Abstract
Objective:
Intravascular ultrasound (IVUS) is not routinely performed in the real-world practice, and the benefits of IVUS-guided drug-eluting stent (DES) implantation in patients with complex coronary lesions remains unclear. This updated meta-analysis attempts to evaluate the clinical outcomes of the IVUS guidance in these patients.
Methods:
We searched potential eligible citations from the PubMed, EMBASE, Medline, and other internet sources. The primary endpoint were major adverse cardiovascular events (MACE), including cardiac death, myocardial infarction (MI), and target vessel revascularization (TVR). The risk of definite/probable stent thrombosis (ST) was chosen as the safety endpoint.
Results:
Nine randomized trials including a total of 3,612 patients with complex coronary lesions were finally analyzed. Compared to angiography guidance, IVUS-guided DES implantation was associated with significantly lower incidence of MACE [odds ratios (OR) 0.57, 95% confidence intervals (CI): 0.45–0.72, p<0.001; I2=0.0%, p=0.674], cardiac death (OR 0.42, 95%CI:0.21–0.82, p=0.010; I2=0.0%, p=0.961), MI (OR 0.65, 95%CI:0.44–0.95, p=0.027; I2=41.8%, p=0.089), TVR (OR 0.55, 95%CI:0.38–0.79, p=0.001; I2=0.0%, p=0.916), target lesion revascularization (TLR) (OR 0.58, 95%CI:0.41–0.82, p=0.002; I2=0.0%, p=0.888), and ST (OR 0.48, 95%CI:0.24–0.93, p=0.029; I2=0.0%, p=0.733).
Conclusion:
The updated meta-analysis demonstrates that DES implantation under IVUS guidance leads to a significant reduction in MACE, cardiac death, MI, TVR, TLR, and ST among patients with complex coronary lesions.
Keywords: intravascular ultrasound, angiography, drug-eluting stent, complex coronary lesions
Introduction
At present, the bare metal stent (BMS) has been widely replaced by the drug-eluting stent (DES), mainly because of its relevant superiority in the reduced rate of stent restenosis, which would significantly decrease the risk of repeat revascularization, and then lead to better stenting outcomes (1, 2). Certainly, the associated beneficial efficacy will get strengthened if a high resolution in evaluating the lesion characteristics is available.
Intravascular ultrasound (IVUS) has been developed as a matured technique, which will provide more accurate details on the vessel size, lesion length, or plaque burden, serving as a powerful approach for optimizing the stenting procedures (3, 4). When receiving DES implantation under IVUS guidance, the relevant changes would mainly manifest as usage of larger or longer stents/balloons, as well as a greater frequency of post-dilation, which would result in a larger post-procedural minimal lumen diameter (MLD) and subsequently reduced incidence of adverse cardiovascular events (5). Recently, the ULTIMATE trial (6) (intravascular ultrasound-guided versus angiography-guided implantation of drug-eluting stent in all-comers) demonstrated that DES implantation under IVUS guidance could significantly reduce the target vessel failure (TVF) in all-comers, compared to angiography guidance. In fact, several large registries (7, 8) and randomized controlled trials (RCT) (9, 10), as well as several meta-analyzes (11, 12), also indicated the benefits of IVUS-guided DES implantation in patients with complex coronary lesions, which were mainly in terms of the reduced risk of major adverse cardiovascular events (MACE). In contrast, other several large RCTs (13-15) indicated that IVUS guidance did not improve long-term MACE rates. These controversial data and the real-high expense would strictly limit IVUS to be routinely performed in clinical practice. Besides, a new RCT (16) focusing on exploring the efficacy of the IVUS-guided DES implantation in patients with unprotected left main (LM) disease has been published recently. As a result, we updated the present meta-analysis to identify the benefits of IVUS-guided DES implantation in patients with complex coronary lesions.
Methods
Literature search
These clinical trials comparing IVUS versus coronary-angiography-guided DES implantation (described as the CAG group) in patients with complex coronary lesions (defined as long coronary artery lesions, chronic total occlusion [CTO] lesions, unprotected LM disease, bifurcation lesions, multiple overlapping stents, or the composite of all the above-mentioned) were searched from the Medline, EMBASE, and the Cochrane Controlled Trials Registry, as well as several other internet sources (last search was in March 2019). To make sure all the potential eligible citations were screened, several relevant keywords and medical subjects were combined, including “intravascular ultrasound or IVUS”, “CTO, LM, bifurcation, long lesions, multiple overlapping stents, or small vessel”, and “drug-eluting stent or DES”. In addition, the potentially relevant references listed in these published reviews or meta-analyzes were also screened for eligibility.
