Abstract
Objective
Several anti-programmed cell death 1 (anti-PD-1) antibodies have demonstrated potential efficacy in the treatment of advanced esophageal squamous cell cancer (ESCC). However, the response to subsequent chemotherapy after the failure of PD-1 blockade in ESCC patients has not been reported, and the optimal sequencing of immunotherapy and chemotherapy remains controversial. The aim of the present study was to evaluate responses to irinotecan-based subsequent chemotherapy in advanced ESCC patients who had progressed after treatment with camrelizumab (SHR-1210), a novel anti-PD-1 antibody.
Methods
We retrospectively reviewed the medical records of patients with advanced ESCC treated with camrelizumab at a single institution. Consecutive patients who received subsequent irinotecan-based chemotherapy were selected for data collection and analysis.
Results
Overall, a total of 28 patients were included. All patients had received at least two lines of systemic treatment prior to irinotecan salvage. The most common regimen that was administered after PD-1 blockade was irinotecan in combination with 5-fluorouracil (5-Fu) (or its derivatives), which was given to 19 patients. The objective response rate (ORR) and disease control rate (DCR) were 17.9% (5/28) and 64.3% (18/28), respectively, with 5 (17.9%) patients achieving a partial response and 13 (46.4%) having stable disease. The median progression-free survival (PFS) was 3.18 [95% confidence interval (95% CI), 2.48−3.88] months and the median overall survival (OS) was 6.23 (95% CI, 4.71−7.75) months. No new safety issues, either immune-related or otherwise, were observed.
Conclusions
Our results suggested that the response to irinotecan-based chemotherapy after PD-1 blockade in advanced ESCC patients appeared similar to that previously observed in patients who had not received PD-1 antibodies, and further study in larger cohorts or randomized trials is warranted to verify our observation.
Keywords: Esophageal squamous cell carcinoma, irinotecan, immunotherapy, treatment outcomes
Introduction
Esophageal cancer ranks the seventh in terms of incidence and the sixth in terms of mortality worldwide, and Eastern Asia is among the regions with the highest prevalence (1). Esophageal squamous cell carcinoma (ESCC) remains the predominant pathological subtype in China and other East Asian countries (2), imposing tremendous burdens both epidemiologically and financially (3). Over one third of esophageal cancer patients present with metastatic disease at the time of diagnosis (4), indicating that there is an imperative need for effective systemic therapies.
Active anti-tumor agents for the treatment of advanced ESCC are extremely limited, especially in patients whose disease progresses after first-line chemotherapy. As part of the effort to discover new treatment measures for ESCC patients, human epidermal growth factor receptor (EGFR) has been investigated as a potential therapeutic target. Compared to placebo, gefitinib, an EGFR-tyrosine kinase inhibitor (TKI), did not prolong overall survival (OS) in a randomized phase 3 trial in which both esophageal adenocarcinoma and ESCC patients were enrolled, and the benefit in terms of PFS was only marginal (5). Likewise, icotinib, another EGFR-TKI, showed limited efficacy in a selected population of pretreated ESCC patients with EGFR overexpression or amplification (6). Based on the fundamental understanding of the interactions of programmed cell death 1 (PD-1) with its ligands and their role in tumor escape mechanisms, immune checkpoint inhibitors (ICIs) have emerged as novel treatment options for a variety of solid tumors. Despite the encouraging efficacy of several anti-PD-1 antibodies in patients with advanced esophageal cancer, only 17%−33% of the patients responded to PD-1 blockade without reliable predictive biomarkers (7-9), and a substantial number of patients would suffer disease progression and require subsequent systemic treatments. Given the lack of effective targeted agents, rechallenge chemotherapy, preferably with regimens not received in previous lines of therapy, is a reasonable choice in the post PD-1 blockade setting.
