Abstract
Objective
To evaluate preoperative serum calcium concentration and investigate the association between calcium level and positive peritoneal cytology in endometrial carcinoma (EC).
Methods
A total of 510 patients who were diagnosed with EC and had surgery were initially enrolled in this study at Peking University People’s Hospital between January 2012 and December 2016. Clinical characteristics and preoperative serum calcium, albumin, carbohydrate antigen (CA)125, CA19-9, carcinoembryonic antigen (CEA) were extracted from patient records and evaluated according to postoperative peritoneal cytology. Predictive factors were assessed with Cox univariate and multivariate analyses. Factors selected from multivariate analysis results were used to build a predictive model.
Results
A total of 510 patients are identified in our database and 444 patients who fulfilled inclusion and exclusion criteria are included in this study. Univariate analysis revealed that ionized calcium concentration was closely related to positive peritoneal cytology, tumor grade and lymph-vascular space invasion (LVSI). Moreover, peritoneal cytology was significantly associated with hypertension, tubal ligation, serum CA125, CA19-9, CEA and ionized calcium level. Multivariate analysis revealed that albumin-adjusted calcium level, CA125 and tubal ligation were independent predictive factors of positive peritoneal cytology (P<0.05). A combination of ionized calcium level with the other two indexes yielded significantly great area under the curve (AUC=0.824).
Conclusions
This study enhanced the value of preoperative ionized calcium level. We also identified several potential biomarkers to predict positive peritoneal cytology in EC patients before surgery.
Keywords: Endometrial carcinoma, serum calcium, peritoneal cytology, logistic regression, predictive model
Introduction
Endometrial carcinoma (EC) is the most common gynecologic cancer in developed countries (1). EC ranked top 10 incidence of female patients in China (2,3). The incidence of EC reaches 63.4 per 10,000 in China in 2015 (4) and is responsible for 74,000 deaths per year worldwide (5). Though the incidence and mortality rates of EC in China were relatively low compared with other countries, the significant increase in incidence rate and marked decrease in patient age suggest the urgency to prevention and control (6). In 2009, the Federation International of Gynecology and Obstetrics (FIGO) revised EC staging system (7). Positive peritoneal cytology from the current staging qsystem is lack of evidence regarding the prognostic impact on EC and is removed from the EC stage. However, there are mounting evidences showing that there is a decreased survival in women with EC who have abnormal peritoneal cytology with EC, including a 2013 report of 14,704 women with stage I/II EC from the Surveillance, Epidemiology and End Results (SEER) database shows that positive cytology predicted decreased survival (8), and the number is increasing (9). Despite many diagnostic modalities, none has proved to be high predictive value and it still remains difficult to reach a precise diagnosis of peritoneal dissemination. Peritoneal dissemination is usually diagnosed with an operation. Although intraoperative peritoneal washing cytology has been regarded as the golden standard to detect peritoneal cytology, there might be false negative rate in the diagnosis. It has been known that positive peritoneal cytology is associated with decreased overall survival in women with stage III. Treatment of these patients with adjuvant chemotherapy increases survival significantly (10). Therefore, identifying positive peritoneal cytology in EC is essential for predicting prognosis and conducting reasonable adjuvant therapy preoperatively.
Several molecular biomarkers have shown values in predicting the positive peritoneal cytology in gastric and colorectal cancer (11,12). However, to our knowledge, risk factors for positive peritoneal cytology in EC have been examined in few studies and are still unclear. Novel biomarkers are urgently needed for predicting abnormal peritoneal cytology in EC. Previously we found that ionized calcium influx to EC cells promotes their proliferation and migration (13). Serum calcium is playing an increasingly crucial role in many types of cancers such as breast cancer (14), ovarian cancer (15) and prostate cancer (16). It is also a novel parameter to assess metabolic syndrome in EC (17). Approximately 50% of calcium in blood is bound to albumin and not physiologically active. An estimate of the active fraction of serum calcium adjusts the serum calcium for the level of serum albumin (18). We therefore examined the association between tumor metastatic ability such as peritoneal cytology, lymph-vascular space invasion (LVSI), tumor grade, lymphanode metastasis, FIGO stage and preoperative serum calcium level or albumin-corrected calcium level (ionized calcium). Finally we established a risk prediction model by a combination of ionized calcium level with some clinical features or biomarkers from multivariate analysis for abnormal peritoneal cytology.
