that investigated and compared the effects of high-dose potent statins on the serum endocan levels. According our findings, both statins significantly reduced the serum endocan levels at the end of 4-week therapy, and these results may be another important evidence for understanding the pathophysiological mechanisms of the pleiotropic effects of statins in patients with AMI.
The effects of atorvastatin (80 mg/day) and rosuvastatin (40 mg/day) therapy on chemerin levels
Chemerin is a novel adipokine that regulates adipogenesis and the adipocyte metabolism (12, 13). It is associated with obesity, metabolic syndrome, and inflammation. Recent studies have shown the association between the increased chemerin levels and the presence and severity of CAD, ACS, and non-dipper hypertension (14-17). Aksan et al. (15) reported that the chemerin level is associated with the presence and severity of CAD in patients with metabolic syndrome. In a recent study, Xiong et al. (40) described the stimulating effects of chemerin on vascular smooth muscle cell proliferation and carotid neointimal hyperplasia. There is no study in the literature investigating and comparing the effects of statins on the levels of chemerin. To the best of our knowledge, we showed that for the first time the effects of high-dose atorvastatin and rosuvastatin on chemerin levels. According to our findings, the effect of atorvastatin 80 mg/day on chemerin levels was limited, while rosuvastatin 40 mg/day significantly reduced the serum chemerin levels at the end of 4-week therapy (Table 2). However, there was no statistically significant difference between groups at the end of 1-month therapy in terms of chemerin change according to baseline in terms of absolute and percentage changes (Table 3). Even there is not any knowledge about the effects of statins chemerin levels, some studies have investigated the effects of statins on other adipokines, and conflicting results have emerged. Krysiak et al. (41) reported a beneficial effect of simvastatin and simvastatin plus ezetimibe combination on the some adipokines like leptin and visfatin. They showed that simvastatin and simvastatin plus ezetimibe combination decreased the levels of adipokines. On the other hand, in a meta-analysis, statin therapy failed to show favorable effects on the leptin levels (42). Although our study is the first investigation that demonstrates the positive effects of rosuvastatin on the chemerin levels in patients with AMI, we think that there is a need for further large-scale studies.
The effects of atorvastatin (80 mg/day) and rosuvastatin (40 mg/day) therapy on galectin-3 levels
Galectin-3 is a 26 kDa, soluble ß-galactoside-binding lectin, mainly secreted by macrophages and has effects on phagocytosis, apoptosis, cell growth/proliferation, and adhesion. Higher galectin-3 levels are associated with an increased risk for incident heart failure and mortality (21, 43). Galectin-3 has been found to be associated with carotid intima media thickness and cardiovascular mortality (44). Winter et al. (45) demonstrated the association between the galectin-3 levels and premature myocardial infarction, and they suggested an interaction between galectin-3 levels and plaque formation and plaque rupture. McKinnon et al. (46) showed that deletion of galectin-3 in the apolipoprotein E (−/−) knockout mice resulted in a markedly reduced volume of atherosclerotic plaques, and the authors concluded that strategies that inhibit galectin-3 may be a new approach in the treatment of atherosclerotic diseases. In a small study, 15 statin-naive patients with atherosclerosis were given atorvastatin 40 mg/day for 12 weeks, and at the end of study, galectin-3 levels decreased non-significantly (47). But in our study, atorvastatin 80 mg/day seems to have no effects on galectin-3 levels in patients with AMI at the end of 4-week therapy.
A substudy of CORONA study showed that patients with systolic heart failure due to ischemic etiology who have galectin-3 levels lower than 19 ng/mL may benefit from rosuvastatin 10 mg treatment (48). However, to the best of our knowledge, there is no study investigating the possible role of baseline galecin-3 levels on response to statin treatment in patients with ischemic heart disease and AMI. In our study, rosuvastatin 40 mg decreased the galectin-3 levels from 18.25 (12.82–23.82) ng/mL to 16.60 (10.60–20.15) ng/mL, and further studies are needed to clarify the association between the reduction of the galectin-3 levels with rosuvastatin 40 mg and long-term clinical endpoints. On the other hand, it may be another research interest whether the reduction of galectin-3 levels provided by rosuvastatin 40 mg have any contribution to the total favorable effects of rosuvastatin treatment in patients with AMI and stable CAD.
