Abstract
Formation of fused pyrazoles via intramolecular 1,3-dipolar cycloadditions of diazo intermediates with pendant alkynes are described. A subsequent thermal [1s, 5s] sigmatropic shift of these pyrazole systems resulted in a ring contraction to form spirocyclic pyrazoles. The limitations of this rearrangement were explored by chang-ing the substituents on the non-migrating aromatic ring and by using substrates lacking an aromatic linkage to the propargyl group.
Keywords: dipolar cycloaddition, spirocycles, pyrazole, sigmatropic rearrangement
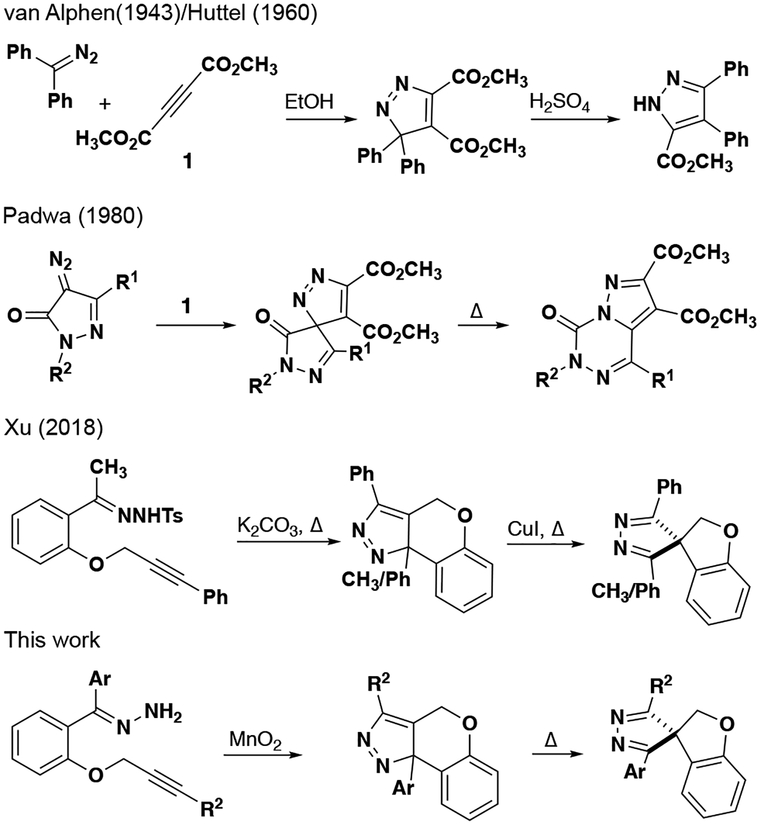
Pyrazoles are common heterocyclic cores in many approved drugs and pharmaceutical lead compounds.1–3 The majority of these heterocycles are made by one of two methods: 1) the Knorr condensation of a hydrazine with a 1,3-diketone or 2) dipolarcycloaddition (DPC) of a diazoalkanes with alkynes.4–7 Pyrazoles resulting from the latter method with two carbon substituents on an sp3-hybridized carbon adjacent to nitrogen can undergo migration to form either 1H-or 4H-pyrazoles. This process was first noted by van Alphen and the mechanism later explored by Hüttel (Figure 1).8–15 The most heavily explored variant involves cyclic acceptor-substituted diazo compounds, which predictably undergo migration of the acyl group to the neighboring nitrogen.16–19 Recently, focus has turned to similar migrations of alkyl groups on bicyclic or fused pyrazole systems.17,20–25 Padwa showed that spirocyclic pyrazoles could be formed, and then a thermal rearrangement would cause a ring expansion to a fused system.26 Valdés has recently explored the migration process in pyrazoles not fused to another ring.20,27 Methods to synthesize spiropyrazoles remain limited, with many requiring the use of transition metal catalysts.28–32 We discovered an approach to this core structure involving ring contraction following an intramolecular DPC reaction. During the final stages of our work, a similar sequence was reported by Xu using an alternative precursor to the requisite diazo substrates.21
Figure 1.
Dipolar cycloaddition rearrangement reactions.
