Abstract
Functional dyspepsia (FD) is a chronic upper gastrointestinal (GI) symptom complex that routine diagnostic work-up, such as endoscopy, blood laboratory analysis, or radiological examination, fails to identify a cause. It is highly prevalent in the World population, and its response to the various available therapeutic strategies is only modest because of the heterogenous nature of its pathogenesis. Therefore, FD represents a heavy medical burden for healthcare systems. We constituted a guideline development committee to review the existing guidelines on the management of functional dyspepsia. This committee drafted statements and conducted a systematic review and meta-analysis of various studies, guidelines, and randomized control trials. External review was also conducted by selected experts. These clinical practice guidelines for FD were developed based on evidence recently accumulated with the revised version of FD guidelines released in 2011 by the Korean Society of Neurogastroenterology and Motility. These guidelines apply to adults with chronic symptoms of FD and include the diagnostic role of endoscopy, Helicobacter pylori screening, and systematic review and meta-analyses of the various treatment options for FD (proton pump inhibitors, H.pylori eradication, and tricyclic antidepressants), especially according to the FD subtype. The purpose of these new guidelines is to aid the understanding, diagnosis, and treatment of FD, and the targets of the guidelines are clinicians, healthcare workers at the forefront of patient care, patients, and medical students. The guidelines will continue to be revised and updated periodically.
Keywords: Dyspepsia, Endoscopy, Evidence-based medicine, Guideline, Proton pump inhibitors
Introduction
Functional dyspepsia (FD) is a chronic and recurrent manifestation of gastrointestinal (GI) symptoms in the absence of an organic disease such as peptic ulcer, GI malignancy, gastroesophageal reflux disease, or pancreatitis. Symptoms of FD include epigastric pain, epigastric burning, postprandial fullness, and postprandial satiation. FD is considered a heterogenous condition. According to the Rome III criteria revised in 2006, FD was divided into 2 subtypes, namely, epigastric pain syndrome (EPS) and postprandial distress syndrome (PDS),1 and these definition was also adopted by the Rome IV criteria.2 Two subtypes of FD were expected to have different pathophysiologies and drug responses. Since the revision of Rome III, many studies have been conducted on this basis, and more research on subtypes of FD has been conducted. The pathophysiology of FD is also heterogenous, and different underlying mechanisms contribute to diverse patterns of symptoms. Impaired gastric accommodation to a meal, delayed gastric emptying, and visceral hypersensitivity are involved in both EPS and PDS, and some patients have an overlap of both subtypes.2 Although the Kyoto consensus3 suggested Helicobacter pylori eradication as a primary treatment for dyspepsia, accepting this consensus in Korea will require careful consideration because H.pylori prevalence in Korea is higher than 50% in adults4 with high resistance rate of antibiotics,5 and the efficacy of H.pylori eradication therapy for FD symptom resolution is modest.
In 2005, the Korean Society of Neurogastroenterology and Motility (KSNM) published evidence-based guidelines for the diagnosis and treatment of FD.6 In 2011, the guidelines were revised through a systematic review that focused on the treatment of FD and have been used in the clinical field.7 We introduced the new guidelines for diagnosis and treatment. This included a systematic review of the diagnosis and treatment and meta-analysis, which were performed with regard to FD treatment options such as proton pump inhibitors (PPIs), H. pylori eradication, and tricyclic antidepressants (TCAs). These guidelines will be helpful for understanding and treatment of FD.
Revision Process
Guideline Development Committee
The steering committee of the KSNM in 2017 undertook the revision of the guidelines. The Working Group for Guidelines Development was formed from 2 of the 12 committees of the KSNM (ie, the FD Research Group and the Clinical Practice Guideline Group). The FD Research Group consisted of 1 institute board member (J.G.K.), 1 staff member (J.H.O.), and 6 general members (C.M.S., J.K.P., K.B.B., J.Y.L, K.J., and C.H.T.). The Clinical Practice Guideline Group consisted of 1 institute board member (H.K.J.), 1 staff member (K.H.S.), and 6 general members (J.E.S., J.S.K, S.J.K, M.K.B., H.C.I., and S.E.K.). The chairman of the Clinical Practice Guideline Group (H.K.J.) supervised and monitored the development process, while a methodologist expert in formulation of guidelines (E.S.S.) conducted the workshop on systematic review and meta-analysis.
Guideline Development Process
Principles of drafting statements
The population, intervention, comparator, outcome, and healthcare setting principles were used as the basis of the statements. Current guidelines consist of 2 main topics: diagnosis and treatment of FD. These guidelines were developed by the de novo method that conducted systematic review and meta-analysis for acid suppressants (including PPIs and histamine receptor 2 antagonists [H2RA]), H. pylori eradication, and TCAs in the management of FD. Subgroup analysis was also performed for the subtypes of FD. Recommendations that were made in previous guidelines but were not supported by medical evidence were revised in English by reinforcing the recent literature.
Systematic review
Electronic databases, including MEDLINE, Embase, Web of Science, Cochrane Library, and KoreaMed, were searched for relevant literature. Data extraction tables for the main topics (acid suppressants, H. pylori) are provided in the supplementary Table (Supplementary Tables 1 and 2). In the review of antisecretory agents, the search keywords were dyspepsia-related index works (Dyspepsia*; Dyspepsie*; Dyspeptic; Indigestion*; Upset stomach; Stomach upset; Apepsy; Apepsia) AND (Proton pump Inhibitor*; Inhibitors, proton pump; Antagonists, histamine H2; Antihistaminics, H2; Blockaders, H2 receptor; Blockaders, histamine H2 receptor; Blockers, histamine H2; H2 antagonists, histamine; H2 antihistamin*; H2 blockers, histamine; H2 receptor blockader*; H2RA; Histamine 2 antagonist*; Histamine H2 antagonist*; Histamine H2 blocker*; Histamine H2 receptor antagonist*; Histamine H2 receptor blockader*; Receptor antagonists, histamine H2; Receptor blockaders, H2). In the review of H. pylori, the search key words were as follows: (Helicobacter Pylori, H. Pylori, Campylobacter Pylori, Helicobacter Infect*, Infect* Helicobacter, Eradicat*, Eradicant*, Eliminat*, Anti Bacterial*, Antibacterial*, Bacteriocid*, Anti-Helicobacter Pylori, Anti-Campylobacter Pylori, Anti-Ulcer Agent*). In the review of TCAs, the search key words were as follows: Antidepressive agents, Serotonin uptake inhibitors, Sulpiride, Mianserin, Desipramine, Imipramine, Trimipramine, Doxepin, Dothiepin, Notriptyline, Amitriptyline, Paroxetine, Sertraline, Fluoxetine, Citalopram, Venlafaxine, Duloxetine, Ecitalopram, Levosulpiride, Mirtazapine, Tricyclic, Desimipramine, Buspirone, Tandospirone). The full literature search strategy is provided as supplementary. The inclusion criteria were: adult (over 19 years of age), literature from 2005 to the present, literature written in English or Korean, and randomized controlled studies. The exclusion criteria were: outcome of interest not reported; narrative review, editorial, guidelines, or not randomized trial; and studies conducted in healthy volunteers. We critically appraised the quality of selected studies using risk of bias tools. We used the Cochrane Risk of bias for randomized controlled trials (RCTs).8 Researchers independently assessed the studies and disagreement were resolved by discussions and 3rd member of opinion (Supplementary Fig. 1–8). We also conducted a literature search on the patients’ preferences. Results of symptom survey of FD patients via computer-assisted personal interview, which results in drug use in FD patients, were in order of “epigastric pain,” “acid reflux/indigestion,” and “time to onset of action.”9
Level of evidence and grade of recommendation
This revision defines 4 levels of evidence and evaluates the level of evidence for each statement based on the modified Grading of Recommendations Assessment, Development, and Evaluation (GRADE) (Table 1).10 Grading of recommendation also modified GRADE methodology as 5 levels including; strong for, weak for, weak against, strong against, and no recommendation (Table 2).10 We considered evidence level, clinical applicability, benefit and harm as recommendation factors. The Committee reviewed the draft of the working group then discussed for consensus.
Table 1.
Level of Evidence
| Class | Explanation |
|---|---|
| High | At least one RCT or SR/meta-analysis with no concern of study quality |
| Moderate | At least one RCT or SR/meta-analysis with minor concern of study quality or |
| At least one cohort/case-control/diagnostic test design study with no concern of study quality | |
| Low | At least one cohort/case-control/diagnostic test study with minor concern of study quality or |
| At least one single arm before-after study, cross-sectional study with no concern of study quality | |
| Very low | At least one cohort/case-control/diagnostic test design study with serious concern of study quality or |
| At least one single arm before-after study, cross-sectional study with minor/severe concern of study quality |
RCT, randomized controlled trials; SR, systemic review.
Table 2.
Grading of Recommendations
| Grade classification | Explanation |
|---|---|
| Strong for | The benefit of intervention is greater than harm with high or moderate level of evidence, which can be strongly recommended in most clinical practice |
| Weak for | The benefit and harm of intervention may vary depending on the clinical situation or patient/social value. It is recommended conditionally according to the clinical situation. |
| Weak against | The benefit and harm of intervention may vary depending on the clinical situation or patient/social value. Intervention may not be recommended in clinical practice |
| Strong against | The harm of intervention is greater than the benefit with high or moderate level of evidence, intervention should not recommended in clinical practice |
| No recommendation | It is not possible to determine the recommendation direction owing to a lack of evidence or discrepancy of result. Thus further evidence is needed. |
Expert consensus by Delphi agreement process
To adopt the core recommendations of the guidelines, the Delphi technique, which is a panel of experts on FD, was used. The panel was selected by former or current members of the KSNM Steering Committee and the faculty of gastroenterology departments of university hospitals. The first Delphi round was conducted on the 9 newly updated recommendations, including 2 diagnostic methods (endoscopy and H. pylori screening) and 7 treatment modalities (including PPIs, prokinetics, eradication of H. pylori infection, antipsychotics, gastromucosal protective agents, and simethicone). The definition of agreement or other methods are the same as the previous guidance development method.11 A total of 27 doctors participated in the first round of Delphi consensus. Specific methods, such as the criteria for consent, were the same as for the previous guidelines. Out of a total of 14 statements, we voted on 9 covering recent research, of which, 2 statements about gastromucosal protective agents and simethicone were not accepted, while the other 7 were.
Internal and external reviews
Guideline development committee members conducted internal reviews through online and offline meetings. KSNM executives completed internal review by creating additional amendments. The Korean Society of Internal Medicine recommended members who acted as external judges (S.C.C. and M.I.P). According to the external review, the definition of FD and the change in terminology were pointed out and modified. In addition, an explanation on the difference between the international trend including the Kyoto consensus and Korean guidelines was added.
Dissemination of the guidelines and revision plans
The developed guidelines will be listed in the “Clinical practice guidelines” on the official website of the Korean Society of Gastroenterology. In addition, these latest guidelines will be presented at medical symposia, conferences, and hospitals. Amendment to these new guidelines is scheduled to be made in about 5 years if the data are accumulated. The relevant committee of KSNM will be responsible for the revision.
Editorial independence
These guidelines were developed independently by KSNM without external funding, and KSNM did not have any specific impact on the development process of working teams. No other organization or individual influenced the content of the guidelines. No member of the working team has a conflict of interest, which has been documented.
Definition of Dyspepsia
Dyspepsia is defined as any symptom that refers to the upper GI tract, and it is one of the most common GI symptom. FD is defined by the Rome IV criteria as a syndrome with one or more of the following symptoms present over the past 3 months, with at least 6 months of onset: bothersome postprandial fullness, early satiation, epigastric pain, and epigastric burning, with no evidence of structural disease, as seen in upper endoscopy that is likely to explain the symptoms.2 The Rome criteria have the advantage of selecting the more homogenous subset from various dyspeptic patients in actual clinical practice. In the National Institute for Health and Care Excellence guidelines and American College of Gastroenterologists & Canadian Association of Gastroenterologists clinical guidelines on dyspepsia management, dyspepsia is defined as predominant epigastric pain lasting at least 1 month and associated with any other upper GI symptom such as epigastric fullness, nausea, vomiting, or heartburn.12,13 These guidelines are intended to be used in clinical practice and are intended to target a wide range of dyspeptic patients beyond those that meet the definition of FD by Rome criteria. Thus, in the presently proposed guideline, dyspepsia is defined as pain or discomfort in the upper abdomen, postprandial fullness, early satiation, bloating, nausea, or vomiting that has lasted more than 1 month.
Refractory FD refers to FD that has continuous symptoms for at least 8 weeks and has been unresponsive to at least 2 medical treatments.14 The guidelines for FD in the Asia-Pacific region and the United States of America (USA) recommend changing to a different drug if adequate therapeutic efficacy has not been achieved after 4 weeks of treatment.15,16 Recent Japanese guideline for dyspepsia suggested that refractory FD is one that did not respond to initial treatment with acid suppressants and prokinetics, and second step of treatment with traditional medicine, anxiolytics or antidepressants, and H. pylori eradication.14 Before diagnosing refractory FD, other organic diseases, including pancreatic diseases, gall bladder dysfunction or other biliary diseases, should be also excluded.
Diagnosis of Functional Dyspepsia
Esophagogastroduodenoscopy
Statement 1: Prompt upper GI endoscopy is recommended in dyspeptic patients aged 40 years or older to exclude organic disorder, including upper gastrointestinal malignancy.