Materials and methods
Patients
We conducted a retrospective, observational, non-interventional cohort study and included all EC patients who had surgical operation and identified from the comprehensive management system of medical record statistics from January 2012 to December 2016 in Peking University People’s Hospital (Beijing, China). For preoperative diagnosis, all patients underwent diagnostic curettage or hysteroscopy examination. The confirmation as primary EC was based on the histopathology of specimens after the surgery. Peritoneal washing lavage fluid was centrifuged at 1,500 r/min for 10 min to collect intact cells. The remaining precipitate was smeared on to four slides, fixed with acetone and stained with conventional haematoxylin and eosin. An experienced cytopathologist interpreted the samples. Detection of free tumor cells after the histopathological examination of the peritoneal washing fluid was accepted as positive cytology. We excluded the cases according to the following criteria: 1) patients with a history of other malignancies; 2) patients with hyperparathyroidism or other parathyroid diseases; 3) patients with chronic kidney disease; or 4) missing clinical data or calcium and albumin values prior to surgery. The biologically active fraction of serum calcium was calculated by the following formula: serum albumin-corrected calcium (mg/dL) = total calcium (mg/dL) + 0.8 × [4 − albumin (g/dL)].
Data collection
Patients’ demographics and clinical-pathological parameters include age, body mass index (BMI), menopausal status, diabetes, hypertension, tubal ligation history and hormone treatment. Laboratory findings, such as serum carbohydrate antigen (CA)125, CA19-9, carcinoembryonic antigen (CEA), calcium and albumin level, were retrospectively reviewed before surgery. To explore serum calcium level in different metastatic ability tumors, we collected some postoperative clinical features including ascites cytology, tumor grade, FIGO stage, lymphanode metastasis, LVSI, living status and recurrence. This study was approved by the Institutional Review Board of Peking University People’s Hospital (2015PHB116-01).
Statistical analysis
All analysis was performed by IBM SPSS Statistics (Version 21.0; IBM Corp., New York, USA). Qualitative variables were expressed as numbers and percentages and were analyzed by the Chi-square test. Quantitative variables were reported as
 with 95% confidence interval (95% CI) and analyzed by Student’s t-test. Multivariate logistic regression model was used to identify the independent risk factors for positive peritoneal cytology in EC. Receiver operating characteristic (ROC) curve was used to assess the predictive power of risk factors for abnormal ascites. Statistical significance was set as P value less than 0.05.
with 95% confidence interval (95% CI) and analyzed by Student’s t-test. Multivariate logistic regression model was used to identify the independent risk factors for positive peritoneal cytology in EC. Receiver operating characteristic (ROC) curve was used to assess the predictive power of risk factors for abnormal ascites. Statistical significance was set as P value less than 0.05.
Results
Patients’ selection
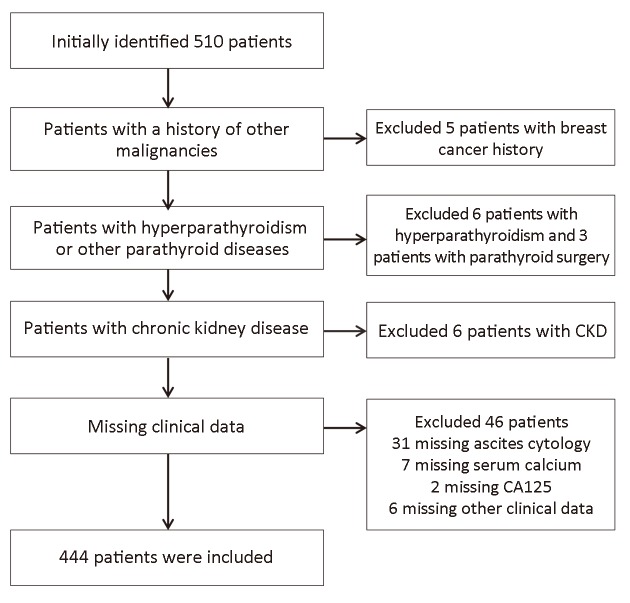
We selected the patients according to the criteria mentioned above and the flowchart detailing patient selection was shown in Figure 1. Table 1 showed the demographics of patients with EC. The mean age of the group was 55.7±9.6 years old. The mean BMI was 26.2±4.7 kg/m2. Calcium concentration decreased compared with pre-adjustment calcium concentration. Over the 444 patients, the majority people were in menopausal state, and less people had complications such as hypertension, diabetes. The minority of the patients had tubal ligation history. Peritoneal cytology accounts for 10.8% of all the participants. The most common FIGO stage and grade was stage I and grade 2, which occupied the majority (79.7% and 43.5%, respectively). There were far less people who had lymph node metastasis (43, 9.7%) than those who had not.
