Abstract
Objective
To explore the intraperitoneal free cancer cell (IFCC) detection value of negative enrichment and immune fluorescence in situ hybridization (NEimFISH) on chromosomes (CEN) 8/17.
Methods
To verify the reliability of NEimFISH, 29 gastric cancer tumors, their adjacent tissues and greater omental tissues were tested. Our study then included 105 gastric cancer patients for IFCC. We defined patients as IFCC-positive if a signal was detected, regardless of the detailed cancer cell numbers. A comparison of clinicopathological features was conducted among IFCC groups. We also compared the diagnosis value and peritoneal recurrence predictive value among different detection methods. The comparison of IFCC number was also conducted among different groups.
Results
A cutoff of 2.5 positive cells could distinguish all benign tissue samples and 97% of malignant tissue samples in our study. Compared to intestinal gastric cancer, patients with diffuse gastric cancer tended to have more IFCCs (6 vs. 4, P=0.002). The IFCC counts were often higher in the lymphovascular invasion positive group than negative group (3 vs. 1, P=0.022). All IFCC samples that were considered positive using conventional cytology were also found to be positive using NEimFISH. When compared to conventional cytology and paraffin pathology, NEimFISH had a higher IFCC positive rate (68.9%) and higher one-year peritoneal recurrence predictive value with area under the curve (AUC) of 0.922.
Conclusions
Gastric cancer could be effectively diagnosed by NEimFISH. The IFCC number found using NEimFISH on CEN8/17 is closely associated with Lauren type and vascular invasion of cancer. NEimFISH is a reliable detection modality with a higher positive detection rate, higher one-year peritoneal recurrence predictive value and quantitative features for IFCC of gastric cancer.
Keywords: NEimFISH, gastric cancer, peritoneal metastasis, conventional cytology, intraperitoneal free cancer cells
Introduction
Gastric cancer is one of the most common gastrointestinal malignant cancers, with approximately 990,000 new patients and 738,000 patients succumbing to the disease (1). The incidence of gastric cancer ranks second of all types of cancer, with the third highest death rate related to cancer in China (2,3). Recent advances in treating gastric cancer have improved the clinical outcomes, however, a substantial proportion of patients are initially diagnosed with unresectable locally advanced or metastatic disease, and more than one-third of these patients have peritoneal metastasis (4). Patients with gastric cancer peritoneal metastasis tend to have a poor prognosis and an advanced oncological stage. Accordingly, the 7th Edition of the American Joint Committee on Cancer (AJCC) Cancer Staging Manual describes the criteria for unresectable disease, which include distant metastasis or peritoneal seeding (5), and the latter includes positive peritoneal cytology indicating peritoneal metastasis. Therefore, how to precisely detect the peritoneal cancer cells becomes a big challenge for doctors and scientists.
Conventional cytology is widely used as a standard criterion for intraperitoneal free cancer cell (IFCC) detection, however, it has been reported that the positive rate from peritoneal wash cytology ranges from 5% to 41% (6-8). Regarding the accuracy of peritoneal recurrence prediction, the sensitivity and specificity of conventional cytology could reach to 34% and 85%, respectively (9). The usefulness of conventional cytology for the prediction of peritoneal metastasis is still controversial (10). Conventional cytology is qualitative, has low sensitivity and depends on the pathologists (11). Therefore, the chances of radical resections may be lowered by the low sensitivity of conventional cytology, which makes this method unreliable when choosing treatment modalities. Another question is whether the different number of cancer cells is associated with various peritoneal recurrent probabilities. Accordingly, a quantified detection method is urgently needed.
With the development of diagnostic technologies, many new methods for detecting IFCC have been put forward. Compared with conventional cytology, reverse transcription-polymerase chain reaction (RT-PCR), with a higher sensitivity, has demonstrated a positive association with peritoneal recurrence and prognosis (12-14). However, the high expense, time-consuming feature and lack of standard processing methods preclude its wider clinical use. Detection of carcinoembryonic antigen (CEA) RNA by RT-PCR of peritoneal washes also proved to be a more sensitive method than conventional cytology, with a 14% increase in detection rate (15). However, the avoidance of false positive results is still a great challenge.
It is widely known that carcinogenesis is a process with a large amount of mutation accumulations and chromosomal variants. The chromosome instability plays an important role in the process of tumorigenesis (16,17). Chromosomes (CEN) 1, 2, 3, 5, 7, 8, 11, 12, 17, and 20 have been widely reported (16,18). Copy number gains of CEN8 have been found in 77.3% of gastric cancer cases (19). CEN8 is famous because of the c-myc gene (18). Gains at CEN17 have also been frequently observed in gastric cancers. CEN17 is known for its suppressor gene TP53 (20). The widespread instability of CEN8 and CEN17 provides us with a solid mechanism foundation for IFCC detection of gastric cancer.
Based on these findings, the IFCC detection value of negative enrichment and immune fluorescence in situ hybridization (NEimFISH) on CENs 8 and 17 amplifications deserves exploration.
