Abstract
The potential advantages and unique challenges of the early life immune system for the development of HIV-specific broadly neutralizing antibodies were discussed during a workshop entitled “Immunological Mechanisms of Inducing HIV Immunity in Infants” sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) in conjunction with the 2018 HIVR4P Conference held in Madrid, Spain. A safe and effective HIV vaccine remains a critical need in the fight against the HIV pandemic, especially to prevent emerging infections in infants, adolescents, and young adults. To successfully target these populations, a vaccine should ideally induce protective immune responses during childhood. Interestingly, several recent studies highlighting differences in immune responses between adults and children have suggested that the early life immune system could present advantages for the elicitation of broadly neutralizing antibodies (bnAbs), a response highly desired for an HIV vaccine. Notably, HIV-infected children develop bnAbs responses earlier and more frequently than infected adults; with emerging evidence that the pathways of elicitation of bnAb lineages may differ between adults and children. Moreover, there is precedent for the prevention of lifelong infections with pediatric immunization, and early life provides a unique window of opportunity for the administration of a multi-dose HIV vaccine that will likely be needed to achieve protective immunity. Further understanding of how the distinct early life immune system can be harnessed to trigger bnAb lineages for induction of durable and polyfunctional HIV-specific immunity is warranted. This strategy will include testing promising HIV vaccine candidates in pediatric populations in preclinical and clinical studies. Novel approaches to identify molecular markers of protection are also key to guide and accelerate pediatric HIV vaccine development.
Keywords: Early life, immune system, HIV, vaccine, broadly neutralizing antibodies
WHY IS A PEDIATRIC HIV VACCINE CRITICALLY NEEDED?
Every year it is estimated that ~1.8 million new HIV infections occur worldwide, including > 150,000 infections among children < 15 years of age that are mainly due to vertical transmission (1). In addition, adolescents and young adults (15 to 24 years) account for almost one-third of yearly HIV infections, making them a key target population for prevention strategies. The burden of adolescent HIV infections is especially high among young women. In 2017, ~460 adolescent girls became infected with HIV every day; and ~50 adolescent girls died of AIDS-related illness every day (2). In sub-Saharan Africa, young women are disproportionally affected by the pandemic: they account for 1 in 5 new HIV infections although they only represent 10% of the population. Though HIV prevention and treatment services are becoming available to an increasing number of people, the tailored comprehensive prevention options are not yet available to a significant number of adolescents and young adults. The implementation of an HIV vaccine early in life may afford an opportunity to protect prior to the vulnerable period of adolescence and sexual debut. Moreover, early life immunization may provide the option to integrate HIV immunization into the current Expanded Program on Immunization and reach infants and young children with high coverage (3).
A major goal for an HIV vaccine is the elicitation of bnAbs. While vaccine candidates tested to date have been unsuccessful in inducing bnAbs in human adults, recent animal studies have provided proof of principle that HIV immunogens can elicit neutralization breadth in certain immune environments. For example, it was demonstrated that immunization with SOSIP, a recombinant protein that mimic the native HIV envelope glycoprotein, results in rapid elicitation of broad and potent serum antibody (Ab) responses in young cows (e.g. calves), animals that possess an Ab repertoire with long third heavy chain complementary determining regions (HCDR3) (4). In addition, vaccination with HIV envelope glycoprotein gp120 can induce bnAbs in autoimmune mice with breached peripheral tolerance (5). These findings highlight the importance of specific criteria of the immune landscape for tailored immune responses towards the development of bnAb lineages.
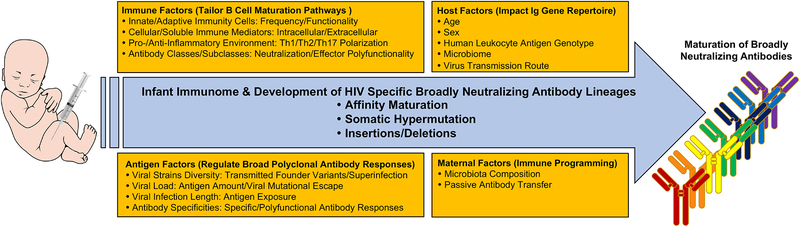
A number of factors suggest that the early life immune landscape may present an opportunity to direct immune development for optimal vaccine-elicited immune responses, including (a) distinct immune regulation/tolerance mechanisms as compared to the adult immune system and (b) less immune preprograming by the environment. The hypothesis that the distinct pediatric immune landscape provides a unique setting for B cell maturation pathways is supported by the early development of broad HIV neutralization in HIV-infected young children. Herein we summarize the presentations from a satellite meeting entitled “Immunological Mechanisms of Inducing HIV Immunity in Infants” sponsored by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health (NIH) in conjunction with the 2018 HIVR4P Conference that took place on October 22, 2018 in Madrid, Spain. Key points of the presentations and discussion included the mechanisms by which the pediatric immunome could provide a distinct immune landscape for rapid development of HIV bnAb lineages and how the understanding of evolutionary pathways of bnAbs in the HIV- infected early life immune system can inform the design of HIV vaccine strategies (Figure 1).