Irinotecan, a semisynthetic topoisomerase I inhibitor, is widely used in the treatment of various solid tumors. The activity of irinotecan, either as a single agent or in combination regimens, was evaluated in pretreated esophageal cancer patients in phase 2 studies and the response rates ranged from 12.5% to 35.7% (10-15). However, evidence of the anti-tumor activity of chemotherapy after the failure of ICIs in esophageal cancer is lacking. In this report, we aim to investigate the efficacy of irinotecan-based salvage chemotherapies after treatment with camrelizumab (SHR-1210), a novel anti-PD-1 antibody, in advanced ESCC patients.
Materials and methods
Patients and study design
We reviewed the medical records and follow-up data for all patients with refractory or metastatic ESCC who were enrolled in two clinical trials at National Cancer Center/National Clinical Research Center for Cancer and treated with camrelizumab monotherapy between May 11 2016 and Oct 30 2018. We included consecutive ESCC patients who received irinotecan-based systemic therapies after the failure of camrelizumab in the analysis performed in the present study.
Of the two clinical trials from which patients were screened, one involved a phase 1 study of camrelizumab. Eligible patients had advanced solid tumors and the disease had progressed after at least one systemic treatment. The other trial was a phase 3 study comparing single-agent camrelizumab therapy with a chemotherapy of the investigator’s choice as the second-line systemic therapy in patients with advanced ESCC. Eligible patients had documented ESCC, and they had been treated with a first-line systemic therapy that had failed. Key exclusion criteria for both trials included a history of active autoimmune disease, ongoing systemic immunosuppressive therapy, a history of organ transplantation or previous anti-PD-1/PD-L1 treatments.
The demographic and clinical characteristics of the patients and the details regarding their subsequent therapies after camrelizumab treatment, including the regimen of choice, dosage and schedule, investigator-assessed best response according to the Response Evaluation Criteria in Solid Tumors (RECIST v1.1), the time of disease progression and death and descriptions of adverse events graded according to the Common Terminology Criteria for Adverse Events (CTCAE v4.03) were retrieved from the archived medical records from the two clinical trials. The protocol used for the current study conforms to the provisions of the Declaration of Helsinki, and has been approved by the independent ethics committee of the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences. Informed consent was waived as the study was of retrospective design.
Outcomes and statistical analysis
The primary endpoints of this study were the objective response rate (ORR) and the disease control rate (DCR) of advanced ESCC patients receiving irinotecan-based salvage chemotherapy after the failure of PD-1 blockade. We also investigated progression-free survival (PFS), OS and safety in this patient population. The ORR was defined as the percentage of patients achieving a best response of complete response (CR) or partial response (PR) as per RECIST v1.1, whereas the DCR was defined as the percentage of patients achieving a best response of CR, PR or stable disease (SD). PFS was defined as the time period between the start of the subsequent systemic therapy after PD-1 blockade and the first documented disease progression or death from any cause, with censoring of patients who were alive and progression-free at the data cut-off point. OS was defined as the time period between treatment initiation and death from any cause, or censoring of patients who were alive at the data cut-off point. Survival analysis was performed using the Kaplan-Meier method. The statistical analysis was performed using IBM SPSS Statistics (Version 22.0; IBM Corp., New York, USA).
Results
Patient characteristics
As of January 7, 2019 (data cut-off point), 68 out of all 69 consecutive ESCC patients enrolled in the two clinical trials had discontinued camrelizumab treatment due to either disease progression or intolerable toxicity, and 37 patients had received subsequent systemic treatments. Irinotecan-based therapies were administered in 28 patients. The regimens, dosages and schedules used for irinotecan-based chemotherapy in all patients were determined at the discretion of the treating physician.
The median age of the 28 patients was 62 (range: 38−69) years old, and the majority of the patients were male (27/28, 96.4%). The patients were heavily pretreated, and eight patients (8/28, 28.6%) had received two or more lines of treatment before treatment with camrelizumab. In addition, 19 patients (19/28, 67.9%) had undergone radiotherapy before PD-1 blockade and 12 patients (12/28, 42.9%) had received esophagectomy before the onset of refractory disease. The investigator-assessed best response to the last chemotherapy regimen received before anti-PD-1 antibody was available in 23 of the patients; the ORR was 14.3% (4/28), and 3 patients achieved PR and 1 patient achieved CR.