Inclusion and exclusion criteria
All eligible citations should meet the following criteria: (1) enrolled adult patients (age from 18 to 90 years) with complex coronary lesions as defined above; (2) randomized clinical trials comparing IVUS versus CAG-guided DES implantation and performing ≥1-year follow-up; and (3) reported relevant results of adverse clinical events. The exclusion criteria were as follows: (1) nonrandomized or non-English trials; (2) BMS implantation only or patients implanted with both BMS and DES, while the relevant data of DES were not provided; (3) duplicated studies, or different studies using the same sample origin.
Data extraction, synthesis, and quality assessment
The standardized data-abstraction forms were used by two independent investigators (FZG and XMN) to review all relevant studies for assessing their eligibility. Disagreements were resolved by a third investigator (X.Y.). The following data were extracted from each included study: the name or the first author of the trial, publication year, baseline demographics, characteristics of lesions, details of stenting procedures, and the clinical outcomes during follow-up. The Jadad score (17) was used to assess the quality of each included randomized study.
Study endpoints
The primary endpoint was the incidence of MACE, and other clinical outcomes were also analyzed as follows: (1) cardiovascular death and all-cause death, (2) myocardial infarction (MI), (3) target vessel revascularization (TVR), and target lesion revascularization (TLR). The definition of MACE differed slightly across each study, and we analyzed all the data following each trial-specific definition as appreciate. The risk of definite/probable stent thrombosis (ST) was chosen as the safety endpoint following the definition by the Academic Research Consortium (18).
Statistical analysis
The meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statements (19). We used the STATA 12.0 (Stata Corp LP, College Station, TX, USA) for the entire statistical analysis, and all of the p-values were two-tailed. All the endpoints were recorded as dichotomous variables, and relevant comparisons were estimated with odds ratios (OR) and 95% confidence intervals (CI). When a p-value was <0.05, statistical significance would be confirmed. If the p-value of Cochrane’s Q test was <0.10, and/or the I2 statistic was ≥0%, significant heterogeneity was indicated, and a random-effect model was selected, or the fixed-effects model with the Mantel–Haenszel method would be preferred instead. Egger’s test was performed for assessing the publication bias, and significant asymmetry had to be considered when the p-value was <0.1 (20). The sensitivity analyzes (exclude one study at a time) were performed to assess the stability of the overall treatment effects.
Results
Eligible studies and patient characteristics
After screening 570 initial articles through the electronic database and another 20 references listed in several published meta-analyzes, a total of nine RCTs (9, 10, 13-16, 21-23) were finally enrolled in the present meta-analysis (Fig. 1). Among these included citations, there were two trials in each subset, including long lesions (9, 15), CTO (10, 21), unprotected LM disease (16, 22), or complex coronary lesions (13, 14). Only 1 paper was for a de novo lesion in a small vessel (diameter 2.25–2.75 mm) (23). Detailed baseline demographics and characteristics of lesions and stenting procedures are summarized in Tables 1–3. The follow-up duration in these studies ranged from 1 year to 2 years, and the quality assessment based on the Jadad score for each study was good except for the two (22, 23).
Figure 1.
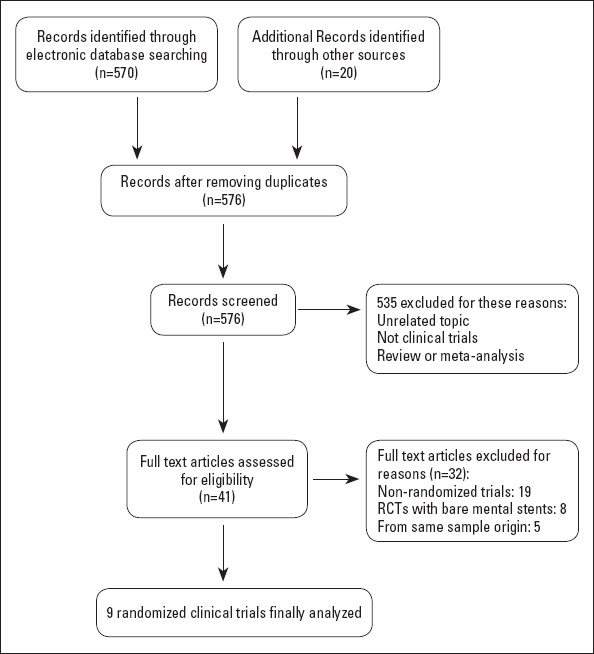
Flow chart depicting the selection of studies enrolled in this meta-analysis
Table 1.