Grade of recommendation: strong
Level of evidence: low
Experts’ opinions: completely agree, 70.4%; mostly agree, 29.6%; partially agree, 0.0%; mostly disagree, 0.0%; completely disagree, 0.0%; and not sure, 0.0%.
Several studies have shown no significant differences in controlling dyspeptic symptoms between “H. pylori test and treat approach” and performing early upper GI endoscopy in patients with dyspepsia, and the former option is more cost-effective.17–19 Studies comparing empiric acid suppression therapy with early endoscopy also showed similar outcomes.20,21 However, most of these studies were conducted in Western countries. In Asia, the incidence of gastric cancer is very high, and the age of onset is younger than in Western countries. A retrospective study of 14 101 patients who have had endoscopy for dyspepsia in China reported that 13 cases (72.2%) among 18 gastric cancers were missed when the H. pylori test and treat strategy was applied in dyspeptic patients under 45 years of age and without alarm symptoms (such as weight loss, dysphagia, GI bleeding, iron deficiency anemia, abdominal mass or persistent vomiting).22 A prospective Taiwan study of 17 894 patients who had upper endoscopy because of uninvestigated dyspepsia reported that 5.3% (12/225) of the gastric cancer patients discovered were less than 45 years of age and did not have alarm symptoms. The study thus recommended that age of 40 might be an optimal age threshold for screening endoscopy for uninvestigated dyspepsia.23
The incidence of gastric cancer in Korea is the highest in the world. In the 2008 Korea Central Cancer Registry, the age-specific gastric cancer incidence per 100 000 persons were 16.7 males and 16.4 females within the ages of 34–39 years, and 36.3 males and 28.8 females within the ages of 40–44 years.24 The age-adjusted incidence rates per 100 000 person-year from 1999 to 2010 were 7.40 males and 8.33 females within the ages of 20–39 years, and these increased up to 73.11 males and 35.13 females within the ages of 40–54 years.25 Data from GLOBOCAN 2012, produced by the International Agency for Research on Cancer (IARC), showed that the age-standardized incidence rates per 100 000 for gastric cancer in Korea were 5.7 within the ages of 15–39 years, and it increased to 30 within the ages of 40–44 years. There is no study to evaluate the incidence of gastric cancer by age in patients with dyspepsia in Korea. There was no gastric cancer among the 308 patients under 40 years old in a study done to determine the usefulness of H. pylori test before endoscopy in 615 Korean patients with dyspepsia.26
A systematic review with meta-analysis was recently done to evaluate the appropriateness of prompt endoscopy as an initial strategy for uninvestigated dyspepsia in Asia, giving the high prevalence of H. pylori infection and malignancy. Gastric cancer patients younger than 45 years and 35 years were 17.8% and 3.0%, respectively, among cancer patients. The review thus concluded that the optimal age threshold for endoscopy screening in patients with uninvestigated dyspepsia in Asia might be 35 years.23 According to annual report of cancer statistics in Korea in 2014, the percentage of gastric cancer was 1.2% (353) for cancer patients aged < 35 years, 3.1% (932) for those aged < 40 years, and 7.5% (2–230) for those < 45 years among the 29 854 patients with gastric cancer. Therefore, because of the high probability of gastric malignancy in patients with dyspepsia and over 40 years of age, we suggest that early upper GI endoscopy should be performed in patients with dyspepsia who are aged 40 or over in order to exclude organic causes, including gastric cancer.
Diagnosis of Helicobacter pylori
Statement 2: Test for H. pylori infection is recommended in dyspeptic patients who are not responding to acid suppressants or prokinetics.
Grade of recommendation: weak
Level of evidence: very low
Multiple factors may be associated with the pathophysiology of FD. These factors can include impaired gastric accommodation, delayed gastric emptying, hypersensitivity, social factors, H. pylori infection, gastric acid secretion, genetic factors, psychological factors, history of infectious colitis, lifestyle, and morphology of the stomach.27 The role of H. pylori infection in FD has not been fully identified. It has been speculated that H. pylori infection causes inflammation of the gastric mucosa, and is associated with specific disturbances of gastric secretory or motor function, which, in turn, may contribute to dyspeptic symptoms.28 Approximately 50.0% of FD patients also have gastritis associated with H. pylori. As H. pylori infection has been found to be related with GI diseases, many investigators have tried to treat FD by eradication of the infection. A systematic review concluded that there was a small but statistically significant benefit of treating H. pylori infection in patients with FD.29 In 17 RCTs comprising over 3500 patients, the relative risk (RR) reduction seen with treatment of H. pylori infection was 10.0% (95% confidence interval [CI], 6.0–14.0%) and the number needed to treat (NNT) to cure one patient with FD was 14 (95% CI, 10–25). In the United Kingdom (UK), the Bristol Helicobacter Project randomized 1517 H. pylori-positive adults under 60 years of age to undergo treatment for H. pylori infection or placebo and followed them up prospectively.30 Among those treated for the infection, of whom over 90.0% achieved successful eradication, there was a small but statistically significant (P < 0.05) reduction in subsequent consultations at the primary care level for dyspeptic complaints. In Korea, the prevalence of H. pylori decreased significantly from 59.6% in 2005 to 51.0% in 2015, and this change was more pronounced among younger age groups under forties.31
The prevalence in Western countries is around 20.0% and 40.0% among young adults and at older ages, respectively.32 The prevalence of gastric cancer is high in Asian countries, including Korea, particularly among young people. Long-term empirical drug treatment as the initial approach to dyspepsia is not advisable because of delay in the diagnosis of organic disease, especially in patients with dyspepsia over 40 years of age, in whom there is high probability of gastric cancer. Therefore, we suggest that “test and treat approach” for H. pylori may be considered in dyspeptic patients under 40 years without alarm symptoms.
Urea breath tests and stool antigen tests are the most widely used noninvasive tests for H. pylori infection, whereas serology is useful in screening and epidemiological studies. The 13C-urea breath test and the stool antigen test provide 90.0% sensitivity and specificity, and the 13C-urea breath test is widely used in clinical practice. Serologic tests detecting IgG antibodies against H. pylori should be used and interpreted with caution as the antibodies can persist sometimes for years, and a positive test may only indicate past infection. In addition, when upper GI endoscopy is undertaken in patients with dyspepsia, rapid urease tests or histological examination using gastric biopsy samples could also be performed to check for H. pylori infection.
Alarm Symptoms
Statement 3: When dyspepsia is accompanied by the following alarm symptoms, signs or medical history, further evaluation should be considered: age > 40 years, abnormal weight loss, progressive dysphagia, bleeding signs, persistent vomiting, family history of gastric cancer, and recent non-steroidal anti-inflammatory drug (NSAID)/anticoagulant/antiplatelet agent use.
Grade of recommendation: strong
Level of evidence: low
FD is diagnosed by both the presence of typical symptoms and the exclusion of organic diseases such as gastric cancer. Several guidelines have recommended evaluation of organic causes when alarm features (eg, weight loss, bleeding signs, progressive dysphagia, persistent vomiting, and recent NSAID/anticoagulant/ antiplatelet use) are present.14,16,33,34 However, a recent systematic review found that alarm symptoms were of limited value in detecting organic pathology.35 This has led to a revised guideline, which recommends further evaluation in patients with alarm symptoms and over the age of 60 years or those with symptoms that suggest a pancreatic or biliary source.36
However, this fails to take into account the epidemiologic differences in organic disease between the East and West. Gastric cancer is highly prevalent in Korea and is projected to be the most commonly diagnosed cancer in men between 35 years and 50 years of age and the third most diagnosed cancer in women of the same age range.37 Furthermore, a recent systematic review found that alarm symptoms in young Asians were of significant diagnostic value.38 Therefore, we suggest that dyspeptic patients with alarm symptoms undergo further evaluation such as upper GI endoscopy, regardless of age. However, we agree with previous guidelines that suggest no further evaluation for patients with intractable dyspepsia without alarm signs as such evaluation may be of limited clinical benefit.36
Patients with continued symptoms of dyspepsia should be carefully reassessed, paying specific attention to the type of ongoing symptoms, and the degree to which symptoms have improved or worsened. If clinically indicated, complete blood count (CBC) and blood chemistry should be performed in dyspeptic patients to identify organic diseases that can cause dyspepsia.14,39 The development of iron deficiency anemia has been found to be a predisposing factor for the diagnosis of pathologic GI diseases. Pathologic GI diseases was diagnosed in 23.0% of Korean adult population who presented with iron deficiency, and GI malignancy was diagnosed in 1.0% of that population, mainly in elderly patients.40 In that study, it was suggested that patients with iron deficiency should undergo endoscopic evaluation of the GI tract, irrespective of whether or not they have anemia.39 Anemia is also associated with H. pylori infection. Qu et al conducted a meta-analysis of 15 case-control studies to investigate the relationship between H. pylori infection and iron deficiency anemia (IDA). The data showed an increased risk of IDA in patients with H. pylori infection with an odds ratio (OR) of 2.2 (95% CI, 1.5–3.2).41 Therefore, current international and national guidelines recommend eradication of H. pylori infection in patients with unexplained IDA.42,43
Patients with unresponsive to empirical medical therapy should undergo blood tests, such as CBC and blood chemistry, if not conducted at the time of initial diagnosis. Upper abdominal ultra-sonography or abdominal CT scan may be employed, especially in areas with high prevalence of liver44 or pancreatic cancers that present with dyspeptic symptoms.14,36,39,44
Treatments
Acid Suppressants
Proton pump inhibitors
Statement 4: PPIs are recommended for the treatment of FD.
Grade of recommendation: strong
Level of evidence: high
Experts’ opinions: completely agree, 44.5%; mostly agree, 37.0%; partially agree, 14.8%; mostly disagree, 3.7%; completely disagree, 0.0%; and not sure, 0.0%.
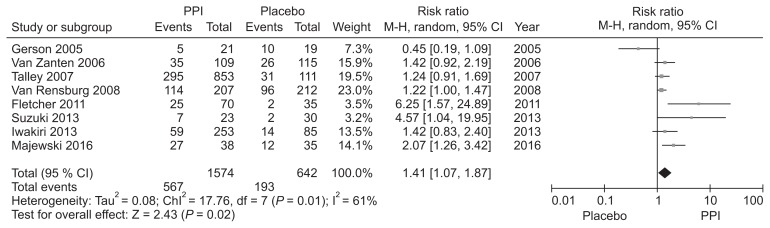
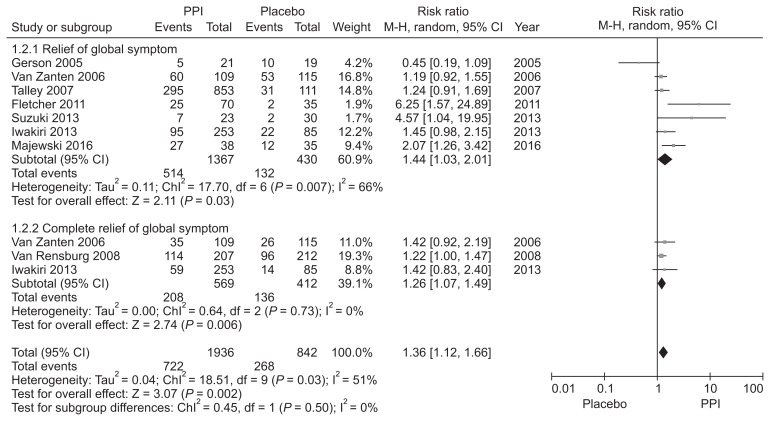
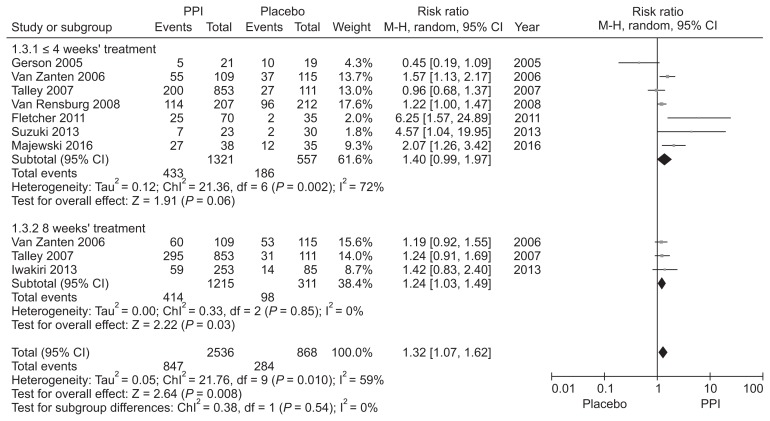
Hypersensitivity to acid, reduced duodenal acid clearance, and altered gastric motility induced by duodenal acid have been suggested as putative roles of acid in FD and justification for the use of acid suppressive therapy.45–47 PPIs have been the mainstay treatment for FD, but the reported efficacy is marginal and controversy exists.48 We performed a new meta-analysis to investigate the efficacy of PPI therapy (Supplementary Fig. 1–3). We identified 8 RCTs involving 2216 FD patients to compare the global symptom improvement between PPI and placebo.49–56 The treatment period was 2–8 weeks. PPI was more effective than placebo for overall global symptom improvement, with symptom improvement seen in 36.0% of the PPI group and 30.0% of the placebo group (RR, 1.41; 95% CI, 1.07–1.87). However, there was heterogeneity between studies (χ2 = 17.76; P = 0.01; I2 = 61%) (Fig. 1). The NNT was 9 (95% CI, 6–24). In subgroup analysis, PPI was effective in terms of symptom relief (RR, 1.44; 95% CI, 1.03–2.01) and complete relief of global symptom (RR, 1.26; 95% CI, 1.07–1.49) (Fig. 2). Subgroup analyses were performed according to the treatment duration. Three RCTs evaluating the efficacy of PPI after 8 weeks of treatment demonstrated statistically significant symptom relief with PPI therapy in FD patients (RR, 1.24; 95% CI, 1.03–1.49) with no heterogeneity (χ2 = 0.33; P = 0.85; I2 = 0%). However, there was no significant difference in efficacy between PPI and placebo in ≤ 4 weeks treatment duration (RR, 1.40; 95% CI, 0.99–1.97) (Fig. 3).