In addition to 3 patients who received irinotecan monotherapy as the salvage treatment after the failure with camrelizumab, 19 patients received irinotecan in combination with 5-fluorouracil (5-Fu) (or one of the 5-Fu derivatives, i.e., S-1 or capecitabine), 3 patients received irinotecan with either apatinib or anlotinib, both are vascular endothelial growth factor receptor-2 (VEGFR-2) TKIs, 2 patients received irinotecan with raltitrexed, and 1 patient received irinotecan with S-1 and anlotinib. Four patients had been treated with irinotecan-based regimens before PD-1 blockade, and all of them had achieved objective response. Of the 19 patients who received irinotecan with 5-Fu (or its derivative), 8 of them had received 5-Fu-containing regimens in previous lines treatment with systemic therapies.
The doses and schedules used for the administration of irinotecan were available for 25 of the patients. The initial dose ranged from 129.8 mg/m2 to 163.0 mg/m2, with a median dose of 147.1 mg/m2. All treatments were administered in 2-week cycles. The patient characteristics are summarized in Table 1.
1.
Patients’ baseline characteristics (N=28)
| Characteristics | n (%) |
| PD-1, programmed cell death 1; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; CNS, central nervous system; VEGFR, vascular endothelial growth factor receptor; TKI, tyrosine kinase inhibitor; *, at salvage chemotherapy after PD-1 blockade. | |
| Age* [median (range)] (year) | 62 (38−69) |
| Gender | |
| Male | 27 (96.4) |
| Female | 1 (3.6) |
| Histologic grade at the time of diagnosis | |
| G1 | 3 (10.7) |
| G2 | 12 (42.9) |
| G3 | 9 (32.1) |
| GX | 4 (14.3) |
| Esophagectomy before systemic therapy | |
| Yes | 12 (42.9) |
| No | 16 (57.1) |
| Radiotherapy before PD-1 blockade | |
| Yes | 19 (67.9) |
| No | 9 (32.1) |
| Number of previous lines of systemic therapy before PD-1 blockade | |
| 1 | 20 (71.4) |
| 2 | 6 (21.4) |
| >2 | 2 (7.1) |
| Best response of PD-1 blockade | |
| CR | 1 (3.6) |
| PR | 7 (25.0) |
| SD | 8 (28.6) |
| PD | 12 (42.9) |
| Irinotecan-based chemotherapy before PD-1 blockade | |
| Yes | 4 (14.3) |
| No | 24 (85.7) |
| Staging at initiation of salvage chemotherapy | |
| III | 1 (3.6) |
| IV | 27 (96.4) |
| Site of metastasis at initiation of salvage chemotherapy | |
| Distant lymph node | 21 (75.0) |
| Liver | 4 (14.3) |
| Lung | 10 (35.7) |
| Bone | 4 (14.3) |
| Adrenal gland | 2 (7.1) |
| Pleura | 2 (7.1) |
| CNS | 2 (7.1) |
| Regimen of salvage chemotherapy | |
| Single agent irinotecan | 3 (10.7) |
| FOLFIRI, or irinotecan with S-1 or capecitabine | 19 (67.9) |
| Irinotecan with raltitrexed | 2 (7.1) |
| Irinotecan with a VEGFR2 TKI | 3 (10.7) |
| Irinotecan with S-1 and a VEGFR2 TKI | 1 (3.6) |
Clinical outcomes
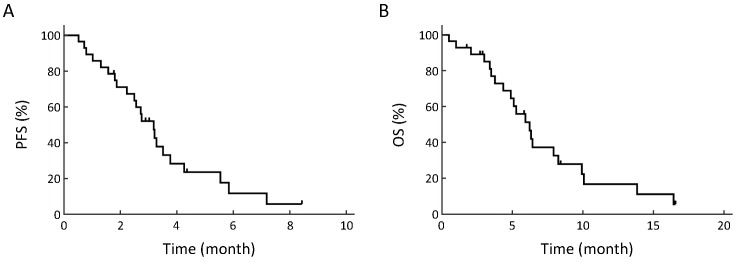
The median follow-up duration from the start of subsequent irinotecan-based chemotherapy was 158 (range: 16−505) d. The investigator-assessed best response was available for 24 out of the 28 patients. The ORR and DCR were 17.9% (5/28) and 64.3% (18/28), respectively, and 5 (17.9%) patients achieved PR and 13 (46.4%) had SD, while 6 (21.4%) patients presented with progressive disease (PD) at their first radiological evaluation. The median PFS was 3.18 (95% CI: 2.48−3.88) months and the median OS was 6.23 (95% CI: 4.71−7.75) months. The survival curves are shown inFigure 1.