To the best of our knowledge, our investigation is the first study that assessed and compared the effects of high-dose statin therapies on galectin-3 levels in patients with AMI. Our results indicate that, although not significant, rosuvastatin 40 mg/day seems to be more effective on galectin-3 levels when compared with atorvastatin 80 mg/day.
Study limitations
Our study has some limitations. First, relatively small differences between the two different drug groups may not have reached statistical significance due to the limited number of patients, and our study is a substudy of another investigation and carries the common disadvantages of substudies. Second, we could not show and compare the effects of these potent statins on other inflammatory markers such as hs-CRP, TNF-α, IL-1, and IL-6. Third, our study was designed to investigate and compare the effects of potent statins on endocan, chemerin, and galectin-3 at the end of the 1st month. For this reason, we do not know any information whether there are any long-term effects of statins on these biomarkers, and whether these effects are associated with hard endpoints, such as death and myocardial infarction. In addition, our study population comprised the patients with AMI, and our results cannot necessarily be applied to a general CAD population.
Conclusion
Both statins have similar favorable effects on endocan, whereas rosuvastatin 40 mg/day seems to be more effective in terms of ability to decrease chemerin and galectin-3 levels. Based on these findings, we concluded that rosuvastatin 40 mg may have better pleiotropic and metabolic effects than atorvastatin 80 mg in patients with AMI.
Acknowledgements
Abstract of this article presented as poster abstract at the 23rd International Congress of Clinical Chemistry and Laboratory Medicine Congress, helded at the Durban International Convention Center in Durban, South Africa on October 22-25, 2017. This study was supported by Scientific Research Project Coordinator of Selcuk University and the founding source did not involve in study design, in the collection, analysis, and interpretation of data; in the writing of the report; in the decision to submit the article for publication.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.T., B.B.A.; Design – A.T., B.B.A., M.U.Y.; Supervision – A.T., H.T., N.A., K.D.; Funding – A.T., C.A., N.A., K.D., F.A.; Materials – A.T., B.Ö., M.S.A., H.T., C.A., E.C.K., F.A.; Data collection and/or processing – A.T., B.Ö., M.S.A., H.T., C.A., E.C.K., F.A.; Analysis and/or interpretation – A.T., M.S.A., E.C.K., M.U.Y., N.A., K.D.; Literature search – A.T., B.Ö., C.A.; Writing – A.T., B.B.A., B.Ö.; Critical review – A.T., B.B.A., M.U.Y.
References
- 1.The top 10 causes of death 2017 [cited 2017 07.03.2017] Available from:URL: http://www.who.int/mediacentre/factsheets/fs310/en/
- 2.White HD, Chew DP. Acute myocardial infarction. Lancet. 2008;372:570–84. doi: 10.1016/S0140-6736(08)61237-4. [DOI] [PubMed] [Google Scholar]
- 3.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, et al. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 4.Boekholdt SM, Hack CE, Sandhu MS, Luben R, Bingham SA, Wareham NJ, et al. C-reactive protein levels and coronary artery disease incidence and mortality in apparently healthy men and women:the EPIC-Norfolk prospective population study 1993-2003. Atherosclerosis. 2006;187:415–22. doi: 10.1016/j.atherosclerosis.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 5.Neunteufl T, Katzenschlager R, Hassan A, Klaar U, Schwarzacher S, Glogar D, et al. Systemic endothelial dysfunction is related to the extent and severity of coronary artery disease. Atherosclerosis. 1997;129:111–8. doi: 10.1016/s0021-9150(96)06018-2. [DOI] [PubMed] [Google Scholar]