Materials and methods
Tissue fluorescence in situ hybridization (FISH)
First, we conducted FISH on 29 samples of gastric cancer tumors and their corresponding greater omental paraffin section tissues to verify the reliability of NEimFISH. All patients were diagnosed with gastric adenocarcinoma by two independent pathologists. We analyzed the status of CEN8/17 amplifications between benign and malignant tissues of gastric cancer. We also conducted FISH on the 29 corresponding cancer adjacent tissues. The cells with CEN amplifications were calculated on 50 cells.
Peritoneal lavage and NEimFISH of IFCC
Based on the preliminary results of tissue experiments, we started to collect peritoneal lavage fluid from gastric cancer patients undergoing surgery in Center of Gastrointestinal Surgery, Peking University Cancer Hospital & Institute ranging from July 2017 to June 2018. All patients received peritoneal lavage with 1,000 mL 0.9% saline, half of which was taken out for detection. And 250 mL saline was used for conventional cytology, paraffin pathology and NEimFISH detection, respectively. First, we collected 250 mL lavage fluid, which was placed into centrifuge tubes. After 5 min of centrifugation, we disposed of the supernatants and standardized the sample volumes to 5 mL. Second, we reduced the white cells using immunomagnetic beads. Third, we chose 100,000 cells as a standard level. The imFISH with CEN8/17 was performed to identify IFCCs. Finally, all regions were scanned with a fluorescence microscope and we marked the site of positive cells with CEN+CD45−/DAPI+. CEN8 and CEN17 positive cells were defined as DAPI+/CD45−/CEN8+ or DAPI+/CD45−/CEN17+, while normal cells are 8/17 diploid structures. CEN8+ and CEN17+ were detected with 3 or more signals. We then recalculated the standard IFCC number by 100 mL. All pathology diagnoses and cell calculations were conducted by two independent pathologists or investigators. We conducted one-year follow-up for patients undergoing radical surgery. All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (Peking University Cancer Hospital) and with the Helsinki Declaration of 1964 and later versions. Informed consent to be included in the study, or the equivalent, was obtained from all patients.
Statistical analysis
We constructed a receiver operating characteristics (ROC) curve to evaluate the diagnostic value of NEimFISH and the one-year peritoneal recurrence predictive value of different detection methods. The cutoff value was calculated using the Youden index by the most valuable diagnostic marker. We defined the samples as IFCC positive regardless of the detailed cancer cell numbers. The comparisons of clinicopathological features were conducted among IFCC groups using a Chi-square test, Kruskal-Wallis test and nonconditional logistic regression analysis. The comparison of IFCC number was also conducted among different groups using a Mann-Whitney U test and Kruskal-Wallis test. P<0.05 was regarded as statistically significant. We performed all statistical analyses using IBM SPSS Statistics (Version 23.0; IBM Corp., New York, USA).
Results
Identification of cancer cells with CEN8/17 amplification
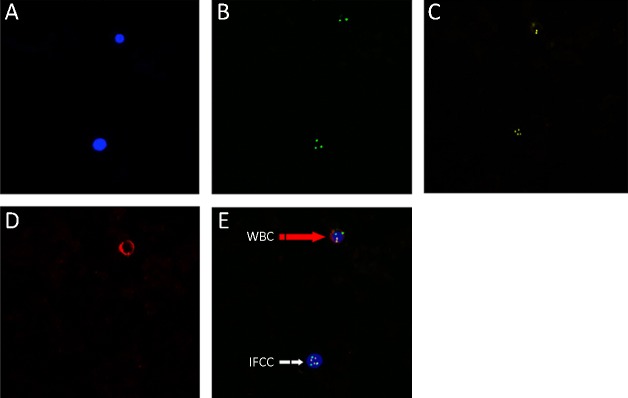
To precisely identify cancer cells with CEN amplifications, we used many markers including DAPI, CD45, CEN8 and CEN17 probes. The gastric cancer cells were confirmed according to the signals of different markers. Cells with DAPI+/CEN8 or CEN17≥3/CD45− were defined as cancer cells. Cells with DAPI+/CD45+ were evaluated as white cells (Figure 1). In tissues, we measured cells with DAPI+/CEN8 or CEN17≥3 as cancer cells (Figure 2).
1.
Immune fluorescence in situ hybridization on chromosomes (CEN)8/17 of intraperitoneal free cancer cell (IFCC). (A) 4’,6-diamidino-2-phenylindole (DAPI); (B,C) Detection of CEN8 and 17 amplifications; (D) CD45; (E) Merged signals. The white arrow indicates IFCC with CEN8, CEN17 amplifications and CD45−. The red arrow represents white blood cell (WBC) with CD45+ and CEN8, CEN17 amplifications negative.
