Abstract
The perivascular adipose tissue (PVAT) has been recently recognized as an important factor in vascular biology, with implications in the pathogenesis of cardiovascular diseases. The cell types and the precursor cells of PVAT appear to be different according to their location, with the component cell type including white, brown, and beige adipocytes. PVAT releases a panel of adipokines and cytokines that maintain vascular homeostasis, but it also has the ability of intervention in the pathogenesis of the atherosclerotic plaques development and in the vascular tone modulation. In this review, we summarize the current knowledge and discuss the role of PVAT as a major contributing factor in the pathogenesis of ischemic coronary disease, hypertension, obesity, and diabetes mellitus. The new perspective of PVAT as an endocrine organ, along with the recent knowledge of the mechanisms involved in dysfunctional PVAT intervention in local vascular homeostasis perturbations, nowadays represent a new area of research in cardiovascular pathology, aiming to discover new therapeutic methods.
Keywords: perivascular adipose tissue, atherosclerosis, obesity, hypertension
Introduction
The traditional concept of the adipose tissue includes three types, according to its structure, function, and location. The white adipose tissue (WAT), composed predominantly of cells exhibiting unilocular lipid inclusions, is mainly located in the hypodermis and perivisceral location. The brown adipose tissue (BAT), temporarily occurring in humans in the interscapular and mediastinal regions, is involved in the maintenance of a consistent body temperature, containing adipocytes with multiple cytoplasmic inclusions, of variable size, associated with a rich capillary network (1, 2). The beige or brite (brown-in-white) adipose tissue (BeAT) may be disposed in between WAT cells and is able to develop a brown-like phenotype (1).
A new type of adipose tissue was added in 1991, by Soltis and Cassis, namely perivascular adipose tissue (PVAT). This variety was described as a neurohumoral regulator of vascular responsiveness, by decreasing the aortic wall contraction, as a response to noradrenaline (3). Later on, in 2001, Okamoto and his team noticed an increase in the leukocytes number in PVAT, as a response to coronary angioplasty, taking into consideration, for the first time, a possible association between the PVAT inflammatory profile and cardiovascular diseases (4).
The interest regarding the origin and the involvement of this tissue in vascular homeostasis has been exponentially growing, as PVAT is currently considered more than a vascular tissue support, being an active contributor to vascular tonus regulation and a dual endocrine and paracrine organ, producing a panel of vasoactive and pro-inflammatory substances (2, 5). The endocrine function is mainly performed by the release of adipokines into blood flow and their circulation to specific receptors. In contrast, the paracrine activity is characterized by a direct local release of vasoactive and pro-inflammatory substances, resulting in both insulin resistance (IR) and microvascular dysfunction (2, 5).
The spectrum of active substances synthesized by PVAT comprises adipokines (adiponectin, visfatin, omentin-1, leptin, apelin, and resistin); nitric oxide (NO); methyl palmitate; hydrogen sulfide (H2S); angiotensin 1-7 (Ang 1-7); reactive oxygen species (ROS); and cytokines, such as interleukin-6 (IL-6), monocyte chemoattractant protein-1 (MCP-1), or tumor necrosis-α (TNF-α), which are involved in atherosclerosis plaques development or in the vascular alterations associated with hypertension and diabetes mellitus (5, 6).
The most accurate imagistic methods for the PVAT proportion quantification are computed tomography and magnetic resonance imaging (6).
Nonetheless, the differences between PVAT and classic types of the adipose tissue are partially known, and its role in vascular homeostasis and cardiovascular pathogeny remains undetermined, as more of its large structural and functional heterogeneity according to the location has been noticed (1, 7). The identification of the PVAT mechanisms of involvement in the vascular tonus maintenance, in endothelial homeostasis, and in IR may lead to a new therapeutic approach in obesity, diabetes mellitus, and cardiovascular diseases.
Based on the current trend in the research of histo-physiology and pathogeny of diseases associated with this tissue, an update of structure, origin, and involvement of PVAT in pathology is provided in our review of the literature.
Structure and locations
The term PVAT denotes the adipose tissue located around arteries and large veins, arterioles, and small vessels disposed in the striated skeletal muscle, and around the renal artery and vein, in renal pelvis (8). PVAT is not associated with the nervous tissue vessels (5, 9).
Structurally, PVAT is composed of adipocytes, fibroblasts, and rare T lymphocytes and macrophages, while WAT contains unmyelinated nerve fibers and numerous mast cells associated with white adipocytes, and BAT has numerous capillaries and unmyelinated, noradrenergic sympathetic nerve fibers mixed with brown fat cells.
In large vessels, an anatomical barrier composed by collagen and elastic fibers, fibroblasts, vasa vasorum, and sympathetic nervous fibers is located between PVAT and the vascular wall (10). In small vessels, PVAT is an intrinsic component of the vascular wall, without a barrier between this tissue and vascular adventitia (8). The substances secreted by PVAT influence the cells of the vascular media and endothelium by direct diffusion or by vasa vasorum (8, 9).
In experimental animals, the standard microscopy has shown a morphological similarity between PVAT associated with the thoracic aorta (tPVAT) and PVAT located around the abdominal aorta (aPVAT). However, electron microscopy reveals numerous lipid inclusions and mitochondria in tPVAT, resembling brown adipocytes. The molecular phenotype of tPVAT involves the Ucp-1, CIDE-A, and PRDM16 positivity, along with the TCF-1 negativity, in a similar manner to that of BAT (5, 11). In contrast, aPVAT adipocytes have a phenotype similar to WAT cells (5, 12), expressing a spectrum of genes codifying characteristic markers such as adiponectin, resistin, lipoprotein lipase (LPL), and glyceraldehyde 3-phosphate dehydrogenase (G3PDH) (7, 11, 12).
Although the studies regarding human PVAT are limited, according to the data that have been published by now, a series of differences between tPVAT and aPVAT have been described in humans. In rodents, tPVAT is similar to BAT throughout their entire their lifespan. In contrast, newborn tPVAT associated with large thoracic vessels is phenotypically similar to BAT, being progressively replaced by BeAT, a clusters of brown-like adipocytes in WAT (Table 1) (13).
Table 1.