- 6.Menon P, Kocher ON, Aird WC. Endothelial Cell Specific Molecule-1 (ESM-1), a Novel Secreted Proteoglycan Stimulates Vascular Smooth Muscle Cell Proliferation and Migration. Circulation. 2011;124(Suppl 21):A15455. [Google Scholar]
- 7.Scherpereel A, Depontieu F, Grigoriu B, Cavestri M, Tsicopoulos A, Gentina T, et al. Endocan, a new endothelial marker in human sepsis. Crit Care Med. 2006;34:532–7. doi: 10.1097/01.ccm.0000198525.82124.74. [DOI] [PubMed] [Google Scholar]
- 8.Roudnicky F, Poyet C, Wild P, Krampitz S, Negrini F, Huggenberger R, et al. Endocan is upregulated on tumor vessels in invasive bladder cancer where it mediates VEGF-A-induced angiogenesis. Cancer Res. 2013;73:1097–106. doi: 10.1158/0008-5472.CAN-12-1855. [DOI] [PubMed] [Google Scholar]
- 9.Kose M, Emet S, Akpinar TS, Kocaaga M, Cakmak R, Akarsu M, et al. Serum Endocan Level and the Severity of Coronary Artery Disease:A Pilot Study. Angiology. 2015;66:727–31. doi: 10.1177/0003319714548870. [DOI] [PubMed] [Google Scholar]
- 10.Kundi H, Balun A, Cicekcioglu H, Karayigit O, Topcuoglu C, Kilinckaya MF, et al. Admission Endocan Level may be a Useful Predictor for In-Hospital Mortality and Coronary Severity Index in Patients With ST-Segment Elevation Myocardial Infarction. Angiology. 2017;68:46–51. doi: 10.1177/0003319716646932. [DOI] [PubMed] [Google Scholar]
- 11.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–53. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bozaoglu K, Bolton K, McMillan J, Zimmet P, Jowett J, Collier G, et al. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology. 2007;148:4687–94. doi: 10.1210/en.2007-0175. [DOI] [PubMed] [Google Scholar]
- 13.Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem. 2007;282:28175–88. doi: 10.1074/jbc.M700793200. [DOI] [PubMed] [Google Scholar]
- 14.Meric M, Soylu K, Avci B, Yuksel S, Gulel O, Yenercag M, et al. Evaluation of plasma chemerin levels in patients with non-dipper blood pressure patterns. Med Sci Monit. 2014;20:698–705. doi: 10.12659/MSM.890784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aksan G, İnci S, Nar G, Soylu K, Gedikli Ö, Yüksel S, et al. Association of serum chemerin levels with the severity of coronary artery disease in patients with metabolic syndrome. Int J Clin Exp Med. 2014;7:5461–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Xiaotao L, Xiaoxia Z, Yue X, Liye W. Serum chemerin levels are associated with the presence and extent of coronary artery disease. Coron Artery Dis. 2012;23:412–6. doi: 10.1097/MCA.0b013e3283576a60. [DOI] [PubMed] [Google Scholar]
- 17.Ji Q, Lin Y, Liang Z, Yu K, Liu Y, Fang Z, et al. Chemerin is a novel biomarker of acute coronary syndrome but not of stable angina pectoris. Cardiovasc Diabetol. 2014;13:145. doi: 10.1186/s12933-014-0145-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharma UC, Pokharel S, van Brakel TJ, van Berlo JH, Cleutjens JPM, Schroen B, et al. Galectin-3 marks activated macrophages in failure-prone hypertrophied hearts and contributes to cardiac dysfunction. Circulation. 2004;110:3121–8. doi: 10.1161/01.CIR.0000147181.65298.4D. [DOI] [PubMed] [Google Scholar]
- 19.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, et al. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–98. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raimond J, Zimonjic DB, Mignon C, Mattei MG, Popescu NC, Monsigny M, et al. Mapping of the galectin-3 gene (LGALS3) to human Chromosome 14 at region 14q21-22. Mamm Genome. 1997;8:706–7. doi: 10.1007/s003359900548. [DOI] [PubMed] [Google Scholar]