FIGURE 1.
Summary of factors that could contribute to regulate the development of HIV specific broadly neutralizing antibody lineages in the setting of the early life immune system
WHAT DRIVES THE DEVELOPMENT OF BROADLY NEUTRALIZING ANTIBODIES IN HIV-INFECTED CHILDREN?
HIV infected children develop neutralization breadth earlier and at a higher frequency than adults. Notably, cross-clade neutralizing Abs were detected in perinatally HIV-infected children within the first year of life (6); and non-progressing clade C HIV-infected children >5 years of age demonstrated greater neutralization breadth and potency against a cross-clade panel of relatively difficult to neutralize (tier 2 and tier 3) viruses compared to adults (7). Extensive mapping of bnAb specificities in the aforementioned cohort of HIV clade C infected children showed that 63% of children developed polyclonal bnAb responses targeting multiple epitopes on HIV envelope, including the variable loop 2 (V2)-glycan, variable loop 3 (V3)-glycan, CD4 binding site, and interface of envelope glycoproteins gp120 and gp41 (8). This finding contrasts with the observation that plasma neutralization breadth in adults is generally driven by Ab of limited specificities (9) and raises the possibility that neutralization breadth is achieved by distinct mechanisms in adults and children.
Several clinical factors have been associated with the development of neutralization breadth in adults, including time since infection, high virus load and early decline in circulating CD4+T cells (10, 11). While an association between persistent high viremia and bnAb development has also been observed in children, this association is not unequivocal (7). Moreover, time since infection appears to be less critical in children than in adults as broad neutralization can be achieved within 1–2 years of infection in children. Interestingly, about 7% of HIV infected pediatric patients, remain clinically healthy and have normal for age CD4+T cell counts in the absence of antiretroviral treatment, despite maintaining high levels of plasma viremia (Paul, 2004). The normal CD4+T cell counts in these pediatric non-progressors is possibly due to reduced HIV infection of long-lived central memory CD4+T cell subsets that may enable absolute CD4+ T cell counts to be maintained despite persistent high viremia. In addition, pediatric non-progressors show low levels of CD4+T cell activation (7). The fact that these children develop broad neutralization suggests that extended exposure to high antigenic load, in the context of high viral load, high CD4 T cell counts, and low immune activation of CD4+ T cells may contribute to drive the activation of multiple B cell lineages and breadth in HIV-infected children.
Due to distinct cellular (e.g., suppressor cells) and soluble (e.g., anti-inflammatory adenosine) factors, the early life immune system exhibits a Th2-polarized anti-inflammatory environment (12). Accordingly, newborn antigen-presenting cells tend to express high levels of IL-6 and IL-23 suggesting a robust ability to mount Th17 and follicular helper T (TFH) cells at similar or even higher levels than adults (12). This skewing of the infant immune system may allow for more robust humoral immunity and could favor the development of broad neutralization. Interestingly, high frequency of IL-21-secreting HIV-specific circulating effector TFH cells in blood and lymphoid tissues of HIV infected, treatment-naïve children (aged 8–13 years) correlates with neutralization breadth (13). Furthermore, the enrichment of IL-21-secreting HIV-specific TFH in pediatric lymphoid tissue is accompanied by increased TFH regulation via abundant regulatory follicular T-cells and HIV-specific CXCR5+CD8+ T cells compared to adults. The distinction between infected children and adults in the magnitude, quality and regulation of HIV-specific TFH responses is consistent with the superior ability of children to develop high frequency, potent bnAbs.
The monoclonal bnAbs isolated from adults have specific immunogenetic characteristics including auto/polyreactivity, long HCDR3 and high level of somatic hypermutation (SHM) (14). To date only two bnAbs isolated from infected children have been described. These include BF520.1 that was isolated from a clade A perinatally infected infant ~ 1 year after infection (15), and AIIMS- P01 that was recently isolated from a 9 year old ART-naïve clade C chronically infected pediatric elite neutralizer (16). These two pediatric bnAbs target the N332 envelope supersite and they both show lower levels of SHM than adult bnAbs against the same region, demonstrating that in the setting of the pediatric immune system, broad neutralization can be achieved without extensive SHM. Interestingly, not all the mutations acquired during Ab maturation of adult bnAbs are necessary for the development of neutralization breadth (17). Wiehe and collaborators have recently developed a computational approach that identified bnAbs being enriched in improbable mutations, and their results suggest that neutralization breadth can be achieved through shorter affinity maturation pathways involving a critical subset of improbable mutations (18). Immune settings that enable the rapid accumulation of critical improbable mutations may therefore accelerate the development of broad neutralization as observed in the infant BF 520.1 bnAb lineage. The developmental pathway of BF520.1 indicates that heterologous cross-clade neutralizing activity evolved in the infant within six months of infection and only 2% SHM was needed to achieve the full breadth of the mature antibody (19). Analysis of heavy and light chains sequences from the two reported pediatric bnAbs have also indicated that pediatric bnAbs may have features that are distinct from the ones described in adults bnAbs. For example, mutations important for BF520.1 functional activity are primarily found in the complementarity-determining regions HCDR2 and LCDR1, in contrast to many HIV bnAbs where major determinants of breadth reside in the HCDR3 region (19). In the case of AIIMS-P01, the presence of indels in the framework region HFR3, that have not been observed in adult bnAbs, appear to be critical for neutralization breadth (16). The emerging knowledge of mutational changes that are critical for bnAb activity and of the molecular networks related to functional immune system dynamics in the pediatric immune landscape is critical to design effective vaccines. Moreover, characterization of additional infant bnAbs will be key to determine if the early life immune system is more suitable for the acquisition of mutations critical for neutralization breadth development.