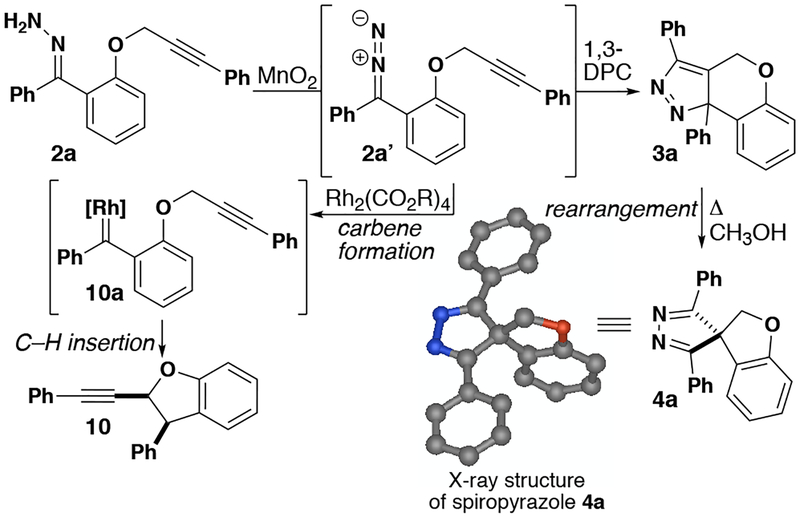
During investigations into C–H insertion reactions of donor/donor metal carbenes, we noticed a competing pathway of an intramolecular 1,3-dipolar cycloaddition of the diazo intermediate in the absence of a rhodium catalyst (Scheme 1).33 It was previously known that diazo intermediates could be trapped in the presence of unsaturated alkenes and alkynes.34–38 Following our standard procedure, hydrazone 2a was made by condensing hydrazine onto the corresponding ketone. Once the hydrazone was oxidized by manganese dioxide, the resulting diazo compound (2a’) reacted immediately with the propargylic ether (Scheme 1).39–41 The new cycloaddition product 3a was isolated and the structure was determined by NMR spectroscopy. Compound 3a then underwent partial conversion to 4a during recrystallization at ambient temperature.8–11
Scheme 1.
Reactions of propargylic hydrazones.
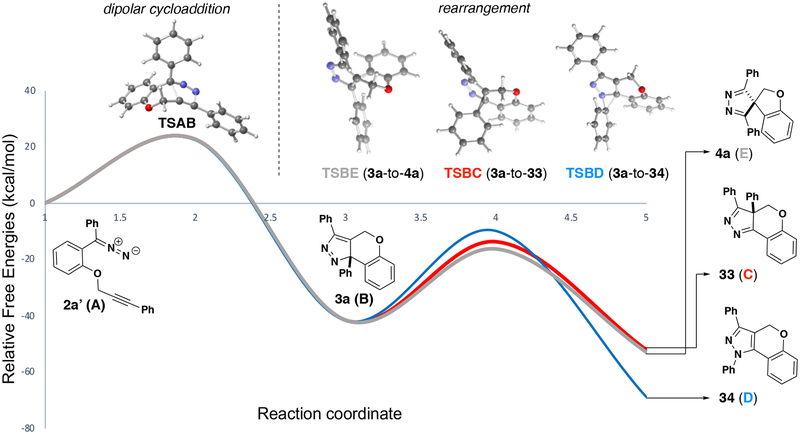
Computed transition states for the various migration pathways are consistent with the observed ring contraction. In previous studies, similar [1s, 5s] sigmatropic shifts proceed to give products of ring expansion.42 The rearrangement of 3a favors formation of the spirobicyclic pyrazole 4a over phenyl migration to either nitrogen or carbon (33 and 34; Figure 2). Results of density functional theory calculations (M062X/6–31+G(d,p)43) reveal that after the dipolar cycloaddition has taken place, 3a forms spirocycle 4a with an energy penalty of only 25.3 kcal/mol (TSBE) compared to the barriers of 27.8 and 31.8 kcal/mol for TSBC and TSBD, respectively. This preference is likely a result of orbital alignment leading to the newly formed σ-bond, this alignment is poorest at TSBD where the phenyl substituent migrates towards the nitrogen atom; in this system the phenyl group moves into the plane of the heterocycle rather than migrating over it44.