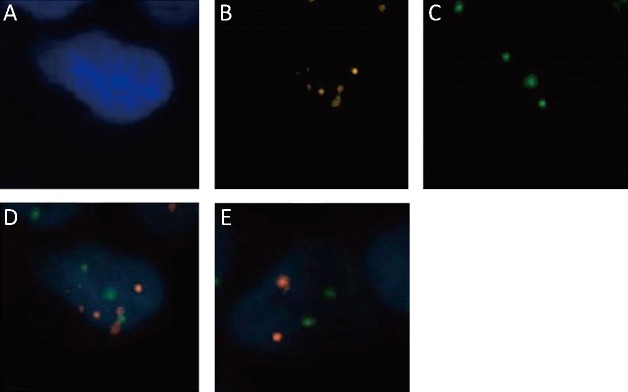
2.
Immune fluorescence in situ hybridization of cells from benign and malignant tissues. (A) 4’,6-diamidino-2-phenylindole (DAPI); (B,C) Signals from CEN8 and CEN17; (D) Merged signals. A−D are for malignant tissues; (E) Signals of benign tissues.
Comparison of CEN8/17 amplification between benign and malignant tissues of gastric cancer
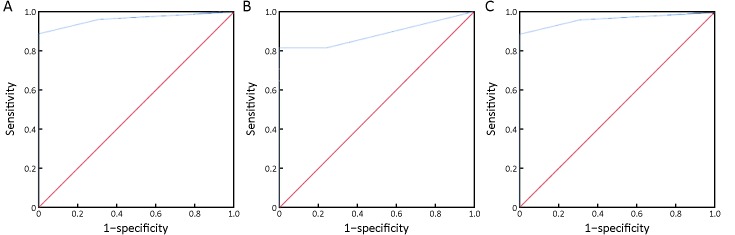
To evaluate the amplification difference, we conducted tissue FISH of CEN8 and CEN17 amplifications for samples from 29 gastric cancer patients. ROC curves were constructed on the diagnostic value of CEN8, CEN17 and CEN8/17. The area under the curve (AUC) is 0.964 and 0.885 for CEN8 and CEN17, respectively (Figure 3A,B). The AUC could reach 0.973 for CEN8/17 (Figure 3C). According to the ROC curve, we calculate the cutoff value as 2.5 for CEN8/17 amplifications. Therefore, we defined tissues with ≤2 CEN8/17 amplified cells as benign tissues. In contrast, tissues with ≥3 CEN8/17 amplified cells were regarded as malignant tissues. Following these guidelines, none of the 29 benign tissues was classified as cancer tissues, and only one cancer sample was misdiagnosed as benign tissues.
3.
Diagnostic value of negative enrichment and immune fluorescence in situ hybridization (NEimFISH) for benign and malignant tissues. (A) CEN8, AUC=0.964; (B) CEN17, AUC=0.885; (C) CEN8/17, AUC=0.973. CEN, chromosome.
The fundamental experiments support the high accuracy of NEimFISH in the differentiation of benign and malignant tissues for gastric cancer.
Comparison of CEN8 and CEN17 amplification distributions between cancer and adjacent tissues of gastric cancer
To evaluate the differences in CEN amplification between cancer and adjacent tissues of gastric cancer, we also conducted tissue FISH for the two CENs amplification from the same 29 gastric cancer patients. The medians of CEN8 amplification are significantly different between cancer and adjacent tissues groups (9 vs. 1, P<0.001). The numbers of CEN17 amplification for cancer and adjacent tissues are five and one cells, respectively, with a significant difference (P<0.001). The amplification status of CEN8/17 are also different between the two groups (10vs. 1, P<0.001) (Table 1). According to the cutoff we calculated, 7/29 samples of adjacent tissues were similar to cancer with obvious CEN8/17 amplifications.
1.
Amplification number between cancer and normal tissues
| CEN | Cancer tissues | Adjacent tissues | P |
| CEN, chromosome. | |||
| CEN8 | 9 | 1 | <0.001 |
| CEN17 | 5 | 1 | <0.001 |
| CEN8/17 | 10 | 1 | <0.001 |
The distinct differences in chromosome amplifications between cancer and adjacent tissues make NEimFISH a preferable option for the identification of IFCC from gastric cancer.
Patients enrollment of gastric cancer for NEimFISH
A total of 105 patients were included in our study. Two patients were not analyzed due to lack of clinical information and four patients were not diagnosed with detailed IFCC numbers. Therefore, we were only able to analyzed the distributions of IFCC numbers for 101 patients.
Comparison of clinicopathological features among both IFCC groups
Free cells were classified as IFCC once CEN8/17 amplifications were detected, regardless of the total number of amplified cells. We performed a comparison of different clinicopathological characteristics between IFCC positive and negative groups. The age, gender and body mass index (BMI) were similar between the two groups (Table 2). The pathological characteristics, such as tumor differentiation, vascular invasion status, tumor stage and node lymph metastasis, had no significant difference between IFCC positive and negative groups (Table 2 ). However, vascular invasion was closely associated with IFCC using a nonconditional logistic regression analysis (Table 3). In addition, the distribution of Lauren classification between the two groups is significantly different (P=0.004) (Table 2). When comparing intestinal types, the diffused gastric cancer had a higher positive rate (Table 3). The expression of proteins such as human epidermal growth factor receptor 2 (HER2), Ki-67, programmed cell death ligand 1 (PD-L1) and mismatch repair (MMR) were also similar within the two groups.