CAN THE INFANT IMMUNE SYSTEM BE MODULATED FOR INDUCTION OF PROTECTIVE IMMUNE RESPONSES?
In order for the fetus to survive and develop in its hemi-allogenic mother, the early life immune system is rich in regulatory processes and in soluble mediators that work in concert at maternal-fetal interface to create a tolerogenic immune environment. Following birth, there is a period of adaptation from the relatively low pathogen environment in the uterus to the need of developing immune responses against pathogenic microbes and environmental antigens, while at the same time establishing a normal microbial flora. As a result, immune responses to bacterial and viral infections during infancy can significantly differ from that of adults. Recent systems-level approaches to investigate immune ontogeny in early life have noted a convergence on a developmental trajectory of immune parameters around 3 months of life, indicating a critical set period from which human immune system variation is shaped by environmental exposures over the course of life (20). It is possible that consistent and robust molecular and cellular changes leading to developmental convergence occurs even earlier as multi-OMICS studies of the dynamics of immune ontogeny within the first week of life have revealed a stable developmental trajectory shared by multiple cohorts of newborns (21). These key pathways of molecular ontogeny are centered around interconnected interferon signaling, complement cascade, and neutrophil activity modules. Interestingly, sequencing of the B cell repertoire in a longitudinal birth cohort has determined that IgG and IgA class-switched B cells progressively gain mutations reaching about 60–75% of adult SHM frequencies by three years of age (22). Clonality of B cells also increased with age and was significantly different between children and adults, with changes in BCR repertoires influenced by infectious diseases and environmental exposures in early life. In addition, the first months of life are critical for the establishment of the repertoire of the gut microflora that involves compositional and functional changes in the microbial community through interactions with the developing immune system in the gut (23). Thus, there is a period of immune plasticity in early life that can be harnessed to modulate lifelong immunity via targeted age-specific interventions.
A cardinal way to enhance vaccine responses is through the use of adjuvants. Recent studies by Ofer Levy and collaborators have demonstrated that adjuvants distinctly modulate vaccine responses across different age groups, suggesting it is critical to understand how adjuvants may best optimize vaccine efficacy by considering early life immune ontogeny. Notably, adjuvantation with the Toll like receptor (TLR) 7/8 agonist, 3M-052, overcomes hypo-responsiveness to pneumoccocoal conjugate vaccine in neonatal non-human primates (NHPs) by enhanced generation of antigen specific CD4+ cells, activation of B cells and serotype specific Ab titers (24). A recent study in neonatal NHPs similarity demonstrated a potential advantage for TLR adjuvantation with AS01 (TLR4) and 3M-052 (TLR7/8) in the induction of breadth, durability and functionality of HIV-specific antibody responses (25). Further understanding of age-specific adjuvantation and formulation strategies along with an increased appreciation of immune ontogeny may inform the rational design of pediatric HIV vaccines to elicit durable and protective immune responses. While alum has been the adjuvant of choice for pediatric vaccines, some licensed vaccines, such as Cervarix (Human Papilloma Virus vaccine adjuvanted with AS04) and the RTS,S (malaria vaccine adjuvanted with AS01) that contain TLR agonists, have now been approved for use in pediatric populations. These new vaccines provide important proof of concept of innovation in adjuvanted pediatric vaccines and suggest that novel adjuvanted vaccine formulations targeted to a given population should enter in the clinical development pipeline to help overcome barriers in eliciting protective immune responses against HIV. Interestingly, combination adjuvant systems such as TLR- and C-type lectin receptor- agonists have been shown to enhance Th1 responses of dendritic cells and demonstrated higher synergy in newborns than in adults or elders (26). It will therefore be of interest to explore if specific adjuvants or adjuvant combinations favor the development of neutralization breadth in the early life immune landscape.