Figure 1.
Forest plot of randomized controlled trials comparing proton pump inhibitors (PPIs) with placebo in patients with functional dyspepsia.
Figure 2.
Forest plot of randomized controlled trials comparing proton pump inhibitors (PPIs) with placebo in patients with functional dyspepsia according to the relief of global symptom.
Figure 3.
Forest plot of randomized controlled trials comparing proton pump inhibitors (PPIs) with placebo in functional dyspepsia patients according to the treatment duration.
Statement 5: PPIs should be recommended as a first-line treatment for FD in patients with EPS.
Grade of recommendation: strong
Level of evidence: moderate
Experts’ opinions: completely agree, 25.9%; mostly agree, 55.6%; partially agree, 18.5%; mostly disagree, 0.0%; completely disagree, 0.0%; and not sure, 0.0%.
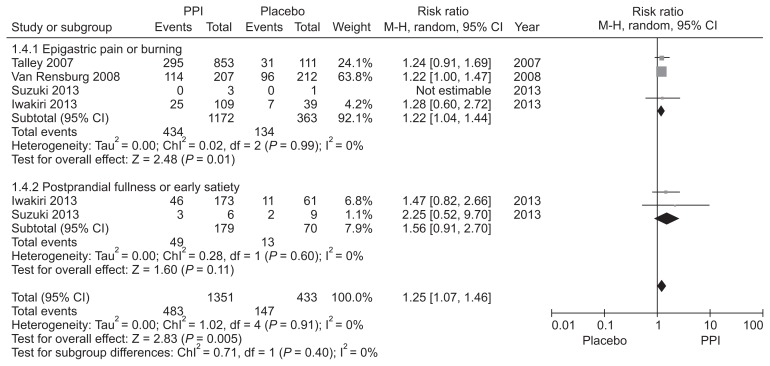
PPIs are believed to be effective for FD patients with ulcer or reflux-like symptoms,57 and several guidelines suggest tailored PPIs treatment according to FD subtype.58–60 However, this is controversial.61–63 Although the Rome III criteria subdivided FD into EPS and PDS, dyspepsia forms a symptom complex with overlap between EPS and PDS.27,64 We identified 4 RCTs that evaluated the efficacy of PPI in FD patients with epigastric pain or burning as the predominant symptom, including 2 involving the EPS subtype based on the Rome III criteria.51,52,54,55 PPI was more effective than placebo for the treatment of predominant epigastric pain or burning (RR = 1.22; 95% CI = 1.04–1.44) with no observed heterogeneity (χ2 = 0.02, P = 0.99, I2 = 0%). Regarding postprandial fullness or early satiation as the predominant symptom, there were 2 RCTs that defined the PDS subtype.54,55 No significant difference was observed between the PPI and placebo groups in patients with the PDS subtype (RR = 1.56; 95% CI = 0.91–2.70) without heterogeneity (Fig. 4). However, further studies are required due to the paucity and heterogeneity of the included studies. We downgraded the quality of evidence due to the application of inconsistent diagnostic criteria for FD among the included studies. Although the level of evidence is not high, it could be useful as first-line treatment for patients with EPS symptoms.
Figure 4.
Forest plot of randomized controlled trials comparing proton pump inhibitors (PPIs) with placebo in functional dyspepsia patients according to the predominant symptom.
Histamine type 2 receptor antagonists
Statement 6: H2RAs are reasonable treatment in functional dyspepsia, especially for short-term use.
Grade of recommendation: weak
Level of evidence: moderate
H2RAs, as acid suppressants, are another option for the treatment of FD. A Cochrane meta-analysis of 12 RCTs involving 2456 non-ulcer dyspeptic patients reported an RR of persistent symptoms of 0.77 (95% CI, 0.65–0.92) and an NNT of 7. However, the evidence justifying the use of H2RAs is limited. The overall quality of the trials was low, and they were conducted before the introduction of the Rome III criteria.65 Regarding the efficacy of H2RAs compared with PPI, there was no statistical difference in terms of symptom relief in a recent meta-analysis of 7 RCTs evaluating 2456 dyspeptic patients (RR, 0.93; 95% CI, 0.76–1.16). However, there was a trend toward greater symptom relief with a PPI among the included studies.36 Moreover, repeat dosing of H2RAs led to the development of tachyphylaxis or tolerance,66 thus attenuating their efficacy but without further reduction in efficacy once tachyphylaxis had been developed.67 Tachyphylaxis possibly limits the use of H2RAs as maintenance therapy for FD due to attenuated efficacy.68 Although H2RAs are considered safe, they can cause adverse drug reactions (ADRs), including anaphylaxis. In a study evaluating 584 patients (694 cases) with ADRs caused by ranitidine, anaphylaxis was occasionally observed.69 Besides, ranitidine metabolite from several manufacturers has been reported to have a carcinogenic property in September 2019, then, many drugs including ranitidine have been withdrawn from the market. Thus, while short-term use of H2RAs may be indicated in the management of FD, caution may need to be taken.
Prokinetics
Statement 7: Prokinetics are effective in the treatment of FD.
Grade of recommendation: strong
Level of evidence: moderate
Experts’ opinions: completely agree, 35.7%; mostly agree, 53.6%; partially agree, 10.7%; mostly disagree, 0.0%; completely disagree, 0.0%; and not sure, 0.0%.
Prokinetic drugs are classified as dopamine D2 receptor antagonists (D2 antagonists), 5-hydroxytryptamine 4 (HT4) receptor agonists, or motilin agonists, based on their mechanism of action. Domperidone, a D2 antagonist, was found in a meta-analysis to be effective for the treatment of bloating and early satiation symptoms as compared to placebo when used for 2–4 weeks.70 Metoclopramide and levosulpiride have also been shown to improve dyspeptic symptoms.71 However, metoclopramide, levosulpiride, and domperidone, which are effective D2 antagonists, may cause extrapyramidal symptoms, and this limits their dosing duration in Korea.
Itopride is a D2 antagonist that works peripherally, avoiding the central receptor-related extrapyramidal side effects and resulting in a minimal elevation of prolactin hormone levels. It was found to be effective for global symptom improvement, postprandial fullness, and early satiation when compared to placebo in a meta-analysis comprising 9 studies that included a total of 2620 patients.72 Although this drug is not available in the USA or UK owing to negative phase III study results,73 it can be used in Korea.
Representative 5-HT4 receptor agonists, cisapride and tegaserod, have been reported to induce arrhythmias and cardiovascular disease. Therefore, these drugs have been excluded from the current market. Mosapride, another 5-HT4 receptor agonist, does not induce arrhythmia, and promotes GI motility and gastric emptying.74 Similar to itopride, mosapride did not show superior results compared with placebo in a RCT of FD, but in one study it was found to improve overall quality of life.75,76 Furthermore, in a recent meta-analysis with 13 RCTs, mosapride did not show a consistent benefit, although the diagnostic criteria were different for each study.77 Conversely, in a sensitivity meta-analysis of 4 high-quality RCTs, mosapride was found to be an effective drug for FD (RR, 1.114; 95% CI, 1.011–1.227; P =0.029).77 Therefore, considering that serotonin receptor agonists have been effective in previous studies of FD and that mosapride continues to be used in clinical practice with no side effects related to arrhythmia, it could be a good option for the treatment of patients with FD.78–80
Recently, a sustained-release, once-daily mosapride formulation was developed (a reduction from the conventional thrice-daily dosing regimen). According to a recent report, 138 patients were enrolled and divided into 2 groups (a study group and a conventional control group). When the improvement of GI symptoms and side effects were compared, the once-daily sustained-release group was not inferior to the conventional mosapride dosing group. Considering the ease of compliance, the once-dosing medication regimen will be a good choice in the future.81
A meta-analysis in 2007, which tested various prokinetics, showed a 30% higher probability of obtaining a treatment effect with prokinetics as compared with placebo (95% CI, 0.208–0.382; P < 0.001). However, the most widely tested drug was cisapride, which has been discontinued due to its side effects.82 Similarly, in a recent meta-analysis of 38 studies, although with low quality of evidence, prokinetics significantly reduced overall symptoms of FD compared to placebo (NNT = 7). However, there was no difference in quality of life or adverse effect.83 In addition, in a recently reported meta-analysis with 4473 patients in 25 RCTs,83 it was demonstrated that currently used prokinetics were more effective than placebo, using a Bayesian method network analysis. In particular, metoclopramide, trimebutine, mosapride, and domperidone were shown to have better efficacy than itopride or acotiamide, using a league-to-league analysis.84
In addition, DA-9701 (motilitone) is a newly developed prokinetic product that is formulated from the plant extracted of Pharbitidis semen and Corydalis tuber. In a study in which 389 patients were divided into 3 groups (motilitone-treated, PPI-treated, and PPI with motilitone-treated), all 3 groups showed significant improvement in dyspeptic symptoms and in quality of life measurements.85 However, there was no difference among the groups except for the status of H. pylori. Of the H. pylori-positive patients, the improvement of dyspeptic symptoms was significantly higher in the PPI alone group or in the PPI with the motilitone group than in the motilitone alone group.85 For patients with poor symptom control, increasing the dose of prokinetics or combining 2 types of prokinetic agents, such as metoclopramide and domperidone, was found to be effective.86,87 Mechanisms, doses, special comments, and adverse effects of prokinetics for FD are summarized in Table 3.
Table 3.
Prokinetic Drugs Used in the Treatment of Functional Dyspepsia
| Drug | Mechanism of action | Dose | Special comments | Side effects |
|---|---|---|---|---|
| Levosulpiride | Dopamine D2 receptor antagonist, 5-HT4 receptor agonist, weak 5-HT3 receptor antagonist | 25 mg tid | Limited to short duration use for avoiding side effect | Menstrual abnormalities and galactorrhea, drug induced parkinsonism |
| Metoclopramide | Dopamine D2 receptor antagonist, 5-HT4 receptor agonist | 5–10 mg tid (max. 30 mg per day) | Limited to only 5 day- use/ treatment, maximal dose: 0.5 mg/kg per day (both adult and child) | Extrapyramidal symptom, gynecomastia, galactorrhea, menstrual irregularities |
| Domperidone | Dopamine D2 receptor antagonist | 10 mg tid (max. 30 mg in a day) | Limited to one-week use/ treatment | Gynecomastia, galactorrhea, menstrual irregularities |
| Itopride | Dopamine D2 receptor antagonist, inhibition of acetylcholinesterase | 50 mg tid | Not available in the US, UK | Rash, diarrhea, giddiness |
| Mosapride | 5-HT4 receptor agonist | 5 mg tid, 15 mg qd | Sustained-release mosapride (once daily) is available | |
| DA9701 (Motilitone) | 5-HT4, 5-HT1A, and 5-HT1B receptors agonistic effect Dopamine D2 receptor antagonistic effect | 30 mg tid | Plant extract | |
| Acotiamide | M1 and M2 muscarinic receptors antagonist | 100 mg tid | Not available in Korea | Headache, diarrhea |
| Erythromycin | Motilin receptor agonist | 250–500 mg tid | Not available in Korea | Arrhythmia, reversible deafness, abdominal pain, diarrhea |
5-HT, 5-hydroxytryptamine; tid, 3 times a day; qd, once a day.
Statement 8: Prokinetics can be useful as a first-line treatment for FD in patients with PDS.
Grade of recommendation: strong
Level of evidence: low
Experts’ opinions: completely agree, 32.2%; mostly agree, 60.7%; partially agree, 7.1%; mostly disagree, 0.0%; completely disagree, 0.0%; and not sure 0.0%.
According to the recent Rome IV classification, a large number of FD patients have PDS, which causes discomfort after meals or a feeling of early satiation.88 In a large-scale study of mosapride in Japan,78 patients with FD were divided into a gastric stasis symptom group and an EPS group. Administration of mosapride led to a significant improvement in the gastric stasis symptom group. However, the control group, which was treated with teprenone, also showed symptomatic improvement, and this is a limitation of the study. In addition, a recent study that used sustained-release mosapride81 showed no significant difference in GI symptom score improvement in PDS when patients were sub-grouped by EPS and PDS. A meta-analysis of the previously mentioned itopride showed a significant effect on postprandial fullness and early satiation when compared to domperidone. However, no statistical difference was noted when compared to placebo.72
Acotiamide is a newly developed drug that exerts its gastroprokinetic activity by increasing acetylcholine release through antagonism of the M1 and M2 muscarinic receptors in the enteric nervous system and inhibition of acetylcholinesterase activity.89 In one RCT, 4 weeks of acotiamide treatment was more effective compared to placebo for the relief of symptom severity and diet-related symptoms.90 In another study using acotiamide, a significant effect on gastric accommodation and gastric emptying was noted in 34.8% of patients.91 Additionally, in a meta-analysis of 7 RCT studies, acotiamide was shown to be more effective against PDS-related symptoms compared to placebo.92 Acotiamide was also reported to have a significant clinical effect on the quality of life and postprandial symptoms in an open, 3-phase study for long-term outcome and safety.93 Recently, a study comparing PDS- and EPS-type patients with FD showed that acotiamide significantly improved symptoms in both groups, but in a subgroup analysis, it was found to be more effective against the PDS type.94 Based on these results, acotiamide would be a good therapeutic agent for patients who primarily have PDS symptoms. However, acotiamide is not available in Korea, and studies using other prokinetics have been lacking. In the future, randomized or large-scale studies are needed to determine the effects of other prokinetic agents on PDS subtypes.