2.
Comparison of clinicopathological features among both of IFCC groups
| Clinicopathological features | Positive | Negative | P |
| IFCC, intraperitoneal free cancer cell; BMI, body mass index; HER2, human epidermal growth factor receptor; PDL, programmed cell death ligand; MMR, mismatch repair. | |||
Age (
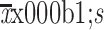 ) (year) ) (year)
|
58±12 | 57±11 | 0.664 |
BMI (
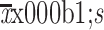 ) (kg/m2) ) (kg/m2)
|
22.49±3.53 | 22.41±3.56 | 0.954 |
| Gender | 0.770 | ||
| Male | 49/71 | 23/32 | |
| Female | 22/71 | 9/32 | |
| Differentiation | 0.606 | ||
| High | 2/70 | 0/30 | |
| Middle | 15/70 | 5/30 | |
| Low | 43/70 | 20/30 | |
| Undifferentiation | 10/70 | 5/30 | |
| Lauren type | 0.004 | ||
| Intestinal | 31/66 | 6/30 | |
| Diffused | 24/66 | 10/30 | |
| Mixed | 11/66 | 14/30 | |
| Vascular invasion | 0.179 | ||
| Positive | 35/60 | 13/30 | |
| Negative | 25/60 | 17/30 | |
| Perineural invasion | 0.648 | ||
| Positive | 37/60 | 17/30 | |
| Negative | 23/60 | 13/30 | |
| Tumor stage | 0.454 | ||
| T1a | 4/60 | 2/0 | |
| T1b | 7/60 | 5/30 | |
| T2 | 10/60 | 2/30 | |
| T3 | 21/60 | 8/30 | |
| T4a | 16/60 | 12/30 | |
| T4b | 2/60 | 1/30 | |
| Node lymph metastasis | 0.728 | ||
| N0 | 22/60 | 13/31 | |
| N1 | 10/60 | 6/31 | |
| N2 | 14/60 | 4/31 | |
| N3a | 10/60 | 5/31 | |
| N3b | 4/60 | 3/31 | |
| Conventional cytology | 0.415 | ||
| Positive | 10/71 | 2/32 | |
| Negative | 61/71 | 30/32 | |
| Pathology | 0.063 | ||
| Positive | 18/71 | 3/32 | |
| Negative | 53/71 | 29/32 | |
| Neoadjuvant group | 0.562 | ||
| Positive | 14/68 | 8/31 | |
| Negative | 54/68 | 23/31 | |
| HER2 expression | 0.295 | ||
| 0 | 33/58 | 12/29 | |
| 1+ | 10/58 | 10/29 | |
| 2+ | 9/58 | 2/29 | |
| 3+ | 6/58 | 5/29 | |
| Ki67 index (%) | 0.502 | ||
| 0−25 | 4/58 | 1/29 | |
| 26−50 | 7/58 | 4/29 | |
| 51−75 | 26/58 | 17/29 | |
| 76−100 | 21/58 | 7/29 | |
| PDL1 expression | 0.543 | ||
| Positive | 8/50 | 6/24 | |
| Negative | 42/50 | 18/24 | |
| MMR | 1.000 | ||
| Positive | 57/58 | 29/29 | |
| Negative | 1/58 | 0/29 | |
3.
Risk factors of IFCC positive using nonconditional logistic regression analysis
| Factors | OR | 95% CI | P |
| IFCC, intraperitoneal free cancer cell; OR, odds ratio; 95% CI, 95% confidence interval. | |||
| Lauren type | |||
| Diffused | 11.95 | 2.55−56.12 | 0.002 |
| Mixed | 2.87 | 0.79−10.40 | 0.109 |
| Vascular invasion | 3.16 | 0.69−18.39 | 0.012 |
| T stage | |||
| T1b | 13.20 | 0.23−750.79 | 0.211 |
| T2 | 1.98 | 0.05−74.01 | 0.712 |
| T3 | 5.31 | 0.12−229.64 | 0.385 |
| T4a | 4.99 | 0.16−154.14 | 0.358 |
| T4b | 1.70 | 0.06−45.36 | 0.753 |
| N stage | |||
| N1 | 0.59 | 0.05−7.03 | 0.674 |
| N2 | 0.72 | 0.07−7.34 | 0.782 |
| N3a | 2.39 | 0.25−23.39 | 0.454 |
| N3b | 1.02 | 0.13−7.99 | 0.979 |
We also conducted comparisons of IFCC positive rates among three different detection methods. The detection rate of IFCC using NEimFISH was obviously higher than that using conventional cytology and paraffin pathology (Table 4).
4.