The characterization and elicitation of bnAbs is at the center of current approaches to inform the HIV vaccine development. The major strategies to elicit bnAbs include lineage-based immunogens, germline targeting vaccine candidates and epitope-based vaccine approaches (reviewed in (27)). The lineage-based immunogen design approach employs a sequential immunization scheme using HIV envelope sequences from patients who developed bnAbs with the goal to reproduce the natural development of a specific bnAb lineage (28). Following proof-of-principle in preclinical studies (3, 29, 30), this strategy is currently under testing in adult clinical trials. Because in most cases, the natural evolution of a B cell lineage is unknown, an alternative approach is to design envelope constructs with specific antigenic features to target bnAb germline (31). Epitope-based vaccine approach consists in designing envelope constructs that incorporate a portion of a bnAb epitope (minimal immunogen) for a focused immune response. Importantly, these strategies rely on the activation of a pool of bnAb precursors cells, and while recent studies have demonstrated the existence of bnAb precursor cells in healthy adults (32, 33), their frequency in pediatric populations remains unknown. The assessment of strategies aiming at elicitation of bnAbs in pediatric animal models will be critical to accelerate evaluation of bnAb immunogens and define if the pediatric immune system presents advantages for induction of neutralization breadth. Also, it will be compelling to define surrogate markers that allow to reliably predict bnAb evolution for early assessment of vaccine efficacy. Vaccine strategies designed to elicit Abs against multiple bnAb epitopes are especially relevant as neutralization breadth in children is generally mediated by a polyclonal response.
DOES AGE AT VACCINATION IMPACT THE DURABILITY OF ANTIBODY RESPONSES?
Targeting vaccine durability is key for population-level impact and cost-effectiveness of HIV vaccination. Immunization in early life may be beneficial in this regard. This potential advantage was recently demonstrated by comparing the durability of humoral responses following adult and childhood primary small pox vaccination (34). Individuals immunized during childhood still had detectable Ab levels more than 30 years after vaccination whereas the neutralizing antibody responses to vaccinia waned after 5–10 years in individuals immunized as adults. Similarly, vaccination of infants with a MF59-adjuvanted HIV Env vaccine induced more durable responses than immunization of adults with the same vaccine (35). An association between younger age at time of vaccination and enhanced Ab responses has also been reported for Hepatitis B and Human Papilloma vaccines (36, 37). Thus, it is possible that HIV immunization early in life could afford lifetime protection. Identifying the immunogen-adjuvant combination that would achieve this goal is therefore a priority in the field.
WHAT ARE THE KEY NEXT STEPS FOR PEDIATRIC HIV VACCINE DEVELOPMENT?
The HIVR4P satellite meeting “Immunological Mechanisms of Inducing HIV Immunity in Infants” (October 22, 2018; Madrid, Spain) provided a forum to identify and address the key research gaps in our understanding of the immune obstacles to HIV vaccine development in the context of pediatric immunity. It was recognized by the group that induction of broadly neutralizing and protective anti-HIV humoral immune responses may be more readily achieved in children than adults. Nevertheless, there still remain critical challenges in our understanding of immune obstacles to the development of an efficacious pediatric HIV vaccine.
The development of infant immune responses should not be considered in isolation and should integrate maternal determinants of infant immunity. The maternal-infant dyad is increasingly recognized as an immunologic unit and studies have shown that maternal HIV infection impacts immunity to pathogens in young infants despite the prevention of HIV transmission (38, 39). Maternal antibodies transferred through the placenta could be particularly relevant to infant HIV immunization. Indeed, immunization with HIV Env proteins complexed with monoclonal Abs alters the immunogenic properties of HIV vaccines and influences the quality of the vaccine-elicited Ab responses. (40). Moreover, passive immunization of neonatal rhesus macaques with a suboptimal dose of monoclonal bnAb b12 (simulating perinatal setting) led to enhanced B cell responses and rapid development of neutralizing Ab responses (41). Maternal antibodies may also contribute to the selection of transmitted virus and several studies have demonstrated that infant transmitted/founder (T/F) viruses are generally resistant to maternal plasma neutralization (42, 43). Whether these infant T/F viruses have specific characteristics that favor the development of neutralization breadth remains unknown. Studies of virus-antibody co-evolution in longitudinal samples from HIV-infected adults who developed neutralization breadth have been instrumental to provide insights on key steps in the evolution of bnAb lineages (28). However, it is also unclear if the presence of maternal antibodies has an impact on the virus-Ab co-evolution and conducting these studies in infants will provide fresh insights into the mechanisms important for early bnAb development.
Current WHO guidelines recommend ART initiation in all HIV-infected children irrespective of their clinical status. Consequently, mechanistic studies of bnAb development in humans can only be conducted with existing pediatric samples. Studies in NHP models can therefore be very useful to further our understanding of neutralization breadth development in early life. Nelson and collaborators recently compared the development nAb responses in infant and adult rhesus macaques infected with T/F SHIV CH505 (44), and observed comparable kinetics in the two groups of animals. However, their study was restricted to the first few months following infection, before the development of neutralization breadth. Further investigations of bnAb development in infant NHPs infected with distinct SHIVs will be key to define if the early development of neutralization breath is recapitulated. NHP models as a pivotal platform for testing HIV vaccine candidates would also help define the potential benefit of early life immunization. Nevertheless, differences in B cell repertoires between humans and NHPs can present an important caveat in the preclinical testing of immunogens aiming at inducing bnAb lineages (30).
