Abstract
The beneficial effects of exercise on the brain are well known. However, the underlying molecular mechanisms are much less well understood. Interestingly, myokine, hormones secreted by muscle in response to exercise, gained attention as such beneficial mediators. In this review, we will focus on FNDC5 and its secreted form, the newly discovered myokine “irisin”. We will discuss their role in the beneficial effects of exercise and its potential application in neurodegenerative disorders.
Keywords: Exercise, FNDC5, irisin, neurodegeneration, cognition, hippocampus, physical activity
Physical activity (PA) has long been associated with tremendous benefits for the human body. In particular, increased PA has been correlated with improved health outcomes 1. Furthermore, aerobic exercise, also known as endurance exercise, has been shown to have beneficial effects on cognitive function and overall brain plasticity 2–4. This exercise-induced improvement in cognitive function has been particularly notable in older adults 2, 5. Exercise has also been shown to improve brain-related outcomes in particular neurodegenerative disorders, such as Parkinson’s Disease ( PD) 6 and Alzheimer’s Diseases (AD) 7 as well neurovascular trauma such as stroke 8 (Figure 1). The hippocampus, an area of the brain associated with cognitive function and spatial awareness, is the main benefactor of the beneficial effects of exercise on the brain. Specific beneficial effects of exercise in the brain include increases in the blood flow to the hippocampus and increases in its size in humans. In rodent models, exercise results in morphological changes in dendrites and dendritic spines, increased synapse plasticity, and importantly, enhanced de novo neurogenesis in the dentate gyrus 4, 9. Other beneficial effects of exercise include a reduction in neuroinflammation 10. For a long time, the adult brain was thought to lack regenerative capabilities. Now it is known that two distinct regions of the brain seem to undergo de novo neurogenesis: the subgranular zone of the dentate gyrus of the hippocampus and a region connecting the rostral migratory stream to the olfactory bulb, the subventricular zone 11. Moreover, exercise is one of the few stimuli known to provoke de novo neurogenesis in the adult mammalian brain as first shown by Van Praag et al. 12. In this study using BrdU to label dividing cells, the group demonstrated that voluntary free-wheel exercise in mice was sufficient to induce neurogenesis in the mouse dentate gyrus region of the hippocampus 12 In this study, bromodeoxyuridine-positive cells, a marker for cell division, was used to quantify neurogenesis in the dentate gyrus. Later that year, van Praag also established that this exercise induced neurogenesis lead to both improvements in synaptic plasticity and spatial learning 13. Interestingly, these effects were noted only with voluntary exercise and were not seen with an enriched environment lacking a running wheel 14, 15.
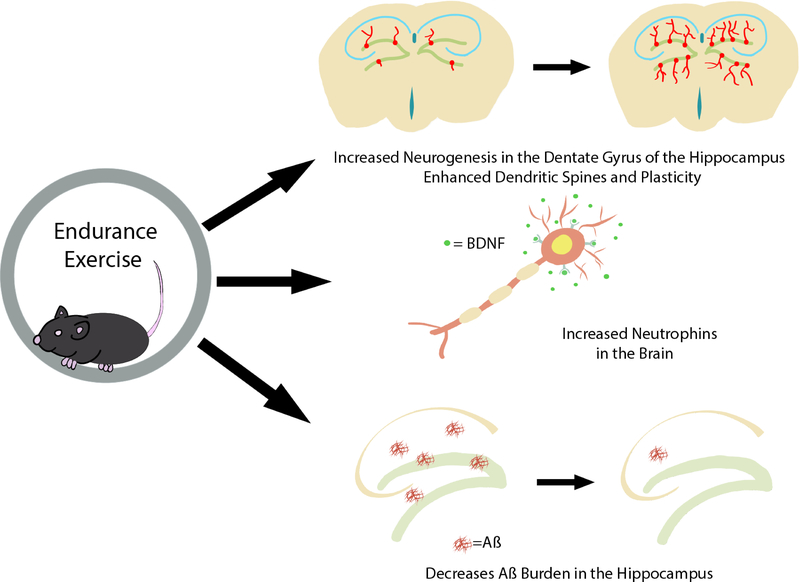
Fig 1.
The beneficial effects of exercise on the brain. Exercise has many beneficial effects on the brain ranging from neurogenesis to improvements in memory function. These include a reduction in neuroinflammation, improvements in synaptic plasticity, increased adult hippocampal neurogenesis, and improvements in memory, mainly spatial learning and memory as well as pattern separation.
Although these beneficial effects of exercise on cognition are widely accepted, the actual mediator of these effects remains to be uncovered. Despite this, there has been considerable evidence regarding the gene FNDC5, along with its secreted form irisin, and its role in improving cognitive function in the brain following exercise. The goal of this review is to shed light on FNDC5/irisin and its role in the beneficial effects of exercise on cognitive function and its potential application in neurodegenerative disorders.