Main characteristics of different types of perivascular adipose tissue
| Body location | Microscopy (experimental model) | Microscopy (humans) | Gene expression | Adipokines and cytokines | Normal functions | References | |
|---|---|---|---|---|---|---|---|
| Periaortic PVAT | Thoracic periaortic adipose tissue | • BAT-like features | • BAT-like features in newborns • BeAT features in adults |
• Ucp-1 • CIDE-A • PRDM16 • Scl27a • Dio2 • Adipoq • C/EBPα • PPAR-γ |
• H2S • ROS • TXA2 • NO • IL-6 • TNF-α • MCP-1 • Angptl2 • leptin • methyl palmitate • irisin • CCL5 (RANTES) • angiotensin 1-7 |
• lipolysis • heat transport throughout the body • vascular relaxation |
(5, 6, 12, 36, 40, 49) |
| Abdominal periaortic adipose tissue | • WAT-like features • few brown adipocytes • less vascularized |
NA | • Ob-Rb • Leptin • Sncg • Nnat • Hox8 • Mest • PPAR-γ |
• TNF-α • TNFR2 • MCP-1 • IL-6 • IL-6R • IL-18 • MMP-2 • angiotensin 1-7 • CCL5 (RANTES) |
• pro-inflammatory • development of aortic aneurysms • atherosclerosis development |
(5, 6, 7, 13, 36) | |
| Perirenal PVAT | • WAT and BAT features | NA | • Ucp-1 • CIDE-A • TCF-1 • Adipoq • C/EBPα • PPAR-γ |
• norepinephrine • adiponectin |
• regulates renal artery tonus | (11, 45) | |
| Mesenteric PVAT | • WAT-like features • four times larger than periaortic adipocytes |
NA | • LP (high level) • LPL • Ucp-1 and CIDE-A (low level) • TCF-1 • Adipoq • C/EBPα |
• leptin • adiponectin • H2S • NO • ROS • SSAO • TNF-α • Ang II • chemerin |
• increases vascular relaxation | (12, 14, 42) | |
| Coronary PVAT | • BAT-like features | • WAT-like features | • low gene WAT expression | • leptin • adiponectin • MCP-1 • IL-1b • IL-6 • TNF-α • chemerin • resistin • visfatin • FABP • LCN2 • omentin-1 • apelin • vaspin • Angptl2 |
• attenuates endothelial- dependent relaxation • increases vascular contractility • protects coronary arteries against wave torsion and the heart against hypothermia • atherosclerosis development |
(6, 7, 32, 34, 37) | |
| Intramuscular PVAT | • WAT features | NA | • WAT-gene expression | • leptin • resistin • adiponectin • TNF-α |
• regulation of muscle perfusion • insulin sensitivity |
(17) | |
| Saphenous vein PVAT | • more WAT-like features | NA | NA | • NO • leptin • PGE2 • PGI2 • MCP-1 • Il-6 • Il-8 |
• vasodilatory • anti-inflammatory • beneficial role in coronary artery bypass |
(18) | |
| Ileo-femoral PVAT | • WAT and BAT | NA | NA | NA | NA | (7) | |
| Tibial and popliteal PVAT | • subcutaneous like WAT features | NA | NA | • less leptin and adiponectin • TNF-α • MCP-1 • Il-6 • Il-8 |
• protective against peripheral artery diseases | (19) | |
| Skin PVAT | • large adipocytes | • adiponectin • HGF • MCP-1 • IGFBP-3 • TPL 1 • PAI-1 |
• vasorelaxation | (22) | |||
| Brachial artery PVAT | NA | NA | • regulation of insulin sensitivity | (21) | |||
| Internal mammary artery PVAT | NA | NA | • leptin (low level) | • pro-inflammatory | (20) |
Adipoq - adiponectin gene; Ang II - angiotensin II; Angptl2 - angiopoietin-like protein 2; BAT - brown adipose tissue; BeAT - beige adipose tissue; CCL5/RANTES - chemokine (C-C motif) ligand 5; CIDE-A - cell death activator
CIDE-A; C/EBPα - CCAAT enhancer binding protein alpha; FABP - fatty acid binding protein; H2S - hydrogen sulfide; HGF - hepatic growth factor; IL - interleukin; IL-6R - interleukin 6 receptor; IGFBP-3 - insulin-like growth factor-binding protein 3; LCN2 - lipocalin-2; LP - leptin gene; LPL - lipoprotein lipase; MCP-1 - monocyte chemoattractant protein; MMP-2 - matrix metalloproteinase-2; NA - non available; Ob-Rb - leptin receptor b; PAI-1 - plasminogen activator inhibitor-1; PGE2 - prostaglandin E2; PGI2 - prostaglandin I2; PPAR-γ - peroxisome proliferator-activated receptor gamma; PRDM16 - PR domain zinc finger protein 16; PVAT - perivascular adipose tissue; ROS - reactive oxygen species;
SSAO - semicarbazide-sensitive amine oxidase; TCF-1 - transcription factor T-cell factor 1; TNF-α - tumor necrosis; TNFR2 - tumor necrosis factor receptor 2; TPL 1 - thromboplastin 1; TXA2 - thromboxane A2; Ucp-1 - uncoupling protein 1; WAT - white adipose tissue
The different phenotypical features of PVAT are also associated with functional differences in the activity of the two subtypes of PVAT. Hence, tPVAT performs lipolysis of lipids accumulated in cytoplasm, facilitates the generated heat transport in the entire organism and facilitates vascular relaxation by H2S synthesis, while aPVAT is involved in cytoplasmic lipid storage (5, 6). Supplementary, aPVAT is less vascularized compared to tPVAT, synthesizes a panel of cytokines, and contains more fibroblasts, macrophages, and other immune cells, in physiological conditions (6). Hence, aPVAT is more pro-inflammatory and more atherogenic than tPVAT, corresponding to its high concentration in the abdominal region of the aorta (12).
PVAT associated with mesenteric vessels has been found only in laboratory animals. These cells resemble WAT, their size being four times larger than that of murine periaortic adipocytes. PVAT associated with mesenteric vessels exhibits a reduced expression of Ucp-1 and CIDE-A, both of them being BAT markers (12). They produce NO, adiponectin, semicarbazide-sensitive amine oxidase (SSAO), or H2S, resulting in a protective role of the entire mesenteric bed (14). In murine models, they intensely express white adipocytes genes, such as TCF-1, Adipoq, and C/EBPα (11).
PVAT around the coronary arteries represents a part of the epicardial adipose tissue, without a clear delimitation between them, although it seems that coronary PVAT acts independently of normal epicardial WAT. The adipose tissue located in the epicardial tissue is more abundant in the atrioventricular region and in interventricular grooves. This tissue is directly involved in the cardiovascular diseases pathogeny, and its volume may represent an indicator of the cardiovascular diseases risk (15).
PVAT, including the epicardial adipose tissue, represents only 0.3% of total adipose tissue mass, while the subcutaneous and visceral adipose tissue represents 82%–97% and 10%–15% of total adipose tissue mass, respectively (16).
The adipose tissue associated with coronary arteries is composed of smaller adipocytes, with an irregular shape, smaller lipid droplets accumulation, and a reduced state of differentiation compared to perirenal and subcutaneous adipocytes. In laboratory animals, coronary PVAT seems to be composed of a mixture of white and brown adipocytes with more similarities to BAT characteristics compared to WAT, while it is more analogous to WAT in humans (7). Moreover, an expression of adipocyte-associated genes, such as PPARγ, C/EBPα, FABP4, FAS, GPDH, LPL, perilipin, ATLG, leptin, peptide levels, and adiponectin, are lower in coronary PVAT than in subcutaneous and perirenal adipose tissues (7).
PVAT associated with renal vessels (rPVAT) is composed of a mixture of white and brown adipocytes. These tissue cells express both white adipogenic (TCF-1 or Adipoq, C/EBPα or PPAR-γ) and brown-like genes (e.g., Ucp-1, CIDE-A), and they function as a local regulator of the vascular tonus (11).
The relatively limited data regarding the new type of PVAT associated with intramuscular vessels showed that it was mainly composed of white adipocytes, cells that secrete several adipokines, such as leptin, resistin, adiponectin, or cytokines, for example, TNF-α, that contribute to the regulation of muscular perfusion and IR control (17).