Defining if early life immunization can induce protective immune responses will ultimately require conducting pediatric HIV vaccine trials. A pediatric HIV vaccine protocol (HVTN 135) is currently in development to evaluate the safety and immunogenicity of HIV CH505 T/F gp120 adjuvanted with TLR4 agonist GLA-SE and to determine, if compared to adults (HVTN 115), infants demonstrate a distinct response to the same vaccine immunogen. Of note, infant vaccination can induce high magnitude and durable antibody responses against the V1V2 region associated with protection in the RV144 vaccine trial (45). Moreover, infants immunized with a MF59 adjuvanted gp120 vaccine developed higher magnitude antibodies than adults immunized with the same vaccine (35) indicating that vaccine adjuvants differently modulate gp120-specific antibody responses in adults and infants; and that infants can robustly respond to HIV Env immunization. From a regulatory perspective, age de-escalation studies do not require prior efficacy testing in adults. Accordingly, the safety and immunogenicity results of HVTN 135 should lead the way to other pediatric HIV vaccine clinical studies.
Identification of early markers of vaccine immunogenicity and molecular signatures correlating with protective immunity will also be essential to help guide future pediatric HIV vaccine trials. Notably, precision vaccinology through applying multidisciplinary technologies has the potential to dissect the human immune response to HIV vaccines that may in turn accelerate, enhance, and de-risk HIV vaccine development.
ACKNOWLEDGMENTS
We thank the workshop panelists for their contributions: Drs. Rama Rao Amara, Barton F. Haynes, Thomas J. Hope, Andrea Hulse, Richard A. Koup, M. Anthony Moody, Penny L. Moore, Maximilian Muenchhof, Nina Russell, Rogier W. Sanders, Stuart Z. Shapiro, Leonidas A. Stamatatos, Georgia D. Tomaras, Richard T. Wyatt, and Wilton B. Williams. We are also extremely grateful to Dr. Ashley Nelson for her help with manuscript preparation. Dr. Ofer Levy’s research is in part funded by the NIH, NIAID Human Immunology Project U19AI118608 as well as Adjuvant Development Program contract HHSN272201800047C and internal awards from the Boston Children’s Hospital Department of Pediatrics and Chief Scientific Office.
Footnotes
CONFLICTS OF INTEREST
Dr. Ofer Levy is a named inventor on several vaccine adjuvant patents. Dr. Sallie Permar serves as a consultant for Moderna, Merck, Sanofi, and Pfizer vaccines and leads sponsored programs from Merck and Moderna. All other authors declare no conflict of interest.
REFERENCES
- 1.UNAIDS. 2017. UNAIDS DATA 2017. https://www.aidsdatahub.org/sites/default/files/publication/UNAIDS_Global_AIDS_Update_2017_Data_book_2017_en.pdf. Accessed
- 2.UNAIDS. 2019. Women and HIV - A spotlight on adolescent girls and young women. Accessed
- 3.Escolano A, Steichen JM, Dosenovic P, Kulp DW, Golijanin J, Sok D, Freund NT, Gitlin AD, Oliveira T, Araki T, Lowe S, Chen ST, Heinemann J, Yao KH, Georgeson E, Saye-Francisco KL, Gazumyan A, Adachi Y, Kubitz M, Burton DR, Schief WR, Nussenzweig MC. 2016. Sequential Immunization Elicits Broadly Neutralizing Anti-HIV-1 Antibodies in Ig Knockin Mice. Cell 166:1445–1458 e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sok D, Le KM, Vadnais M, Saye-Francisco KL, Jardine JG, Torres JL, Berndsen ZT, Kong L, Stanfield R, Ruiz J, Ramos A, Liang CH, Chen PL, Criscitiello MF, Mwangi W, Wilson IA, Ward AB, Smider VV, Burton DR. 2017. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 548:108–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schroeder KMS, Agazio A, Strauch PJ, Jones ST, Thompson SB, Harper MS, Pelanda R, Santiago ML, Torres RM. 2017. Breaching peripheral tolerance promotes the production of HIV-1-neutralizing antibodies. J Exp Med 214:2283–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goo L, Chohan V, Nduati R, Overbaugh J. 2014. Early development of broadly neutralizing antibodies in HIV-1-infected infants. Nat Med 20:655–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muenchhoff M, Adland E, Karimanzira O, Crowther C, Pace M, Csala A, Leitman E, Moonsamy A, McGregor C, Hurst J, Groll A, Mori M, Sinmyee S, Thobakgale C, Tudor-Williams G, Prendergast AJ, Kloverpris H, Roider J, Leslie A, Shingadia D, Brits T, Daniels S, Frater J, Willberg CB, Walker BD, Ndung’u T, Jooste