Comparison of detection rate among three methods
| Methods | Positive | Negative | Positive rate (%) |
| NEimFISH, negative enrichment and immune fluorescence in situ hybridization. | |||
| Conventional cytology | 12 | 91 | 11.7 |
| Paraffin pathology | 21 | 82 | 20.4 |
| NEimFISH | 71 | 62 | 68.9 |
Comparison of CEN8/17 amplification and IFCC number among different groups of patients
To evaluate the distributions of IFCC number, we conducted a comparison of CEN8/17 amplifications within different groups of patients. The IFCC number had no significant difference in both gender and neoadjuvant groups of patients. The patients with T≥2 stages have more IFCC than patients at the T1 stage (2 vs. 1, P=0.271). The IFCC number is similar in the groups of lymph node stages (2 vs. 1, P=0.328). Patients of positive groups with vascular invasion are detected having more IFCC compared with patients of negative groups (3 vs. 1, P=0.022). However, the IFCC number is uniformly distributed between perineural invasion positive and negative groups (P=0.441). Diffused gastric cancer has more IFCCs than intestinal and mixed gastric cancer with a significant difference (6 vs. 4 vs. 1, P=0.002). As we speculated, for the patients at Borrmann IV, we detected a median of 10 IFCCs, which is significantly more than that in other patients. However, the difference is not statistically significant (P=0.666) (Table 5).
5.
Comparison of IFCC number among patients
| Variables | IFCC | P |
| IFCC, intraperitoneal free cancer cells; PDL, programmed cell death ligand. | ||
| Gender | 0.611 | |
| Male | 2 | |
| Female | 3 | |
| Neoadjuvant group | 0.895 | |
| Yes | 3 | |
| No | 2 | |
| Tumor stage | 0.271 | |
| T1 | 1 | |
| ≥T2 | 2 | |
| Node lymph | 0.328 | |
| Positive | 2 | |
| Negative | 1 | |
| Vascular invasion | 0.022 | |
| Positive | 3 | |
| Negative | 1 | |
| Perineural invasion | 0.441 | |
| Positive | 2 | |
| Negative | 2 | |
| Lauren type | 0.002 | |
| Intestinal | 4 | |
| Diffused | 6 | |
| Mixed | 1 | |
| Differentiation | 0.406 | |
| High | 8 | |
| Middle | 4 | |
| Low | 3 | |
| Undifferentiation | 1 | |
| Borrmann classification | 0.666 | |
| I | 2 | |
| II | 2 | |
| III | 2 | |
| IV | 10 | |
| PDL1 expression | 1.000 | |
| Positive | 3 | |
| Negative | 3 | |
| Ki67 index (%) | 0.650 | |
| 0−25 | 7 | |
| 26−50 | 2 | |
| 51−75 | 1 | |
| 76−100 | 3 | |
| Pathology | 0.012 | |
| Positive | 6 | |
| Negative | 2 | |
| Conventional cytology | 0.022 | |
| Positive | 8 | |
| Negative | 2 | |
In different groups of conventional cytology, 8 and 2 IFCCs were found for positive and negative groups of patients, respectively, which was a significant difference (P=0.022). The IFCC number was also significantly different between positive and negative groups of paraffin pathology (6 vs. 2, P=0.012) (Table 5).
Predictive value of peritoneal recurrence among different detection modalities
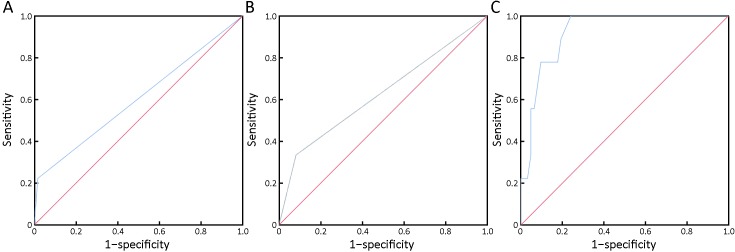
After follow-up, we included 71 patients for one-year peritoneal recurrence rate analyses. Thirty-two patients did not receive radical surgery and two patients were lost. To evaluate the one-year peritoneal recurrence predictive value of different methods, we constructed ROC. The AUC is 0.603 and 0.626 for conventional cytology and paraffin pathology, respectively (Figure 4A,B). However, the AUC could reach 0.922 for NEimFISH (Figure 4C). The cutoff 5.5 could get the optimal predictive value with 100% of sensitivity and 75.8% of specificity.
4.
Peritoneal recurrence predictive value of different detection modalities. (A) Conventional cytology, area under the curve (AUC)=0.603, P=0.32; (B) Paraffin pathology, AUC=0.626, P=0.225; (C) Negative enrichment and immune fluorescence in situ hybridization (NEimFISH), AUC=0.922, P<0.001.
Therefore, NEimFISH could predict one-year peritoneal recurrence more effectively than conventional cytology and paraffin pathology.