Factors Involved in Brain Health
Factors mediating the beneficial effects of PA/exercise on the brain can be broadly divided in two categories: (1) factors locally produced in the brain, so-called neurotrophins and (2) systemic factors circulating in the blood, for example, produced by skeletal muscle (myokines). During the last years, systemic factors released from skeletal muscle have gained a lot interest as regulators of the beneficial effects of exercise on the brain among them FNDC5 and its secreted form “irisin”. Other systemic factors are being discussed in detail in this issue by Tari et al. Neurotrophins play a major role in the growth and development of neurons, ranging from synaptic plasticity to survival. One of these neurotrophic factors is brain-derived neurotrophic factor (BDNF). Interestingly, as we will discuss later in this review, FNDC5 seems to be an important regulator of BDNF and therefore, we will briefly introduce BDNF. For an in-depth review on BNDF and exercise, please see 9, 16, 17. BDNF has been shown to enhance neuronal survival, migration, and dendritic outgrowth 18, 19. In addition, it regulates synaptic plasticity and cognitive function 20–22. BDNF has also been shown to be a crucial regulator of the beneficial effects of exercise in the brain. For example, Vaynman et al. has shown that blocking the tropomyosin receptor kinase B (TrkB), the BDNF receptor, with anti-TrkB antibodies leads to diminished expression of important synaptic proteins as well as decreased retention in spatial learning tasks, both of which have previously been seen to be part of the exercise-induced benefits on the brain. 23, 24.
Origins of FNDC5/Irisin
FNDC5 (fibronectin type III domains containing protein 5) was originally described in 2002 independently by two separate laboratories. Teufel et al. found the FRCP2 gene while searching and characterizing different fibronectin type III domains25, and Ferrer- Martinez et al. discovered FRCP2, also known as the PeP protein, during peroxisomal studies 26. FRCP2/PeP was later renamed FNDC5. The cleaved and secreted form of FNDC5 was discovered in 2012 and named “irisin” after the Greek goddess Iris, the messenger of the gods 27.
FNDC5 is a glycosylated type I membrane protein. It contains a N-terminal signal peptide [amino acid (aa) 1–28], a FNIII domain (aa 33–124), a transmembrane domain (aa 150–170), and a cytoplasmic tail (aa 171–209) (www.uniporot.org) (Figure 2). Irisin is generated via proteolytic cleavage of FNDC5 and contains 112 amino acids (aa 29– 140). The enzyme that cleaves FNDC5 has yet to be uncovered. However, the crystal structure of irisin has been resolved 28. Intriguingly, the FNIII-like domain shows an unusual confirmation, with a continuous intersubunit beta-sheet dimer, that has not been previously described for any other FNIII protein. Subsequent biochemical experiments confirmed the existence of irisin (bacterial recombinant) as a homodimer. In a recent paper investigating the receptor for irisin, biochemical and biophysical studies identified interactions between irisin and αV/β5 integrin 29. Chemical inhibition of the αV integrin blocked irisin signaling in both osteocytes and fat cells.
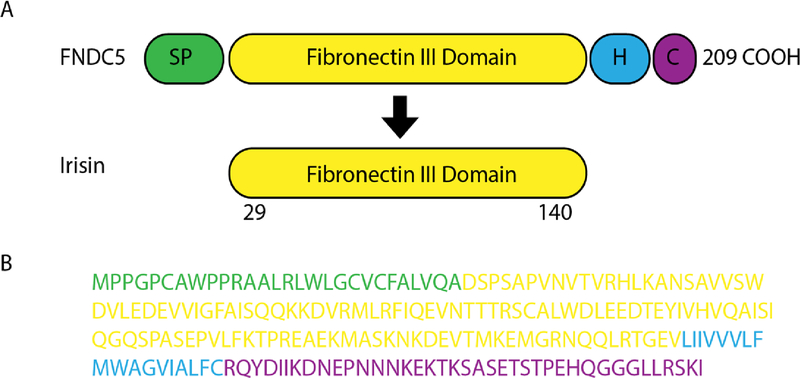
Fig 2.
Analysis of Irisin Peptides by Mass Spectrometry. (a) Scheme of the murine FNDC5 protein structure (top) and murine irisin protein structure (bottom). SP signal peptide, H hydrophobic domain, C cytoplasmic domain. (b) Murine FNDC5 amino acid sequence with corresponding domains colored. The irisin sequence is in yellow.