1.
Flowchart detailing patient selection. CKD, chronic kidney disease; CA, carbohydrate antigen.
1.
Demographics of patients with endometrial carcinoma
| Characteristics | n (%) |
| BMI, body mass index; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; FIGO, Federation International of Gynecology and Obstetrics; LVSI, lymph-vascular space invasion. | |
Age (year) (
 ) )
|
55.7±9.6 |
BMI (kg/m2) (
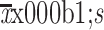 ) )
|
26.2±4.7 |
CA125 (U/mL) (
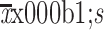 ) )
|
38.0±68.8 |
CA19-9 (U/mL) (
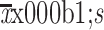 ) )
|
34.5±32.0 |
CEA (ng/mL) (
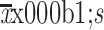 ) )
|
5.0±10.8 |
Calcium concentration (mg/dL) (
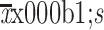 ) )
|
9.1±0.7 |
Adjusted calcium concentration (mg/dL) (
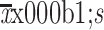 ) )
|
8.8±0.7 |
Albumin (g/L) (
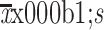 ) )
|
43.5±4.2 |
| Menopausal status | |
| No | 163 (36.7) |
| Yes | 281 (63.3) |
| Diabetes | |
| No | 335 (75.5) |
| Yes | 109 (24.5) |
| Hypertension | |
| No | 261 (58.8) |
| Yes | 183 (41.2) |
| Tubal ligation | |
| No | 366 (82.4) |
| Yes | 78 (17.6) |
| Hormone therapy | |
| No | 410 (92.3) |
| Yes | 34 (7.7) |
| Peritoneal cytology | |
| Negative | 396 (89.2) |
| Positive | 48 (10.8) |
| Tumor grade | |
| 1 | 149 (33.6) |
| 2 | 193 (43.5) |
| 3 | 102 (23.0) |
| FIGO stage | |
| I | 354 (79.7) |
| II | 21 (4.7) |
| III | 57 (12.8) |
| IV | 12 (2.7) |
| Lymph node metastasis | |
| Negative | 401 (90.3) |
| Positive | 43 (9.7) |
| LVSI | |
| Negative | 363 (81.8) |
| Positive | 81 (18.2) |
| Recurrence | |
| No | 407 (91.7) |
| Yes | 37 (8.3) |
Association between serum calcium and metastatic capability
In this univariate logistic analysis, we assessed the relationship between low/high serum total/ionized calcium level and clinical outcomes such as tumor grade, FIGO stage, LVSI, lymph node metastasis and recurrence. This result revealed that the distribution of peritoneal cytology (P<0.001), tumor grade (P=0.007) and LVSI (P=0.012) were obviously different distributed in ionized calcium level group and what’s more, abnormal ascites showed the most significant difference. There were no statistically significant differences on FIGO stage, lymph node metastasis, recurrence between the two groups in this study (Table 2).
2.
Association between serum total/ionized calcium level and clinical parameters
| Variables | Calcium concentration [n (%)] | Adjusted calcium concentration [n (%)] | |||||
| Low* | High* | P | Low* | High* | P | ||
| FIGO, Federation International of Gynecology and Obstetrics; LVSI, lymph-vascular space invasion. *, Low and High were defined according to the means of calcium concentration (9.1 mg/dL) and adjusted calcium concentration (8.8 mg/dL). | |||||||
| Peritoneal cytology | 0.247 | <0.001 | |||||
| Negative | 200 (90.9) | 196 (87.5) | 213 (94.2) | 183 (83.9) | |||
| Positive | 20 (9.1) | 28 (12.5) | 13 (5.8) | 35 (16.1) | |||
| Tumor grade | 0.059 | 0.007 | |||||