Saphenous veins PVAT contain adipocytes with morphological traits that are more similar to those of white than brown adipocytes. These are producing leptin, prostaglandin E2 (PGE2), prostacyclin (PGI2), and NO, which have a local vasodilator and anti-inflammatory effect (18).
In ileo-femoral vessels, PVAT seems to be composed of an equal proportion of the two phenotypes of adipocytes (7). Although similar in morphology, the PVAT of tibial and popliteal vessels has a less inflammatory phenotype than subcutaneous adipose tissue and, consequently, shows a decreased level of synthesis of leptin, adiponectin, TNF-α, MCP-1, IL-6, and interleukin-8 (IL-8), being most probably involved in peripheral artery diseases occurrence (19).
The mammary artery PVAT has a local vasodilatory effect (20). The PVAT disposed around the brachial artery appears to resemble intramuscular PVAT, modulating the IR, while the PVAT associated with deep dermis has adipocytes larger than hypodermis, and they determine a local pro-inflammatory effect, in animals (21, 22).
These structural characteristics correlated to the location are closely linked to PVAT variable intervention in different diseases pathogeny. Thus, PVAT associated with coronary arteries contributes to atheromatous plaques development and to the development of ischemic myocardial disease, while arteriolar PVAT intervenes in hypertension and intermittent claudication, renal artery PVAT is associated with microalbuminuria, and the striated muscular tissue PVAT is associated with the increase of IR and diabetes mellitus (8).
Origin
The traditional concept has been that mesoderm is the place of origin for mesenchymal cells differentiation, as the same cells of origin of adipocytes precursors. During the last years, studies have shown that each type of adipose cell has, at a certain moment, a specific precursor and, moreover, these being differentiated at separate times during embryogenesis (1, 23). Most white adipocytes are differentiated from Myf5+ and PAX3+ precursors or Myf5−/Pax3+ (24). In contrast, in obesity, WAT hyperplasia is achieved from the PDGFRα+ precursor cells residing in the adipose tissue blood vessels, although their identity has been imprecisely determined (25). The brown adipocytes are developing from the paraxial mesoderm Myf5+/Pax3+/Pax7+/En1+, common precursor of myocytes, which progresses along the adipocytes lineage due to the intervention of BMP7 and PRDM16 (1, 24).
Beige cells are often described as inducible brown adipocytes, although there is no consensus concerning their embryonic origin. The transdifferentiation of mature white adipocytes, the differentiation and maturation of pre-existing white preadipocytes, the maturation of pre-existing brown preadipocytes from WAT, and the differentiation from vascular precursors, likewise to that occurring during WAT hyperplasia represent the four possible development pathways of beige adipocytes, which are currently accepted (1).
In mice, a direct comparison of gene expression of thoracic PVAT, along with interscapular BAT and WAT, has revealed that only 228 genes (i.e., 0.79%) are significantly different between thoracic PVAT and classical BAT, while no significant difference has been noted in the expression of BAT specific genes, such as CIDE-A, Ucp-1, or PPARγ (11). Regarding the PVAT composing adipocytes, most studies suggest that they develop from a mesenchymal precursor SM22α+, with a periaortic location, a common source for vascular smooth muscular cells (1, 23).
PPARγ is a central regulator of the adipocyte gene expression and differentiation. The PPARγ deletion in BAT adipogenesis is also blocking the PVAT development, without any implication in the vascular smooth muscular fibers development. The insufficient PVAT development is associated with the increase of local inflammation, a feature which facilitates the occurrence and the development of atheromatous plaques (13).
The studies performed in experimental models have demonstrated that thoracic aorta PVAT express BAT specific genes, such as Ucp-1, Slc27a, PRDM16, Dio2, or CIDE-A, while PVAT located around the abdominal aorta expresses WAT specific genes, such as Sncg, Hox8, Nnat, and Mest (5, 12). These differences are also supported by the higher expression of several transcription factors involved in brown adipocytes adipogenesis in tPVAT compared to PVAT, such as BMP7, Ehmt1, Ebf2, PRDM16, and PGC-1a (12).
tPVAT originates as BAT from a common Myf5−Pax3+/Pax3− precursor. Moreover, in a study performed on animal models, most of the periaortic PVAT develops from a Myf5−Pax3+ precursor, in females, while in males, it develops from a Myf5−Pax3− precursor (1, 26).
These data are contradictory to those of Ye et al. (27), stating that PVAT is organized in laboratory animals as three long strip-shaped fat depots around the thoracic artery, as follows: an anterior one, a lateral-left one, and another lateral right. According to the same study, PVAT localized in the anterior part of the thoracic aorta is developing from SM22α+ progenitors, while the adipocytes located on the lateral parts of the thoracic aorta are developing from both SM22α+ and Myf5+ cells. Moreover, the same authors observed that the adipocytes located on the lateral faces of the aorta have more Ucp1+ expression and have a stronger wall relaxation effect compared to perivascular adipocytes of the anterior part of the aorta (27).
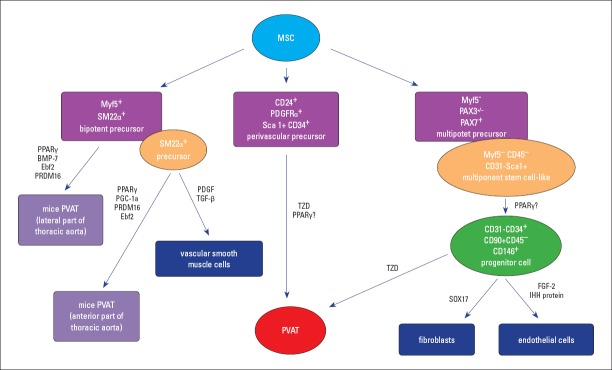
Literature data support the white adipocyte of PVAT development from a perivascular PDGFRα+ precursor or from a CD31−CD34+ precursor cell, the latter being also the origin for both fibroblasts and endothelial cells (Fig. 1) (1, 28).
Figure 1.
Perivascular adipose tissue development
Perivascular adipose tissue develops from a perivasculary PDGFRα+ precursor or from a Myf5-/Pax3+/− precursor cell for both fibroblasts and endothelial cells, a process regulated by SOX17, FGF-2, and IHH proteins. In experimental animals, PVAT from the lateral and anterior part of the thoracic aorta (tPVAT) originates from Myf5+ SM22α+, followed by preadipocytes differentiation into mature adipocytes, a process regulated by stimulatory factors, such as PPARγ, BMP-7, Ebf2, PRDM16, and PGC-1a. Another possibility of differentiation of the SM22α+ precursor is toward vascular smooth muscle cells, under the influence of PDGF and TGF-β.
BMP-7 - bone morphogenetic protein 7; Ebf2 - early B-cell factor 2; FGF-2 - fibroblast growth factor 2; IHH protein - Indian hedgehog homolog; Myf5 - myogenic factor 5; MSC - mesenchymal stem cell; PDGF - platelet-derived growth factor; PGC-1a - peroxisome proliferator-activated receptor gamma coactivator 1 alpha; PPARγ - peroxisome proliferator-activated receptor gamma; PRDM16 - PR domain containing 16; PVAT - perivascular adipose tissue; SOX17 - transcription factor SOX-17; TGF-β - transforming growth factor beta; tPVAT - PVAT associated to thoracic aorta; TZD- thiazolidinedione
Consequently, the PVAT origin may be considered as having own precursors, different from those developing from classic adipocyte lineages (1, 12). Furthermore, according to the location, it is most probable that there are different precursors for PVAT cells corresponding to their phenotypic and functional diversity. The identification of PVAT specific adipocyte precursors may represent the pivotal key of their manipulation in future toward an amelioration of the metabolic and vascular health.