Quantification of Irisin
Irisin is a unique polypeptide: the sequence is 100% conserved between humans and mice. It has an atypical start codon in humans (ATA not ATG). In addition, it circulates at very low levels in the blood 27, 30. These features of irisin led to a controversy whether irisin indeed was a circulating hormone. The atypical start codon of human FNDC5 sparked debate, despite previous studies establishing that a small percentage of functional eukaryotic mRNAs do indeed begin translation with this atypical ATA 31, 32. Some believed that the atypical start codon of human FNDC5 meant it was a “null mutation” or pseudogene and thus functional circulating irisin would not exist 33, 34. This was exacerbated by the lack of a defined antibody, ELISA, or other method of detecting or quantifying circulating irisin at the time 35, 36.
To address this issue, in 2015 we developed a method to quantify circulating irisin in human plasma via targeted mass spectrometry with control peptides enriched with stable heavy isotopes as internal standards 30. This method was both state-of-the-art and precise; it demonstrated that the non-canonical ATA start codon of human irisin was in fact the main site of initiating translation. Further, we were able to quantify circulating irisin in sedentary individuals at a concentration of ~3.6 ng/ml. This circulating concentration was found to be significantly higher in individuals that participated in endurance exercise. This study determined that in fact human irisin does exist and can be regulated via aerobic exercise. Recently, another group using this targeted mass spectrometry approach with heavy internal control peptides found irisin to be present in human cerebrospinal fluid at approximately 0.26–1.86 ng/ml in men over 80 years of age with various diseases 37. However, a knock-out validated antibody for irisin is still missing and would be a valuable resource for the scientific community.
Irisin in the Central Nervous System- Neural Development Effects
Fndc5 is known to be profoundly expressed in the brain in many regions, including cerebellar Purkinje cells25, 26, 38, the hypothalamus 39, as well as the hippocampus 40. Further, the presence of irisin in the cerebrospinal fluid of humans was identified via Western blot and mass spectrometry 37, 41. During the maturation of primary mouse embryonic cortical neuron cultures or the differentiation of human embryonic stem cell- derived neural cells into neurons, FNDC5 levels are increasing 40, 42. High levels of FNDC5 are also found in the heart and oxidative skeletal muscle 40. Forced overexpression of FNDC5 during the formation of neuronal precursors from a mouse embryonic stem cell population lead to increased BDNF, GFAP as well as Map2, b- tubulinIII, and Neurocan, all three being markers of neuronal maturation. However, when FNDC5 was overexpressed in an undifferentiated mouse embryonic stem cell population, the aforementioned effects were not detected 43. Knockdown of FNDC5 in neuronal precursor cells has been shown to impair the maturation process of neurons and astrocytes 44. Pharmacological dose of recombinant irisin in the mouse H19–7 hippocampal cell line led to increased cell proliferation 45. We have previously shown that overexpression of FNDC5 in primary cortical neurons increased cell survival in culture whereas knockdown of FNDC5 reduces the cell survival 40. Pharmacological dose of recombinant irisin in the mouse H19–7 hippocampal cell line led to increased cell proliferation 45. Together, these data suggest that FNDC5/irisin play a developmental role in regulating the process of neuronal differentiation and maturation.
The Role of FNDC5/Irisin in PA/Exercise
Skeletal muscle composes 40% of the body mass in humans. Skeletal muscle is an endocrine organ and secretes myokines, muscle-derived secretory proteins with a wide array of biological functions 17. Endurance exercise activates on a central transcriptional coactivator known as PGC-1α. Irisin, the cleaved form of FNDC5 was shown to be a PGC-1α-dependent myokine that was secreted from skeletal muscle during exercise by Bostrom et. al in 2012 27. Some of the major metabolic benefits of PA/exercise were shown to be promoted by irisin; these include increased glucose tolerance as well as the “browning” of white adipose tissue. 27, 46. Upregulation of circulating irisin by overexpression of FNDC5 in the liver, via adenoviral vector, led to an increase in both 27. Many studies have confirmed the increase of Fndc5 mRNA in skeletal muscle during exercise in mice 40, 47–49 as well as humans 34, 36, 50–52 using RNA sequencing or qPCR. However, there are studies, which have not found an increase of FNDC5 expression in muscle after exercise, suggesting that FNDC5 is not induced by any and all exercise interventions. Of note, several of these exercise regimens that did not see an upregulation of FNDC5 also did not observe an increase in PGC1a, its transcriptional regulator 52, 53 or did not assess changes of PGC-1a 54–56. Other factors that could explain the differences between the different studies are: exercise regimen used, mode of exercise, time point of sampling, and study population.