| 1 | 78 (35.5) | 71 (31.7) | 76 (33.6) | 73 (33.5) | |||
| 2 | 102 (46.4) | 91 (40.6) | 111 (49.1) | 82 (37.6) | |||
| 3 | 40 (18.2) | 62 (27.7) | 39 (17.3) | 63 (28.9) | |||
| FIGO stage | 0.128 | 0.088 | |||||
| I | 181 (82.3) | 173 (77.2) | 186 (82.3) | 168 (77.1) | |||
| II | 10 (4.5) | 11 (4.9) | 9 (4.0) | 12 (5.5) | |||
| III | 27 (12.3) | 30 (13.4) | 29 (12.8) | 28 (12.8) | |||
| IV | 2 (0.9) | 10 (4.5) | 2 (0.9) | 10 (4.6) | |||
| Lymph node metastasis | 0.289 | 0.354 | |||||
| Negative | 202 (91.8) | 199 (88.8) | 207 (91.6) | 194 (89.0) | |||
| Positive | 18 (8.2) | 25 (11.2) | 19 (8.4) | 24 (11.0) | |||
| LVSI | 0.309 | 0.012 | |||||
| Negative | 184 (83.6) | 179 (79.9) | 195 (86.3) | 168 (77.1) | |||
| Positive | 36 (16.4) | 45 (20.1) | 31 (13.7) | 50 (22.9) | |||
| Recurrence | 0.819 | 0.330 | |||||
| No | 201 (91.4) | 206 (92.0) | 210 (92.9) | 197 (90.4) | |||
| Yes | 19 (8.6) | 18 (8.0) | 16 (7.1) | 21 (9.6) | |||
Risk factors for peritoneal metastasis in patients with EC
In the former univariate analysis, we found that ionized calcium concentration had the most significant impact on peritoneal cytology. Furthermore, we detected the risk factors of positive peritoneal cytology. The univariate analysis revealed that there were no statistically significant differences on age, BMI, menopausal status, diabetes, hormone therapy, calcium and albumin level. However, preoperative ionized serum calcium level [odds ratio (OR)=6.061, 95% CI: 3.358−10.939, P<0.001] were significantly higher in patients with positive peritoneal cytology patients. Meanwhile, positive ascites were closely related to hypertension (OR=0.499, 95% CI: 0.256−0.972, P=0.041), tubal ligation history (OR=0.088, 95% CI: 0.012−0.649, P=0.017), CA125 (OR=1.007, 95% CI: 1.004−1.011, P<0.001), CA19-9 (OR=1.008, 95% CI: 1.003−1.013, P=0.018) and CEA (OR=1.021, 95% CI: 1.002−1.040, P=0.032) (Table 3). Multivariate logistic regression analysis indicated that serum ionized calcium level (OR=7.912, P<0.001), CA125 (OR=1.009, P<0.001) and tubal ligation (OR=0.060, P=0.010) were the independent risk factors correlated with peritoneal metastasis in patients with EC (Table 4).
3.
Univariate analysis of clinicopathological parameters between patients with positive or negative peritoneal cytology
| Variables | Univariate analysis | P | |
| OR | 95% CI | ||
| BMI, body mass index; CA, carbohydrate antigen; CEA, carcinoembryonic antigen; OR, odds ratio; 95% CI, 95% confidence interval. | |||
| Age | 0.998 | 0.967−1.030 | 0.898 |
| BMI | 0.968 | 0.902−1.040 | 0.376 |
| Menopausal status | 0.963 | 0.518−1.788 | 0.905 |
| Diabetes | 0.584 | 0.265−1.290 | 0.183 |
| Hypertension | 0.499 | 0.256−0.972 | 0.041 |
| Tubal ligation | 0.088 | 0.012−0.649 | 0.017 |
| Hormone therapy | 1.472 | 0.541−4.001 | 0.449 |
| CA125 | 1.007 | 1.004−1.011 | <0.001 |
| CA19-9 | 1.008 | 1.003−1.013 | 0.018 |
| CEA | 1.021 | 1.002−1.040 | 0.032 |
| Calcium | 1.198 | 0.745−1.927 | 0.457 |
| Adjusted calcium | 6.061 | 3.358−10.939 | <0.001 |
| Albumin | 0.947 | 0.887−1.010 | 0.096 |
4.