PVAT and atherosclerosis
PVAT is involved in atherosclerosis pathogeny via several possible mechanisms, such as the release of pro-inflammatory substances, NO, H2S, and adipokines, along with leukocytes, vascular smooth muscle cells (VSMCs), and macrophages intervention.
Pro-inflammatory substances released by PVAT exhibit a pro-atherogenic role, facilitating the atheromatous plaque stability, being counterbalanced by their protective role in endothelial dysfunction (29). Moreover, as a consequence of its thermogenic effect, tPVAT physiologically contributes to the reduction of the concentration of plasma lipids from vasculature, while aPVAT participates in free fatty acids uptake from circulatory flow, both of which represent anti-atherogenic mechanisms (6).
PVAT becomes dysfunctional in obesity, resulting in an increased production of pro-inflammatory factors and cytokines targeting the vascular wall, inducing endothelial dysfunction and inflammation, suggesting a correlation of PVAT with atherosclerosis. In supporting this observation, the Öhman et al. (30) study demonstrates that visceral adipose tissue transplantation in the vicinity of the carotid artery is associated with endothelial dysfunction and that it facilitates the development of atherosclerotic structural alterations, compared to the subcutaneous adipose tissue transplant in the same location.
NO is produced both by endothelial synthase and by PVAT, having an anti-atherogenic effect via platelets aggregation inhibition and vascular smooth muscle regulation (6, 31). The NO anti-atherogenic action is also boosted by adiponectin, released by PVAT, which stimulates NO produced by endothelial synthase (32).
H2S synthesis represents another possible mechanism of the anti-atheromatous PVAT action (6). This observation is also supported by a study on the ApoE−/− mice consuming high-fat diet, showing that H2S may inhibit the atheromatous lesions progression, by regulating the expression of CX3CR1 and C-X3-C CX3CL1, in atheromatous plaques macrophages. However, the H2S anti-atheromatous action may involve several mechanisms, such as vasorelaxation, endothelium preservation, anti-inflammatory responses, and the regulation of ion channels or antioxidative action (33), so further research is necessary to ensure validation.
Adiponectin is one of the most important adipokines synthesized by PVAT adipocytes, exhibiting an anti-atheromatous effect, performed due to the suppression of ROS generation, apoptosis inhibition, initiation of cholesterol release from macrophage, and modulation of the immune system by decreasing the release of pro-inflammatory factors and regulation of the TLR4 expression and AMP-activated protein kinase, promoting macrophage autophagy via the Akt/FOXO3 signaling pathway, and NO production by eNOS stimulation via PI3/Akt phosphorylation (6, 32).
Other adipokines with anti-atheromatous effects released by PVAT are vaspin, an inhibitor of the ROS generation, apelin, which increases the cholesterol efflux, and omentin-1, which diminishes macrophages activation (34). Chemerin, leptin, resistin, lipocalin-2 (LCN2), visfatin, and fatty acid binding proteins are other PVAT adipokines that are counterbalancing the activity of anti-atheromatous factors (6, 32). These stimulate angiogenesis, ROS genesis, endothelial cell proliferation, and endothelial dysfunction through the protein kinase C-beta pathway, the expression of some adhesion molecules, macrophages infiltration of atheromatous plaques, proliferation of vascular smooth muscle cells via a p38 MAPK-dependent pathway, leukocytes recruitment, or release of TNF-α and IL-6 (6, 32).
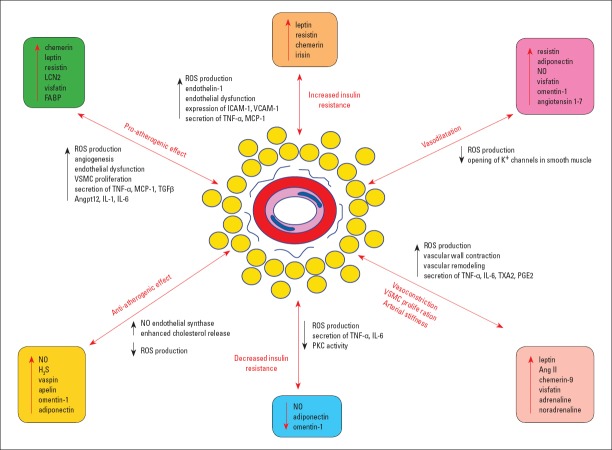
PVAT normally produces a spectrum of agents that support the normal function of the endothelium, adiponectin, NO, and H2S representing the most remarkable among them. Conversely, dysfunctional PVAT produces leptin, TNF-α, and IL-6, molecules that stimulate the production of VCAM-1, ICAM-1, and MCP-1, which activate the monocytes migration to the intimal subendothelial layer (6). Here monocytes being transformed into macrophages become able to secrete pro-inflammatory factors, such as IL-6, IFN-γ, and TNF-α (35). These substances endorse the recruitment of inflammatory cells in the vascular wall and the subintimal accumulation of cholesterol (Fig. 2).
Figure 2.
Perivascular adipose tissue implications in pathology
Perivascular adipose tissue implications in pathology are mainly directed to the following pathways: increased insulin resistance (by an increased ROS and endothelin-1 production, endothelial dysfunction, the ICAM-1 and VCAM-1 expression, and the secretion of TNF-α and MCP-1) vs. decreased insulin resistance, vasoconstriction vs. dilatation, VSMC proliferation, arterial stiffness (by increased ROS production, strong vascular wall contraction, amplified remodeling, and enhanced secretion of TNF-α, IL-1, TXA2, and PGE2), and pro-atherogenic effects (by enhanced ROS genesis, angiogenesis stimulation, increased endothelial dysfunction, VSMC proliferation, and increased release of TNF-α, MCP-1, TGF-β, Angptl2, IL-1, and IL-6) vs. anti-atherogenic effects.
Ang II - angiotensin II; Angptl2 - adipose tissue-specific angiopoietin-like 2; ICAM-1 - intercellular adhesion molecule 1; H2S - hydrogen sulfide; IL - interleukin; FABP - fatty acid binding protein 4; LCN2 - lipocalin-2; MCP-1 - monocyte chemoattractant protein-1; NO - nitric oxide; PGE2 - prostaglandin E2; PKC - protein kinase C; ROS - reactive oxygen species; TGF-β - transforming growth factor beta; TNF-α - tumor necrosis alpha; TXA2 - thromboxane A2; VCAM-1 - vascular cell adhesion protein 1; VSMC - vascular smooth muscle cells
The homeostasis of anti-atherosclerosis adipokines and pro-atheromatous adipokines serum levels is perturbed in people with coronary disease, all of these factors being potential future biomarkers useful in the follow up of the atheromatous plaque development (34).