- 21.Imran TF, Shin HJ, Mathenge N, Wang F, Kim B, Joseph J, et al. Meta-Analysis of the Usefulness of Plasma Galectin-3 to Predict the Risk of Mortality in Patients With Heart Failure and in the General Population. Am J Cardiol. 2017;119:57–64. doi: 10.1016/j.amjcard.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention &Rehabilitation (EACPR) Atherosclerosis. 2016;253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Altunkeser BB, Tuncez A, Ozturk B, Tezcan H, Ates MS, Yilmaz C, et al. Comparative effects of high-dose atorvastatin versus rosuvastatin on lipid parameters, oxidized low-density lipoprotein, and proprotein convertase subtilisin kexin 9 in acute coronary syndrome. Coron Artery Dis. 2019;30:285–90. doi: 10.1097/MCA.0000000000000715. [DOI] [PubMed] [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, et al. Third Universal Definition of Myocardial Infarction. Circulation. 2012;126:2020–35. doi: 10.1016/j.gheart.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jr, et al. 2014 AHA/ACC Guideline for the Management of Patients With Non-ST-Elevation Acute Coronary Syndromes:Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;130:2354–94. doi: 10.1161/CIR.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 26.Weng TC, Yang YH, Lin SJ, Tai SH. A systematic review and meta-analysis on the therapeutic equivalence of statins. J Clin Pharm Ther. 2010;35:139–51. doi: 10.1111/j.1365-2710.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 27.LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, et al. Treating to New Targets (TNT) Investigators. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–35. doi: 10.1056/NEJMoa050461. [DOI] [PubMed] [Google Scholar]
- 28.Nissen SE, Nicholls SJ, Sipahi I, Libby P, Raichlen JS, Ballantyne CM, et al. ASTEROID Investigators. Effect of very high-intensity statin therapy on regression of coronary atherosclerosis:the ASTEROID trial. JAMA. 2006;295:1556–65. doi: 10.1001/jama.295.13.jpc60002. [DOI] [PubMed] [Google Scholar]
- 29.Pitt B, Loscalzo J, Monyak J, Miller E, Raichlen J. Comparison of lipid-modifying efficacy of rosuvastatin versus atorvastatin in patients with acute coronary syndrome (from the LUNAR study) Am J Cardiol. 2012;109:1239–46. doi: 10.1016/j.amjcard.2011.12.015. [DOI] [PubMed] [Google Scholar]
- 30.Balta S, Mikhailidis DP, Demirkol S, Ozturk C, Celik T, Iyisoy A. Endocan:A novel inflammatory indicator in cardiovascular disease?Atherosclerosis 2015. 243:339–43. doi: 10.1016/j.atherosclerosis.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 31.Balta S, Mikhailidis DP, Demirkol S, Ozturk C, Kurtoglu E, Demir M, et al. Endocan--a novel inflammatory indicator in newly diagnosed patients with hypertension:a pilot study. Angiology. 2014;65:773–7. doi: 10.1177/0003319713513492. [DOI] [PubMed] [Google Scholar]
- 32.Qiu CR, Fu Q, Sui J, Zhang Q, Wei P, Wu Y, et al. Serum Endothelial Cell-Specific Molecule 1 (Endocan) Levels in Patients With Acute Myocardial Infarction and Its Clinical Significance. Angiology. 2017;68:354–9. doi: 10.1177/0003319716651349. [DOI] [PubMed] [Google Scholar]
- 33.Xiong C, Zhao ZW, Chen ZY, Wu LZ, Luo YK, Hu FD, et al. Elevated Human Endothelial Cell-Specific Molecule-1 Level and Its Association With Coronary Artery Disease in Patients With Hypertension. J Invest Med. 2015;63:867–70. doi: 10.1097/JIM.0000000000000219. [DOI] [PubMed] [Google Scholar]
- 34.Almuti K, Rimawi R, Spevack D, Ostfeld RJ. Effects of statins beyond lipid lowering:potential for clinical benefits. Int J Cardiol. 2006;109:7–15. doi: 10.1016/j.ijcard.2005.05.056. [DOI] [PubMed] [Google Scholar]
- 35.Packard CJ, Shepherd J, Cobbe SM, Ford I, Isles CG, McKillop JH, et al. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS) Circulation. 1998;97:1440–5. doi: 10.1161/01.cir.97.15.1440. [DOI] [PubMed] [Google Scholar]