P, Moore PL, Morris L, Goulder P. 2016. Nonprogressing HIV-infected children share fundamental immunological features of nonpathogenic SIV infection. Sci Transl Med 8:358ra125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ditse Z, Muenchhoff M, Adland E, Jooste P, Goulder P, Moore PL, Morris L. 2018. HIV-1 Subtype C-Infected Children with Exceptional Neutralization Breadth Exhibit Polyclonal Responses Targeting Known Epitopes. J Virol 92: e00878–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walker LM, Simek MD, Priddy F, Gach JS, Wagner D, Zwick MB, Phogat SK, Poignard P, Burton DR. 2010. A limited number of antibody specificities mediate broad and potent serum neutralization in selected HIV-1 infected individuals. PLoS Pathog 6:e1001028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Locci M, Havenar-Daughton C, Landais E, Wu J, Kroenke MA, Arlehamn CL, Su LF, Cubas R, Davis MM, Sette A, Haddad EK, International AVIPCPI, Poignard P, Crotty S. 2013. Human circulating PD-1+CXCR3-CXCR5+ memory Tfh cells are highly functional and correlate with broadly neutralizing HIV antibody responses. Immunity 39:758–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow P, Moody MA. 2017. Immunologic characteristics of HIV-infected individuals who make broadly neutralizing antibodies. Immunol Rev 275:62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kollmann TR, Kampmann B, Mazmanian SK, Marchant A, Levy O. 2017. Protecting the Newborn and Young Infant from Infectious Diseases: Lessons from Immune Ontogeny. Immunity 46:350–363. [DOI] [PubMed] [Google Scholar]
- 13.Roider J, Maehara T, Ngoepe A, Ramsuran D, Muenchhoff M, Adland E, Aicher T, Kazer SW, Jooste P, Karim F, Kuhn W, Shalek AK, Ndung’u T, Morris L, Moore PL, Pillai S, Kloverpris H, Goulder P, Leslie A. 2018. High-Frequency, Functional HIV-Specific T-Follicular Helper and Regulatory Cells Are Present Within Germinal Centers in Children but Not Adults. Front Immunol 9:1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sadanand S, Suscovich TJ, Alter G. 2016. Broadly Neutralizing Antibodies Against HIV: New Insights to Inform Vaccine Design. Annu Rev Med 67:185–200. [DOI] [PubMed] [Google Scholar]
- 15.Simonich CA, Williams KL, Verkerke HP, Williams JA, Nduati R, Lee KK, Overbaugh J. 2016. HIV-1 Neutralizing Antibodies with Limited Hypermutation from an Infant. Cell 166:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar S, Panda H, Makhdoomi MA, Mishra N, Safdari HA, Chawla H, Aggarwal H, Reddy ES, Lodha R, Kumar Kabra S, Chandele A, Dutta S, Luthra K. 2019. An HIV-1 Broadly Neutralizing Antibody from a Clade C-Infected Pediatric Elite Neutralizer Potently Neutralizes the Contemporaneous and Autologous Evolving Viruses. J Virol 93: e01495–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jardine JG, Sok D, Julien JP, Briney B, Sarkar A, Liang CH, Scherer EA, Henry Dunand CJ, Adachi Y, Diwanji D, Hsueh J, Jones M, Kalyuzhniy O, Kubitz M, Spencer S, Pauthner M, Saye-Francisco KL, Sesterhenn F, Wilson PC, Galloway DM, Stanfield RL, Wilson IA, Burton DR, Schief WR. 2016. Minimally Mutated HIV-1 Broadly Neutralizing Antibodies to Guide Reductionist Vaccine Design. PLoS Pathog 12:e1005815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wiehe K, Bradley T, Meyerhoff RR, Hart C, Williams WB, Easterhoff D, Faison WJ, Kepler TB, Saunders KO, Alam SM, Bonsignori M, Haynes BF. 2018. Functional Relevance of Improbable Antibody Mutations for HIV Broadly Neutralizing Antibody Development. Cell Host Microbe 23:759–765.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simonich CA, Doepker L, Ralph D, Williams JA, Dhar A, Yaffe Z, Gentles L, Small CT, Oliver B, Vigdorovich V, Mangala Prasad V, Nduati R, Sather DN, Lee KK, Matsen Iv FA, Overbaugh J. 2019. Kappa chain maturation helps drive rapid development of an infant HIV-1 broadly neutralizing antibody lineage. Nat Commun 10:2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olin A, Henckel E, Chen Y, Lakshmikanth T, Pou C, Mikes J, Gustafsson A, Bernhardsson AK, Zhang C, Bohlin K, Brodin P. 2018. Stereotypic Immune System Development in Newborn Children. Cell 174:1277–1292.