In addition to the beneficial effects of irisin on metabolism, we later showed that endurance exercise induced irisin expression not only in skeletal muscle but also in the hippocampus, a region of the brain involved in memory and spatial awareness 40. We demonstrated that neuronal Fndc5 gene expression is also regulated by PGC-1α. In addition, we identified FNDC5 as an important regulator of BDNF. Forced expression of FNDC5 in primary cortical neurons increased Bdnf expression and Bdnf expression was markedly reduced following RNAi-mediated knockdown of FNDC5. Similarly, the common human BDNF Val66Met polymorphism was shown to impair both BDNF and FNDC5 expression in the brain following exercise.49 Likewise, Choi et al. showed that exercise-induced adult hippocampal neurogenesis was associated with increases in both BDNF and FNDC5, helping improve cognition in a mouse model of AD 57. Importantly, peripheral delivery of FNDC5 to the liver via adenoviral vectors, which elevated blood irisin levels, induced the expression of Bdnf and other neuroprotective genes in the hippocampus. This finding implies that irisin, or factor induced by irisin, can cross the blood-brain barrier to affect gene expression in the brain. This discovery encouraged the idea that irisin has great therapeutic promise prompting further research on the role of irisin, the hippocampus, and improvements in cognitive function. Since then, there have been a few studies measuring the serum levels of irisin in relation to the cognitive function of either aging individuals 58, young athletes 59 or obese and morbidly obese patients 60. Two out of the three studies found that irisin positively correlates with better cognitive function 58, 59, particularly in the active individuals, whereas the other found a negative correlation of irisin with cognitive function 60. In a study investigating diabetic mild cognitive impairment, irisin overexpression showed elevated levels of BDNF in the serum and the hippocampus and lower levels of inflammatory advanced glycated end products and glycosylated hemoglobinA1c 61. In primary hippocampal nerve cell culture from these diabetic rats, the overexpression of irisin produced higher levels of BDNF and cellular metabolic NAD(P)H-dependent activity following exposure to increasing levels of glucose. These results indicate that irisin may regulate the expression of BDNF and glycometabolism in diabetic rats. Taken together, these findings point toward the relationship of FNDC5 and BDNF expression in the brain that is linked to endurance exercise and subsequently the important metabolic mediators PGC-1α. Further it indicates that irisin seems to be a key peptide at the interface between metabolism and brain function.
Of note, exercise can consist of either endurance or resistance exercise training, the former an aerobic, cardiovascular form of exercise and the latter focusing more muscle strength and hypertrophy. Currently, evidence suggest that irisin is not involved in resistance training. This is to be expected as endurance exercise activates PGC-1α1, an upstream transcriptional regulator of FNDC5, while resistance training utilizes the PGC-1α4 isoform, inducing hypertrophy in exercised muscle 62. Similarly, a study found no differences in serum irisin levels between control and exercised individuals after high-intensity interval and resistance training 63.
Irisin and Neuroprotection in Neurodegeneration
There is a vast body of literature demonstrating the beneficial effects of PA/exercise on neurodegenerative diseases (Figure 3), including AD (as reviewed by Tari et al. in this issue), PD6, and Huntington’s disease (HD)64. As discussed above, the neurotrophin BDNF plays an important role in the homeostatic function and maintenance of the neurons; particularly in synaptic plasticity and neurogenesis. Decreased levels of BDNF have been identified in serum, as well as in hippocampal and cortex samples of AD and PD patients 65–67. To establish novel blood-based biomarkers of cognition and stress, Küster et al. investigated the serum levels of BDNF, irisin and molecules from the kynurenine pathway as potential biomarkers of cognitive decline and dementia in older adults at risk of dementia. Following PA/exercise or cognitive training compared to a control group, both BDNF and irisin serum levels positively correlated with global cognition scores and memory 58. Previous studies have shown that administration of recombinant BDNF in AD and HD animal models improves cognitive function and disease pathogenesis 66–68. Choi et. al demonstrated that exercise provided cognitive benefit to 5×FAD mice, a mouse model of AD, by inducing adult hippocampal neurogenesis (AHN) and elevating levels of BDNF and FNDC5. They successfully mimicked the beneficial effects of exercise on AD mice by genetically and pharmacologically inducing AHN in combination with elevating BDNF levels 57. However, directly increasing levels of hippocampal IL-6 or FNDC5 via lentivirus failed to improve cognition or increase BDNF in 5×FADProAHN mice (5xFAD injected with P7C3 and lentiviral Wnt3 to promote adult hippocampal neurogenesis). Recently, Lourenco et al. published that by delivering FNDC5 adenovirus either by intracerebroventricular injection or tail vein injection they were able to rescue memory impairments and synaptic plasticity in a mouse model of AD 69. Interestingly, an in vitro study carried out by Wang et al. illustrated the potential neuroprotective effects of irisin in a primary neuron culture following a β-amyloid peptide (Aβ) insult. Notably, the effects of irisin were not observed by direct treatment of the neuronal cell culture with irisin and Aβ peptide (25–35); but only with the treatment of conditioned medium from astrocyte culture treated with irisin and Aβ peptide. Following treatment with astrocyte conditioned medium, there was significant improvement in neuronal cell viability after Aβ peptide exposure. The astrocyte cultures showed a reduction in inflammatory cytokine production of IL-6, IL-1β and COX-2 via the Akt/NFκB pathway upon Aβ peptide exposure. Another study has illustrated increased levels of glucose transport and phosphorylation by the AMPK pathway in astrocyte cell culture following treatment of recombinant irisin 70. These findings indicate that irisin may be mediating neuroprotective effects via astrocytes. Mitochondrial dysfunction has been implicated with multiple neurodegenerative diseases such as PD 71. This link between the metabolic effects of irisin and its effects on the brain warrant further investigation for potential therapeutic applications of irisin in the future.