Discussion
Our study demonstrated the diagnostic value of NEimFISH using CEN8/17 on gastric cancer. The cutoff of 2.5 could distinguish all of the benign tissue samples and 97% of the malignant tissue samples in our study. Carcinogenesis is a multi-step process that includes an accumulation of genetic mutations and a wide range of genetic changes, including point mutations, chromosome level changes and genes amplifications, which are found in gastric cancer (21,22). DNA ploidy has been identified as having a close relationship with proliferating activity, metastatic potential and prognosis (23,24). Gastric cancers with DNA aneuploidy are often more sensitive to anticancer drugs (24). Accordingly, The Cancer Genome Atlas (TCGA) classifies tumors with chromosome instability as one of important genetic types of gastric cancer (17). Our results also support the imperative role of chromosome amplifications in gastric cancer. We also validate the reliability of NEimFISH in the differentiation of gastric cancer. The high diagnostic accuracy makes it a prospective method for the detection of IFCC.
Many studies have contributed to the treatment modalities of gastric cancer with peritoneal metastasis. The Adjuvant Chemotherapy Trial of TS-1 for Gastric Cancer (ACTS-GC) has shown the potential to reduce peritoneal metastasis of Tegafur-gimeuracil-oxo (S-1) (25). The CCOG0301 study (26) included patients with visible peritoneal deposits despite their cytology status, and the results showed that there was no statistically significant difference between patients with or without invisible peritoneal metastasis. Although the prognosis of gastric cancer patients with IFCC is disappointing, approximately 25% of these patients could live for more than 5 years following surgery and chemotherapy (27). Consequently, combined cytoreductive surgery and hyperthermic intraperitoneal chemotherapy could obviously improve overall survival for gastric cancer patients with peritoneal metastasis (28-30), especially for those patients with P1 and P2 disease (31). Therefore, positive strategies should be taken for these patients. In this case, the methods of detecting patients with potentially recurrence of peritoneal metastasis become important so that clinicians can choose the best treatment options.
As the standard method, conventional cytology is used to detect IFCC and predict peritoneal metastasis. However, some studies (32) found that IFCC using conventional cytology was not an independent prognostic factor for recurrence of peritoneal metastasis. They indicated that conventional cytology was not an effective tool for the staging of gastric cancer. These contradictory results may come from the low positive rate and qualitative features of conventional cytology. In our study, we found that the positive rate is higher in NEimFISH groups than in conventional cytology groups. Meanwhile, the IFCC in positive cytology groups is more than that in negative groups, which also reminds us of the importance of quantitative IFCC. Accordingly, we believe that the NEimFISH method described in our study could avoid the defects of conventional cytology. However, its false negative rate is an important factor we must take into consideration, and the real status of IFCC is hard to evaluate owing to the lack of a gold standard. Therefore, we correlated it with peritoneal recurrence to guide clinical strategies. Comparing with other modalities, NEimFISH could predict the peritoneal recurrence after radical surgery more accurately, which may help us avoid peritoneal recurrence or metastasis with more positive strategies. However, its value for predicting peritoneal metastasis still deserves studying owing to the lack of enough follow-up time.
In our study, we detected more IFCC with CEN8/17 amplifications in T2+ groups than in T1 groups of gastric cancer patients. The most important theory for peritoneal metastasis of gastric cancer is the seed and soil theory. Tumor cells exfoliate from the primary tumor mass to the abdominal cavity, attaching to the peritoneal surface, and finally invading into subperitoneal tissues (33,34). IFCCs are positively associated with serosal invasion, lymph node invasion, peritoneal recurrence and mortality (35,36). However, no statistically significant difference was found between the two groups, which may be attributed to the limited patient number. Transmesothelial and translymphatic metastases are widely proposed as two different processes of peritoneal metastasis formation (37). Additionally, lymph node metastasis is regarded as another pathway to peritoneal metastasis. In our study, however, we did not find a statistically significant difference in IFCC number between positive and negative lymph node metastases. This may be attributed to the limited sample number.
Apart from the dissemination from tumor mass, IFCCs could also originate from transected lymphatic channels and tumor-contaminated blood lost in the surgical field (38). In our research, we also found that patients with vascular invasion have more IFCC than patients without vascular invasion. The IFCC number was identified using Lauren classification, and the diffused type of gastric cancer tends to be found with more IFCC than the intestinal and mixed cell types of gastric cancer. This finding is consistent with the results of other studies. The diffused type of gastric cancer is usually diagnosed with peritoneal metastasis, whereas the intestinal type of gastric cancer is diagnosed with liver metastasis. Peritoneal metastasis is identified more frequently in gastric cancer patients with a diffuse infiltrative growth pattern than in patients without that pattern (10,39).
Although we demonstrated that NEimFISH is a promising IFCC detection method with a higher positive rate, this study had many limitations. The limited number of samples and lack of enough recurrent information make our detection technology hard to promote. Therefore, more prospective clinical trials and recurrent survival analyses are needed.
Conclusions
Gastric cancer could be effectively diagnosed by NEimFISH. The IFCC number found using NEimFISH on CEN8/17 is closely associated with Lauren type and vascular invasion of cancer. NEimFISH is a reliable detection modality with a high positive detection rate, high one-year peritoneal recurrence predictive value and quantitative features for IFCC analysis of gastric cancer.