Leukocytes afflux and their supplementary secretion of pro-inflammatory cytokines are characteristics of the initial stage of the atheromatous lesions. Recent data have shown that vascular adventitia and PVAT inflammation occur in the early stages of atheromatous lesions, before the endothelial dysfunction and atheromatous plaques development, macrophages infiltration being even stronger in the adventitia than in intima (36). PVAT adipocytes and inflammatory cells recruited in the adventitia, along with PVAT, release an excess of cytokines and chemokines, such as MCP-1 (monocytes chemoattractant), IL-6 (stimulates superoxide production, endothelium dysfunction, and VSMCs migration and proliferation), TNF-α (induces endothelial lesions, stimulates ROS production, and increases VCAM-1 and ICAM-1, facilitating the initiation of atheromatous plaques formation), and CCL5/RANTES (induces T cells and monocytes recruitment in the vascular wall, VSMCs proliferation, endothelial dysfunction, enhanced secretion of pro-inflammatory adipokines, and suppressed release of anti-inflammatory adipokines) (36).
VSMCs and the accumulation of extracellular matrix produced by them form the neointima (6). PVAT participates in its accelerated formation by producing MPC-1, angiopoietin-like protein 2 (Angptl2), and TGF-β, TNF-α, IL-6, IL-8, leptin, visfastin, as stimulators of the VSMC proliferation and migration (6).
A special reminder is that most of the literature data are the result of animal experiments, so these have to be interpreted in this context, considering that possible differences of the in vivo PVAT activity, in a complex human microenvironment, may occur.
Although the data related to the relationship between human PVAT and atheromatosis are more limited, a recent study performed on 44 patients with a coronary bypass revealed that the adipocytes associated with the thoracic aorta and coronary arteries are significantly smaller than those comprising the subcutaneous tissue (37).
The saphenous vein is the most useful vessel in coronary artery bypass grafting (38). This is performed by conventional or intermediate saphenous vein harvesting techniques for coronary artery bypass graft surgery. A new method was added, named a “no-touch” technique. This method involves the vascular wall harvesting along with all associated structures, including PVAT, considering their recently demonstrated role in endothelial homeostasis. However, the advantages of the no-touch method are controversial. Although a higher rate of vascular permeability has been noticed in cases that benefited from the no-touch technique, a higher number of interventions are necessary for the validation of this method (38).
Macrophage phenotype is particular in coronary PVAT, being different from thoracic aorta and subcutaneous tissue associated PVAT (37). Thus, they exhibit a lower ratio of CD206-positive M2 to CD11c-positive M1 macrophages, along with a significantly larger extent of CD11c-positive M1 macrophages, and an enhanced gene expression of a panel of inflammatory cytokines, such as IL-6, IL-1b, TNF-α, and MCP-1 (37). Moreover, the differences are related to an increased fibrosis, along with a decreased gene expression of anti-inflammatory adipokines (e.g., adiponectin), and an increased activation of inflammasomes and endoplasmic reticulum stress (37). Nowadays, all of these observations support the PVAT’s active role in the initiation and progression of atheromatous coronary lesions and of ischemic coronary disease, encompassing the vascular support role that has been previously attributed to this tissue.
PVAT role in obesity and diabetes mellitus
Obesity is mainly represented by an excessive accumulation of adipose tissue, which is frequently accompanied by mild, chronic, and systemic inflammation. PVAT is involved in the obesity and diabetes mellitus pathogeny by several possible mechanisms, such as the release of free fatty acids, hyperglycemia, adipokines synthesis, and metabolic inflammation.
Free fatty acids are abundantly released in obesity by the adipose tissue, including PVAT. These free fatty acids are bound to TLRs, with the phosphorylation of insulin receptor substrate-1 (IRS-1), resulting in the down-regulation of the glucose transporter-4 (GLUT-4) and, hence, IR (2). IRS-1 phosphorylation activates PI3-kinase and Akt, which induce the NO synthesis by endothelial cells. Moreover, intracellular oxidation of free fatty acids leads to the PKC activation and to ROS production, with pro-atherogenic effects (39).
Hyperglycemia is also perturbing endothelial homeostasis, by the NO reduced synthesis and increased ROS production, via PKC activation, and NF-κB-mediated inflammation. PKC also increases the synthesis of endothelin-1, a molecule that stimulates the platelet aggregation and induces vasoconstriction (39).
However, only hyperglycemia and the increased IR do not explain the amplitude of vascular lesions associated with diabetes, the glycemia normalization being unassociated with their decrease. These findings support the PVAT involvement in disease pathogenesis, by adipokines and cytokines, which enhance the endothelial dysfunctions and vascular lesions (6), in diabetes patients. Moreover, PVAT dysfunction has been correlated with decreased glucose transport due to diminished muscle perfusion (17).
Adiponectin synthesized by PVAT is physiologically improving the insulin sensitivity, and it decreases hyperglycemia and the serum level of fatty acids. PVAT hypertrophy in obesity is accompanied by diminished adiponectin synthesis, an effect promoted by the amplified synthesis of pro-inflammatory cytokines (TNF-α and IL-6), by leptin release, which leads to the development of IR via the sympathetic nervous system intervention, and via resistin production, followed by an enhanced ICAM-1 and VCAM-1 expression (6).
Irisin is a recently added adipokine, a hormone released mainly by skeletal muscular fibers after an intense effort. This paracrine adipokine induces the WAT to adopt a brown adipose tissue-like phenotype, but, concomitantly, it may lead to endothelial dysfunction and atheromatous lesions in patients with diabetes. Hence, irisin and its receptor may be new therapeutic targets in diabetes mellitus and obesity (40).
Omentin-1 is another recently described adipokine, being mainly released by white adipocytes in the visceral adipose tissue, with an anti-inflammatory and anti-hyperglycemic effect, by improved IR (6). In coronary ischemic disease, an increased level of serum omentin-1 has been reported (34).
PVAT metabolic inflammation has been identified during the last decade, in correlation to obesity, named metflammation or metabolic inflammation. This type of inflammatory reaction is not induced by an antigen, but it is rather orchestrated by metabolic cells in response to excess nutrients and energy (41). In this direction, macrophages and other lymphoid cells, such as mast cells, lymphocytes (CD4+ and CD8+ T cells, natural killer cells), eosinophils, and dendritic cells have been identified in PVAT. These cells secrete paracrine factors that actively participate in the vascular homeostasis and glucide metabolism (15).
A trigger for metflammation is the result of adipocytes hypertrophy, which leads to local hypoxia in obesity (36). This is manifested by immune cells chemoattraction in vascular adventitia and in PVAT, followed by the release of cytokines with a vascular homeostasis effect. PVAT releases chemoattractants for monocytes, granulocytes, and T lymphocytes, an action mainly mediated by the secretion of IL-8 and MCP-1. These in fact act as pro-atherogenic cytokines, and their synthesis is amplified in obesity. Pro-inflammatory cytokines released by PVAT are also, most probably, associated with an IR increase, by its action on several important sites of insulin action (6).
The PVAT metflammation is correlated to perturbation of the release of adipokines and other factors with vasorelaxation effect. Thus, the secretion of adiponectin, an anti-inflammatory adipokine, is diminished, while pro-inflammatory cytokines, such as IL-6, IL-8, and MCP-1 register an increased production in PVAT. A pro-apoptotic, pro-inflammatory, and vascular cells proliferation stimulatory role has been attributed in humans, to chemerin, by Nox activation and redox-sensitive mitogen-activated protein kinases signaling. Experimentally, this adipokine produced by PVAT decreases the NO-dependent cGMP signaling, resulting in diminished aortic wall relaxation in mice, associated with an increased O2– synthesis (2).