Erythromycin, a motilin receptor agonist, was shown to be effective in facilitating gastric emptying, and one study showed that it caused improvements in bloating-related symptoms.95 However, a randomized study96 did not show any statistical difference compared to placebo, and erythromycin is not currently being used in Korea for this indication. Although well-planned RCT studies are lacking, prokinetics are considered important for symptomatic improvement in patients with PDS symptoms in Korea.
Statement 9: It is reasonable to determine the administration of dopamine antagonists carefully, because prolonged use or administration of these drugs in some vulnerable patients can cause irreversible adverse events.
Grade of recommendation: strong
Level of evidence: low
Prokinetics that have proven effective over placebo are mostly D2 antagonists. They act on the excitatory motor neurons in the digestive tract. There are issues of clinical safety for these D2 antagonists, and these require the attention of prescribing physicians. Metoclopramide is the most well-known neuroleptic D2 antagonist. It can cause extrapyramidal symptoms and is characterized by an acute dystonic reaction within the first 24 hours to 48 hours with a typical adult dose. The risk of extrapyramidal adverse effects increases with duration of treatment and total cumulative dose. These effects are generally irreversible and more prevalent in adolescent patients.97
Domperidone may cause QT prolongation and thus cardiac arrhythmias may occur consequently. In 2 case-control studies in 2010, domperidone exposures were significantly higher in patients with sudden death or severe ventricular arrhythmias than in controls.98,99 Based on this, the European Medicines Agency recommended limited use of domperidone. In 2014, the Korean Ministry of Food and Drug Safety also sent a letter of safety regarding the use of the drug: the ministry recommended limited use for alleviating nausea or vomiting, emphasizing not to use beyond a dose of 10 mg 3 times a day, for at most 1 week. Concomitant administration of cardiac medications, quinolones, clarithromycin, isoniazid, anti-fungal agents, and fluoxetine, which may cause QT prolongation, was prohibited.
Data on 132 drug-related movement disorders in one institution showed that the majority (68.9%) were related to levosulpiride. Most of the patients were relatively elderly patients, aged 60 years or older, and they had Parkinsonism, face dyskinesia, and isolated tremor after 5–10 months of medication. Symptoms continued in about half of the patients even after discontinuation of the drug.100
Helicobacter pylori Eradication
Statement 10: H. pylori eradication is a reasonable treatment for dyspeptic patients because eradication therapy can provide long-term relief of dyspeptic symptoms.
Grade of recommendation: weak
Level of evidence: high
Experts’ opinions: completely agree, 18.5%; mostly agree, 55.6%; partially agree, 25.9%; mostly disagree, 0.0%; completely disagree, 0.0%; and not sure 0.0%.
In 2 meta-analyses of the RCTs, a small but statistically significant improvement in the long-term (6 months to 12 months) was observed in the H. pylori eradication group,17,101 but the effect was not significant in the short-term (3 months).101 One, including 14 RCTs showed that the improvement in dyspeptic symptoms was better in the eradication group than that in the control group after 12 months;17 the other meta-analysis, including 25 RCTs demonstrated the conflicting results.101 In addition, the side effects were significantly higher in the eradication group and the quality of life was not significantly improved.101 The European, USA, and Canadian guidelines strongly recommend eradicating H. pylori as the primary treatment for dyspepsia.12,36
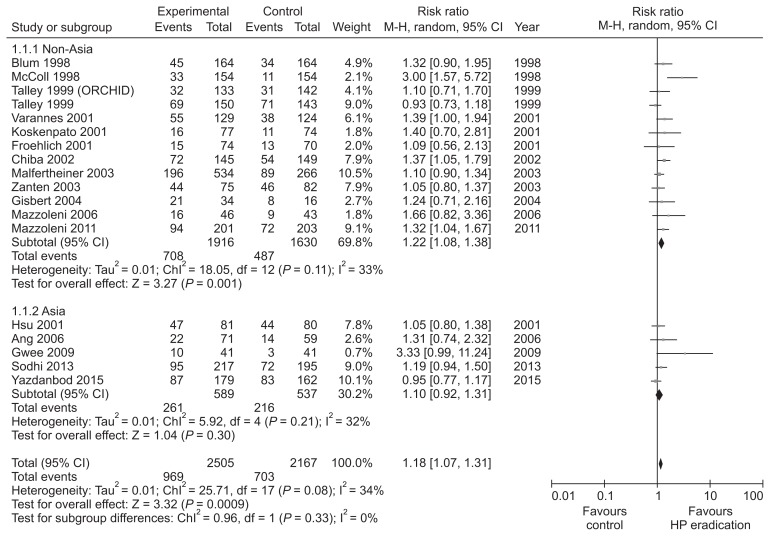
We performed meta-analysis of 18 RCTs from January 1997 to December 2017, evaluating the long-term (more than 6 months) effect of H. pylori eradication in dyspeptic patients (Table 4 and Supplementary Fig. 4 and 5).102–119 There was a statistically significant improvement of dyspeptic symptoms in H. pylori eradication group (RR dyspepsia improvement = 1.18; 95% CI, 1.07–1.31). Heterogeneity among studies was moderate but significant (χ2 = 25.7; P = 0.08; I2 = 34%) (NNT = 14) (Fig. 5). Because of significant heterogeneity among studies, we performed subgroup analysis according to the regions; 5 RCTs from Asia and 13 RCTs from regions outside Asia. H. pylori eradication therapy induced statistically significant improvement of dyspeptic symptom (RR dyspepsia improvement = 1.22; 95% CI, 1.08–1.38) without significant heterogeneity (χ2 = 18.1; P = 0.11; I2 = 33%) in studies from regions outside Asia. However, meta-analysis of studies from Asia revealed that impact of H. pylori eradication therapy on dyspeptic symptom was not statistically significant (RR dyspepsia improvement = 1.10; 95% CI, 0.92–1.31) and heterogeneity among studies was insignificant (χ2 = 5.9; P = 0.21; I2 = 32%). Overall, the effectiveness of H. pylori eradication in FD patients was small but statistically significant. Therefore, eradication therapy can be considered for dyspeptic patients. However, NNT was not small and subgroup analysis from studies conducted in Asia was not statistically significant. The prevalence of H. pylori in Korea is estimated as 54% (95% CI, 50.1–57.8).4 In areas with high H. pylori prevalence, the cost and side effects associated with eradication therapy, the risk of emergence of resistant bacteria, and the risk of reinfection are expected to be higher than in areas with low H. pylori prevalence. Therefore, further well-designed RCTs on the efficacy of H. pylori eradication in FD patients and the benefit and risk of eradication therapy in high H. pylori prevalence areas should be performed.
Table 4.
The Characteristics of the Studies Included in the Meta-analysis of Effect of Helicobacter pylori Eradication
| Studies | Country | H. pylori prevalence | Arms (regimens) | No. of patients | Mean age (yr) | Eradication rate (%) | Follow-up | Adverse event (n [%]) |
|---|---|---|---|---|---|---|---|---|
| McColl et al,102 1998 | UK | 35.5% | OAM | 160 | 42.0 ± 12.0 | 85.0 | 12 mo | NA |
| O | 158 | 42.2 ± 13.0 | 12.0 | 12 mo | NA | |||
| Blum et al,103 1998 | Austria, Canada, Germany, Iceland, Ireland, Sweden, South Africa | 41.6% (mean) | OAC | 164 | 47.0 | 79.0 | 12 mo | 12 (7.0) |
| O | 164 | 47.0 | 2.0 | 12 mo | 2 (1.0) | |||
| Talley et al,104 1999 | US | 35.6% | OAC | 150 | 46.3 | 90.0 | 12 mo | NA |
| Placebo | 143 | 46.5 | 2.0 | 12 mo | NA | |||
| Talley et al,105 1999 (ORCHID) | Australia, New Zealand, Europe | 28.0% (mean) | OAC | 133 | 51.0 | 85.0 | 12 mo | NA |
| Placebo | 142 | 47.0 | 4.0 | 12 mo | NA | |||
| Koskenpato et al,106 2001 | Finland | 56.8% | OAM | 77 | 51.5 ± 9.5 | 82.0 | 12 mo | NA |
| O | 74 | 51.8 ± 11.8 | 1.0 | 12 mo | NA | |||
| Varannes et al,108 2001 | France | 46.9% | RAC | 129 | 50.0 ± 16.0 | 69.0 | 12 mo | 36 (28.0) |
| Placebo | 124 | 52.0 ± 14.0 | 18.0 | 12 mo | 12 (10.0) | |||
| Froehlich et al,109 2001 | Switzerland | 18.9% | LAC | 92 | 43.6 ± 12.4 | 75.0 | 12 mo | NA |
| L | 88 | 45.6 ± 14.2 | 4.0 | 12 mo | NA | |||
| Hsu et al,107 2001 | Taiwan | 53.9% | LMTe | 81 | 50.3 ± 15.1 | 78.0 | 12 mo | NA |
| L | 80 | 51.6 ± 16.4 | 0.0 | 12 mo | NA | |||
| Chiba et al,110 2002 | Canada | 38.0% | OCM | 145 | 50 (18–82) | 75.0 | 12 mo | 61 (42.0) |
| O | 149 | 49 (19–81) | 14.0 | 12 mo | 62 (42.0) | |||
| Zanten et al,111 2003 | Canada | 38.0% | LAC | 75 | 47.0 ± 13.0 | 82.0 | 12 mo | NA |
| Placebo | 82 | 49.0 ± 13.0 | 6.0 | 12 mo | NA | |||
| Malfertheiner et al,112 2003 | Germany | 35.3% | LAC | 247 (30 mg, n-124; 15 mg, n = 123) | 46.1 ± 12.8 (30 mg) | 65.6 (30 mg) | 12 mo | 18 (7.0) (30 mg) |
| 46.9 ± 12.0 (15 mg) | 62.1 (15 mg) | 13 (5.0) (15 mg) | ||||||
| L | 133 | 45.5 ± 12.6 | 4.5 | 12 mo | 15 (6.0) | |||
| Gisbert et al,113 2004 | Spain | 54.9% | OAC | 34 | 42.0 | 76.0 | 12 mo | NA |
| R | 16 | 41.0 | 0.0 | 12 mo | NA | |||
| Mazzoleni et al,114 2006 | Brazil | 71.2% | LAC | 46 | 43.2 ± 11.9 | 91.3 | 12 mo | NA |
| L | 45 | 39.2 ± 13.8 | 0.0 | 12 mo | NA | |||
| Ang,115 2006 | Singapore | 40.8% | LAC | 71 | 38.6 | 73.2 | 52 wk | 4 (6.0) |
| Prokinetic 6wk | 59 | 38.4 | 0.0 | 52 wk | 3 (5.0) | |||
| Gwee,116 2009 | Singapore | 40.8% | OCTi | 41 | 44.7 ± 11.4 | 68.3 | 12 mo | NA |
| Placebo | 41 | 36.1 ± 12.1 | 4.9 | 12 mo | NA | |||
| Mazzoleni et al,117 2011 | Brazil | 71.2% | OAC | 201 | 46.1 ± 12.4 | 88.6 | 12 mo | 172 (93.0) |
| 203 | 46.0 ± 12.2 | 7.4 | 12 mo | 146 (82.0) | ||||
| Sodhi et al,118 2013 | India | 63.5% | OAC | 259 | 46 (25–65) | 69.9 | 12 mo | NA |
| O | 260 | 43 (20–68) | 5.0 | 12 mo | NA | |||
| Yazdanbod et al,119 2015 | Iran | 59.0% | OACB | 186 | 36.8 | 87.1 | 12 mo | NA |
| Omeprazole | 173 | 36.8 | 2.9 | 12 mo | NA |
H. pylori, Helicobacter pylori; O, omeprazole; A, amoxicillin; M, metronidazole; C, clarithromycin; R, ranitidine; L, lansoprazole; Te, Tetracycline; Ti, tinidazole; B, bismuth; NA, not applicable.
Data of mean age are presented as mean ± SD, mean, or mean (range).
Figure 5.
Forest plot of randomized controlled trials comparing Helicobacter pylori eradication with placebo antibiotics in H. pylori infected patients with functional dyspepsia. HP, Helicobacter pylori.
Among dyspeptic patients, factors that predict good response to H. pylori eradication include an onset of indigestion within 5 years, erosive gastritis or erosive duodenitis, and microscopic gastritis above moderate degree.102,103 However, the relationships between these factors and effectiveness of eradication therapy are discordant among studies. Therefore, further researches are also needed in this area.
Fundic Relaxants
Statement 11: Fundic relaxant drugs may be effective in improving generalized dyspeptic symptoms, postprandial fullness, and early satiation.