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee AH, Shannon CP, Amenyogbe N, Bennike TB, Diray-Arce J, Idoko OT, Gill EE, Ben-Othman R, Pomat WS, van Haren SD, Cao KL, Cox M, Darboe A, Falsafi R, Ferrari D, Harbeson DJ, He D, Bing C, Hinshaw SJ, Ndure J, Njie-Jobe J, Pettengill MA, Richmond PC, Ford R, Saleu G, Masiria G, Matlam JP, Kirarock W, Roberts E, Malek M, Sanchez-Schmitz G, Singh A, Angelidou A, Smolen KK, Consortium E, Brinkman RR, Ozonoff A, Hancock REW, van den Biggelaar AHJ, Steen H, Tebbutt SJ, Kampmann B, Levy O, Kollmann TR. 2019. Dynamic molecular changes during the first week of human life follow a robust developmental trajectory. Nat Commun 10:1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nielsen SCA, Roskin KM, Jackson KJL, Joshi SA, Nejad P, Lee JY, Wagar LE, Pham TD, Hoh RA, Nguyen KD, Tsunemoto HY, Patel SB, Tibshirani R, Ley C, Davis MM, Parsonnet J, Boyd SD. 2019. Shaping of infant B cell receptor repertoires by environmental factors and infectious disease. Sci Transl Med 11:eaat2004.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka M, Nakayama J. 2017. Development of the gut microbiota in infancy and its impact on health in later life. Allergol Int 66:515–522. [DOI] [PubMed] [Google Scholar]
- 24.Dowling DJ, van Haren SD, Scheid A, Bergelson I, Kim D, Mancuso CJ, Foppen W, Ozonoff A, Fresh L, Theriot TB, Lackner AA, Fichorova RN, Smirnov D, Vasilakos JP, Beaurline JM, Tomai MA, Midkiff CC, Alvarez X, Blanchard JL, Gilbert MH, Aye PP, Levy O. 2017. TLR7/8 adjuvant overcomes newborn hyporesponsiveness to pneumococcal conjugate vaccine at birth. JCI Insight 2:e91020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips B, Van Rompay KKA, Rodriguez-Nieves J, Lorin C, Koutsoukos M, Tomai M, Fox CB, Eudailey J, Dennis M, Alam SM, Hudgens M, Fouda G, Pollara J, Moody A, Shen X, Ferrari G, Permar S, De Paris K. 2018. Adjuvant-Dependent Enhancement of HIV Env-Specific Antibody Responses in Infant Rhesus Macaques. J Virol 92: e01051–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Haren SD, Dowling DJ, Foppen W, Christensen D, Andersen P, Reed SG, Hershberg RM, Baden LR, Levy O. 2016. Age-Specific Adjuvant Synergy: Dual TLR7/8 and Mincle Activation of Human Newborn Dendritic Cells Enables Th1 Polarization. J Immunol 197:4413–4424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andrabi R, Bhiman JN, Burton DR. 2018. Strategies for a multi-stage neutralizing antibody-based HIV vaccine. Curr Opin Immunol 53:143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonsignori M, Liao HX, Gao F, Williams WB, Alam SM, Montefiori DC, Haynes BF. 2017. Antibody-virus co-evolution in HIV infection: paths for HIV vaccine development. Immunol Rev 275:145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders KO, Verkoczy LK, Jiang C, Zhang J, Parks R, Chen H, Housman M, Bouton-Verville H, Shen X, Trama AM, Scearce R, Sutherland L, Santra S, Newman A, Eaton A, Xu K, Georgiev IS, Joyce MG, Tomaras GD, Bonsignori M, Reed SG, Salazar A, Mascola JR, Moody MA, Cain DW, Centlivre M, Zurawski S, Zurawski G, Erickson HP, Kwong PD, Alam SM, Levy Y, Montefiori DC, Haynes BF. 2017. Vaccine Induction of Heterologous Tier 2 HIV-1 Neutralizing Antibodies in Animal Models. Cell Rep 21:3681–3690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams WB, Zhang J, Jiang C, Nicely NI, Fera D, Luo K, Moody MA, Liao HX, Alam SM, Kepler TB, Ramesh A, Wiehe K, Holland JA, Bradley T, Vandergrift N, Saunders KO, Parks R, Foulger A, Xia SM, Bonsignori M, Montefiori DC, Louder M, Eaton A, Santra S, Scearce R, Sutherland L, Newman A, Bouton-Verville H, Bowman C, Bomze H, Gao F, Marshall DJ, Whitesides JF, Nie X, Kelsoe G, Reed SG, Fox CB, Clary K, Koutsoukos M, Franco D, Mascola JR, Harrison SC, Haynes BF, Verkoczy L. 2017. Initiation of HIV neutralizing B cell lineages with sequential envelope immunizations. Nat Commun 8:1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stamatatos L, Pancera M, McGuire AT. 2017. Germline-targeting immunogens. Immunol Rev 275:203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yacoob C, Pancera M, Vigdorovich V, Oliver BG, Glenn JA, Feng J, Sather DN, McGuire AT, Stamatatos L. 2016. Differences in Allelic Frequency and CDRH3 Region Limit the Engagement of HIV Env Immunogens by Putative VRC01 Neutralizing Antibody Precursors. Cell Rep 17:1560–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jardine JG, Kulp DW, Havenar-Daughton C, Sarkar A, Briney B, Sok D, Sesterhenn F, Ereno-Orbea J, Kalyuzhniy O, Deresa I, Hu X, Spencer S, Jones M, Georgeson E, Adachi Y, Kubitz M, deCamp AC, Julien