- 36.Nissen SE, Tuzcu EM, Schoenhagen P, Crowe T, Sasiela WJ, Tsai J, et al. Reversal of Atherosclerosis with Aggressive Lipid Lowering (REVERSAL) Investigators. Statin therapy, LDL cholesterol, C-reactive protein, and coronary artery disease. N Engl J Med. 2005;352:29–38. doi: 10.1056/NEJMoa042000. [DOI] [PubMed] [Google Scholar]
- 37.Rikitake Y, Kawashima S, Takeshita S, Yamashita T, Azumi H, Yasuhara M, et al. Anti-oxidative properties of fluvastatin, an HMG-CoA reductase inhibitor, contribute to prevention of atherosclerosis in cholesterol-fed rabbits. Atherosclerosis. 2001;154:87–96. doi: 10.1016/s0021-9150(00)00468-8. [DOI] [PubMed] [Google Scholar]
- 38.Williams JK, Sukhova GK, Herrington DM, Libby P. Pravastatin has cholesterol-lowering independent effects on the artery wall of atherosclerotic monkeys. J Am Coll Cardiol. 1998;31:684–91. doi: 10.1016/s0735-1097(97)00537-8. [DOI] [PubMed] [Google Scholar]
- 39.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–35. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 40.Xiong W, Luo Y, Wu L, Liu F, Liu H, Li J, et al. Chemerin Stimulates Vascular Smooth Muscle Cell Proliferation and Carotid Neointimal Hyperplasia by Activating Mitogen-Activated Protein Kinase Signaling. PLoS One. 2016;11:e0165305. doi: 10.1371/journal.pone.0165305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krysiak R, Zmuda W, Okopien B. The effect of simvastatin-ezetimibe combination therapy on adipose tissue hormones and systemic inflammation in patients with isolated hypercholesterolemia. Cardiovasc Ther. 2014;32:40–6. doi: 10.1111/1755-5922.12057. [DOI] [PubMed] [Google Scholar]
- 42.Sahebkar A, Giua R, Pedone C. Impact of statin therapy on plasma leptin concentrations:a systematic review and meta-analysis of randomized placebo-controlled trials. Br J Clin Pharmacol. 2016;82:1674–84. doi: 10.1111/bcp.13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ho JE, Liu CY, Lyass A, Courchesne P, Pencina MJ, Vasan RS, et al. Galectin-3, a Marker of Cardiac Fibrosis, Predicts Incident Heart Failure in the Community. J Am Coll Cardiol. 2012;60:1249–56. doi: 10.1016/j.jacc.2012.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Madrigal-Matute J, Lindholt JS, Fernandez-Garcia CE, Benito-Martin A, Burillo E, Zalba G, et al. Galectin-3, a biomarker linking oxidative stress and inflammation with the clinical outcomes of patients with atherothrombosis. J Am Heart Assoc. 2014;3:e000785. doi: 10.1161/JAHA.114.000785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winter MP, Wiesbauer F, Alimohammadi A, Blessberger H, Pavo N, Schillinger M, et al. Soluble galectin-3 is associated with premature myocardial infarction. Eur J Clin Invest. 2016;46:386–91. doi: 10.1111/eci.12605. [DOI] [PubMed] [Google Scholar]
- 46.MacKinnon AC, Liu XJ, Hadoke PWF, Miller MR, Newby DE, Sethi T. Inhibition of galectin-3 reduces atherosclerosis in apolipoprotein E-deficient mice. Glycobiology. 2013;23:654–63. doi: 10.1093/glycob/cwt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chang YY, Wu YW, Lee JK, Lin YM, Lin YT, Kao HL, et al. Effects of 12 weeks of atorvastatin therapy on myocardial fibrosis and circulating fibrosis biomarkers in statin-naive patients with hypertension with atherosclerosis. J Investig Med. 2016;64:1194–9. doi: 10.1136/jim-2016-000092. [DOI] [PubMed] [Google Scholar]
- 48.Gullestad L, Ueland T, Kjekshus J, Nymo SH, Hulthe J, Muntendam P, et al. CORONA Study Group. Galectin-3 predicts response to statin therapy in the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) Eur Heart J. 2012;33:2290–6. doi: 10.1093/eurheartj/ehs077. [DOI] [PubMed] [Google Scholar]