1.
Survival curves of patients receiving irinotecan-based salvage chemotherapy. Progression-free survival (PFS) (A) and overall survival (OS) (B) from initiation of subsequent irinotecan-based salvage chemotherapy after treatment with camrelizumab.
All 5 patients who responded to irinotecan-based salvage chemotherapy were treated with irinotecan in combination with 5-Fu or S-1. The ORR to subsequent chemotherapy in responders and non-responders to PD-1 blockade was 12.5% (1/8) and 20.0% (4/20), respectively, whereas the DCR in the two subgroups was 62.5% (5/8) and 65.0% (13/20), respectively. Three of the four patients who had received irinotecan-based chemotherapy prior to camrelizumab presented with PD during rechallenge, while the remaining patient was not assessed.
Safety
Leukopenia (13/28, 46.4%) and neutropenia (12/28, 42.9%) were the most common hematological adverse events, followed by anemia (7/28, 25.0%) and thrombocytopenia (1/28, 3.6%). The most frequently observed nonhematological adverse events were increased ALT/AST/bilirubin (11/28, 39.3%) and nausea/vomiting (11/28, 39.3%). No treatment-related deaths occurred, and no new immune-related adverse events (irAEs) or deterioration in terms of previous irAEs were observed. Most adverse events, including those preexisting irAEs, were grade 1−2 and were managed with appropriate supportive care. The treatment-related adverse events are summarized inTable 2.
2.
Summary of treatment related toxicities (N=28)
| TRAEs | n (%) | |
| Grade 1−2 | Grade 3−4 | |
| TRAE, treatment related adverse event. | ||
| Neutropenia | 10 (35.7) | 2 (7.1) |
| Leukopenia | 11 (39.3) | 2 (7.1) |
| Anemia | 7 (25.0) | 0 (0) |
| Thrombocytopenia | 1 (3.6) | 0 (0) |
| Increased ALT/AST/bilirubin | 10 (35.7) | 1 (3.6) |
| Nausea/Vomiting | 11 (39.3) | 0 (0) |
| Diarrhea | 3 (10.7) | 0 (0) |
| Fatigue | 4 (14.3) | 0 (0) |
Discussion
The results of this retrospective study showed that irinotecan-based chemotherapy subsequent to treatment failure achieved an ORR of 17.9% and a DCR of 64.3% in 28 consecutive ESCC patients who had been previously enrolled in clinical trials of an anti-PD-1 antibody. To the best of our knowledge, this is the first report on the efficacy of subsequent chemotherapy after ICI treatment in advanced esophageal cancer patients.