Figure 2.
Predicted (M062X/6–31+G(d,p)) reaction profile for compound 2a’, based on free energies of minima and transition state structures.
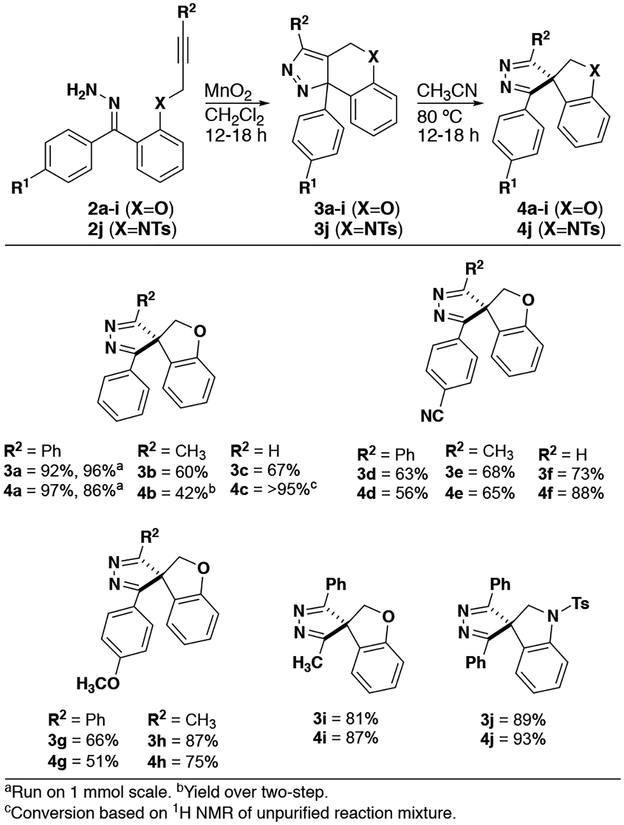
While the oxidation and DPC reactions occur readily at room temperature, various conditions were explored for the subsequent rearrangement. Heating to 80 °C for 12–18h provided full conversion. The temperature and time required were insensitive to the polarity of the solvent, including protic solvents. Acetonitrile was optimal for solubility and appropriate reflux temperature. These optimized conditions were applied to a variety of substrates (Figure 3). Changing R2 from a phenyl to methyl (4e and 4h, Figure 3) produced only small changes in yield relative to 4d and 4g, suggesting that this substituent has little impact on the migration step. When a terminal alkyne was used (R2=H), the rearrangement product 4c was not stable to purification. When R1 was changed from H to CN, the rearrangement proceeded smoothly and the product (4f) was isolated in 88% yield. Alternatively, when R1 was changed to OCH3, the terminal alkyne (R2=H) did not survive the harsher conditions needed for hydrazone formation. Changing the heteroatom from O to NTs (3a to 3j) did not negatively impact the DPC or rearrangement reactions.
Figure 3.
Benzophenone derived spiropyrazoles.
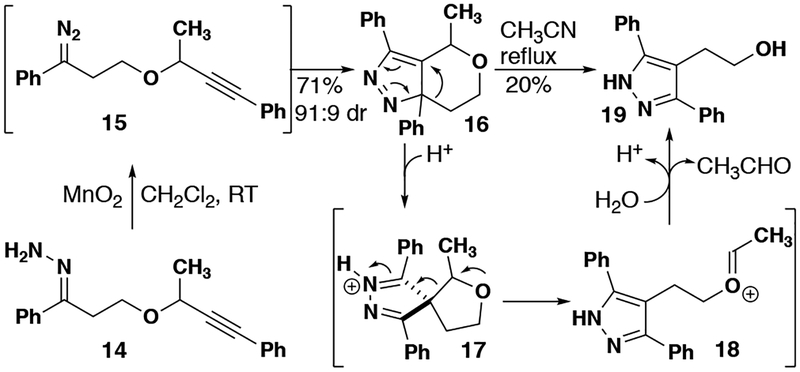
Substrates that would produce heterocycles lacking a fused benzene ring were also explored. Although the oxidation and DPC both proceed smoothly, producing 16 in 71% yield (Figure 4) as a 91:9 mixture of diastereomers, the rearrangement required much higher temperature when compared to a similar fused substrate (3b). Upon heating to reflux in acetonitrile, only alcohol 1945 was isolated, which is attributed to the expected rearrangement to 17 followed by acid-catalyzed hydrolysis via oxocarbenium ion 18.