The details of the included RCTs
| Study | Center | Enrolled patients | Sample size, n IVUS/Control | Follow-Up | MACE | Type of DES | Study quality |
|---|---|---|---|---|---|---|---|
| RESET trial (2013) | Multicenter | Long lesions | 269/274 | 1 year | Cardiac death, MI, TVR, or ST | Zotarolimus/Everolimus | 5 |
| IVUS-XPL trial (2016) | Multicenter | Long lesions | 700/700 | 1 year | Cardiac death, MI, or TLR | Everolimus | 5 |
| CTO-IVUS trial (2015) | Multicenter | CTO | 201/201 | 1 year | Cardiac death, MI, or TVR | Zotarolimus/Nobori biolimus | 5 |
| Air-CTO trial (2015) | Multicenter | CTO | 115/115 | 2 years | Death, MI, TLR, ST | First/Second-generation | 4 |
| Tan et al. (2015) | Single center | Unprotected LM | 61/62 | 2 years | Death, MI, or TLR | Sirolimus | 2 |
| Liu et al. (2019) | Single center | Unprotected LM | 167/169 | 1 year | Cardiac death, MI, and TVR | First /Second-generation | 3 |
| Home DES IVUS (2010) | Single center | Complex lesions | 105/105 | 18 months | Death, MI, TLR | TAXUS/CYPHER | 4 |
| AVIO trial (2013) | Multicenter | Complex lesions | 142/142 | 2 years | Cardiac death, MI, TVR | NA | 4 |
| Zhang et al. (2016) | Single center | De novo lesion in a small vessel (diameter 2.25–2.75 mm) | 42/42 | 1 year | Cardiac death, MI, or TVR | NA | 2 |
Note: The qualities of included randomized trials were assessed by the Jadad score. CTO - chronic total occlusion; DES - drug-eluting stent; IVUS - intravascular ultrasound; LM - left main disease; MACE - major averse cardiovascular events; MI - myocardial infarction; mm - millimeter; n - number; NA - not available; RCT - randomized controlled trials; ST - stent thrombosis; TLR - target lesion revascularization; TVR - target vessel revascularization
Table 2.
Characteristics of the past medical histories among the included RCTs
| Study | Age, years | Male, % | Hypertension, % | Diabetes, % | Dyslipidemia, % | Smoker, % | Prior MI, % | Prior PCI, % | LVEF, % |
|---|---|---|---|---|---|---|---|---|---|
| RESET trial (2013) | 62.8/64.3 | 65.8/54.7 | 61.3/65.8 | 31.6/29.9 | 61.3/61.7 | 21.6/17.2 | 1.1/2.9 | NA | 55.3/54.0 |
| IVUS-XPL trial (2016) | 64/64 | 69.0/68.7 | 64.9/63.4 | 35.7/36.6 | 67.3/65.4 | 22.1/25.9 | 4.9/4.1 | 10.9/9.9 | 62.9/62.4 |
| CTO-IVUS trial (2015) | 61.0/61.4 | 80.6/80.6 | 62.7/63.7 | 34.8/33.8 | NA | 35.3/34.3 | 8.0/8.0 | 15.4/15.9 | 56.9/56.7 |
| Air-CTO trial (2015) | 67/66 | 88.7/80.0 | 74.8/70.4 | 29.6/27.0 | 21.7/27.8 | 39.1/39.1 | 20.9/30.4 | NA | 55/56 |
| Tan et al. (2015) | 76.5/75.6 | 62.3/69.4 | 41.0/46.8 | 34.4/29.5 | NA | 44.3/46.8 | 16.4/21.0 | NA | 55.3/53.3 |
| Liu et al. (2019) | 65.3/64.9 | 63.5/63.9 | 69.5/72.2 | 33.5/30.8 | 37.7/37.9 | 37.1/35.5 | 17.4/14.2 | 19.8/16.6 | 55.6/58.4 |
| HOME DES IVUS (2010) | 59.4/60.2 | 73.3/71.4 | 66.7/71.4 | 41.9/44.8 | 62.9/65.7 | 40.0/35.2 | 37.1/32.4 | 17.1/14.3 | NA |
| AVIO trial (2013) | 63.9/63.6 | 82.4/76.8 | 70.4/66.9 | 23.9/26.8 | 70.4/76.8 | 34.5/31.0 | NA | NA | 55.3/55.9 |
| Zhang et al. (2016) | 63/60 | 50.0/59.5 | 64.3/59.5 | NA | 47.6/59.5 | 52.4/52.4 | NA | NA | 58/57 |
Data are recorded as intravascular ultrasound guidance vs. angiography guidance. LVEF - left ventricular ejection fraction; MI - myocardial infarction; NA - not available; PCI - percutaneous coronary intervention
Table 3.