Accordingly, PVAT is characterized by a cellular and metabolic plasticity, corresponding to its quality of source of active factors in the pathogeny of diabetes mellitus and obesity (10, 42). A possible increased proportion of its brown adipocytes, known as browning of the adipose tissue, accompanied by local thermogenesis intensification, may represent a hypothetical protective mechanism against metabolic diseases.
PVAT and hypertension
PVAT is involved in hypertension pathogeny by intervention of several possible mechanisms, such as the local renin–angiotensin–aldosterone system (RAAS), sympathetic innervation, norepinephrine, adipokines, cytokines, H2O2, NADPH, and inflammation.
Although it has been previously considered an inert component of the vascular wall, displaying a structural role, PVAT is currently seen as a key role tissue in vascular homeostasis and in the maintenance of the arterial tension (2).
If, in physiological conditions, the vasoactive substances released by PVAT have an anti-contractile and anti-inflammatory effect in the vascular wall, in pathologic conditions, a dysfunctional PVAT produces a panel of pro-oxidative, pro-inflammatory, and vasoconstrictor factors, which support and potentiate vascular remodelation (7, 43).
RAAS expressed by PVAT adipocytes comprises angiotensinogen and angiotensin-converting enzyme, for the synthesis of angiotensin II, which is a potent vasoconstrictor. This has been demonstrated in experiments showing that angiotensin II produced by PVAT adipocytes induces mesenteric artery contractions due to the AT1 receptors activation (44).
Angiotensin II induces vasoconstriction and the release of aldosterone in zona glomerulosa of the adrenal cortex, in physiological status, and by this, sodium and water retention in kidney, with secondary arterial tension increase. Another mechanism of the angiotensin II action may be that of indirect stimulation of the vascular wall contraction, by increase in superoxide free radicals in PVAT adipocytes, or even in the vascular wall.
Sympathetic innervation involvement in vascular tonus maintenance has been recognized as an increased sympathetic activity being associated with hypertension. Such nervous endings have been identified in the laboratory animals PVAT structure, while in humans, they have been identified in PVAT associated with saphenous veins (18). However, the relationship between these sympathetic nerve fibers from PVAT is evident, both physiologically and pathologically (10). The catecholamines released following the sympathetic stimulation, via α-and β-adrenoceptors, are stimulators of adipocyte lipolysis.
Norepinephrine has been experimentally identified in rPVAT, as a consequence of considering its location and possible involvement in the renal vessels tonus regulation, with its independent release on local sympathetic innervation and which may influence the renal vessels tonus (11). These observations have been supported by another study that revealed norepinephrine production in PVAT, suggesting the existence of an independent adrenergic system in PVAT regardless of its location, still incompletely described, which may uptake 1 and 2 monoamine (VMAT1 and VMAT2) by a vesicular transporter, and accumulate, metabolize, and possibly produce norepinephrine. Moreover, considering that no VMAT1 and VMAT2 have been identified by immunofluorescence in retroperitoneal adipocytes, the possibility that PVAT adipocytes may represent a specific type of adipocytes able to accumulate norepinephrine may be suggested (45).
Chemerin-9 is an adipokine with vasoconstrictive abilities that decreases the endothelial-dependent vascular relaxation. Thus, Darios et al. have shown that chemerin produced by PVAT is stimulating the sympathetic contraction via its receptor, which is co-localized with tyrosine hydrolase in sympathetic nerves of the rat superior mesenteric artery, as a possible factor involved in some types of hypertension and obesity (42).
Other cytokines with vasoconstrictive capacity, in addition to chemerin, are released by PVAT, such as TNF-α and IL-6, which amplify the vascular tonus by NO and endothelial relaxation decrease, mainly in obese individuals. In obese mice, PVAT also releases contractile cyclooxygenase (COX) products, including thromboxane A2 (TXA2) and PGE2, although significant amounts of these substances have been identified in controls (Fig. 2) (10).
Thus, PVAT has the ability to release a panel of vasoconstrictive factors that act on the vascular wall directly or indirectly (via sympathetic or endothelial-dependent relaxation regulation). The serum levels of angiotensin II, superoxide, chemerin, catecholamines, leptin, and contractile prostanoids have been noticed in patients diagnosed with diabetes mellitus, obesity, and hypertension, with PVAT being currently identified as a source of all these factors (10).
Although PVAT is one of the key elements in the arterial tension control, the PVAT role in vascular remodelation, induced by the contractile factors produced by them, is still disputed (10).
Vasodilatory substances produced by PVAT adipocytes are counteracting these vasoconstrictor factors, such as resistin, adiponectin, leptin, NO, H2O2, TNF-α, MCP-1, CCL5/RANTES, visfatin, omentin-1, and angiotensin 1-7. Among these, leptin, TNF-α, IL-6, and H2O2 have a dual action, vasoconstrictory and vasodilatory, their balance being perturbed in the pathological status (2, 10, 36, 46). Supplementary, other types of cells, in addition to adipocytes, found in the PVAT structure, such as lymphocytes and macrophages, are sources of vasoactive factors and of substances that modulate the adipocyte activity (47).
However, hypertrophied PVAT in obesity shows hypoxia resulting in the adipocyte MCP-1 synthesis, followed by the monocyte production and release of TNF-α acting on vascular tonus (47).
H2O2 is a vasoactive substance that induces vasoconstriction and relaxation by different mechanisms, according to its concentration. By its direct action on the endothelium, H2O2 also induces the NO release and vascular wall relaxation in an endothelium-independent manner, involving the direct opening of K+ channels in smooth muscle fibers. The vascular tonus increase caused by H2O2 is a result of a direct COX stimulation and Ca2+ intracellular increase (2). In addition, H2O2 is also able to induce the Rho kinase pathway activation, resulting both in the vascular smooth muscle contraction and in cellular proliferation and migration in cardiovascular diseases. The vascular relaxation induced by an endothelial-dependent mechanism is correlated with the ROS production in PVAT. Subsequently, an increased ROS production in PVAT is an important factor that leads to a vascular tonus increase (48).
Results from a recent study performed on the thoracic aorta of Balb/c mice demonstrated that PVAT exerts an endothelium-independent vasodilatory effect, involving the PI3K/Akt pathway activation via the Mas and AT2 receptors stimulation. This pathway culminates in the NO and H2O2 production by neuronal nitric oxide synthase (49).
The NADPH activity increased in PVAT induces an increased production of O2– and a diminished synthesis of NO by the eNOS inhibition, a feature that contributes to vascular oxidative stress. This mechanism is also involved in PVAT dysfunctional contribution to endothelial disturbance secondary to the NO biodisponibility decrease (31). The mentioned effect is counterbalanced by angiotensin 1-7 produced by PVAT adipocytes, which by specific endothelial receptors stimulate the release of NO and the opening of voltage-dependent K+ (Kv) channels, resulting in a vascular tonus reduction (2).