Multivariate analysis of risk factors for endometrial carcinoma patients with positive peritoneal cytology
| Variables | Multivariate analysis | P | |
| OR | 95% CI | ||
| CA, carbohydrate antigen; CEA, carcinoembryonic antigen; OR, odds ratio; 95% CI, 95% confidence interval. | |||
| Hypertension | 0.436 | 0.184−1.304 | 0.060 |
| Tubal ligation | 0.060 | 0.007−0.503 | 0.010 |
| CA125 | 1.009 | 1.005−1.101 | <0.001 |
| CA19-9 | 1.305 | 0.396−4.304 | 0.662 |
| CEA | 1.023 | 0.976−1.073 | 0.345 |
| Adjusted calcium | 7.912 | 4.033−15.520 | <0.001 |
Diagnosed accuracy of risk factors for predicting positive peritoneal cytology in EC patients
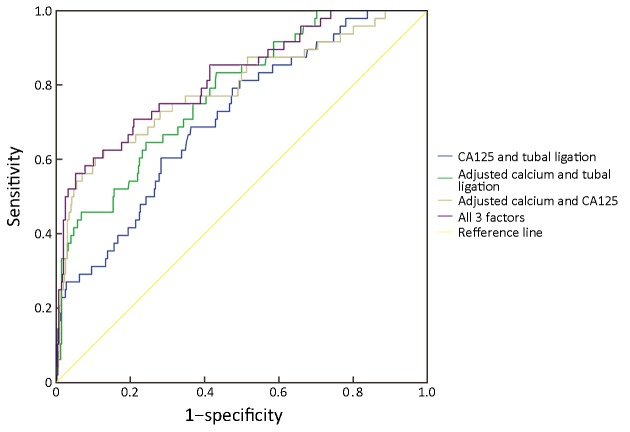
In order to identify the accuracy for diagnosing positive peritoneal cytology, we analyzed the ROC curves for different combinations of risk factors. Figure 2 showed the ROC curve of any two of the three factors and the combined three factors for predicting the risk of positive peritoneal cytology in patients with EC. Based on the analysis, the results indicated that combined serum CA125, tubal ligation with ionized calcium level had the highest diagnostic accuracy for predicting abnormal peritoneal cytology in EC (AUC=0.821, P<0.001) (Table 5).
2.
Receiver operating characteristic (ROC) curves for predicting risk of positive peritoneal cytology in endometrial carcinoma (EC) patients. CA, carbohydrate antigen.
5.
AUC and P-value of risk factors for predicting positive peritoneal cytology with endometrial carcinoma
| Factors | AUC | 95% CI | P | |
| Lower bound | Upper bound | |||
| CA, carbohydrate antigen; AUC, area under receiver operating characteristic curve; 95% CI, 95% confidence interval. | ||||
| Adjusted calcium + tubal ligation | 0.779 | 0.712 | 0.846 | <0.001 |
| Adjusted calcium + CA125 | 0.791 | 0.714 | 0.869 | <0.001 |
| Tubal ligation + CA125 | 0.712 | 0.637 | 0.787 | <0.001 |
| Adjusted calcium + tubal ligation + CA125 | 0.821 | 0.753 | 0.888 | <0.001 |
Discussion
In our study, we had observed a statistically significant correlation between positive peritoneal cytology and preoperative ionized serum calcium level. The correlation remained significant after multivariate analysis. Furthermore, we established a prognostic model of ionized calcium to predict abnormal peritoneal cytology. This study reinforced the potential benefit of using preoperative serum calcium to assess patients with EC and found that positive peritoneal cytology was associated with calcium for the first time.
Abnormal peritoneal cytology was found in about 11% of EC patients (19). Microscopic peritoneal metastasis was suspected when cytopathologic examination of pelvic washings demonstrated malignant cells. Although the FIGO 2009 stage removed peritoneal cytology as staging criteria from the EC staging system (7), positive cytology is still an independent risk factor of survival in multiple studies in EC (20-22). There were many studies focusing on CA125 (23), stages (24), BMI (25), tumor grade (26) to predict peritoneal tumor cell in colorectal and prostate cancer. However, the biomarkers for prediction in EC were still unknown. Identifying available and inexpensive indexes of positive peritoneal cytology had a meaningful benefit for timely intervention and prevention. Thus, we carried out this study to determine the risk factors for patients’ clinicopathological parameters of abnormal peritoneal cytology.
Based on the analysis of this study, hypertension, tubal ligation, serum CA125, CA19-9, CEA levels and ionized serum calcium were found to be significantly correlated with abnormal ascites results. Li et al. revealed that tubal ligation was associated with lower possibility of peritoneal metastasis, which is in line with our results (27). Our group had also early found that serum calcium is also a biomarker for metabolic syndrome in EC. Calcium and its related channels were found to promote migration in EC, indicating that EC cells may require a high Ca2+ microenvironment and more active Ca2+ entry into cells. The association between higher serum calcium and different cancers had long been reported. Total and ionized serum calcium could elevate risk for fatal prostate cancer (16) and act as a risk factor in bone metastasis in bladder cancer (28), breast cancer (29) and discriminate malignant pelvic masses from benign ones (30), which agreed with our results that elevated calcium value was a negative prognostic factor for positive cytology in peritoneum. In this study, the multivariate analysis results showed that ionized serum calcium was an independent risk factor for positive peritoneal tumor cell (OR=7.912, 95% CI: 4.033−15.520, P<0.001). It meant that the risk of developing positive peritoneal cytology was higher when the serum calcium elevated in patients with EC.