Angiographic and procedural characteristics
| RESET | IVUS-XPL | CTO-IVUS | Air-CTO | Tan et al. | Liu et al. | HOME DES IVUS | AVIO | Zhang et al. | |
|---|---|---|---|---|---|---|---|---|---|
| LM, % | 0/0 | 0/0 | 0/0 | 0/2.6 | 100/100 | 100/100 | 2.9/3.8 | NA | 0/0 |
| LAD, % | 62.1/67.5 | 65.0/59.9 | 41.8/46.8 | 44.3/36.5 | NA | 55.7/52.7 | 56.2/54.3 | 53.3/48.6 | 47.6/52.4 |
| LCX, % | 15.2/12.8 | 13.7/15.4 | 14.4/15.9 | 20.9/14.8 | NA | 44.3/49.7 | NA | NA | 47.6/50.0 |
| RCA, % | 22.7/19.7 | 21.3/24.7 | 43.8/37.3 | 34.8/46.1 | NA | 62.3/58.0 | 28.6/23.8 | NA | 54.8/42.9 |
| MVD, % | 40.5/37.6 | NA | 10.4/7.5 | 48.7/57.4 | 88.5/83.9 | 82.6/84.6 | 15.0/17.0 | NA | NA |
| Lesion length, mm | 29.6/30.6 | 34.7/35.2 | 36.3/35.5 | 29.0/30.6 | NA | NA | 18.1/17.6 | 27.4/25.5 | 17.0/15.1 |
| Reference vessel diameter, mm | 2.82/2.80 | 2.89/2.85 | 2.69/2.64 | 2.65/2.60 | NA | NA | 3.17/2.95 | 2.7/2.6 | 2.40/2.39 |
| Stent length, mm | 32.4/32.3 | 39.3/39.2 | 43.6/41.5 | 55/52 | 21.5/18.2 | 32.6/33.3 | 23.6/22.1 | 23.9/23.2 | 26.1/23.3 |
| Stent number, n | NA | 1.3/1.3 | 1.7/1.6 | 1.6/1.5 | 1.4/1.4 | 2.2/2.4 | 1.3/1.3 | NA | NA |
| Stent diameter, mm | NA | NA | 2.91/2.85 | 3.05/2.86 | 3.43/3.44 | 3.46/3.29 | NA | 2.95/2.86 | 2.64/2.45 |
| Post-dilation, % | 54.6/44.5 | 76.3/57.4 | 51.2/41.3 | NA | 37.7/14.5 | NA | 31.4/0 | 88.3/68.4 | NA |
| Max balloon diameter, mm | 3.1/3.1 | 3.1/3.0 | NA | NA | NA | 3.53/3.45 | 3.3/3.1 | 3.4/3.2 | 2.9/2.6 |
| Max balloon pressure, atm | 13.5/13.5 | 16.5/15.9 | 14.6/13.8 | NA | NA | 15.3/13.9 | 16.4/15.2 | 20.3/19.6 | 15.9/14.1 |
| Pre-procedural MLD, mm | 0.95/0.93 | 0.83/0.82 | NA | NA | 1.90/1.92 | NA | 1.1/0.97 | 0.76/0.65 | 0.91/0.90 |
| Post-procedural MLD, mm | 2.55/2.55 | 2.64/2.56 | 2.64/2.56 | 2.62/2.40 | 3.44/3.43 | NA | 2.94/2.87 | 2.55/2.39 | 2.77/2.53 |
| Pre-procedural DS, % | NA | 71.1/71.4 | 100/100 | 100/100 | NA | NA | 82.3/79.2 | 71.6/75.5 | 77.8/77.8 |
| Post-procedural DS, % | NA | 12.8/13.7 | 9.0/10.2 | 11.0/13.8 | NA | NA | 14.6/15.3 | 13.9/15.5 | 6.7/7.9 |
Data are recorded as intravascular ultrasound guidance vs. angiography guidance. DS - diameter stenosis; LAD - left anterior descending artery; LCX - left circumflex artery; LM - left main coronary artery; mm - millimeters; MLD - minimal lumen diameter; MVD - multi-vessel disease; n - number; RCA - right coronary artery; NA - not available
Study endpoints
All nine trials reported MACE and MI results, while eight trials (9, 10, 14-16, 21-23) reported cardiac death data, four trials (10, 13, 15, 21) reported all-cause death data, six trials (10, 14-16, 21, 23) reported TVR data, seven trials (9, 10, 13, 14, 16, 21, 22) reported TLR data, and eight trials (9, 10, 13-16, 21, 22) reported ST results. No significant heterogeneity was observed, and the fixed-effects model with the Mantel–Haenszel method was applied for all result analyzes.