Grade of recommendation: weak
Level of evidence: moderate
Impaired relaxation of the proximal part of the stomach to accommodate a meal is present in 40.0% of patients with FD and is a pathophysiological mechanism that is associated with symptoms such as early satiation and weight loss.120 Buspirone and tandospirone are 5-HT1 receptor agonists and have fundic relaxation effects.121 In a randomized, double-blind, crossover study, buspirone significantly increased gastric accommodation after 4 weeks of treatment. It significantly reduced the overall severity of dyspeptic symptoms and individual symptoms of postprandial fullness, early satiation, and abdominal bloating.122 In a double-blind, placebo-controlled study, tandospirone significantly improved total abdominal symptom scores and upper abdominal pain in patients with FD.123 5-HT4 receptor agonists such as cisapride, tegaserod, and mosapride citrate can enhance meal-induced gastric accommodation and improve the symptoms of some patients with FD.74,124–126 Acotiamide acts as a muscarinic receptor antagonist and cholinesterase antagonist, improves gastric emptying, and enhances fundic relaxation. In an RCT that used real-time ultrasonography, acotiamide significantly enhanced postprandial gastric accommodation reflex in patients with FD.127 A placebo-controlled study that used gastric scintigraphy demonstrated that acotiamide significantly increased gastric accommodation and improved overall GI symptoms and anxiety scores.91 Some antidepressants also have fundic relaxant activity and improve gastric accommodation. In a double-blind, placebo-controlled study, amitriptyline and escitalopram were administered for 12 weeks, and single-photon emission computed tomography imaging was used to measure the gastric accommodation. These drugs significantly improved gastric accommodation; however, further studies of the precise mechanism of action are needed.128
Antidepressants
Statement 12: TCAs may be effective in functional dyspeptic patients who do not respond to conventional therapy.
Grade of recommendation: weak
Level of evidence: moderate
Experts’ opinions: completely agree, 34.6%; mostly agree, 53.9%; partially agree, 11.5%; mostly disagree, 0.0%; completely disagree, 0.0%; and not sure, 0.0%.
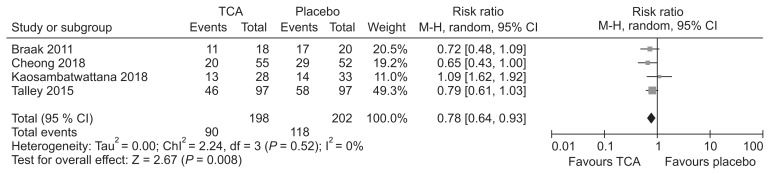
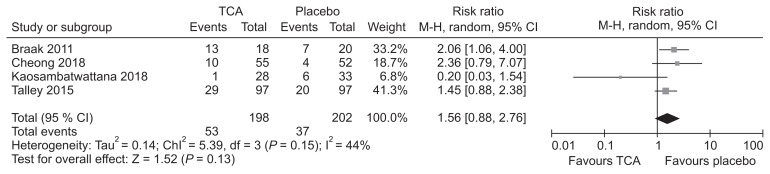
TCAs are used clinically to improve symptoms in patients with functional GI disorders. A recent guideline from Korea recommended that TCAs can be considered for symptom improvement in IBS patients.11 Recently, 2 systematic reviews compared TCAs with placebo in patients with FD.129,130 The first review included 4 RCTs and found that administration of TCAs was associated with reduced number of patients showing no improvement in symptoms compared with placebo (RR, 0.76; 95% CI, 0.62–0.94) with an NNT of 7 (95% CI, 4–26).129 The second review included 3 RCTs and found TCAs to be effective in reducing dyspeptic symptoms (RR, 0.74; 95% CI, 0.61–0.91) with an NNT of 6 (95% CI, 6–18).130 Our systematic review identified one RCT131 that was not included in the previous review. Meta-analysis including this study found TCAs to be effective in symptom improvement compared to placebo (RR, 0.78; 95% CI, 0.64–0.93; P = 0.008) (Fig. 6 and Supplementary Fig. 6–8). Data pooled from these studies found that the incidence of adverse effects were not higher among those taking TCAs (RR, 1.56; 95% CI, 0.88–2.76; P = 0.13) (Fig. 7).
Figure 6.
Forest plot of randomized controlled trials comparing tricyclic antidepressants (TCAs) with placebo in patients with functional dyspepsia.
Figure 7.
Forest plot of adverse events in randomized controlled trials comparing tricyclic antidepressants (TCAs) with placebo in patients with functional dyspepsia.
Two RCTs from Asia compared TCAs with placebo for patients with refractory FD.131,132 The first study found imipramine to be effective in relief of dyspeptic symptoms after 12 weeks of administration, compared with placebo.131 However, the other study reported that nortriptyline failed to achieve reduction in dyspeptic symptoms after 8 weeks of treatment.132 There was a research showing that TCAs were more effective in treating ulcer-like FD, corresponding to the EPS type, than in dysmotility-like FD.133 In summary, TCAs may be effective in treatment of refractory FD, especially for patients with the EPS type. Although there was no statistically significant difference in adverse events between TCAs and placebo in our study, patients should be cautioned about the adverse event profiles. In addition, further studies are needed to evaluate the efficacy of TCAs in Asian patients with FD.
Gastroprotective Agents and Others
In a recent meta-analysis of 17 RCTs, rebamipide, which is a quinolone derivative, improved dyspeptic symptoms by 23.0% compared to placebo or the control group when the symptomatic improvement was expressed as dichotomous outcomes. Especially in patients with organic dyspepsia, symptoms decreased by 28.0%, which was statistically significant, but there was no significant improvement in FD.134 The mechanism by which rebamipide decreases dyspeptic symptoms seems to be that it improves chronic gastritis. When symptom score was used as the outcome variable, symptoms were improved in FD as well as in organic dyspepsia.
Sucralfate is an antacid, and 2 placebo-controlled studies have been reported for the management of non-ulcer dyspepsia with sucralfate. Sucralfate administration for 3 weeks was not effective in improving symptoms when compared to placebo in one study.135 In contrast, sucralfate administration for 4 weeks showed significant symptom improvement compared to placebo in another study.136 In an analysis of 2 studies, sucralfate improved symptoms but the improvement was not statistically significant.137
There were 2 studies on simethicone, an anti-flatulence agent. In a 4-week randomized comparison of simethicone (80 mg 3 times a day) and cisapride (10 mg 3 times a day), simethicone was more effective than cisapride for relieving bloating at 2 and 4 weeks and for relieving reflux only at 2 weeks.138 Based on this study, a placebo-controlled study was conducted, which was an 8-week clinical trial with a slight increase in simethicone dose (105 mg 3 times daily).139 Simethicone and cisapride improved symptoms when compared with placebo in FD patients, and simethicone showed better symptom improvement in the first 2 weeks as compared with cisapride. Simethicone has been known as a drug that reduces GI gas. Although its mechanism of action on FD is not known precisely, simethicone seems to decrease the surface tension of air bubbles that are not absorbed in the intestine.
Psychological Therapies
Statement 13: Psychological therapies can be considered when drug therapies are ineffective in improving symptoms in patients with FD.
Grade of recommendation: weak
Level of evidence: moderate
Patients of FD had higher psychological problems such as anxiety, depression, and psychological distress than those with no FD as found in a study.140 Psychological therapies for patients with FD have included dynamic psychotherapy, hypnotherapy, behavioral treatments, and cognitive-behavioral therapy.141–143 A systematic review showed insufficient evidence on the efficacy of psychotherapies in nonulcer dyspepsia although in one trial, hypnotherapy was significantly superior to the control.141,144
A prospective RCT showed that 4 month-intensified medical management (medical therapy with testing-for and targeting-of abnormalities of motor and sensory function) with psychological intervention was significantly beneficial in reducing dyspepsia symptoms that did not respond to conventional therapy. Additional cognitive behavioral therapy may be especially effective for control of concomitant anxiety and depression.145 Another RCT of a 10-week group psychotherapy in combination with standard medical treatment showed significantly improved dyspeptic symptoms and dyspepsia-related quality of life, compared with medical therapy alone.146
A recent systematic review showed a significant benefit of psychological therapies in reducing dyspepsia symptoms (RR, 0.53; 95% CI, 0.44–0.65) with an NNT of 3. The review included studies that described the outcome in terms of a dichotomous improvement in dyspepsia symptoms in 789 FD patients.36 The outcomes of psychological intervention had negative association with poor sleep quality (OR, 7.68; 95% CI, 1.83–32.25) and bad marriage status (OR, 1.22; 95% CI, 1.10–1.36), but positive association with extroversion in personality traits (OR, 0.86; 95% CI, 0.76–0.96).147 Considering all these, psychological therapies can be considered in severely affected FD patients that are not responding to drug therapies, especially if the symptoms may be related to psychological factors.
Diet
Statement 14: Dietary modification may be effective for symptom relief in patients with FD.
Grade of recommendation: weak
Level of evidence: low
Although dietary factor is considered to have an important role in patients with FD, studies on the causal relationship of specific foods with FD are still lacking and inconsistent. Generally, it is desirable to avoid foods that induce dyspeptic symptoms. Fatty foods particularly exacerbate or induce dyspeptic symptoms. Intraduodenal lipid increases sensitivity to gastric distension and induces abdominal fullness and discomfort in patients with FD.148 There was a trend for a reduction in fat intake in FD patients, and post-prandial fullness and bloating were related directly to high fat intake.149 High fat intake was also found to induce nausea and abdominal pain in FD patients more than in healthy controls.150 Milk and dairy food, wheat-containing food and spicy food may also provoke dyspeptic symptoms.151,152 Carbonated drinks and coffee are associated with dyspeptic symptoms.152,153 However, there are no clear evidences of a role for dietary intervention with respect to these specific foods for the purpose of dyspeptic symptom relief, and well-designed studies are needed.
Conclusions
Dyspepsia is a common clinical problem. GI endoscopy should be performed in patients older than 40 years to rule out organic cause, especially gastric cancer. If the symptoms become chronic or repeat after the elimination of the underlying disease, patients with FD should be treated with PPIs, especially in EPS subtypes. Although H2RAs were shown to be as effective, their effectiveness is unknown for long-term use. Prokinetics such as dopamine D2 antagonists and 5-HT4 receptor agonists could be used for patients with FD, especially in PDS subtypes. Some D2 antagonists need to be used carefully as they can cause adverse reactions during long-term use. Meta-analysis shows significant but modest efficacy of H. pylori eradication on long-term resolution of FD symptom. H. pylori eradication therapy can be applied in case if PPIs and prokinetics are not effective, or in young patients with chronic dyspeptic symptoms in Korea. TCAs may be effective in treatment of non-responder to conventional management of FD, especially for patients with the EPS type. Since the pathophysiology of FD is diverse, it is necessary to elaborate on drug therapy, stress management, and dietary education in order to avoid the recurrence of symptoms. In addition, detailed guidance and education on the disease will be helpful for proper treatment and long-term management.
Supplementary Materials
Note: To access the supplementary tables and figures mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm19209.
Supplementary Information
Acknowledgements
We are grateful to all who participated in this study. We thank Prof. Moo In Park and Prof. Suck Chei Choi for working as external judges. In addition, we also thank Prof. Ein-Soon Shin for helping us develop the guidelines.
Footnotes
Financial support: None.
Conflicts of interest: None.
Author Contributions: Jung Hwan Oh, Joong Goo Kwon, and Hye-Kyung Jung have contributed in writing and editing the paper as the first author and the corresponding author; Kyung Ho Song, Seung Joo Kang, Sung Eun Kim, Joon Sung Kim, Jong Kyu Park, Ki Bae Bang, Myong Ki Baeg, Jeong Eun Shin, Cheol Min Shin, Ju Yup Lee, Hyun Chul Lim, Kyoungwon Jung, and Chung Hyun Tae have contributed in the systematic review, the extraction of recommendations, and writing the paper; and Hye-Kyung Jung has designed the development of guideline as the chairman of the committee.
References
- 1.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 2.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology. 2016;150:1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 3.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooi JKY, Lai WY, Ng WK, et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153:420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 5.Kim BJ, Kim HS, Song HJ, et al. Online registry for nationwide database of current trend of Helicobacter pylori eradication in Korea: interim analysis. J Korean Med Sci. 2016;31:1246–1253. doi: 10.3346/jkms.2016.31.8.1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bak YT. [Evidence based guideline for diagnosis and treatment: Therapeutic guideline for GERD]. Kor J Neurogastroenterol Motil. 2005;11:13–17. [Korean] [Google Scholar]
- 7.Jee SR, Jung HK, Min BH, et al. [Guidelines for the treatment of functional dyspepsia]. Korean J Gastroenterol. 2011;57:67–81. doi: 10.4166/kjg.2011.57.2.67. [Korean] [DOI] [PubMed] [Google Scholar]
- 8.Higgins JP, Altman DG, Gøtzsche PC, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mühlbacher AC, Sadler A, Kaczynski A. Patients’ preferences in functional dyspepsia and motility disorder: a discrete-choice experiment. Value in Health. 2016;19:A316. doi: 10.1016/j.jval.2016.03.963. [DOI] [Google Scholar]
- 10.Schünemann H, Brożek J, Guyatt G, Oxman A. [accessed 9 December, 2019];GRADE handbook. Available from URL: https://gdt.gradepro.org/app/handbook/hand-book.html.