Figure 4.
Oxygen tethered substrate 14 leading to alcohol 19 by rearrangement and hydrolysis after the DPC reaction.
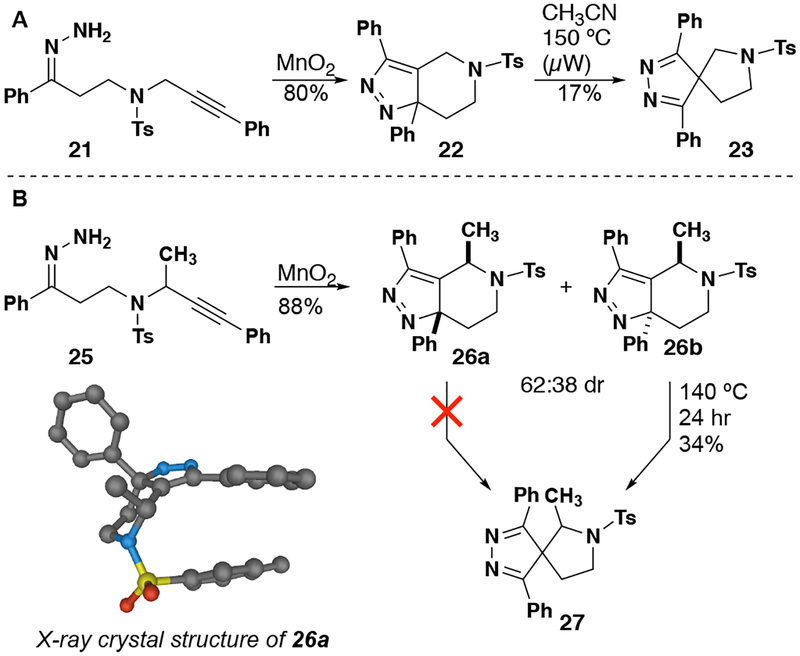
Two nitrogen-tethered substrates lacking a fused benzene ring were also investigated (Figure 5). Upon oxidation, sulfonamide 21 underwent facile dipolar cycloaddition, producing 22 in 80% yield. As with the oxygen-tethered substrate 15, the subsequent rearrangement proved more difficult. Rearrangement product 23 was isolated in only 17% yield from a complex mixture of unidentifiable by-products after employing microwave heating in acetonitrile to 150 °C. The addition of various Lewis acids, including MgBr2, Sc(OTf)3, BF3•OEt and PPTS failed to facilitate the rearrangement at a lower temperature as did the use of several other solvents (methanol, dichloroethane, HFIP and toluene). Substrate 25 was prepared in order to examine the influence of the stereogenic center on the dipolar cycloaddition/rearrangement sequence. As with previous substrates, the cycloaddition occurred readily upon oxidation of hydrazone 25 with modest diastereoselectivity favoring cis isomer 26a relative to trans isomer 26b. Although 26a is formed via a chair like transition state with the methyl group in an axial position, this transition state structure is predicted to be favored by 1 kcal/mol, since a 1,2 diequatorial interaction of the protecting group and the methyl is avoided.43 Isomers 26a and 26b performed differently when heated. Cis isomer 26a did not undergo rearrangement after prolonged heating at 210 °C, whereas trans isomer 26b behaves similarly to 22, eventually producing 27 in modest yield. While the computed barrier for rearrangement of 26a is higher than that for rearrangement of 26b, these differ by <1 kcal/mol at the level of theory used,43 indicating that either a different method is needed to rationalize the experimental result or the experimental situation is more complicate than expected (e.g., explicit solvent effects are important).
Figure 5.
Nitrogen-tethered substrates leading to spirocyclic pyrrolidines.