MACE
As depicted in Figure 2, significant reduction in the risk of MACE was observed with respect to IVUS-guided DES implantation (OR 0.57, 95% CI: 0.45–0.72, p<0.001; I2=0.0%, p=0.674). Egger’s test suggested no publication bias (p=0.251), and the sensitivity analysis proved the superiority of IVUS guidance based on the omission of a single study from the overall analysis at one time. In addition, after excluding the Tan et al. (22) and Zhang et al. (6) trials (potentially high-bias risk studies), the results remained stable (OR 0.60, 95% CI: 0.47–0.77, p<0.001; I2=0.0%, p=0.676, Supplement Fig. 1).
Figure 2.
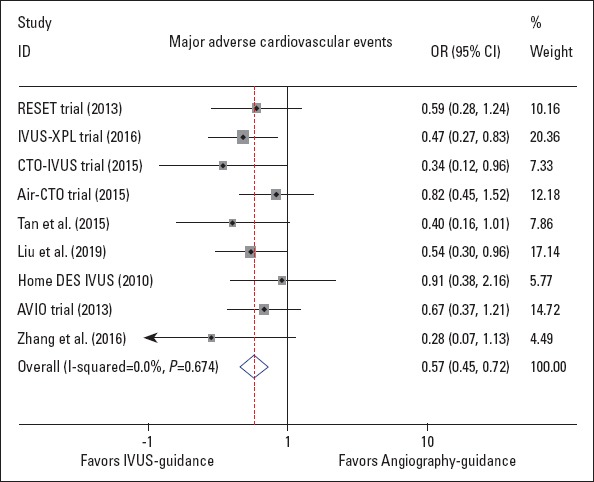
Forest plot of the odds ratio of major adverse cardiovascular events, associated with IVUS guidance compared with angiography guidance
Supplement Figure 1.
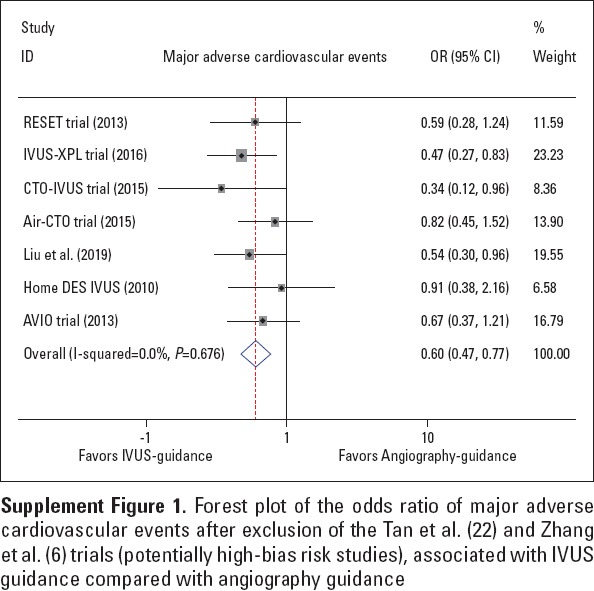
Forest plot of the odds ratio of major adverse cardiovascular events after exclusion of the Tan et al. (22) and Zhang et al. (6) trials (potentially high-bias risk studies), associated with IVUS guidance compared with angiography guidance
Other clinical outcomes
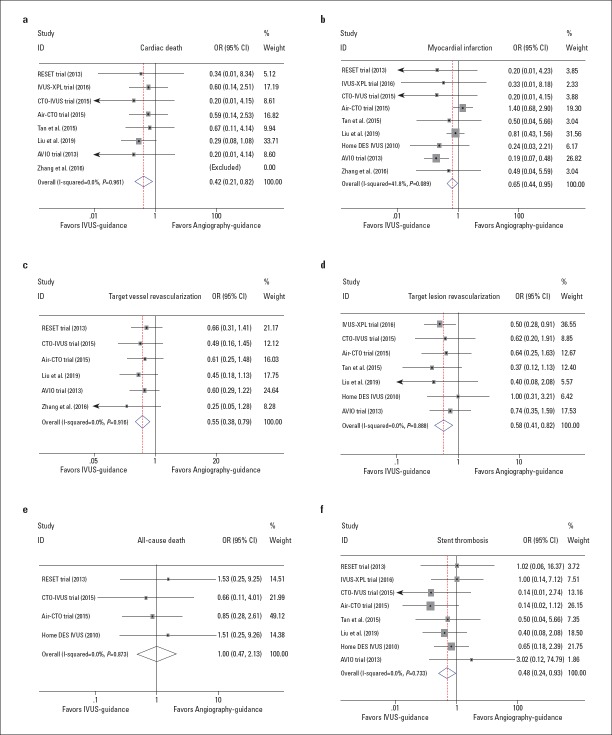
As shown in Figure 3, IVUS-guided DES implantation was associated with a lower incidence of cardiac death (OR 0.42, 95% CI: 0.21–0.82, p=0.010; I2=0.0%, p=0.961, Fig. 3a); MI (OR 0.65, 95% CI: 0.44–0.95, p=0.027; I2=41.8%, p=0.089, Fig. 3b); TVR (OR 0.55, 95% CI: 0.38–0.79, p=0.001; I2=0.0%, p=0.916, Fig. 3c); TLR (OR 0.58, 95% CI: 0.41–0.82, p=0.002; I2=0.0%, p=0.888, Fig. 3d), and ST (OR 0.48, 95% CI: 0.24–0.93, p=0.029; I2=0.0%, p=0.733, Fig. 3f). There was no publication bias determined by Egger’s test (p=0.764, 0.466, 0.133, 1.000, and 0.711 for cardiac death, MI, TVR, TLR, and ST, respectively), and the sensitivity analysis confirmed the stability of these positive results. However, the risk of all-cause death did not differ significantly between the IVUS guidance and angiography guidance (OR 1.00, 95% CI: 0.47–2.13, p=0.993; I2=0.0%, p=0.873, Fig. 3e).