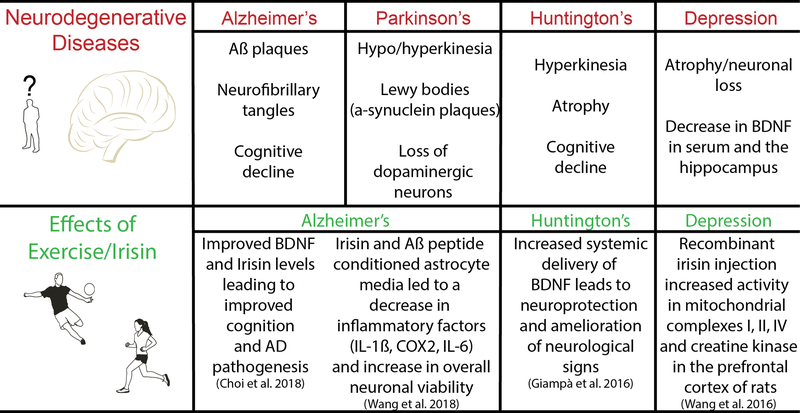
Fig 3.
The effects of both irisin and exercise on neurodegenerative disease Irisin has been shown to have beneficial effects in a variety of neurodegenerative diseases.
The Future of FNDC5/Irisin, PA/Exercise, and Brain Health
A large amount of interest has been generated by the myokine irisin in recent years; a simple PubMed database search of the keyword “irisin” yields over 700 peer-reviewed articles. Although irisin is a myokine secreted from skeletal muscle, it has a large impact on varying regions of the body ranging from lipid metabolism, thermogenesis and browning of white adipose tissue to bone resorption as well as neuronal differentiation and neuroprotection in the brain (Figure 4). As previously discussed, aerobic exercise has been shown to have the greatest impact upon cognitive function in the aging population and in neurodegenerative diseases. Although more research is necessary to determine whether the FNDC5/irisin protein can improve cognitive function in animals, our prior studies suggest that a hormone administered peripherally could induce some of the effects of endurance exercise on the brain. The future for FNDC5/irisin is very exciting, and we look forward to the potential therapeutic uses of this myokine and exercise in ameliorating cognitive deficits associated with neurogenerative disease.
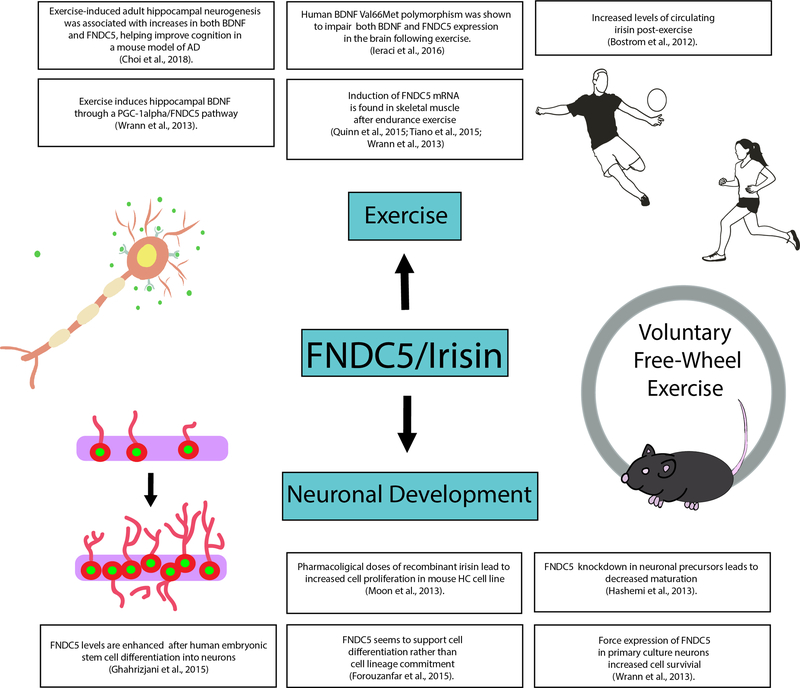
Fig 4.
The role of FNDC5/irisin in exercise and the brain. Exercise has been shown to be a strong stimulus for the upregulation of FNDC5/irisin. In addition to the metabolic benefits of FNDC5/irisin, it has also been shown to play a role in neuronal differentiation and neuroprotection in brain.