We have discovered a tandem DPC/rearrangement sequence that produces spirocyclic pyrazoles from hydrazone precursors. Although three different rearrangement pathways have been observed with acyclic pyrazoles, we observe only one pathway for substrates with benzene-fused tethers leading to spirocyclic products, which aligns well with computed transition state energies. Substrates derived from benzophenones with a variety of substituents perform well with either oxygen or nitrogen linkages to the pendant alkyne. Substrates leading to spirocycles lacking a fused benzene ring undergo smooth oxidation and dipolar cycloaddition while exhibiting significantly higher barriers to rearrangement.
Experimental Section
Experimental procedures and compound characterization can be found in the Supporting Information (PDF). 1H and 13C NMR spectra for all new compounds (PDF). X-ray data for compound 4a (CIF) and 26a (CIF).
Supplementary Material
Acknowledgments
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number R01GM124234. The contents solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Acknowledgment is made to the American Chemical Society Petroleum Research Fund (53767-ND1) for partial support of this research. M.C.R. wants to thank the CESGA (Centro de Supercomputación de Galicia) for the free allocation of computational time and to Carlos Silva López for his helpful discussions. N.C.T. thanks UC Davis for providing a Provost’s Undergraduate Fellowship (PUF). We thank the National Science Foundation (CHE1531193) for the Dual source X-ray diffractometer and the XSEDE program (CHE030089) for computational support.
References:
- (1).Khan MF; Alam MM; Verma G; Akhtar W; Akhter M; Shaquiquzzaman M Eur. J. Med. Chem 2016, 120, 170–201. [DOI] [PubMed] [Google Scholar]
- (2).Xu Z; Gao C; Ren Q-C; Song X-F; Feng L-S; Lv Z-S Eur. J. Med. Chem 2017, 139, 429–440. [DOI] [PubMed] [Google Scholar]
- (3).Faria JV; Vegi PF; Miguita AGC; dos Santos MS; Boechat N; Bernardino AM R. Bioorg. Med. Chem 2017, 25 (21), 5891–5903. [DOI] [PubMed] [Google Scholar]
- (4).Singh MS; Chowdhury S; Koley S Tetrahedron 2016, 72 (13), 1603–1644. [Google Scholar]
- (5).Fustero S; Sánchez-Roselló M; Barrio P; Simón-Fuentes A Chem. Rev 2011, 111 (11), 6984–7034. [DOI] [PubMed] [Google Scholar]
- (6).Kempson J In Name React. Heterocycl. Chem. II; John Wiley & Sons, Inc., 2011; pp 317–326. [Google Scholar]
- (7).Yoon J-Y; Lee S; Shin H Curr. Org. Chem 2011, 15 (5), 657–674. [Google Scholar]
- (8).Hüttel R; Riedl J; Martin H; Franke K Chem. Ber 1960, 93 (6), 1425–1432. [Google Scholar]
- (9).Hüttel R; Franke K; Martin H; Riedl J Chem. Ber 1960, 93 (6), 1433–1446. [Google Scholar]
- (10).van Alphen J Recl. des Trav. Chim. des Pays-Bas 2010, 62 (7), 485–490. [Google Scholar]
- (11).van Alphen J Recl. des Trav. Chim. des Pays-Bas 2010, 62 (7), 491–496. [Google Scholar]
- (12).Jefferson EA; Warkentin JJ Org. Chem 1994, 59 (2), 455–462. [Google Scholar]
- (13).Jefferson EA; Warkentin JJ Am. Chem. Soc 1992, 114 (16), 6318–6325. [Google Scholar]
- (14).Majchrzak MW; Jefferson E; Warkentin JJ Am. Chem. Soc 1990, 112 (6), 2449–2451. [Google Scholar]
- (15).Franck-Neumann M; Dietrich-Buchecker C Tetrahedron Lett 1976, No. 24, 2069–2072. [Google Scholar]