Pro-inflammatory mediators, such as IL-6, MCP-1, and leptin, are highly expressed in human PVAT, compared to subcutaneous adipose tissue, resulting in dysfunctional vascular tonus, as demonstrated by studies which demonstrate that the vasodilator effect of PVAT adipocytes associated to small caliber vessels is diminished in obese people (17). Moreover, it seems that adiponectin plays a more enhanced role in vascular relaxation than in experimental rodent models (17).
Conclusion
The traditional perception of the adipose tissue structure and physiology, regarded as a reservoir tissue, has suffered radical changes during the last couple of decades. The classic types of adipocytes, white and brown, have been progressively supplemented by beige adipocytes and, lately, by the perivascular adipose tissue. Subsequently, the adipose tissue is regarded as a “tissue complex” with a remarkable morphologic and functional diversity, adapted to according to its anatomical location.
The data concerning the PVAT adipocytes origin, structure, and activity are limited, but most probably their specific precursors are different from other types of adipocytes and are correlated to their location. PVAT is not only a vascular support, with a protective role, but is also a real endocrine organ, as its adipocytes are clearly one of the types involved both in vascular homeostasis and in vascular dysfunctions associated with obesity, hypertension, and coronary ischemic disease. The identification of their secretory status in normal and dysfunctional status would open new regulatory pathways of the relationship between PVAT and vascular wall, by modulation of vascular protective factors.
The new concept that PVAT acts as a dynamic sensor of vascular biology led to the intensification of research to exploit its potential in obesity-related cardiovascular disease prevention and treatment. In this regard, potential pharmacological therapies are studied, focusing on different PVAT intracellular pathways related to the VSMCs proliferation inhibition, in vascular tone control, or in the inhibition of endothelial dysfunction. In this regard, the use of several drugs has been proposed, such as metformin/thiazolidinediones, which stimulate the AMP protein kinase, or captopril/telmisartan, which intervenes in the RAAS modulation of atorvastatin for the inhibition of HMG-CoA reductase (hydroxy-methyl-glutaryl-coenzyme A reductase), and of P-Selectin Glycoprotein Ligand-1 binding inhibitors, which reduce PVAT inflammation. The concept of reduction of PVAT inflammation and of serum triglycerides, by their burn in BAT, thoracic PVAT, or WAT beige adipocytes, is probably one of the most promising modalities of therapeutic approach in obesity, atherosclerosis, and other associated cardiovascular diseases.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – A.G., C.A., R.A.B., S.E.G., I.D.C.; Design – I.DC., S.E.G.; Supervision – I.D.C., R.A.B., S.E.G.; Fundings – Not applicable; Materials – Not applicable; Data collection &/or processing – Not applicable; Analysis &/or interpretation – Not applicable; Literature search – A.G., C.A., R.A.B., S.E.G., I.D.C.; Writing – A.G., C.A., R.A.B., S.E.G., I.D.C.; Critical review – A.G., C.A., R.A.B., S.E.G., I.D.C.; Graphic representation design – A.G., C.A., R.A.B.
References
- 1.Hildebrand S, Stümer J, Pfeifer A. PVAT and Its Relation to Brown, Beige, and White Adipose Tissue in Development and Function. Front Physiol. 2018;9:70. doi: 10.3389/fphys.2018.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa RM, Neves KB, Tostes RC, Lobato NS. Perivascular Adipose Tissue as a Relevant Fat Depot for Cardiovascular Risk in Obesity. Front Physiol. 2018;9:253. doi: 10.3389/fphys.2018.00253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soltis EE, Cassis LA. Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin Exp Hypertens A. 1991;13:277–96. doi: 10.3109/10641969109042063. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, et al. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–35. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 5.van Dam AD, Boon MR, Berbée JFP, Rensen PCN, van Harmelen V. Targeting white, brown and perivascular adipose tissue in atherosclerosis development. Eur J Pharmacol. 2017;816:82–92. doi: 10.1016/j.ejphar.2017.03.051. [DOI] [PubMed] [Google Scholar]
- 6.Qi XY, Qu SL, Xiong WH, Rom O, Chang L, Jiang ZS. Perivascular adipose tissue (PVAT) in atherosclerosis:a double-edged sword. Cardiovasc Diabetol. 2018;17:134. doi: 10.1186/s12933-018-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández-Alfonso MS, Somoza B, Tsvetkov D, Kuczmanski A, Dashwood M, Gil-Ortega M. Role of Perivascular Adipose Tissue in Health and Disease. Compr Physiol. 2017;8:23–59. doi: 10.1002/cphy.c170004. [DOI] [PubMed] [Google Scholar]
- 8.Gil-Ortega M, Somoza B, Huang Y, Gollasch M, Fernandez-Alfonso MS. Regional differences in perivascular adipose tissue impacting vascular homeostasis. Trends Endocrinol Metab. 2015;26:367–75. doi: 10.1016/j.tem.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka K, Sata M. Roles of Perivascular Adipose Tissue in the Pathogenesis of Atherosclerosis. Front Physiol. 2018;9:3. doi: 10.3389/fphys.2018.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramirez JG, O'Malley EJ, Ho WSV. Pro-contractile effects of perivascular fat in health and disease. Br J Pharmacol. 2017;174:3482–95. doi: 10.1111/bph.13767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Restini CBA, Ismail A, Kumar RK, Burnett R, Garver H, Fink GD, et al. Renal perivascular adipose tissue:Form and function. Vascul Pharmacol. 2018;106:37–45. doi: 10.1016/j.vph.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran KV, Fitzgibbons T, Min SY, DeSouza T, Corvera S. Distinct adipocyte progenitor cells are associated with regional phenotypes of perivascular aortic fat in mice. Mol Metab. 2018;9:199–206. doi: 10.1016/j.molmet.2017.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiong W, Zhao X, Villacorta L, Rom O, Garcia-Barrio MT, Guo Y, et al. Brown Adipocyte-Specific PPARγ(Peroxisome Proliferator-Activated Receptor γ) Deletion Impairs Perivascular Adipose Tissue Development and Enhances Atherosclerosis in Mice. Arterioscler Thromb Vasc Biol. 2018;38:1738–47. doi: 10.1161/ATVBAHA.118.311367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayala-Lopez N, Thompson JM, Watts SW. Perivascular Adipose Tissue's Impact on Norepinephrine-Induced Contraction of Mesenteric Resistance Arteries. Front Physiol. 2017;8:37. doi: 10.3389/fphys.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li P, Liu S, Lu M, Bandyopadhyay G, Oh D, Imamura T, et al. Hematopoietic-Derived Galectin-3 Causes Cellular and Systemic Insulin Resistance. Cell. 2016;167:973–84. doi: 10.1016/j.cell.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease:what is the link?Nutr Metab Cardiovasc Dis 2010. 20:481–90. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Meijer RI, Bakker W, Alta CL, Sipkema P, Yudkin JS, Viollet B, et al. Perivascular adipose tissue control of insulin-induced vasoreactivity in muscle is impaired in db/db mice. Diabetes. 2013;62:590–8. doi: 10.2337/db11-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dashwood MR, Loesch A. Does perivascular fat influence neural control of the saphenous vein?Implications in coronary artery bypass surgery (CABG) Curr Neurobiol. 2011;2:71–4. [Google Scholar]