Figure 3.
Forest plots of other efficacy endpoints of the included trials. The odds ratios of cardiac death (a), myocardial infarction (b), target vessel revascularization (c), target lesion revascularization (d), all-cause death (e), and stent thrombosis (f), associated with IVUS guidance compared with angiography guidance
Discussion
Based on the whole analysis, the major findings demonstrated that (1) IVUS-guided DES implantation may significantly reduce the risk of MACE, cardiac death, MI, TVR, TLR, and ST among patients with complex coronary lesions; and that (2) there is no significant difference with respect to the incidence of all-cause death.
This updated meta-analysis extended the superior results from our prior citation (11), among which a new RCT (16) focusing on exploring the efficacy of the IVUS guidance in patients suffering from unprotected LM disease was employed. In the recent trial, a total of 336 patients were enrolled (IVUS guidance vs. angiography guidance, 167 vs. 169, respectively), and the results showed that the IVUS guidance was related to a lower incidence of MACE (13.2% vs. 21.9%, p=0.031), which might mainly be derived from the significant reduction in the risk of cardiac death (1.8% vs. 5.9%, p=0.048) (16). In fact, the large ULTIMATE trial (6) (intravascular ultrasound-guided versus angiography-guided implantation of drug-eluting stent in all-comers) has indicated that DES implantation under IVUS guidance could significantly reduce the TVF by analyzing the largest sample size (IVUS guidance vs. angiography guidance, 724 vs. 724, respectively), which will make the dominant position of IVUS guidance in all-comer patients well established. Of note, most occurrences of procedure-related adverse events were mainly because of the under-expansion and malposition of implanted stents. In the BMS era, the IVUS guidance could significantly reduce the risk of restenosis and TVR, but it did not decrease the incidence of death and MI (24), while better results were found with DES (1, 25). However, it would still be much easier for the implanted DES to appear with under-expansion and malposition in complex coronary lesions, which will significantly increase the risk of stent restenosis and subsequently lead to worse clinical outcomes (26). IVUS plays the key role in overcoming several limitations of coronary angiography in the stenting procedures, because not only much more accurate details of the lesion characteristics and stenting procedures are provided for decision making, but because it is also helpful in detecting relevant complications earlier.
In patients with LM disease, the major limit of coronary angiography guidance might be caused by the potential interfering effects of the aortic cusp opacification, particularly in these with distal bifurcation lesions (27). It will be quite difficult for coronary angiography to achieve the precise data of target vessels, because it mainly relies on the visual inspection. Instead, IVUS will make it easier to achieve more accurate details of target vessels, mainly based on the digital inspection of lesion morphology, true luminal size, lumen area, and reference lumen area, and then provide a better approach to selecting an appropriate diameter and length of the implanted stents or applied balloons. As summarized in our meta-analysis, a greater frequency of post-dilation and a lager max balloon diameter and max balloon pressure were used under the IVUS guidance, and they resulted in a lager stent diameter, which could significantly reduce the under-expansion and malposition of implanted stents. Consequently, a 38.6% reduction in MACE and a 60.7% reduction in cardiac death were observed with respect to IVUS guidance in the present study.