The response and survival data in our study were comparable to the results reported for irinotecan-based chemotherapies in ICI-naïve pretreated advanced esophageal cancer patients. Weekly irinotecan monotherapy yielded an ORR of 15.4% in cisplatin-refractory esophageal cancer in a phase 2 trial, and the median PFS and OS were 2 and 5 months, respectively (10). Several phase 2 trials reported the efficacy of irinotecan combination therapies in patients who had been treated previously for advanced esophageal cancer. The ORR achieved with treatment with irinotecan and 5-FU/leucovorin or capecitabine ranged from 17% to 29% (11,12), while the ORR achieved with treatment with irinotecan and docetaxel as a second-line or later therapy ranged from 12.5% to 35.7% (13-15). The OS achieved with both combination therapies ranged from 24 weeks to 11.4 months in chemotherapy-exposed patients (11-15). Notably, all these trials were conducted in the Western hemisphere, and the histological subtype of cancer in most enrolled patients was adenocarcinoma. As with ESCC, the evidence of treatment efficacy was sparse. We previously conducted a retrospective analysis of 27 Chinese patients to evaluate the efficacy of an irinotecan plus fluorouracil-based regimen as a second- or third-line chemotherapy for recurrent or metastatic ESCC, in which an ORR of 29.6% was attained, and the median PFS and OS were 4.8 and 10.5 months, respectively (16). Recently, we reported the results of a prospective randomized, multicenter, phase 3 trial comparing irinotecan plus S-1 vs. S-1 alone in previously treated advanced ESCC patients, the response rate in the irinotecan plus S-1 group was 24.6%, and the median PFS was 3.8 months (17). Although the response and survival data from the previous ESCC trials appeared to suggest that the results of those trials were somewhat superior to those observed in this study, it is important to highlight that the patients in the present study were receiving third-line (at least) systemic therapy, and over half of the regimens consisted of “rechallenge” with either irinotecan or 5-Fu. In contrast, 85% of the patients in the ICI-naïve retrospective study and 83.6% in the irinotecan plus S-1 gruop of the prospective study were receiving irinotecan-based chemotherapy for the first time (16,17). Therefore, we may infer that exposure to ICIs did not render subsequent irinotecan-based salvage chemotherapy less effective in advanced ESCC patients, but this observation requires further validation in larger cohorts or randomized trials.
In our study, chemotherapy after PD-1 blockade was well tolerated with few toxicities. Importantly, none of the adverse events were considered to be immune-related. The toxicity profile was consistent with our previous observations in ICI-naïve ESCC patients (16,17). The results might suggest that previous PD-1 blockade did not seem to affect subsequent chemotherapy in terms of toxicity as no new safety issues, either immune-related or otherwise, were noticed in the present study.
It has been postulated that chemotherapy and immunotherapy might have synergistic effects that could allow cytotoxic agents to enhance the efficacy of immunotherapy by overcoming immunosuppression and facilitating tumor antigen presentation and the migration of immune cells into the tumor core (18). Nevertheless, the precise immunomodulatory effects of ICIs on subsequent chemotherapy treatments are unclear, and the optimal sequencing of immunotherapy and chemotherapy as to maximize clinical benefits remains controversial. A significantly higher ORR was observed for salvage chemotherapy after ICI treatment than for the last chemotherapy treatment before PD-1/PD-L1 blockade for all regimens (53.4% vs. 39.4%) in a retrospective study of patients with non-small cell lung cancer (NSCLC). Based on these counterintuitive findings, a treatment sequence of immunotherapy followed by chemotherapy was considered superior, as ICIs were believed to make tumors more vulnerable to subsequent chemotherapy (19). In another retrospective study of patients with relapsed or refractory Hodgkin lymphoma who failed to respond to anti-PD-1 antibodies, 15 patients were re-exposed to the same chemotherapy agent they had received prior to ICI treatment. Among them, 9 patients responded to chemotherapy before treatment with ICIs, whereas the number of patients achieving a PR or CR increased to 12 during re-exposure chemotherapy, suggesting that the anti-PD-1 antibodies might have restored chemosensitivity (20). In contrast, the ORR to first-line platinum-based chemotherapy before ICI treatment and subsequent chemotherapy after ICIs were 57% and 21%, respectively, in a cohort of patients with metastatic urothelial cancer (MUC); the latter rate was in line with the expected results in patients without ICI exposure (21). In our current analysis, the response rate to irinotecan-based chemotherapy after PD-1 blockade was only slightly higher than the rate of response to the last chemotherapy before PD-1 inhibition (17.9% vs. 14.3%). Meanwhile, the three patients who had initially responded to the irinotecan-based regimen before anti-PD-1 antibody treatment failed to respond a second time post PD-1 therapy in our study. Taking into consideration the variety of regimens used for the last chemotherapy treatment before ICI treatment in our study and the small sample size, it might be too early to conclude that sensitivity to chemotherapy could be either restored or improved after PD-1 blockade. Caution must be taken when interpreting these conflicting findings, which were obtained from different types of solid tumors. The lack of stratification based on specific chemotherapy regimens in both the NSCLC and MUC studies should not be overlooked. In addition, it could be possible that the immunomodulatory effect of ICIs on subsequent systemic therapy might have distinct disease- or drug-specific characteristics.