Calcium acted as a predictive role in many cancers, such as prostate cancer (31), urothelial carcinoma (32), renal cancer (33), ovarian cancer (34) and breast cancer (35) and the combination of risk factors appeared to be more accurate for predicting peritoneal metastasis (36). In the present study, we also analyzed the predictive accuracy of combined risk factors for predicting peritoneal tumor cell in patients with EC. Compared with two-combined factor ROC curves, combination of ionized calcium with serum CA125, tubal ligation had a higher accuracy for predicting positive peritoneal cytology. Therefore, combining serum calcium with other clinical indexes was a good way to predict metastatic peritoneal tumor cells.
The influence the biological mechanism of serum ionized calcium had on peritoneal cytology remained to be determined. We speculated that the metastatic ability of peritoneal tumor cells might stem from calcium influx through calcium channel. An increase in plasma membrane calcium channel expression and activity sustained an elevated calcium entry, promoting higher cell proliferation and migration in most cases (37). For example, T-type voltage-gated calcium channel increased calcium influx and enhanced the metastatic ability of breast cancer cells (38). Piezo2 channel regulated cell cytoskeleton and metastasis by increasing intracellular calcium concentration (39). Calcium changes the cytoskeleton (40) and surface stiffness (41) during the process of migration. Therefore, calcium may play a role in promoting metastasis by mediating cytoskeleton and mechanical characteristics of cancer cells.
Predicting peritoneal tumor cells is of great importance and value in EC patients. First, serum calcium is affordable and easily acquired because no extra examination is needed. Then, although pathological diagnosis is the gold standard for peritoneal cytology, the diagnosis becomes more precise when combined with other preoperative indicators like calcium level because the structure of abdominal cavity is too complex to obtain metastatic peritoneal tumor cells if the amount of tumor cell is very few. What’s more, it will guide the extent of peritoneal lavage during the surgery. If the level of preoperative serum calcium increases dramatically, it indicates that peritoneal cytology is likely to be positive and surgeons should be careful to avoid abdominal dissemination during the operation and flush the abdominal and pelvic cavity cautiously during the operation. Finally, the prediction of peritoneal tumor cells by increased serum calcium indicates that calcium and its channel may cause tumor cells to migrate and adhere, whose molecular mechanism needs further study. This study provides a new idea for the combination of clinical research and basic research.
Although the results of this study are interesting, there are still some limitations in it. First, this is a retrospective study and some of the patients’ records have missed, which leads to patients’ selection bias. Second, the study is conducted in a single medical center with relative small sample, which may not present the generalized characteristics of the whole population with EC. In the future, we plan to carry out a validation study in other centers and explore the mechanism of metastatic peritoneal tumor cells caused by calcium level.
Conclusions
This study suggested that ionized calcium concentration indicated metastatic ability including peritoneal metastasis, tumor grade and LVSI and it was also an independent risk factor for positive peritoneal tumor cells in patients with EC. Combination of ionized calcium with CA125, tubal ligation had the highest diagnostic accuracy for predicting positive peritoneal cytology. However, this study needs to conduct further external validation in clinical practice and the detailed mechanisms still need further study.
Acknowledgements
This study was supported by National Natural Science Foundation of China (No. 81874108, No. 81802607).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Tanaka K, Kobayashi Y, Sugiyama J, et al Histologic grade and peritoneal cytology as prognostic factors in type 1 endometrial cancer. Int J Clin Oncol. 2017;22:533–40. doi: 10.1007/s10147-016-1079-5. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, Chen Q, Quan P, et al Cancer incidence and mortality in Henan province, 2012. Chin J Cancer Res. 2016;28:275–85. doi: 10.21147/j.issn.1000-9604.2016.03.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen W, Sun K, Zheng R, et al Cancer incidence and mortality in China, 2014. Chin J Cancer Res. 2018;30:13–20. doi: 10.21147/j.issn.1000-9604.2018.