In general, the optimal stent deployment was defined as follows: good apposition (apposition of all stent struts to the vessel wall), optimal stent expansion (with minimal stent area of 5 mm2) or cross-sectional area (CSA) >90% of distal reference lumen CSA for small vessel, and no edge dissection (5 mm margins proximal and distal to the stent). However, we did not perform the subgroup analysis because among these included trials, only the IVUS-XPL (9) (IVUS-Xience Prime stent for long coronary lesions) trial reported the relevant data, indicating that patients who did not meet the IVUS criteria had a significantly higher incidence of MACE compared to those meeting the IVUS criteria for stent optimization (4.6% vs. 1.5%, p=0.02). Nonetheless, similar encouraging results pertaining to IVUS-guided DES implantation were also acquired in the present study. Instead, a non-significantly reduced risk of all-cause death was observed in the IVUS guidance group. As a result, more powerful relevant randomized trials performing a further sub-analysis of patients with an IVUS-defined optimal stenting procedure are still warranted to confirm all the benefits with respect to the IVUS guidance in patients with complex coronary lesions.
Study limitations
There were several limitations involved in the current meta-analysis. First, no individual patient data were analyzed, and several included RCTs had small sample sizes, which might affect the evaluation of IVUS guidance’s efficacy. Second, some accurate details of the stenting procedures were still absent from this study mainly because of the inadequacy of relevant data, including the time of procedure, selection of different two-stent techniques for potentially occurred bifurcation lesions, or choice for sheaths with different sizes, etc. Third, the follow-up duration in these included trials was different. Although at least a 1-year follow-up was required in each enrolled study, the longer follow-up was still preferred to compare IVUS guidance to angiography guidance. Finally, no definite maintained durations and dosages of the dual antiplatelet therapy regimen post-stenting procedures for these included patients.
Conclusion
IVUS-guided DES implantation could significantly reduce the risk of MACE, cardiac death, MI, TVR, TLR, and ST in patients with complex coronary lesions. More powerful randomized clinical trials with more precise subgroup analyses are still warranted to confirm the benefits of IVUS guidance for these patients.
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – Z.F.; Design – Z.F., M.X.; Supervision – Z.F., Y.X.; Fundings – B.X., S.H.; Materials – Z.F., H.W.; Data collection &/or processing – Z.F., M.X.; Analysis &/or interpretation – Z.F., M.X., B.X.; Literature search – H.W., B.X.; Writing – Z.F.; Critical review – S.H.
References
- 1.Stone GW, Ellis SG, Cox DA, Hermiller J, O'Shaughnessy C, Mann JT, et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–31. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- 2.Stone GW, Moses JW, Ellis SG, Schofer J, Dawkins KD, Morice MC, et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- 3.Mintz GS. Features and parameters of drug-eluting stent deployment discoverable by intravascular ultrasound. Am J Cardiol. 2007;100:26M–35M. doi: 10.1016/j.amjcard.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 4.Roy P, Steinberg DH, Sushinsky SJ, Okabe T, Pinto Slottow TL, Kaneshige K, et al. The potential clinical utility of intravascular ultrasound guidance in patients undergoing percutaneous coronary intervention with drug-eluting stents. Eur Heart J. 2008;29:1851–7. doi: 10.1093/eurheartj/ehn249. [DOI] [PubMed] [Google Scholar]
- 5.Elgendy IY, Mahmoud AN, Elgendy AY, Bavry AA. Outcomes With Intravascular Ultrasound-Guided Stent Implantation:A Meta-Analysis of Randomized Trials in the Era of Drug-Eluting Stents. Circ Cardiovasc Interv. 2016;9:e003700. doi: 10.1161/CIRCINTERVENTIONS.116.003700. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Gao X, Kan J, Ge Z, Han L, Lu S, et al. Intravascular Ultrasound Versus Angiography-Guided Drug-Eluting Stent Implantation:The ULTIMATE Trial. J Am Coll Cardiol. 2018;72:3126–37. doi: 10.1016/j.jacc.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents:the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463–70. doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- 8.Claessen BE, Mehran R, Mintz GS, Weisz G, Leon MB, Dogan O, et al. Impact of intravascular ultrasound imaging on early and late clinical outcomes following percutaneous coronary intervention with drug-eluting stents. JACC Cardiovasc Interv. 2011;4:974–81. doi: 10.1016/j.jcin.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Hong SJ, Kim BK, Shin DH, Nam CM, Kim JS, Ko YG, et al. Effect of Intravascular Ultrasound-Guided vs Angiography-Guided Everolimus-Eluting Stent Implantation:The IVUS-XPL Randomized Clinical Trial. JAMA. 2015;314:2155–63. doi: 10.1001/jama.2015.15454. [DOI] [PubMed] [Google Scholar]