Acknowledgements
This study was supported by the National Science Foundation for Young Scientists of China (No. 81802735).
Footnote
Conflicts of Interest: Author Yali Bai, Yanwen Xue and Yingjie Yang were employed by company Cyttel Biosciences INC. The authors have no conflicts of interest to declare.
Contributor Information
Zhaode Bu, Email: buzhaode@cjcrcn.org.
Jiafu Ji, Email: jijiafu@hsc.pku.edu.cn.
References
- 1.Karimi P, Islami F, Anandasabapathy S, et al Gastric cancer: descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol Biomarkers Prev. 2014;23:700–13. doi: 10.1158/1055-9965.epi-13-1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torre LA, Bray F, Siegel RL, et al Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 3.Chen W, Zheng R, Zhang S, et al Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29:1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Magge D, Zenati M, Mavanur A, et al Aggressive locoregional surgical therapy for gastric peritoneal carcinomatosis. Ann Surg Oncol. 2014;21:1448–55. doi: 10.1245/s10434-013-3327-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Washington K 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–9. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 6.Bando E, Yonemura Y, Takeshita Y, et al Intraoperative lavage for cytological examination in 1,297 patients with gastric carcinoma. Am J Surg. 1999;178:256–62. doi: 10.1016/s0002-9610(99)00162-2. [DOI] [PubMed] [Google Scholar]
- 7.Ribeiro U Jr., Gama-Rodrigues JJ, Bitelman B, et al Value of peritoneal lavage cytology during laparoscopic staging of patients with gastric carcinoma. Surg Laparosc Endosc. 1998;8:132–5. doi: 10.1097/00019509-199804000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Burke EC, Karpeh MS Jr., Conlon KC, et al Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol. 1998;5:411–5. doi: 10.1007/bf02303859. [DOI] [PubMed] [Google Scholar]
- 9.Hoskovec D, Varga J, Dytrych P, et al Peritoneal lavage examination as a prognostic tool in cases of gastric cancer. Arch Med Sci. 2017;13:612–6. doi: 10.5114/aoms.2016.64044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bentrem D, Wilton A, Mazumdar M, et al The value of peritoneal cytology as a preoperative predictor in patients with gastric carcinoma undergoing a curative resection. Ann Surg Oncol. 2005;12:347–53. doi: 10.1245/ASO.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 11.Kitayama J, Emoto S, Yamaguchi H, et al Flow cytometric quantification of intraperitoneal free tumor cells is a useful biomarker in gastric cancer patients with peritoneal metastasis. Ann Surg Oncol. 2015;22:2336–42. doi: 10.1245/s10434-014-4238-9. [DOI] [PubMed] [Google Scholar]
- 12.Nakanishi H, Kodera Y, Yamamura Y, et al Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT-PCR on the lightcycler. Int J Cancer. 2000;89:411–7. doi: 10.1002/1097-0215(20000920)89:5<411::aid-ijc3>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Schmidt P, Thiele M, Rudroff C, et al Detection of tumor cells in peritoneal lavages from patients with gastrointestinal cancer by multiplex reverse transcriptase PCR. Hepatogastroenterology. 2001;48:1675–9. [PubMed] [Google Scholar]
- 14.Kodera Y, Nakanishi H, Yamamura Y, et al Prognostic value and clinical implications of disseminated cancer cells in the peritoneal cavity detected by reverse transcriptase-polymerase chain reaction and cytology. Int J Cancer. 1998;79:429–33. doi: 10.1002/(sici)1097-0215(19980821)79:4<429::aid-ijc20>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 15.Wang JY, Lin SR, Lu CY, et al Gastric cancer cell detection in peritoneal lavage: RT-PCR for carcinoembryonic antigen transcripts versus the combined cytology with peritoneal carcinoembryonic antigen levels. Cancer Lett. 2005;223:129–35. doi: 10.1016/j.canlet.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 16.Tsukamoto Y, Uchida T, Karnan S, et al Genome-wide analysis of DNA copy number alterations and gene expression in gastric cancer. J Pathol. 2008;216:471–82. doi: 10.1002/path.2424. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Research Network Comprehensive molecular characterization of gastric adenocarcinoma. Nature. 2014;513:202–9. doi: 10.1038/nature13480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buffart TE, van Grieken NC, Tijssen M, et al High resolution analysis of DNA copy-number aberrations of chromosomes 8, 13, and 20 in gastric cancers. Virchows Arch. 2009;455:213–23. doi: 10.1007/s00428-009-0814-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang JU Chromosome 8q as the most frequent target for amplification in early gastric carcinoma. Oncol Lett. 2014;7:1139–43. doi: 10.3892/ol.2014.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oya M, Yao T, Tsuneyoshi M Expressions of cell-cycle regulatory gene products in conventional gastric adenomas: possible immunohistochemical markers of malignant transformation. Hum Pathol. 2000;31:279–87. doi: 10.1016/s0046-8177(00)80239-7. [DOI] [PubMed] [Google Scholar]