- (16).Yamazaki T; Shechter H Tetrahedron Lett. 1972, 13 (44), 4533–4536. [Google Scholar]
- (17).Jones GW; Chang KT; Shechter HJ Am. Chem. Soc 1979, 101 (14), 3906–3916. [Google Scholar]
- (18).Vuluga D; Legros J; Crousse B; Bonnet-Delpon D Green Chem. 2009, 11 (2), 156–159. [Google Scholar]
- (19).Wu LL; Ge YC; He T; Zhang L; Fu XL; Fu HY; Chen H; Li RX Synthesis 2012, 44 (10), 1577–1583. [Google Scholar]
- (20).Pérez-Aguilar MC; Valdés C Angew. Chem. Int. Ed 2015, 54 (46), 13729–13733. [DOI] [PubMed] [Google Scholar]
- (21).Zheng Y; Qiu L; Hong K; Dong S; Xu X Chem. Eur. J 2018, 24 (26), 6705–6711. [DOI] [PubMed] [Google Scholar]
- (22).Hirakawa K; Minami Y; Hayashi SJ Chem. Soc. Perkin Trans 1 1982, No. 0, 577–579. [Google Scholar]
- (23).Braun S; Sturm V; Runzheimer K-O Chem. Ber 1988, 121 (5), 1017–1019. [Google Scholar]
- (24).Huisgen R; Reissig HU; Huber HJ Am. Chem. Soc 1979, 101 (13), 3647–3648. [Google Scholar]
- (25).Mataka S; Ohshima T; Tashiro MJ Org. Chem 1981, 46 (20), 3960–3964. [Google Scholar]
- (26).Padwa A; Woolhouse AD; Blount JJ J. Org. Chem 1983, 48 (7), 1069–1074. [Google Scholar]
- (27).Pérez-Aguilar MC; Valdés C Angew. Chem. Int. Ed 2013, 52 (28), 7219–7223. [DOI] [PubMed] [Google Scholar]
- (28).Masumoto E; Maruoka H; Okabe F; Fujioka T; Yamagata KJ Heterocycl. Chem 2015, 52 (1), 48–53. [Google Scholar]
- (29).Zheng H; Zhu Y; Shi Y Angew. Chem. Int. Ed 2014, 53 (42), 11280–11284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).Yoon H; Rölz M; Landau F; Lautens M Angew. Chem. Int. Ed 2017, 56 (36), 10920–10923. [DOI] [PubMed] [Google Scholar]
- (31).Pérez-Gómez M; García-López JA Angew. Chem. Int. Ed 2016, 55 (46), 14389–14393. [DOI] [PubMed] [Google Scholar]
- (32).Zhao HW; Li B; Pang HL; Tian T; Chen XQ; Song XQ; Meng W; Yang Z; Zhao Y. Di; Liu ,YY Org. Lett 2016, 18 (4), 848–851. [DOI] [PubMed] [Google Scholar]
- (33).Soldi C; Lamb KN; Squitieri RA; González-López M; Di Maso MJ; Shaw JT J. Am. Chem. Soc 2014, 136 (43), 15142–15145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Quiclet-Sire B; Zard SZ Chem. Commun 2006, No. 17, 1831. [DOI] [PubMed] [Google Scholar]
- (35).Zheng Y; Zhang X; Yao R; Wen Y; Huang J; Xu XJ Org. Chem 2016, 81 (22), 11072–11080. [DOI] [PubMed] [Google Scholar]
- (36).Divya KVL; Meena A; Suja TD Synthesis 2016, 48 (23), 4207–4212. [Google Scholar]
- (37).Liu P; Xu QQ; Dong C; Lei X; Lin GQ Synlett 2012, 23 (14), 2087–2092. [Google Scholar]
- (38).Vuluga D; Legros J; Crousse B; Bonnet-Delpon D Green Chem. 2009, 11 (2), 156–159. [Google Scholar]
- (39).Morrison H; Danishefsky S; Yates PJ Org. Chem 1961, 26 (7), 2617–2618. [Google Scholar]
- (40).Severin T; Pehr H Chem. Ber 1979, 112 (11), 3559–3565. [Google Scholar]
- (41).Lévesque É; Laporte ST; Charette AB Angew. Chem. Int. Ed 2017, 56 (3), 837–841. [DOI] [PubMed] [Google Scholar]
- (42).Pérez-Aguilar MC; Valdés C Angew. Chem. Int. Ed 2015, 54 (46), 13729–13733. [DOI] [PubMed] [Google Scholar]
- (43).Information., S. S. .
- (44).See Supporting Information Section 6A2, Figure 2 for further details.
- (45).Wei W; Tang Y; Zhou Y; Deng G; Liu Z; Wu J; Li Y; Zhang J; Xu S Org. Lett 2018, 20 (20), 6559–6563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.