In our current analysis, the similar efficacy of salvage chemotherapy in responders and non-responders that was observed after PD-1 blockade might imply that further clinical benefit from chemotherapy was not associated with prior response to ICIs. These findings were consistent with the results from a retrospective study of advanced urothelial cancer patients, in which the duration of and the response to prior PD-1/PD-L1 inhibitor treatment were not associated with survival in patients receiving subsequent treatment with anti-tumor agents (22). However, these observations remain to be investigated in larger cohorts since both studies were of retrospective design and involved small numbers of patients.
The limitations of our report include the small sample size which precluded the confirmation of any conclusions and further statistical analysis in the subgroups of interest. Moreover, the retrospective nature of the study could result in selection bias. In addition, patients included in the present study were enrolled from two separate clinical trials. As a result, they had received different numbers of prior systemic therapies before irinotecan-based chemotherapy. Such difference could have partly affected the results, because those who had received multiple prior lines of treatments might have inferior outcomes. Nonetheless, this is the only report on advanced esophageal cancer patients treated with chemotherapy after PD-1 inhibition and might shed light on the management of ESCC patients in this specific setting, and the understanding of the interactions between ICIs and cytotoxic agents.
Conclusions
The response to irinotecan-based salvage chemotherapy after the failure of an anti-PD-1 antibody in advanced ESCC patients was similar to that previously reported in patients who had not received ICIs. Further studies are required to verify our findings in larger cohorts, and prospective trials are needed to define the optimal sequencing of immunotherapy and chemotherapy in advanced ESCC patients.
Acknowledgements
This study was partially supported by the Capital’s Funds for Health Improvement and Research (No. CFH2018-4-4024).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Zhang HZ, Jin GF, Shen HB Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin J Cancer. 2012;31:281–6. doi: 10.5732/cjc.011.10390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang Z, Zeng H, Xia R, et al Annual cost of illness of stomach and esophageal cancer patients in urban and rural areas in China: A multi-center study. Chin J Cancer Res. 2018;30:439–48. doi: 10.21147/j.issn.1000-9604.2018.04.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Y Epidemiology of esophageal cancer. World J Gastroenterol. 2013;19:5598–606. doi: 10.3748/wjg.v19.i34.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dutton SJ, Ferry DR, Blazeby JM, et al Gefitinib for oesophageal cancer progressing after chemotherapy (COG): a phase 3, multicentre, double-blind, placebo-controlled randomised trial. Lancet Oncol. 2014;15:894–904. doi: 10.1016/S1470-2045(14)70024-5. [DOI] [PubMed] [Google Scholar]
- 6.Huang J, Fan Q, Lu P, et al Icotinib in patients with pretreated advanced esophageal squamous cell carcinoma with EGFR overexpression or EGFR gene amplification: A single-arm, multicenter phase 2 study. J Thorac Oncol. 2016;11:910–7. doi: 10.1016/j.jtho.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 7.Doi T, Piha-Paul SA, Jalal SI, et al Safety and antitumor activity of the anti-programmed death-1 antibody pembrolizumab in patients with advanced esophageal carcinoma. J Clin Oncol. 2018;36:61–7. doi: 10.1200/JCO.2017.74.9846. [DOI] [PubMed] [Google Scholar]