- 21.Maleki SS, Röcken C Chromosomal instability in gastric cancer biology. Neoplasia. 2017;19:412–20. doi: 10.1016/j.neo.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lengauer C, Kinzler KW, Vogelstein B Genetic instabilities in human cancers. Nature. 1998;396:643–9. doi: 10.1038/25292. [DOI] [PubMed] [Google Scholar]
- 23.Baba H, Korenaga D, Kakeji Y, et al DNA ploidy and its clinical implications in gastric cancer. Surgery. 2002;131:S63–70. doi: 10.1067/msy.2002.119306. [DOI] [PubMed] [Google Scholar]
- 24.Oki E, Hisamatsu Y, Ando K, et al Clinical aspect and molecular mechanism of DNA aneuploidy in gastric cancers. J Gastroenterol. 2012;47:351–8. doi: 10.1007/s00535-012-0565-4. [DOI] [PubMed] [Google Scholar]
- 25.Sakuramoto S, Sasako M, Yamaguchi T, et al Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357:1810–20. doi: 10.1056/NEJMoa072252. [DOI] [PubMed] [Google Scholar]
- 26.Kodera Y, Ito S, Mochizuki Y, et al A phase II study of radical surgery followed by postoperative chemotherapy with S-1 for gastric carcinoma with free cancer cells in the peritoneal cavity (CCOG0301 study) Eur J Surg Oncol. 2009;35:1158–63. doi: 10.1016/j.ejso.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 27.Kodera Y Gastric cancer with minimal peritoneal metastasis: is this a sign to give up or to treat more aggressively? Nagoya J Med Sci. 2013;75:3–10. [PMC free article] [PubMed] [Google Scholar]
- 28.Glockzin G, Piso P Current status and future directions in gastric cancer with peritoneal dissemination. Surg Oncol Clin N Am. 2012;21:625–33. doi: 10.1016/j.soc.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Kulu Y, Müller-Stich B, Büchler MW, et al Surgical treatment of peritoneal carcinomatosis: current treatment modalities. Langenbecks Arch Surg. 2014;399:41–53. doi: 10.1007/s00423-013-1144-8. [DOI] [PubMed] [Google Scholar]
- 30.Ishigami H, Yamaguchi H, Yamashita H, et al Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer. 2017;20:128–34. doi: 10.1007/s10120-016-0684-3. [DOI] [PubMed] [Google Scholar]
- 31.Geng X, Liu H, Lin T, et al Survival benefit of gastrectomy for gastric cancer with peritoneal carcinomatosis: a propensity score-matched analysis. Cancer Med. 2016;5:2781–91. doi: 10.1002/cam4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cotte E, Peyrat P, Piaton E, et al Lack of prognostic significance of conventional peritoneal cytology in colorectal and gastric cancers: results of EVOCAPE 2 multicentre prospective study. Eur J Surg Oncol. 2013;39:707–14. doi: 10.1016/j.ejso.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Ji Z, Sun J, Wu H, et al Assessment of hyperthermic intraperitoneal chemotherapy to eradicate intraperitoneal free cancer cells. Transl Oncol. 2016;9:18–24. doi: 10.1016/j.tranon.2015.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yonemura Y, Endou Y, Fujimura T, et al Diagnostic value of preoperative RT-PCR-based screening method to detect carcinoembryonic antigen-expressing free cancer cells in the peritoneal cavity from patients with gastric cancer. ANZ J Surg. 2001;71:521–8. doi: 10.1046/j.1440-1622.2001.02187.x. [DOI] [PubMed] [Google Scholar]
- 35.Tustumi F, Bernardo WM, Dias AR, et al Detection value of free cancer cells in peritoneal washing in gastric cancer: a systematic review and meta-analysis. Clinics (Sao Paulo) 2016;71:733–45. doi: 10.6061/clinics/2016(12)10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pecqueux M, Fritzmann J, Adamu M, et al Free intraperitoneal tumor cells and outcome in gastric cancer patients: a systematic review and meta-analysis. Oncotarget. 2015;6:35564–78. doi: 10.18632/oncotarget.5595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yonemura Y, Kawamura T, Bandou E, et al The natural history of free cancer cells in the peritoneal cavity. Recent Results Cancer Res. 2007;169:11–23. doi: 10.1007/978-3-540-30760-0_2. [DOI] [PubMed] [Google Scholar]
- 38.Marutsuka T, Shimada S, Shiomori K, et al Mechanisms of peritoneal metastasis after operation for non-serosa-invasive gastric carcinoma: an ultrarapid detection system for intraperitoneal free cancer cells and a prophylactic strategy for peritoneal metastasis. Clin Cancer Res. 2003;9:678–85. [PubMed] [Google Scholar]
- 39.Mezhir JJ, Shah MA, Jacks LM, et al Positive peritoneal cytology in patients with gastric cancer: natural history and outcome of 291 patients. Ann Surg Oncol. 2010;17:3173–80. doi: 10.1245/s10434-010-1183-0. [DOI] [PubMed] [Google Scholar]