- 11.Song KH, Jung HK, Kim HJ, et al. Clinical practice guidelines for irritable bowel syndrome in Korea, 2017 revised edition. J Neurogastroenterol Motil. 2018;24:197–215. doi: 10.5056/jnm17145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection-the maastricht v/florence consensus report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 13. [accessed 9 Desember, 2019];Gastro-oesophageal reflux disease and dyspepsia in adults: investigation and management. Available from URL: https://www.nice.org.uk/guidance/cg184. [PubMed]
- 14.Miwa H, Kusano M, Arisawa T, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol. 2015;50:125–139. doi: 10.1007/s00535-014-1022-3. [DOI] [PubMed] [Google Scholar]
- 15.Talley NJ, Lam SK, Goh KL, Fock KM. Management guidelines for uninvestigated and functional dyspepsia in the Asia-Pacific region: first Asian Pacific working party on functional dyspepsia. J Gastroenterol Hepatol. 1998;13:335–353. doi: 10.1111/j.1440-1746.1998.tb00644.x. [DOI] [PubMed] [Google Scholar]
- 16.Talley NJ, Vakil N. Guidelines for the management of dyspepsia. Am J Gastroenterol. 2005;100:2324–2337. doi: 10.1111/j.1572-0241.2005.00225.x. [DOI] [PubMed] [Google Scholar]
- 17.Zhao B, Zhao J, Cheng WF, et al. Efficacy of Helicobacter pylori eradication therapy on functional dyspepsia: a meta-analysis of randomized controlled studies with 12-month follow-up. J Clin Gastroenterol. 2014;48:241–247. doi: 10.1097/MCG.0b013e31829f2e25. [DOI] [PubMed] [Google Scholar]
- 18.Lassen AT, Hallas J, Schaffalitzky de Muckadell OB. Helicobacter pylori test and eradicate versus prompt endoscopy for management of dyspeptic patients: 6.7 year follow up of a randomised trial. Gut. 2004;53:1758–1763. doi: 10.1136/gut.2004.043570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lassen AT, Pedersen FM, Bytzer P, Schaffalitzky de Muckadell OB. Helicobacter pylori test-and-eradicate versus prompt endoscopy for management of dyspeptic patients: a randomised trial. Lancet. 2000;356:455–460. doi: 10.1016/S0140-6736(00)02553-8. [DOI] [PubMed] [Google Scholar]
- 20.Bytzer P, Hansen JM, Schaffalitzky de Muckadell OB. Empirical H2-blocker therapy or prompt endoscopy in management of dyspepsia. Lancet. 1994;343:811–816. doi: 10.1016/S0140-6736(94)92023-0. [DOI] [PubMed] [Google Scholar]
- 21.Laheij RJ, Severens JL, Van de Lisdonk EH, Verbeek AL, Jansen JB. Randomized controlled trial of omeprazole or endoscopy in patients with persistent dyspepsia: a cost-effectiveness analysis. Aliment Pharmacol Ther. 1998;12:1249–1256. doi: 10.1046/j.1365-2036.1998.00423.x. [DOI] [PubMed] [Google Scholar]
- 22.Li XB, Liu WZ, Ge ZZ, Chen XY, Shi Y, Xiao SD. Helicobacter pylori “test-and-treat” strategy is not suitable for the management of patients with uninvestigated dyspepsia in Shanghai. Scand J Gastroenterol. 2005;40:1028–1031. doi: 10.1080/00365520510023206. [DOI] [PubMed] [Google Scholar]
- 23.Liou JM, Lin JT, Wang HP, et al. The optimal age threshold for screening upper endoscopy for uninvestigated dyspepsia in Taiwan, an area with a higher prevalence of gastric cancer in young adults. Gastrointest Endosc. 2005;61:819–825. doi: 10.1016/S0016-5107(05)00366-4. [DOI] [PubMed] [Google Scholar]
- 24.Shin A, Kim J, Park S. Gastric cancer epidemiology in Korea. J Gastric Cancer. 2011;11:135–140. doi: 10.5230/jgc.2011.11.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Song M, Kang D, Yang JJ, et al. Age and sex interactions in gastric cancer incidence and mortality trends in Korea. Gastric Cancer. 2015;18:580–589. doi: 10.1007/s10120-014-0411-x. [DOI] [PubMed] [Google Scholar]
- 26.Hwang IR, Kim JH, Lee KJ, Cho SW. Can Helicobacter pylori serology predict non-ulcer dyspepsia in young dyspeptic patients? Korean J Gastrointest Endosc. 2000;21:696–703. [Google Scholar]
- 27.Talley NJ, Ford AC. Functional dyspepsia. N Engl J Med. 2015;373:1853–1863. doi: 10.1056/NEJMra1501505. [DOI] [PubMed] [Google Scholar]
- 28.Suzuki H, Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:168–174. doi: 10.1038/nrgastro.2013.9. [DOI] [PubMed] [Google Scholar]
- 29.Delaney B, Ford AC, Forman D, Moayyedi P, Qume M. Initial management strategies for dyspepsia. Cochrane Database Syst Rev. 2005;19:CD001961. doi: 10.1002/14651858.CD001961.pub2. [DOI] [PubMed] [Google Scholar]
- 30.Harvey RF, Lane JA, Nair P, et al. Clinical trial: prolonged beneficial effect of Helicobacter pylori eradication on dyspepsia consultations - the Bristol Helicobacter Project. Aliment Pharmacol Ther. 2010;32:394–400. doi: 10.1111/j.1365-2036.2010.04363.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee JH, Choi KD, Jung HY, et al. Seroprevalence of Helicobacter pylori in Korea: a multicenter, nationwide study conducted in 2015 and 2016. Helicobacter. 2018;23:e12463. doi: 10.1111/hel.12463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eusebi LH, Zagari RM, Bazzoli F. Epidemiology of Helicobacter pylori infection. Helicobacter. 2014;19(suppl 1):1–5. doi: 10.1111/hel.12165. [DOI] [PubMed] [Google Scholar]
- 33.Talley NJ, Vakil NB, Moayyedi P. American gastroenterological association technical review on the evaluation of dyspepsia. Gastroenterology. 2005;129:1756–1780. doi: 10.1053/j.gastro.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuyzen van Zanten SJ, Bradette M, Chiba N, et al. Evidence-based recommendations for short- and long-term management of uninvestigated dyspepsia in primary care: an update of the Canadian Dyspepsia Working Group (CanDys) clinical management tool. Can J Gastroenterol. 2005;19:285–303. doi: 10.1155/2005/674607. [DOI] [PubMed] [Google Scholar]
- 35.Moayyedi P, Talley NJ, Fennerty MB, Vakil N. Can the clinical history distinguish between organic and functional dyspepsia? JAMA. 2006;295:1566–1576. doi: 10.1001/jama.295.13.1566. [DOI] [PubMed] [Google Scholar]
- 36.Moayyedi PM, Lacy BE, Andrews CN, Enns RA, Howden CW, Vakil N. ACG and CAG clinical guideline: management of dyspepsia. Am J Gastroenterol. 2017;112:988–1013. doi: 10.1038/ajg.2017.154. [DOI] [PubMed] [Google Scholar]
- 37.Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in korea, 2018. Cancer Res Treat. 2018;50:317–323. doi: 10.4143/crt.2018.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen SL, Gwee KA, Lee JS, et al. Systematic review with meta-analysis: prompt endoscopy as the initial management strategy for uninvestigated dyspepsia in Asia. Aliment Pharmacol Ther. 2015;41:239–252. doi: 10.1111/apt.13028. [DOI] [PubMed] [Google Scholar]
- 39.Miwa H, Ghoshal UC, Fock KM, et al. Asian consensus report on functional dyspepsia. J Gastroenterol Hepatol. 2012;27:626–641. doi: 10.1111/j.1440-1746.2011.07037.x. [DOI] [PubMed] [Google Scholar]
- 40.Park JS, Park DI, Park SK, et al. Endoscopic evaluation of significant gastrointestinal lesions in patients with iron deficiency with and without anaemia: a Korean association for the study of intestinal disease study. Intern Med J. 2009;39:441–446. doi: 10.1111/j.1445-5994.2008.01785.x. [DOI] [PubMed] [Google Scholar]
- 41.Qu XH, Huang XL, Xiong P, et al. Does Helicobacter pylori infection play a role in iron deficiency anemia? A meta-analysis. World J Gastroenterol. 2010;16:886–896. doi: 10.3748/wjg.v16.i7.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goddard AF, James MW, McIntyre AS, Scott BB. Guidelines for the management of iron deficiency anaemia. Gut. 2011;60:1309–1316. doi: 10.1136/gut.2010.228874. [DOI] [PubMed] [Google Scholar]
- 43.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection--the maastricht IV/ florence consensus report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 44.Ryu E, Kim K, Cho MS, Kwon IG, Kim HS, Fu MR. Symptom clusters and quality of life in Korean patients with hepatocellular carcinoma. Cancer Nurs. 2010;33:3–10. doi: 10.1097/NCC.0b013e3181b4367e. [DOI] [PubMed] [Google Scholar]
- 45.Son HJ, Rhee PL, Kim JJ, Koh KC, Paik SW, Rhee JC. Hypersensitivity to acid in ulcer-like functional dyspepsia. Korean J Intern Med. 1997;12:188–192. doi: 10.3904/kjim.1997.12.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samsom M, Verhagen MA, vanBerge Henegouwen GP, Smout AJ. Abnormal clearance of exogenous acid and increased acid sensitivity of the proximal duodenum in dyspeptic patients. Gastroenterology. 1999;116:515–520. doi: 10.1016/S0016-5085(99)70171-X. [DOI] [PubMed] [Google Scholar]
- 47.Lee KJ, Tack J. Duodenal implications in the pathophysiology of functional dyspepsia. J Neurogastroenterol Motil. 2010;16:251–257. doi: 10.5056/jnm.2010.16.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinto-Sanchez MI, Yuan Y, Hassan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;11:CD011194. doi: 10.1002/14651858.CD011194.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gerson LB, Triadafilopoulos G. A prospective study of oesophageal 24-h ambulatory pH monitoring in patients with functional dyspepsia. Dig Liver Dis. 2005;37:87–91. doi: 10.1016/j.dld.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 50.van Zanten SV, Armstrong D, Chiba N, et al. Esomeprazole 40 mg once a day in patients with functional dyspepsia: the randomized, placebo-controlled “ENTER” trial. Am J Gastroenterol. 2006;101:2096–2106. doi: 10.1111/j.1572-0241.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 51.Talley NJ, Vakil N, Lauritsen K, et al. Randomized-controlled trial of esomeprazole in functional dyspepsia patients with epigastric pain or burning: does a 1-week trial of acid suppression predict symptom response? Aliment Pharmacol Ther. 2007;26:673–682. doi: 10.1111/j.1365-2036.2007.03410.x. [DOI] [PubMed] [Google Scholar]
- 52.van Rensburg C, Berghöfer P, Enns R, et al. Efficacy and safety of pantoprazole 20 mg once daily treatment in patients with ulcer-like functional dyspepsia. Curr Med Res Opin. 2008;24:2009–2018. doi: 10.1185/03007990802184545. [DOI] [PubMed] [Google Scholar]
- 53.Fletcher J, Derakhshan MH, Jones GR, Wirz AA, McColl KE. BMI is superior to symptoms in predicting response to proton pump inhibitor: randomised trial in patients with upper gastrointestinal symptoms and normal endoscopy. Gut. 2011;60:442–448. doi: 10.1136/gut.2010.228064. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki H, Kusunoki H, Kamiya T, et al. Effect of lansoprazole on the epigastric symptoms of functional dyspepsia (ELF study): a multicentre, prospective, randomized, double-blind, placebo-controlled clinical trial. United European Gastroenterol J. 2013;1:445–452. doi: 10.1177/2050640613510904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Iwakiri R, Tominaga K, Furuta K, et al. Randomised clinical trial: rabeprazole improves symptoms in patients with functional dyspepsia in Japan. Aliment Pharmacol Ther. 2013;38:729–740. doi: 10.1111/apt.12444. [DOI] [PubMed] [Google Scholar]
- 56.Majewski M, Sarosiek I, Cooper CJ, et al. Gastric pH and therapeutic responses to exsomeprazole in patients with functional dyspepsia: potential clinical implications. Am J Med Sci. 2016;352:582–592. doi: 10.1016/j.amjms.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 57.Talley NJ, Meineche-Schmidt V, Paré P, et al. Efficacy of omeprazole in functional dyspepsia: double-blind, randomized, placebo-controlled trials (the Bond and Opera studies) Aliment Pharmacol Ther. 1998;12:1055–1065. doi: 10.1046/j.1365-2036.1998.00410.x. [DOI] [PubMed] [Google Scholar]
- 58.Miwa H, Ghoshal UC, Gonlachanvit S, et al. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012;18:150–168. doi: 10.5056/jnm.2012.18.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miwa H, Kusano M, Arisawa T, et al. Evidence-based clinical practice guidelines for functional dyspepsia. J Gastroenterol. 2015;50:125–139. doi: 10.1007/s00535-014-1022-3. [DOI] [PubMed] [Google Scholar]
- 60.Syam AF, Simadibrata M, Makmun D, et al. National consensus on management of dyspepsia and Helicobacter pylori infection. Acta Med Indones. 2017;49:279–287. [PubMed] [Google Scholar]