Acknowledgments
We acknowledge support from a Pathway to Independence (PI) Award (4R00NS087096-03), a NeuroBehavior Laboratory Pilot Project Research Award from the Harvard NeuroDiscovery Center (HNDC), and a Hassenfeld Cardiovascular Scholar Award to C.D.W.
Abbreviations:
- Aβ
amyloid beta protein
- AD
Alzheimer’s disease
- Akt/NFκB
protein kinase B/nuclear factor kappa-light-chain-enhancer of activated B cells
- AMPK
5’ Adenosine monophosphate-activated protein kinase
- BDNF
brain derived neurotrophic factor
- BrdU
5-bromo-2’-deoxyuridine
- COX-2
cyclooxygenase 2
- ELISA
enzyme-linked immunosorbent assay
- FNDC5
fibronectin type III domains containing protein 5
- GFAP
glial fibrillary acidic protein
- HD
Huntington’s disease
- IL-1
interleukin 1
- IL-6
interleukin 6
- Map2
microtubule-associated protein 2
- NADPH
nicotinamide adenine dinucleotide phosphate
- PA
physical activity
- PD
Parkinson’s disease
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- RNAi
RNA interference
- TrkB
tropomyosin receptor kinase b
Footnotes
Statement of conflict of interest: C.D.W. is holder of US Patent Application, WO 2015051007 A1
References
- 1.Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ. 2006;174:801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erickson KI, Gildengers AG, Butters MA. Physical activity and brain plasticity in late adulthood. Dialogues Clin Neurosci. 2013;15:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013;17:525–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell metabolism. 2012;16:706–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. [DOI] [PubMed] [Google Scholar]
- 6.Ahlskog JE. Does vigorous exercise have a neuroprotective effect in Parkinson disease? Neurology. 2011;77:288–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchman AS, Boyle PA, Yu L, Shah RC, Wilson RS, Bennett DA. Total daily physical activity and the risk of AD and cognitive decline in older adults. Neurology. 2012;78:1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quaney BM, Boyd LA, McDowd JM, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabil Neural Repair. 2009;23:879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cotman CW, Berchtold NC, Christie LA. Exercise builds brain health: key roles of growth factor cascades and inflammation. Trends in neurosciences. 2007;30:464–472. [DOI] [PubMed] [Google Scholar]
- 10.Barrientos RM, Frank MG, Crysdale NY, et al. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Praag H, Kempermann G, Gage FH. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nature neuroscience. 1999;2:266–270. [DOI] [PubMed] [Google Scholar]
- 13.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:13427–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kobilo T, Liu QR, Gandhi K, Mughal M, Shaham Y, van Praag H. Running is the neurogenic and neurotrophic stimulus in environmental enrichment. Learning & memory (Cold Spring Harbor, N.Y.). 2011;18:605–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mustroph ML, Chen S, Desai SC, Cay EB, DeYoung EK, Rhodes JS. Aerobic exercise is the critical variable in an enriched environment that increases hippocampal neurogenesis and water maze learning in male C57BL/6J mice. Neuroscience. 2012;219:62–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay CP, Kuys SS, Brauer SG. The Effect of Aerobic Exercise on Brain- Derived Neurotrophic Factor in People with Neurological Disorders: A Systematic Review and Meta-Analysis. Neural plasticity. 2017;2017:4716197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper C, Moon HY, van Praag H. On the Run for Hippocampal Plasticity. Cold Spring Harbor perspectives in medicine. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park H, Poo MM. Neurotrophin regulation of neural circuit development and function. Nat Rev Neurosci. 2013;14:7–23. [DOI] [PubMed] [Google Scholar]
- 19.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: implications in CNS function. J Neurosci. 2009;29:12764–12767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egan MF, Kojima M, Callicott JH, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. [DOI] [PubMed] [Google Scholar]
- 21.Kuipers SD, Bramham CR. Brain-derived neurotrophic factor mechanisms and function in adult synaptic plasticity: new insights and implications for therapy. Curr Opin Drug Discov Devel. 2006;9:580–586. [PubMed] [Google Scholar]
- 22.Hariri AR, Goldberg TE, Mattay VS, et al. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. J Neurosci. 2003;23:6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vaynman SS, Ying Z, Yin D, Gomez-Pinilla F. Exercise differentially regulates synaptic proteins associated to the function of BDNF. Brain Res. 2006;1070:124–130. [DOI] [PubMed] [Google Scholar]
- 24.Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. [DOI] [PubMed] [Google Scholar]
- 25.Teufel A, Malik N, Mukhopadhyay M, Westphal H. Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene. 2002;297:79–83. [DOI] [PubMed] [Google Scholar]