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xi Y, Wang W, Chen W, et al Incidence and mortality of corpus uteri cancer in China, 2008−2012. Chin J Cancer Res. 2019;31:435–42. doi: 10.21147/j.issn.1000-9604.2019.03.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen W, Zheng R, Baade PD, et al Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 6.Ferlay J, Shin HR, Bray F, et al Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 7.Pecorelli S Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105:103–4. doi: 10.1016/j.ijgo.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Seagle BL, Alexander AL, Lantsman T, et al Prognosis and treatment of positive peritoneal cytology in early endometrial cancer: matched cohort analyses from the National Cancer Database. Am J Obstet Gynecol. 2018;218:329.e1–e15. doi: 10.1016/j.ajog.2017.11.601. [DOI] [PubMed] [Google Scholar]
- 9.Matsuo K, Yabuno A, Hom MS, et al Significance of abnormal peritoneal cytology on survival of women with stage I-II endometrioid endometrial cancer. Gynecol Oncol. 2018;149:301–9. doi: 10.1016/j.ygyno.2018.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Milgrom SA, Kollmeier MA, Abu-Rustum NR, et al Positive peritoneal cytology is highly predictive of prognosis and relapse patterns in stage III (FIGO 2009) endometrial cancer. Gynecol Oncol. 2013;130:49–53. doi: 10.1016/j.ygyno.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Hasbahceci M, Malya FU, Kunduz E, et al Use of serum and peritoneal CEA and CA19-9 in prediction of peritoneal dissemination and survival of gastric adenocarcinoma patients: are they prognostic factors? Ann R Coll Surg Engl. 2018;100:257–66. doi: 10.1308/rcsann.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takakura Y, Ikeda S, Imaoka Y, et al An elevated preoperative serum carbohydrate antigen 19-9 level is a significant predictor for peritoneal dissemination and poor survival in colorectal cancer. Colorectal Dis. 2015;17:417–25. doi: 10.1111/codi.12865. [DOI] [PubMed] [Google Scholar]
- 13.Hao J, Bao X, Jin B, et al Ca2+ channel subunit alpha 1D promotes proliferation and migration of endometrial cancer cells mediated by 17beta-estradiol via the G protein-coupled estrogen receptor . FASEB J. 2015;29:2883–93. doi: 10.1096/fj.14-265603. [DOI] [PubMed] [Google Scholar]
- 14.Thaw SS, Sahmoun A, Schwartz GG, et al Serum calcium, tumor size, and hormone receptor status in women with untreated breast cancer. Cancer Biol Ther. 2012;13:467–71. doi: 10.4161/cbt.19606. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz GG, Tretli S, Vos L, et al Prediagnostic serum calcium and albumin and ovarian cancer: A nested case-control study in the Norwegian Janus Serum Bank Cohort. Cancer Epidemiol. 2017;49:225–30. doi: 10.1016/j.canep.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GG, and Skinner HG A prospective study of total and ionized serum calcium and time to fatal prostate cancer. Cancer Epidemiol Biomarkers Prev. 2012;21:1768–73. doi: 10.1158/1055-9965.EPI-12-0585. [DOI] [PubMed] [Google Scholar]
- 17.Lin Y, Zhou J, Cao L, et al Serum calcium is a novel parameter to assess metabolic syndrome in endometrial carcinoma. J Gynecol Oncol. 2019;30:e12. doi: 10.3802/jgo.2019.30.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baird GS Ionized calcium. Clin Chim Acta. 2011;412:696–701. doi: 10.1016/j.cca.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Wethington SL, Barrena Medel NI, Wright JD, et al Prognostic significance and treatment implications of positive peritoneal cytology in endometrial adenocarcinoma: Unraveling a mystery. Gynecol Oncol. 2009;115:18–25. doi: 10.1016/j.ygyno.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 20.Garg G, Gao F, Wright JD, et al Positive peritoneal cytology is an independent risk-factor in early stage endometrial cancer. Gynecol Oncol. 2013;128:77–82. doi: 10.1016/j.ygyno.2012.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fader AN, Java J, Tenney M, et al Impact of histology and surgical approach on survival among women with early-stage, high-grade uterine cancer: An NRG Oncology/Gynecologic Oncology Group ancillary analysis. Gynecol Oncol. 2016;143:460–65. doi: 10.1016/j.ygyno.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Obermair A, Geramou M, Tripcony L, et al Peritoneal cytology: impact on disease-free survival in clinical stage I endometrioid adenocarcinoma of the uterus. Cancer Lett. 2001;164:105–10. doi: 10.1016/S0304-3835(00)00722-9. [DOI] [PubMed] [Google Scholar]