JP, Wilson IA, Burton DR, Crotty S, Schief WR. 2016. HIV-1 broadly neutralizing antibody precursor B cells revealed by germline-targeting immunogen. Science 351:1458–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Slike BM, Creegan M, Marovich M, Ngauy V. 2017. Humoral Immunity to Primary Smallpox Vaccination: Impact of Childhood versus Adult Immunization on Vaccinia Vector Vaccine Development in Military Populations. PLoS One 12:e0169247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McGuire EP, Fong Y, Toote C, Cunningham CK, McFarland EJ, Borkowsky W, Barnett S, Itell HL, Kumar A, Gray G, McElrath MJ, Tomaras GD, Permar SR, Fouda GG. 2018. HIV-Exposed Infants Vaccinated with an MF59/Recombinant gp120 Vaccine Have Higher-Magnitude Anti-V1V2 IgG Responses than Adults Immunized with the Same Vaccine. J Virol 92: e01070–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Giuliano AR, Lazcano-Ponce E, Villa L, Nolan T, Marchant C, Radley D, Golm G, McCarroll K, Yu J, Esser MT, Vuocolo SC, Barr E. 2007. Impact of baseline covariates on the immunogenicity of a quadrivalent (types 6, 11, 16, and 18) human papillomavirus virus-like-particle vaccine. J Infect Dis 196:1153–1162. [DOI] [PubMed] [Google Scholar]
- 37.Ota MO, Vekemans J, Schlegel-Haueter SE, Fielding K, Whittle H, Lambert PH, McAdam KP, Siegrist CA, Marchant A. 2004. Hepatitis B immunisation induces higher antibody and memory Th2 responses in new-borns than in adults. Vaccine 22:511–519. [DOI] [PubMed] [Google Scholar]
- 38.Jennewein MF, Abu-Raya B, Jiang Y, Alter G, Marchant A. 2017. Transfer of maternal immunity and programming of the newborn immune system. Semin Immunopathol 39:605–613. [DOI] [PubMed] [Google Scholar]
- 39.Goetghebuer T, Smolen KK, Adler C, Das J, McBride T, Smits G, Lecomte S, Haelterman E, Barlow P, Piedra PA, van der Klis F, Kollmann TR, Lauffenburger DA, Alter G, Levy J, Marchant A. 2019. Initiation of anti-retroviral therapy before pregnancy reduces the risk of infection-related hospitalization in HIV-exposed uninfected infants born in a high-income country. Clin Infect Dis 68:1193–1203. [DOI] [PubMed] [Google Scholar]
- 40.Hioe CE, Kumar R, Upadhyay C, Jan M, Fox A, Itri V, Peachman KK, Rao M, Liu L, Lo NC, Tuen M, Jiang X, Kong XP, Zolla-Pazner S. 2018. Modulation of Antibody Responses to the V1V2 and V3 Regions of HIV-1 Envelope by Immune Complex Vaccines. Front Immunol 9:2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng CT, Jaworski JP, Jayaraman P, Sutton WF, Delio P, Kuller L, Anderson D, Landucci G, Richardson BA, Burton DR, Forthal DN, Haigwood NL. 2010. Passive neutralizing antibody controls SHIV viremia and enhances B cell responses in infant macaques. Nat Med 16:1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goo L, Milligan C, Simonich CA, Nduati R, Overbaugh J. 2012. Neutralizing antibody escape during HIV-1 mother-to-child transmission involves conformational masking of distal epitopes in envelope. J Virol 86:9566–9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kumar A, Smith CEP, Giorgi EE, Eudailey J, Martinez DR, Yusim K, Douglas AO, Stamper L, McGuire E, LaBranche CC, Montefiori DC, Fouda GG, Gao F, Permar SR. 2018. Infant transmitted/founder HIV-1 viruses from peripartum transmission are neutralization resistant to paired maternal plasma. PLoS Pathog 14:e1006944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson AN, Goswami R, Dennis M, Tu J, Mangan RJ, Saha PT, Cain DW, Curtis AD, Shen X, Shaw GM, Bar K, Hudgens M, Pollara J, De Paris K, Van Rompay KKA, Permar SR. 2019. SHIV.CH505-infected infant and adult rhesus macaques exhibit similar HIV Env-specific antibody kinetics, despite distinct T-follicular helper (Tfh) and germinal center B cell landscapes. J Virol 93:e00168–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fouda GG, Cunningham CK, McFarland EJ, Borkowsky W, Muresan P, Pollara J, Song LY, Liebl BE, Whitaker K, Shen X, Vandergrift NA, Overman RG, Yates NL, Moody MA, Fry C, Kim JH, Michael NL, Robb M, Pitisuttithum P, Kaewkungwal J, Nitayaphan S, Rerks-Ngarm S, Liao HX, Haynes BF, Montefiori DC, Ferrari G, Tomaras GD, Permar SR. 2015. Infant HIV type 1 gp120 vaccination elicits robust and durable anti-V1V2 immunoglobulin G responses and only rare envelope-specific immunoglobulin A responses. J Infect Dis 211:508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]