- 26.Ferrer-Martinez A, Ruiz-Lozano P, Chien KR. Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn. 2002;224:154–167. [DOI] [PubMed] [Google Scholar]
- 27.Bostrom P, Wu J, Jedrychowski MP, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schumacher MA, Chinnam N, Ohashi T, Shah RS, Erickson HP. The structure of irisin reveals a novel intersubunit beta-sheet fibronectin type III (FNIII) dimer: implications for receptor activation. J Biol Chem. 2013;288:33738–33744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Wrann CD, Jedrychowski M, et al. Irisin Mediates Effects on Bone and Fat via alphaV Integrin Receptors. Cell. 2018;175:1756–1768.e1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jedrychowski MP, Wrann CD, Paulo JA, et al. Detection and Quantitation of Circulating Human Irisin by Tandem Mass Spectrometry. Cell metabolism. 2015;22:734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell. 2011;147:789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ivanov IP, Firth AE, Michel AM, Atkins JF, Baranov PV. Identification of evolutionarily conserved non-AUG-initiated N-terminal extensions in human coding sequences. Nucleic Acids Res. 2011;39:4220–4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raschke S, Elsen M, Gassenhuber H, et al. Evidence against a beneficial effect of irisin in humans. PLoS One. 2013;8:e73680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albrecht E, Norheim F, Thiede B, et al. Irisin - a myth rather than an exercise- inducible myokine. Sci Rep. 2015;5:8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perakakis N, Triantafyllou GA, Fernandez-Real JM, et al. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13:324–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huh JY, Mougios V, Kabasakalis A, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. The Journal of clinical endocrinology and metabolism. 2014;99:E2154–2161. [DOI] [PubMed] [Google Scholar]
- 37.Ruan Q, Zhang L, Ruan J, et al. Detection and quantitation of irisin in human cerebrospinal fluid by tandem mass spectrometry. Peptides. 2018;103:60–64. [DOI] [PubMed] [Google Scholar]
- 38.Dun SL, Lyu RM, Chen YH, Chang JK, Luo JJ, Dun NJ. Irisin-immunoreactivity in neural and non-neural cells of the rodent. Neuroscience. 2013;240:155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Varela-Rodriguez BM, Pena-Bello L, Juiz-Valina P, Vidal-Bretal B, Cordido F, Sangiao-Alvarellos S. FNDC5 expression and circulating irisin levels are modified by diet and hormonal conditions in hypothalamus, adipose tissue and muscle. Sci Rep. 2016;6:29898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wrann CD, White JP, Salogiannnis J, et al. Exercise induces hippocampal BDNF through a PGC-1alpha/FNDC5 pathway. Cell metabolism. 2013;18:649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piya MK, Harte AL, Sivakumar K, et al. The identification of irisin in human cerebrospinal fluid: influence of adiposity, metabolic markers, and gestational diabetes. Am J Physiol Endocrinol Metab. 2014;306:E512–518. [DOI] [PubMed] [Google Scholar]
- 42.Ghahrizjani FA, Ghaedi K, Salamian A, et al. Enhanced expression of FNDC5 in human embryonic stem cell-derived neural cells along with relevant embryonic neural tissues. Gene. 2015;557:123–129. [DOI] [PubMed] [Google Scholar]
- 43.Forouzanfar M, Rabiee F, Ghaedi K, et al. Fndc5 overexpression facilitated neural differentiation of mouse embryonic stem cells. Cell Biol Int. 2015;39:629–637. [DOI] [PubMed] [Google Scholar]
- 44.Hashemi MS, Ghaedi K, Salamian A, et al. Fndc5 knockdown significantly decreased neural differentiation rate of mouse embryonic stem cells. Neuroscience. 2013;231:296–304. [DOI] [PubMed] [Google Scholar]
- 45.Moon HS, Dincer F, Mantzoros CS. Pharmacological concentrations of irisin increase cell proliferation without influencing markers of neurite outgrowth and synaptogenesis in mouse H19–7 hippocampal cell lines. Metabolism. 2013;62:1131–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee P, Linderman JD, Smith S, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell metabolism. 2014;19:302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Quinn LS, Anderson BG, Conner JD, Wolden-Hanson T. Circulating irisin levels and muscle FNDC5 mRNA expression are independent of IL-15 levels in mice. Endocrine. 2015;50:368–377. [DOI] [PubMed] [Google Scholar]
- 48.Tiano JP, Springer DA, Rane SG. SMAD3 negatively regulates serum irisin and skeletal muscle FNDC5 and peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1alpha) during exercise. J Biol Chem. 2015;290:7671–7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ieraci A, Madaio AI, Mallei A, Lee FS, Popoli M. Brain-Derived Neurotrophic Factor Val66Met Human Polymorphism Impairs the Beneficial Exercise-Induced Neurobiological Changes in Mice. Neuropsychopharmacology. 2016;41:3070–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lecker SH, Zavin A, Cao P, et al. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ Heart Fail. 2012;5:812–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Norheim F, Langleite TM, Hjorth M, et al. The effects of acute and chronic exercise on PGC-1alpha, irisin and browning of subcutaneous adipose tissue in humans. FEBS J. 2014;281:739–749. [DOI] [PubMed] [Google Scholar]