- 19.Villacorta L, Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Horm Mol Biol Clin Investig. 2015;21:137–47. doi: 10.1515/hmbci-2014-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drosos I, Chalikias G, Pavlaki M, Kareli D, Epitropou G, Bougioukas G, et al. Differences between perivascular adipose tissue surrounding the heart and the internal mammary artery:possible role for the leptin-inflammation-fibrosis-hypoxia axis. Clin Res Cardiol. 2016;105:887–900. doi: 10.1007/s00392-016-0996-7. [DOI] [PubMed] [Google Scholar]
- 21.Rittig K, Staib K, Machann J, Bottcher M, Peter A, Schick F, et al. Perivascular fatty tissue at the brachial artery is linked to insulin resistance but not to local endothelial dysfunction. Diabetologia. 2008;51:2093–9. doi: 10.1007/s00125-008-1128-3. [DOI] [PubMed] [Google Scholar]
- 22.Egawa G, Miyachi Y, Kabashima K. Identification of perivascular adipose tissue in the mouse skin using two-photon microscopy. J Dermatol Sci. 2013;70:139–40. doi: 10.1016/j.jdermsci.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 23.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, et al. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–78. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pellegrinelli V, Carobbio S, Vidal-Puig A. Adipose tissue plasticity:how fat depots respond differently to pathophysiological cues. Diabetologia. 2016;59:1075–88. doi: 10.1007/s00125-016-3933-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vishvanath L, MacPherson KA, Hepler C, Wang QA, Shao M, Spurgin SB, et al. Pdgfrβ+Mural Preadipocytes Contribute to Adipocyte Hyperplasia Induced by High-Fat-Diet Feeding and Prolonged Cold Exposure in Adult Mice. Cell Metab. 2016;23:350–9. doi: 10.1016/j.cmet.2015.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ye M, Ruan CC, Fu M, Xu L, Chen D, Zhu M, et al. Developmental and functional characteristics of the thoracic aorta perivascular adipocyte. Cell Mol Life Sci. 2019;76:777–89. doi: 10.1007/s00018-018-2970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun C, Berry WL, Olson LE. PDGFRαcontrols the balance of stromal and adipogenic cells during adipose tissue organogenesis. Development. 2017;144:83–94. doi: 10.1242/dev.135962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baltieri N, Guizoni DM, Victorio JA, Davel AP. Protective role of perivascular adipose tissue in endothelial dysfunction and insulin-induced vasodilatation of hypercholesterolemic LDL receptor-deficient mice. Front Physiol. 2018;9:229. doi: 10.3389/fphys.2018.00229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Öhman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, et al. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein E deficient mice. Atherosclerosis. 2011;219:33–9. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, et al. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol. 2016;36:78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 32.Sena CM, Pereira A, Fernandes R, Letra L, Seiça RM. Adiponectin improves endothelial function in mesenteric arteries of rats fed a high-fat diet:role of perivascular adipose tissue. Br J Pharmacol. 2017;174:3514–26. doi: 10.1111/bph.13756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang ZJ, Wu J, Guo W, Zhu YZ. Atherosclerosis and the Hydrogen Sulfide Signaling Pathway - Therapeutic Approaches to Disease Prevention. Cell Physiol Biochem. 2017;42:859–75. doi: 10.1159/000478628. [DOI] [PubMed] [Google Scholar]
- 34.Du Y, Ji Q, Cai L, Huang F, Lai Y, Liu Y, et al. Association between omentin-1 expression in human epicardial adipose tissue and coronary atherosclerosis. Cardiovasc Diabetol. 2016;15:90. doi: 10.1186/s12933-016-0406-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabas I, García-Cardeña G, Owens GK. Recent insights into the cellular biology of atherosclerosis. J Cell Biol. 2015;209:13–22. doi: 10.1083/jcb.201412052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skiba DS, Nosalski R, Mikolajczyk TP, Siedlinski M, Rios FJ, Montezano AC, et al. Anti-atherosclerotic effect of the angiotensin 1-7 mimetic AVE0991 is mediated by inhibition of perivascular and plaque inflammation in early atherosclerosis. Br J Pharmacol. 2017;174:4055–69. doi: 10.1111/bph.13685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Numaguchi R, Furuhashi M, Matsumoto M, Sato H, Yanase Y, Kuroda Y, et al. Differential Phenotypes in Perivascular Adipose Tissue Surrounding the Internal Thoracic Artery and Diseased Coronary Artery. J Am Heart Assoc. 2019;8:e011147. doi: 10.1161/JAHA.118.011147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Elshafay A, Bendary AH, Vuong HT, Ahmed AR, Mokhtar MA, Soliman AL, et al. Does No-Touch Technique Better than Conventional or Intermediate Saphenous Vein Harvest Techniques for Coronary Artery Bypass Graft Surgery:a Systematic Review and Meta-analysis. J Cardiovasc Transl Res. 2018;11:483–94. doi: 10.1007/s12265-018-9832-y. [DOI] [PubMed] [Google Scholar]
- 39.Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107:1058–70. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rana KS, Pararasa C, Afzal I, Nagel DA, Hill EJ, Bailey CJ, et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc Diabetol. 2017;16:147. doi: 10.1186/s12933-017-0627-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177–85. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 42.Darios ES, Winner BM, Charvat T, Krasinksi A, Punna S, Watts SW. The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am J Physiol Heart Circ Physiol. 2016;311:H498–507. doi: 10.1152/ajpheart.00998.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Das E, Moon JH, Lee JH, Thakkar N, Pausova Z, Sung HK. Adipose Tissue and Modulation of Hypertension. Curr Hypertens Rep. 2018;20:96. doi: 10.1007/s11906-018-0894-7. [DOI] [PubMed] [Google Scholar]
- 44.Riedel J, Badewien-Rentzsch B, Kohn B, Hoeke L, Einspanier R. Characterization of key genes of the renin-angiotensin system in mature feline adipocytes and during in vitro adipogenesis. J Anim Physiol Anim Nutr (Berl) 2016;100:1139–48. doi: 10.1111/jpn.12392. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad MF, Ferland D, Ayala-Lopez N, Contreras GA, Darios E, Thompson J, et al. Perivascular Adipocytes Store Norepinephrine by Vesicular Transport. Arterioscler Thromb Vasc Biol. 2019;39:188–99. doi: 10.1161/ATVBAHA.118.311720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aghamohammadzadeh R, Unwin RD, Greenstein AS, Heagerty AM. Effects of Obesity on Perivascular Adipose Tissue Vasorelaxant Function:Nitric Oxide, Inflammation and Elevated Systemic Blood Pressure. J Vasc Res. 2015;52:299–305. doi: 10.1159/000443885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nosalski R, Guzik TJ. Perivascular adipose tissue inflammation in vascular disease. Br J Pharmacol. 2017;174:3496–513. doi: 10.1111/bph.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.da Costa RM, Fais RS, Dechandt CRP, Louzada-Junior P, Alberici LC, Lobato NS, et al. Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice. Br J Pharmacol. 2017;174:3527–41. doi: 10.1111/bph.13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nóbrega N, Araújo NF, Reis D, Facine LM, Miranda CAS, Mota GC, et al. Hydrogen peroxide and nitric oxide induce anticontractile effect of perivascular adipose tissuevia renin angiotensin system activation. Nitric Oxide. 2019;84:50–9. doi: 10.1016/j.niox.2018.12.011. [DOI] [PubMed] [Google Scholar]