- 23.Huang CJ, Jiang JK, Chang SC, et al Serum CA125 concentration as a predictor of peritoneal dissemination of colorectal cancer in men and women. Medicine (Baltimore) 216;95:e5177. doi: 10.1097/MD.0000000000005177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Arakawa K, Kawai K, Ishihara S, et al Prognostic Significance of peritoneal metastasis in stage IV colorectal cancer patients with R0 resection: A multicenter, retrospective study. Dis Colon Rectum. 2017;60:1041–9. doi: 10.1097/DCR.0000000000000858. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Nie RC, Ouyang LY, et al Body mass index (BMI) may be a prognostic factor for gastric cancer with peritoneal dissemination. World J Surg Oncol. 2017;15:52. doi: 10.1186/s12957-016-1076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cummins KA, Russell GB, Votanopoulos KI, et al Peritoneal dissemination from high-grade appendiceal cancer treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) J Gastrointest Oncol. 2016;7:3–9. doi: 10.3978/j.issn.2078-6891.2015.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li M, Li M, Zhao L, et al Prior tubal ligation might influence metastatic spread of nonendometrioid endometrial carcinoma. Int J Gynecol Cancer. 2016;26:1092–7. doi: 10.1097/IGC.0000000000000727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang P, Lan M, Peng AF, et al Serum calcium, alkaline phosphotase and hemoglobin as risk factors for bone metastases in bladder cancer. PLoS One. 2017;12:e0183835. doi: 10.1371/journal.pone.0183835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hack CC, Stoll MJ, Jud SM, et al Correlation of mammographic density and serum calcium levels in patients with primary breast cancer. Cancer Med. 2017;6:1473–81. doi: 10.1002/cam4.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly MG, Winkler SS, Lentz SS, et al Serum calcium and serum albumin are biomarkers that can discriminate malignant from benign pelvic masses. Cancer Epidemiol Biomarkers Prev. 2015;24:1593–8. doi: 10.1158/1055-9965.EPI-15-0443. [DOI] [PubMed] [Google Scholar]
- 31.Chen S, Wang L, Qian K, et al Establishing a prediction model for prostate cancer bone metastasis. Int J Biol Sci. 2019;15:208–20. doi: 10.7150/ijbs.27537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sheth KR, Haddad AQ, Ashorobi OS, et al Prognostic serum markers in patients with high-grade upper tract urothelial carcinoma. Urol Oncol. 2016;34:418.e9–e16. doi: 10.1016/j.urolonc.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 33.Silberstein JL, Adamy A, Maschino AC, et al Systematic classification and prediction of complications after nephrectomy in patients with metastatic renal cell carcinoma (RCC) BJU Int. 2012;110:1276–82. doi: 10.1111/j.1464-410X.2012.11103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz GG, Skinner HG Prospective studies of total and ionized serum calcium in relation to incident and fatal ovarian cancer. Gynecol Oncol. 2013;129:169–72. doi: 10.1016/j.ygyno.2012.12.046. [DOI] [PubMed] [Google Scholar]
- 35.Wulaningsih W, Sagoo HK, Hamza M, et al Serum Calcium and the risk of breast cancer: Findings from the Swedish AMORIS study and a Meta-analysis of prospective studies. Int J Mol Sci. 2016;17:pii:E1487. doi: 10.3390/ijms17091487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiao G, Wang X, Zhou L, et al Autologous dendritic cell-cytokine induced killer cell immunotherapy combined with S-1 plus cisplatin in patients with advanced gastric cancer: A prospective study. Clin Cancer Res. 2019;25:1494–504. doi: 10.1158/1078-0432.CCR-18-2360. [DOI] [PubMed] [Google Scholar]
- 37.Déliot N, Constantin B Plasma membrane calcium channels in cancer: Alterations and consequences for cell proliferation and migration. Biochim Biophys Acta. 2015;1848:2512–22. doi: 10.1016/j.bbamem.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 38.Bhargava A, Saha S T-Type voltage gated calcium channels: a target in breast cancer? Breast Cancer Res Treat. 2019;173:11–21. doi: 10.1007/s10549-018-4970-0. [DOI] [PubMed] [Google Scholar]
- 39.Pardo-Pastor C, Rubio-Moscardo F, Vogel-Gonzalez M, et al Piezo2 channel regulates RhoA and actin cytoskeleton to promote cell mechanobiological responses. Proc Natl Acad Sci U S A. 2018;115:1925–30. doi: 10.1073/pnas.1718177115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YT, Chen YF, Chiu WT, et al Microtubule-associated histone deacetylase 6 supports the calcium store sensor STIM1 in mediating malignant cell behaviors. Cancer Res. 2013;73:4500–9. doi: 10.1158/0008-5472.CAN-12-4127. [DOI] [PubMed] [Google Scholar]
- 41.Lee WH, Choong LY, Mon NN, et al TRPV4 regulates breast cancer cell extravasation, stiffness and actin cortex. Sci Rep. 2016;6:27903. doi: 10.1038/srep27903. [DOI] [PMC free article] [PubMed] [Google Scholar]