- 52.Pekkala S, Wiklund PK, Hulmi JJ, et al. Are skeletal muscle FNDC5 gene expression and irisin release regulated by exercise and related to health? The Journal of physiology. 2013;591:5393–5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alvehus M, Boman N, Soderlund K, Svensson MB, Buren J. Metabolic adaptations in skeletal muscle, adipose tissue, and whole-body oxidative capacity in response to resistance training. European journal of applied physiology. 2014;114:1463–1471. [DOI] [PubMed] [Google Scholar]
- 54.Besse-Patin A, Montastier E, Vinel C, et al. Effect of endurance training on skeletal muscle myokine expression in obese men: identification of apelin as a novel myokine. International journal of obesity (2005). 2014;38:707–713. [DOI] [PubMed] [Google Scholar]
- 55.Kurdiova T, Balaz M, Vician M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. The Journal of physiology. 2014;592:1091–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Scalzo RL, Peltonen GL, Giordano GR, et al. Regulators of human white adipose browning: evidence for sympathetic control and sexual dimorphic responses to sprint interval training. PloS one. 2014;9:e90696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Choi SH, Bylykbashi E, Chatila ZK, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer’s mouse model. Science (New York, N.Y.). 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kuster OC, Laptinskaya D, Fissler P, et al. Novel Blood-Based Biomarkers of Cognition, Stress, and Physical or Cognitive Training in Older Adults at Risk of Dementia: Preliminary Evidence for a Role of BDNF, Irisin, and the Kynurenine Pathway. Journal of Alzheimer’s disease : JAD. 2017;59:1097–1111. [DOI] [PubMed] [Google Scholar]
- 59.Belviranli M, Okudan N, Kabak B, Erdogan M, Karanfilci M. The relationship between brain-derived neurotrophic factor, irisin and cognitive skills of endurance athletes. The Physician and sportsmedicine. 2016;44:290–296. [DOI] [PubMed] [Google Scholar]
- 60.Fagundo AB, Jimenez-Murcia S, Giner-Bartolome C, et al. Modulation of Irisin and Physical Activity on Executive Functions in Obesity and Morbid obesity. Scientific reports. 2016;6:30820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huang L, Yan S, Luo L, Yang L. Irisin regulates the expression of BDNF and glycometabolism in diabetic rats. Molecular medicine reports. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ruas JL, White JP, Rao RR, et al. A PGC-1alpha isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151:1319–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He Z, Tian Y, Valenzuela PL, et al. Myokine Response to High-Intensity Interval vs. Resistance Exercise: An Individual Approach. Frontiers in physiology. 2018;9:1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Corrochano S, Blanco G, Acevedo-Arozena A. Skeletal Muscle Modulates Huntington’s Disease Pathogenesis in Mice: Role of Physical Exercise. Journal of experimental neuroscience. 2018;12:1179069518809059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Michalski B, Fahnestock M. Pro-brain-derived neurotrophic factor is decreased in parietal cortex in Alzheimer’s disease. Brain research. Molecular brain research. 2003;111:148–154. [DOI] [PubMed] [Google Scholar]
- 66.Arancibia S, Silhol M, Mouliere F, et al. Protective effect of BDNF against beta- amyloid induced neurotoxicity in vitro and in vivo in rats. Neurobiology of disease. 2008;31:316–326. [DOI] [PubMed] [Google Scholar]
- 67.Giampa C, Montagna E, Dato C, Melone MA, Bernardi G, Fusco FR. Systemic delivery of recombinant brain derived neurotrophic factor (BDNF) in the R6/2 mouse model of Huntington’s disease. PloS one. 2013;8:e64037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang L, Fang Y, Lian Y, et al. Brain-derived neurotrophic factor ameliorates learning deficits in a rat model of Alzheimer’s disease induced by abeta1–42. PloS one. 2015;10:e0122415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lourenco MV, Frozza RL, de Freitas GB, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nature medicine. 2019;25:165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Pan J. Irisin ameliorates depressive-like behaviors in rats by regulating energy metabolism. Biochemical and biophysical research communications. 2016;474:22–28. [DOI] [PubMed] [Google Scholar]
- 71.Park JS, Davis RL, Sue CM. Mitochondrial Dysfunction in Parkinson’s Disease: New Mechanistic Insights and Therapeutic Perspectives. Current neurology and neuroscience reports. 2018;18:21. [DOI] [PMC free article] [PubMed] [Google Scholar]