- 61.Moayyedi P, Delaney BC, Vakil N, Forman D, Talley NJ. The efficacy of proton pump inhibitors in nonulcer dyspepsia: a systematic review and economic analysis. Gastroenterology. 2004;127:1329–1337. doi: 10.1053/j.gastro.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 62.Pinto-Sanchez MI, Yuan Y, Hanssan A, Bercik P, Moayyedi P. Proton pump inhibitors for functional dyspepsia. Cochrane Database Syst Rev. 2017;3:CD011194. doi: 10.1002/14651858.CD011194.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Talley NJ, Goodsall T, Potter M. Functional dyspepsia. Aust Prescr. 2017;40:209–213. doi: 10.18773/austprescr.2017.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tack J, Bisschops R, Sarnelli G. Pathophysiology and treatment of functional dyspepsia. Gastroenterology. 2004;127:1239–1255. doi: 10.1053/j.gastro.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 65.Moayyedi P, Soo S, Deeks J, Delaney B, Innes M, Forman D. Pharmacological interventions for non-ulcer dyspepsia. Cochrane Database Syst Rev. 2006;18:CD001960. doi: 10.1002/14651858.CD001960.pub3. [DOI] [PubMed] [Google Scholar]
- 66.Miner PB, Jr, Allgood LD, Grender JM. Comparison of gastric pH with omeprazole magnesium 20.6 mg (Prilosec OTC) o.m. famotidine 10 mg (Pepcid AC) b.d. and famotidine 20 mg b.d. over 14 days of treatment. Aliment Pharmacol Ther. 2007;25:103–109. doi: 10.1111/j.1365-2036.2006.03129.x. [DOI] [PubMed] [Google Scholar]
- 67.McRorie JW, Kirby JA, Miner PB. Histamine2-receptor antagonists: rapid development of tachyphylaxis with repeat dosing. World J Gastrointest Pharmacol Ther. 2014;5:57–62. doi: 10.4292/wjgpt.v5.i2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hojo M, Nagahara A, Asaoka D, et al. A randomized, double-blind, pilot study of the effect of famotidine on acotiamide treatment for functional dyspepsia. Digestion. 2017;96:5–12. doi: 10.1159/000477345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Park KH, Pai J, Song DG, et al. Ranitidine-induced anaphylaxis: clinical features, cross-reactivity, and skin testing. Clin Exp Allergy. 2016;46:631–639. doi: 10.1111/cea.12708. [DOI] [PubMed] [Google Scholar]
- 70.Veldhuyzen van Zanten SJ, Jones MJ, Verlinden M, Talley NJ. Efficacy of cisapride and domperidone in functional (nonulcer) dyspepsia: a meta-analysis. Am J Gastroenterol. 2001;96:689–696. doi: 10.1016/S0002-9270(00)02314-5. [DOI] [PubMed] [Google Scholar]
- 71.Quigley EM. Prokinetics in the management of functional gastrointestinal disorders. J Neurogastroenterol Motil. 2015;21:330–336. doi: 10.5056/jnm15094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang X, Lv B, Zhang S, Fan YH, Meng LN. Itopride therapy for functional dyspepsia: a meta-analysis. World J Gastroenterol. 2012;18:7371–7377. doi: 10.3748/wjg.v18.i48.7371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Talley NJ, Tack J, Ptak T, Gupta R, Giguère M. Itopride in functional dyspepsia: results of two phase III multicentre, randomised, double-blind, placebo-controlled trials. Gut. 2008;57:740–746. doi: 10.1136/gut.2007.132449. [DOI] [PubMed] [Google Scholar]
- 74.Kusunoki H, Haruma K, Hata J, et al. Efficacy of mosapride citrate in proximal gastric accommodation and gastrointestinal motility in healthy volunteers: a double-blind placebo-controlled ultrasonographic study. J Gastroenterol. 2010;45:1228–1234. doi: 10.1007/s00535-010-0292-7. [DOI] [PubMed] [Google Scholar]
- 75.Hallerbäck BI, Bommelaer G, Bredberg E, et al. Dose finding study of mosapride in functional dyspepsia: a placebo-controlled, randomized study. Aliment Pharmacol Ther. 2002;16:959–967. doi: 10.1046/j.1365-2036.2002.01236.x. [DOI] [PubMed] [Google Scholar]
- 76.Cho YK, Choi MG, Kim SH, et al. [The effect of mosapride on quality of life in functional dyspepsia]. Korean J Gastroenterol. 2004;43:160–167. [Korean] [PubMed] [Google Scholar]
- 77.Bang CS, Kim JH, Baik GH, et al. Mosapride treatment for functional dyspepsia: a meta-analysis. J Gastroenterol Hepatol. 2015;30:28–42. doi: 10.1111/jgh.12662. [DOI] [PubMed] [Google Scholar]
- 78.Hongo M, Harasawa S, Mine T, et al. Large-scale randomized clinical study on functional dyspepsia treatment with mosapride or teprenone: Japan Mosapride Mega-Study (JMMS) J Gastroenterol Hepatol. 2012;27:62–68. doi: 10.1111/j.1440-1746.2011.06949.x. [DOI] [PubMed] [Google Scholar]
- 79.Kinoshita Y, Hashimoto T, Kawamura A, et al. Effects of famotidine, mosapride and tandospirone for treatment of functional dyspepsia. Aliment Pharmacol Ther. 2005;21(suppl 2):37–41. doi: 10.1111/j.1365-2036.2005.02472.x. [DOI] [PubMed] [Google Scholar]
- 80.Otaka M, Jin M, Odashima M, et al. New strategy of therapy for functional dyspepsia using famotidine, mosapride and amitriptyline. Aliment Pharmacol Ther. 2005;21(suppl 2):42–46. doi: 10.1111/j.1365-2036.2005.02473.x. [DOI] [PubMed] [Google Scholar]
- 81.Yoon H, Lee DH, Lee YH, et al. Efficacy and safety of UI05M-SP015CT in functional dyspepsia: a randomized, controlled trial. Gut Liver. 2018;12:516–522. doi: 10.5009/gnl17416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hiyama T, Yoshihara M, Matsuo K, et al. Meta-analysis of the effects of prokinetic agents in patients with functional dyspepsia. J Gastroenterol Hepatol. 2007;22:304–310. doi: 10.1111/j.1440-1746.2006.04493.x. [DOI] [PubMed] [Google Scholar]
- 83.Pittayanon R, Yuan Y, Bollegala NP, et al. Prokinetics for functional dyspepsia: a systematic review and meta-analysis of randomized control trials. Am J Gastroenterol. 2019;114:233–243. doi: 10.1038/s41395-018-0258-6. [DOI] [PubMed] [Google Scholar]
- 84.Yang YJ, Bang CS, Baik GH, et al. Prokinetics for the treatment of functional dyspepsia: Bayesian network meta-analysis. BMC Gastroenterol. 2017;17:83. doi: 10.1186/s12876-017-0639-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jung HK, Lee KJ, Choi MG, et al. Efficacy of DA-9701 (motilitone) in functional dyspepsia compared to pantoprazole: a multicenter, randomized, double-blind, non-inferiority study. J Neurogastroenterol Motil. 2016;22:254–263. doi: 10.5056/jnm15178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Parkman HP, Hasler WL, Fisher RS. American gastroenterological association technical review on the diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1592–1622. doi: 10.1053/j.gastro.2004.09.055. [DOI] [PubMed] [Google Scholar]
- 87.Tatsuta M, Iishi H, Nakaizumi A, Okuda S. Effect of treatment with cisapride alone or in combination with domperidone on gastric emptying and gastrointestinal symptoms in dyspeptic patients. Aliment Pharmacol Ther. 1992;6:221–228. doi: 10.1111/j.1365-2036.1992.tb00265.x. [DOI] [PubMed] [Google Scholar]
- 88.Aziz I, Palsson OS, Törnblom H, Sperber AD, Whitehead WE, Simrén M. Epidemiology, clinical characteristics, and associations for symptom-based Rome IV functional dyspepsia in adults in the USA, Canada, and the UK: a cross-sectional population-based study. Lancet Gastroenterol Hepatol. 2018;3:252–262. doi: 10.1016/S2468-1253(18)30003-7. [DOI] [PubMed] [Google Scholar]
- 89.Tack J, Janssen P. Acotiamide (Z-338, YM443), a new drug for the treatment of functional dyspepsia. Expert Opin Investig Drugs. 2011;20:701–712. doi: 10.1517/13543784.2011.562890. [DOI] [PubMed] [Google Scholar]
- 90.Matsueda K, Hongo M, Tack J, Saito Y, Kato H. A placebo-controlled trial of acotiamide for meal-related symptoms of functional dyspepsia. Gut. 2012;61:821–828. doi: 10.1136/gutjnl-2011-301454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nakamura K, Tomita T, Oshima T, et al. A double-blind placebo controlled study of acotiamide hydrochloride for efficacy on gastrointestinal motility of patients with functional dyspepsia. J Gastroenterol. 2017;52:602–610. doi: 10.1007/s00535-016-1260-7. [DOI] [PubMed] [Google Scholar]
- 92.Xiao G, Xie X, Fan J, et al. Efficacy and safety of acotiamide for the treatment of functional dyspepsia: systematic review and meta-analysis. ScientificWorldJournal. 2014;2014 doi: 10.1155/2014/541950. 541950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tack J, Pokrotnieks J, Urbonas G, et al. Long-term safety and efficacy functional dyspepsia (postprandial distress syndrome)-results from the European phase 3 open-label safety trial. Neurogastroenterol Motil. 2018;30:e13284. doi: 10.1111/nmo.13284. [DOI] [PubMed] [Google Scholar]
- 94.Shinozaki S, Osawa H, Sakamoto H, Hayashi Y, Kawarai Lefor A, Yamamoto H. The effect of acotiamide on epigastric pain syndrome and postprandial distress syndrome in patients with functional dyspepsia. J Med Invest. 2016;63:230–235. doi: 10.2152/jmi.63.230. [DOI] [PubMed] [Google Scholar]
- 95.Arts J, Caenepeel P, Verbeke K, Tack J. Influence of erythromycin on gastric emptying and meal related symptoms in functional dyspepsia with delayed gastric emptying. Gut. 2005;54:455–460. doi: 10.1136/gut.2003.035279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin. Preliminary studies. N Engl J Med. 1990;322:1028–1031. doi: 10.1056/NEJM199004123221502. [DOI] [PubMed] [Google Scholar]
- 97.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011;29:4189–4198. doi: 10.1200/JCO.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Domperidone: ventricular arrhythmia and sudden death (continued) Prescrire Int. 2012;21:183. [PubMed] [Google Scholar]
- 99.Frommeyer G, Fischer C, Ellermann C, et al. Severe proarrhythmic potential of the antiemetic agents ondansetron and domperidone. Cardiovasc Toxicol. 2017;17:451–457. doi: 10.1007/s12012-017-9403-5. [DOI] [PubMed] [Google Scholar]
- 100.Shin HW, Kim MJ, Kim JS, Lee MC, Chung SJ. Levosulpiride-induced movement disorders. Mov Disord. 2009;24:2249–2253. doi: 10.1002/mds.22805. [DOI] [PubMed] [Google Scholar]
- 101.Du LJ, Chen BR, Kim JJ, Kim S, Shen JH, Dai N. Helicobacter pylori eradication therapy for functional dyspepsia: systematic review and meta-analysis. World J Gastroenterol. 2016;22:3486–3495. doi: 10.3748/wjg.v22.i12.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.McColl K, Murray L, El-Omar E, et al. Symptomatic benefit from eradicating Helicobacter pylori infection in patients with nonulcer dyspepsia. N Engl J Med. 1998;339:1869–1874. doi: 10.1056/NEJM199812243392601. [DOI] [PubMed] [Google Scholar]
- 103.Blum AL, Talley NJ, O’Morain C, et al. Lack of effect of treating Helicobacter pylori infection in patients with nonulcer dyspepsia. Omeprazole plus clarithromycin and amoxicillin effect one year after Treatment (OCAY) study group. N Engl J Med. 1998;339:1875–1881. doi: 10.1056/NEJM199812243392602. [DOI] [PubMed] [Google Scholar]
- 104.Talley NJ, Vakil N, Ballard ED, 2nd, Fennerty MB. Absence of benefit of eradicating Helicobacter pylori in patients with nonulcer dyspepsia. N Engl J Med. 1999;341:1106–1111. doi: 10.1056/NEJM199910073411502. [DOI] [PubMed] [Google Scholar]
- 105.Talley NJ, Janssens J, Lauritsen K, Rácz I, Bolling-Sternevald E. Eradication of Helicobacter pylori in functional dyspepsia: randomised double blind placebo controlled trial with 12 months’ follow up. The Optimal Regimen Cures Helicobacter Induced Dyspepsia (ORCHID) study group. BMJ. 1999;318:833–837. doi: 10.1136/bmj.318.7187.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Koskenpato J, Farkkilä M, Sipponen P. Helicobacter pylori eradication and standardized 3-month omeprazole therapy in functional dyspepsia. Am J Gastroenterol. 2001;96:2866–2872. doi: 10.1111/j.1572-0241.2001.04240.x. [DOI] [PubMed] [Google Scholar]
- 107.Hsu PI, Lai KH, Tseng HH, et al. Eradication of Helicobacter pylori prevents ulcer development in patients with ulcer-like functional dyspepsia. Aliment Pharmacol Ther. 2001;15:195–201. doi: 10.1046/j.1365-2036.2001.00903.x. [DOI] [PubMed] [Google Scholar]