- 10.Kim BK, Shin DH, Hong MK, Park HS, Rha SW, Mintz GS, et al. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation:Randomized Study. Circ Cardiovasc Interv. 2015;8:e002592. doi: 10.1161/CIRCINTERVENTIONS.115.002592. [DOI] [PubMed] [Google Scholar]
- 11.Fan ZG, Gao XF, Li XB, Shao MX, Gao YL, Chen SL, et al. The outcomes of intravascular ultrasound-guided drug-eluting stent implantation among patients with complex coronary lesions:a comprehensive meta-analysis of 15 clinical trials and 8,084 patients. Anatol J Cardiol. 2017;17:258–68. doi: 10.14744/AnatolJCardiol.2016.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bavishi C, Sardar P, Chatterjee S, Khan AR, Shah A, Ather S, et al. Intravascular ultrasound-guided vs angiography-guided drug-eluting stent implantation in complex coronary lesions:Meta-analysis of randomized trials. Am Heart J. 2017;185:26–34. doi: 10.1016/j.ahj.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 13.Jakabcin J, Spacek R, Bystron M, Kvasnák M, Jager J, Veselka J, et al. Long-term health outcome and mortality evaluation after invasive coronary treatment using drug eluting stents with or without the IVUS guidance. Randomized control trial. HOME DES IVUS. Catheter Cardiovasc Interv. 2010;75:578–83. doi: 10.1002/ccd.22244. [DOI] [PubMed] [Google Scholar]
- 14.Chieffo A, Latib A, Caussin C, Presbitero P, Galli S, Menozzi A, et al. A prospective, randomized trial of intravascular-ultrasound guided compared to angiography guided stent implantation in complex coronary lesions:the AVIO trial. Am Heart J. 2013;165:65–72. doi: 10.1016/j.ahj.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 15.Kim JS, Kang TS, Mintz GS, Park BE, Shin DH, Kim BK, et al. Randomized comparison of clinical outcomes between intravascular ultrasound and angiography-guided drug-eluting stent implantation for long coronary artery stenoses. JACC Cardiovasc Interv. 2013;6:369–76. doi: 10.1016/j.jcin.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 16.Liu XM, Yang ZM, Liu XK, Zhang Q, Liu CQ, Han QL, et al. Intravascular ultrasound-guided drug-eluting stent implantation for patients with unprotected left main coronary artery lesions:A single-center randomized trial. Anatol J Cardiol. 2019;21:83–90. doi: 10.14744/AnatolJCardiol.2018.21447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials:is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 18.Laskey WK, Yancy CW, Maisel WH. Thrombosis in coronary drug-eluting stents:report from the meeting of the Circulatory System Medical Devices Advisory Panel of the Food and Drug Administration Center for Devices and Radiologic Health, December 7-8, 2006. Circulation. 2007;115:2352–7. doi: 10.1161/CIRCULATIONAHA.107.688416. [DOI] [PubMed] [Google Scholar]
- 19.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions:explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian NL, Gami SK, Ye F, Zhang JJ, Liu ZZ, Lin S, et al. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions:two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10:1409–17. doi: 10.4244/EIJV10I12A245. [DOI] [PubMed] [Google Scholar]
- 22.Tan Q, Wang Q, Liu D, Zhang S, Zhang Y, Li Y. Intravascular ultrasound-guided unprotected left main coronary artery stenting in the elderly. Saudi Med J. 2015;36:549–53. doi: 10.15537/smj.2015.5.11251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang JQ, Shi R, Pang W, Guo Q, Xu Y, Zhang J, et al. Application of intravascular ultrasound in stent implantation for small coronary arteries. J Clin Invasive Cardiol. 2016;3:2–8. [Google Scholar]
- 24.Parise H, Maehara A, Stone GW, Leon MB, Mintz GS. Meta-analysis of randomized studies comparing intravascular ultrasound versus angiographic guidance of percutaneous coronary intervention in pre-drug-eluting stent era. Am J Cardiol. 2011;107:374–82. doi: 10.1016/j.amjcard.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 25.Ahn JM, Kang SJ, Yoon SH, Park HW, Kang SM, Lee JY, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drug-eluting stent implantation in 26,503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338–47. doi: 10.1016/j.amjcard.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 26.Wilensky RL, Selzer F, Johnston J, Laskey WK, Klugherz BD, Block P, et al. Relation of percutaneous coronary intervention of complex lesions to clinical outcomes (from the NHLBI Dynamic Registry) Am J Cardiol. 2002;90:216–21. doi: 10.1016/s0002-9149(02)02457-8. [DOI] [PubMed] [Google Scholar]
- 27.Sano K, Mintz GS, Carlier SG, de Ribamar Costa J, Jr, Qian J, Missel E, et al. Assessing intermediate left main coronary lesions using intravascular ultrasound. Am Heart J. 2007;154:983–8. doi: 10.1016/j.ahj.2007.07.001. [DOI] [PubMed] [Google Scholar]