- 8.Huang J, Xu B, Mo H, et al Safety, activity, and biomarkers of SHR-1210, an anti-PD-1 antibody, for patients with advanced esophageal carcinoma. Clin Cancer Res. 2018;24:1296–304. doi: 10.1158/1078-0432.CCR-17-2439. [DOI] [PubMed] [Google Scholar]
- 9.Kudo T, Hamamoto Y, Kato K, et al Nivolumab treatment for oesophageal squamous-cell carcinoma: an open-label, multicentre, phase 2 trial. Lancet Oncol. 2017;18:631–9. doi: 10.1016/S1470-2045(17)30181-X. [DOI] [PubMed] [Google Scholar]
- 10.Burkart C, Bokemeyer C, Klump B, et al A phase II trial of weekly irinotecan in cisplatin-refractory esophageal cancer. Anticancer Res. 2007;27:2845–8. [PubMed] [Google Scholar]
- 11.Assersohn L, Brown G, Cunningham D, et al Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol. 2004;15:64–9. doi: 10.1093/annonc/mdh007. [DOI] [PubMed] [Google Scholar]
- 12.Leary A, Assersohn L, Cunningham D, et al A phase II trial evaluating capecitabine and irinotecan as second line treatment in patients with oesophago-gastric cancer who have progressed on, or within 3 months of platinum-based chemotherapy. Cancer Chemother Pharmacol. 2009;64:455–62. doi: 10.1007/s00280-008-0893-5. [DOI] [PubMed] [Google Scholar]
- 13.Lordick F, von Schilling C, Bernhard H, et al Phase II trial of irinotecan plus docetaxel in cisplatin-pretreated relapsed or refractory oesophageal cancer. Br J Cancer. 2003;89:630–3. doi: 10.1038/sj.bjc.6601168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burtness B, Gibson M, Egleston B, et al Phase II trial of docetaxel-irinotecan combination in advanced esophageal cancer. Ann Oncol. 2009;20:1242–8. doi: 10.1093/annonc/mdn787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hawkes E, Okines AF, Papamichael D, et al Docetaxel and irinotecan as second-line therapy for advanced oesophagogastric cancer. Eur J Cancer. 2011;47:1146–51. doi: 10.1016/j.ejca.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Wang X, Huang J Irinotecan plus fluorouracil-based regimen as second or third-line chemotherapy for recurrent or metastatic esophageal squamous cell carcinoma. Thorac Cancer. 2016;7:246–50. doi: 10.1111/1759-7714.12323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Xu B, Liu Y, et al Irinotecan plus S-1 versus S-1 in patients with previously treated recurrent or metastatic esophageal cancer (ESWN 01): a prospective randomized, multicenter, open-labeled phase 3 trial. Cancer Commun (Lond) 2019;39:16. doi: 10.1186/s40880-019-0359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Apetoh L, Ladoire S, Coukos G, et al Combining immunotherapy and anticancer agents: the right path to achieve cancer cure? Ann Oncol. 2015;26:1813–23. doi: 10.1093/annonc/mdv209. [DOI] [PubMed] [Google Scholar]
- 19.Park SE, Lee SH, Ahn JS, et al Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non-small cell lung cancer. J Thorac Oncol. 2018;13:106–11. doi: 10.1016/j.jtho.2017.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Rossi C, Gilhodes J, Maerevoet M, et al Efficacy of chemotherapy or chemo-anti-PD-1 combination after failed anti-PD-1 therapy for relapsed and refractory Hodgkin lymphoma: A series from Lysa centers. Am J Hematol. 2018 doi: 10.1002/ajh.25154. [DOI] [PubMed] [Google Scholar]
- 21.Szabados B, van Dijk N, Tang YZ, et al Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol. 2018;73:149–52. doi: 10.1016/j.eururo.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Sonpavde G, Pond GR, Mullane S, et al Outcomes in patients with advanced urothelial carcinoma after discontinuation of programmed death (PD)-1 or PD ligand 1 inhibitor therapy. BJU Int. 2017;119:579–84. doi: 10.1111/bju.13674. [DOI] [PubMed] [Google Scholar]