- 108.Bruley Des Varannes S, Fléjou JF, Colin R, Zaïm M, Meunier A, Bidaut-Mazel C. There are some benefits for eradicating Helicobacter pylori in patients with non-ulcer dyspepsia. Aliment Pharmacol Ther. 2001;15:1177–1185. doi: 10.1046/j.1365-2036.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 109.Froehlich F, Gonvers JJ, Wietlisbach V, et al. Helicobacter pylori eradication treatment does not benefit patients with nonulcer dyspepsia. Am J Gastroenterol. 2001;96:2329–2336. doi: 10.1111/j.1572-0241.2001.04037.x. [DOI] [PubMed] [Google Scholar]
- 110.Chiba N, Van Zanten SJ, Sinclair P, Ferguson RA, Escobedo S, Grace E. Treating Helicobacter pylori infection in primary care patients with uninvestigated dyspepsia: the Canadian adult dyspepsia empiric treatment-Helicobacter pylori positive (CADET-Hp) randomised controlled trial. BMJ. 2002;324:1012–1016. doi: 10.1136/bmj.324.7344.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Veldhuyzen van Zanten S, Fedorak RN, Lambert J, Cohen L, Vanjaka A. Absence of symptomatic benefit of lansoprazole, clarithromycin, and amoxicillin triple therapy in eradication of Helicobacter pylori positive, functional (nonulcer) dyspepsia. Am J Gastroenterol. 2003;98:1963–1969. doi: 10.1016/S0002-9270(03)00432-5. [DOI] [PubMed] [Google Scholar]
- 112.Malfertheiner P, J MOssner J, Fischbach W, et al. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2003;18:615–625. doi: 10.1046/j.1365-2036.2003.01695.x. [DOI] [PubMed] [Google Scholar]
- 113.Gisbert JP, Cruzado AI, Garcia-Gravalos R, Pajares JM. Lack of benefit of treating Helicobacter pylori infection in patients with functional dyspepsia. Randomized one-year follow-up study. Hepatogastroenterology. 2004;51:303–308. [PubMed] [Google Scholar]
- 114.Mazzoleni LE, Sander GB, Ott EA, et al. Clinical outcomes of eradication of Helicobacter pylori in nonulcer dyspepsia in a population with a high prevalence of infection: results of a 12-month randomized, double blind, placebo-controlled study. Dig Dis Sci. 2006;51:89–98. doi: 10.1007/s10620-006-3090-6. [DOI] [PubMed] [Google Scholar]
- 115.Ang TL, Fock KM, Teo EK, et al. Helicobacter pylori eradication versus prokinetics in the treatment of functional dyspepsia: a randomized, double-blind study. J Gastroenterol. 2006;41:647–653. doi: 10.1007/s00535-006-1818-x. [DOI] [PubMed] [Google Scholar]
- 116.Gwee KA, Teng L, Wong RK, Ho KY, Sutedja DS, Yeoh KG. The response of Asian patients with functional dyspepsia to eradication of Helicobacter pylori infection. Eur J Gastroenterol Hepatol. 2009;21:417–424. doi: 10.1097/MEG.0b013e328317b89e. [DOI] [PubMed] [Google Scholar]
- 117.Mazzoleni LE, Sander GB, Francesconi CF, et al. Helicobacter pylori eradication in functional dyspepsia: HEROES trial. Arch Intern Med. 2011;171:1929–1936. doi: 10.1001/archinternmed.2011.533. [DOI] [PubMed] [Google Scholar]
- 118.Sodhi JS, Javid G, Zargar SA, et al. Prevalence of Helicobacter pylori infection and the effect of its eradication on symptoms of functional dyspepsia in Kashmir, India. J Gastroenterol Hepatol. 2013;28:808–813. doi: 10.1111/jgh.12178. [DOI] [PubMed] [Google Scholar]
- 119.Yazdanbod A, Salimian S, Habibzadeh S, Hooshyar A, Maleki N, Norouzvand M. Effect of Helicobacter pylori eradication in Iranian patients with functional dyspepsia: a prospective, randomized, placebo-controlled trial. Arch Med Sci. 2015;11:964–969. doi: 10.5114/aoms.2015.54851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tack J, Piessevaux H, Coulie B, Caenepeel P, Janssens J. Role of impaired gastric accommodation to a meal in functional dyspepsia. Gastroenterology. 1998;115:1346–1352. doi: 10.1016/S0016-5085(98)70012-5. [DOI] [PubMed] [Google Scholar]
- 121.Van Oudenhove L, Kindt S, Vos R, Coulie B, Tack J. Influence of buspirone on gastric sensorimotor function in man. Aliment Pharmacol Ther. 2008;28:1326–1333. doi: 10.1111/j.1365-2036.2008.03849.x. [DOI] [PubMed] [Google Scholar]
- 122.Tack J, Janssen P, Masaoka T, Farré R, Van Oudenhove L. Efficacy of buspirone, a fundus-relaxing drug, in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2012;10:1239–1245. doi: 10.1016/j.cgh.2012.06.036. [DOI] [PubMed] [Google Scholar]
- 123.Miwa H, Nagahara A, Tominaga K, et al. Efficacy of the 5-HT1A agonist tandospirone citrate in improving symptoms of patients with functional dyspepsia: a randomized controlled trial. Am J Gastroenterol. 2009;104:2779–2787. doi: 10.1038/ajg.2009.427. [DOI] [PubMed] [Google Scholar]
- 124.Tack J, Broeckaert D, Coulie B, Janssens J. The influence of cisapride on gastric tone and the perception of gastric distension. Aliment Pharmacol Ther. 1998;12:761–766. doi: 10.1046/j.1365-2036.1998.00366.x. [DOI] [PubMed] [Google Scholar]
- 125.Tack J, Janssen P, Bisschops R, Vos R, Phillips T, Tougas G. Influence of tegaserod on proximal gastric tone and on the perception of gastric distention in functional dyspepsia. Neurogastroenterol Motil. 2011;23:e32–e39. doi: 10.1111/j.1365-2982.2010.01613.x. [DOI] [PubMed] [Google Scholar]
- 126.Amano T, Ariga H, Kurematsu A, et al. Effect of 5-hydroxytryptamine receptor 4 agonist mosapride on human gastric accommodation. Neurogastroenterol Motil. 2015;27:1303–1309. doi: 10.1111/nmo.12623. [DOI] [PubMed] [Google Scholar]
- 127.Kusunoki H, Haruma K, Manabe N, et al. Therapeutic efficacy of acotiamide in patients with functional dyspepsia based on enhanced post-prandial gastric accommodation and emptying: randomized controlled study evaluation by real-time ultrasonography. Neurogastroenterol Motil. 2012;24:540–545. e250–e251. doi: 10.1111/j.1365-2982.2012.01897.x. [DOI] [PubMed] [Google Scholar]
- 128.Lacy BE, Saito YA, Camilleri M, et al. Effects of antidepressants on gastric function in patients with functional dyspepsia. Am J Gastroenterol. 2018;113:216–224. doi: 10.1038/ajg.2017.458. [DOI] [PubMed] [Google Scholar]
- 129.Lu Y, Chen M, Huang Z, Tang C. Antidepressants in the treatment of functional dyspepsia: a systematic review and meta-analysis. PLoS One. 2016;11:e0157798. doi: 10.1371/journal.pone.0157798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ford AC, Luthra P, Tack J, Boeckxstaens GE, Moayyedi P, Talley NJ. Efficacy of psychotropic drugs in functional dyspepsia: systematic review and meta-analysis. Gut. 2017;66:411–420. doi: 10.1136/gutjnl-2015-310721. [DOI] [PubMed] [Google Scholar]
- 131.Cheong PK, Ford AC, Cheung CKY, et al. Low-dose imipramine for refractory functional dyspepsia: a randomised, double-blind, placebo-controlled trial. Lancet Gastroenterol Hepatol. 2018;3:837–844. doi: 10.1016/S2468-1253(18)30303-0. [DOI] [PubMed] [Google Scholar]
- 132.Kaosombatwattana U, Pongprasobchai S, Limsrivilai J, Maneerattanaporn M, Leelakusolvong S, Tanwandee T. Efficacy and safety of nor-triptyline in functional dyspepsia in Asians: a randomized double-blind placebo-controlled trial. J Gastroenterol Hepatol. 2018;33:411–417. doi: 10.1111/jgh.13914. [DOI] [PubMed] [Google Scholar]
- 133.Talley NJ, Locke GR, Saito YA, et al. Effect of amitriptyline and escitalopram on functional dyspepsia: a multicenter, randomized controlled study. Gastroenterology. 2015;149:340–349. e2. doi: 10.1053/j.gastro.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jaafar MH, Safi SZ, Tan MP, Rampal S, Mahadeva S. Efficacy of rebamipide in organic and functional dyspepsia: a systematic review and meta-analysis. Dig Dis Sci. 2018;63:1250–1260. doi: 10.1007/s10620-017-4871-9. [DOI] [PubMed] [Google Scholar]
- 135.Gudjónsson H, Oddsson E, Björnsson S, et al. Efficacy of sucralfate in treatment of non-ulcer dyspepsia. A double-blind placebo-controlled study. Scand J Gastroenterol. 1993;28:969–972. doi: 10.3109/00365529309098293. [DOI] [PubMed] [Google Scholar]
- 136.Kairaluoma MI, Hentilae R, Alavaikko M, et al. Sucralfate versus placebo in treatment of non-ulcer dyspepsia. Am J Med. 1987;83:51–55. doi: 10.1016/0002-9343(87)90828-X. [DOI] [PubMed] [Google Scholar]
- 137.Moayyedi P, Soo S, Deeks J, et al. Systematic review: antacids, H2-receptor antagonists, prokinetics, bismuth and sucralfate therapy for non-ulcer dyspepsia. Aliment Pharmacol Ther. 2003;17:1215–1227. doi: 10.1046/j.1365-2036.2003.01575.x. [DOI] [PubMed] [Google Scholar]
- 138.Holtmann G, Gschossmann J, Karaus M, et al. Randomised double-blind comparison of simethicone with cisapride in functional dyspepsia. Aliment Pharmacol Ther. 1999;13:1459–1465. doi: 10.1046/j.1365-2036.1999.00644.x. [DOI] [PubMed] [Google Scholar]
- 139.Holtmann G, Gschossmann J, Mayr P, Talley NJ. A randomized placebo-controlled trial of simethicone and cisapride for the treatment of patients with functional dyspepsia. Aliment Pharmacol Ther. 2002;16:1641–1648. doi: 10.1046/j.1365-2036.2002.01322.x. [DOI] [PubMed] [Google Scholar]
- 140.Adibi P, Keshteli AH, Daghaghzadeh H, Roohafza H, Pournaghshband N, Afshar H. Association of anxiety, depression, and psychological distress in people with and without functional dyspepsia. Adv Biomed Res. 2016;5:195. doi: 10.4103/2277-9175.190936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Calvert EL, Houghton LA, Cooper P, Morris J, Whorwell PJ. Long-term improvement in functional dyspepsia using hypnotherapy. Gastroenterology. 2002;123:1778–1785. doi: 10.1053/gast.2002.37071. [DOI] [PubMed] [Google Scholar]
- 142.Haug TT, Wilhelmsen I, Svebak S, Berstad A, Ursin H. Psychotherapy in functional dyspepsia. J Psychosom Res. 1994;38:735–744. doi: 10.1016/0022-3999(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 143.Hamilton J, Guthrie E, Creed F, et al. A randomized controlled trial of psychotherapy in patients with chronic functional dyspepsia. Gastroenterology. 2000;119:661–669. doi: 10.1053/gast.2000.16493. [DOI] [PubMed] [Google Scholar]
- 144.Soo S, Forman D, Delaney BC, Moayyedi P. A systematic review of psychological therapies for nonulcer dyspepsia. Am J Gastroenterol. 2004;99:1817–1822. doi: 10.1111/j.1572-0241.2004.30086.x. [DOI] [PubMed] [Google Scholar]
- 145.Haag S, Senf W, Tagay S, et al. Is there a benefit from intensified medical and psychological interventions in patients with functional dyspepsia not responding to conventional therapy? Aliment Pharmacol Ther. 2007;25:973–986. doi: 10.1111/j.1365-2036.2007.03277.x. [DOI] [PubMed] [Google Scholar]
- 146.Orive M, Barrio I, Orive VM, et al. A randomized controlled trial of a 10 week group psychotherapeutic treatment added to standard medical treatment in patients with functional dyspepsia. J Psychosom Res. 2015;78:563–568. doi: 10.1016/j.jpsychores.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 147.Chen Y, Wang C, Wang J, et al. Association of psychological characteristics and functional dyspepsia treatment outcome: a case-control study. Gastroenterol Res Pract. 2016;2016 doi: 10.1155/2016/5984273. 5984273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Barbera R, Feinle C, Read NW. Nutrient-specific modulation of gastric mechanosensitivity in patients with functional dyspepsia. Dig Dis Sci. 1995;40:1636–1641. doi: 10.1007/BF02212683. [DOI] [PubMed] [Google Scholar]
- 149.Pilichiewicz AN, Horowitz M, Holtmann GJ, Talley NJ, Feinle-Bisset C. Relationship between symptoms and dietary patterns in patients with functional dyspepsia. Clin Gastroenterol Hepatol. 2009;7:317–322. doi: 10.1016/j.cgh.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 150.Pilichiewicz AN, Feltrin KL, Horowitz M, et al. Functional dyspepsia is associated with a greater symptomatic response to fat but not carbohydrate, increased fasting and postprandial CCK, and diminished PYY. Am J Gastroenterol. 2008;103:2613–2623. doi: 10.1111/j.1572-0241.2008.02041.x. [DOI] [PubMed] [Google Scholar]
- 151.Feinle-Bisset C, Azpiroz F. Dietary and lifestyle factors in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10:150–157. doi: 10.1038/nrgastro.2012.246. [DOI] [PubMed] [Google Scholar]
- 152.Carvalho RV, Lorena SL, Almeida JR, Mesquita MA. Food intolerance, diet composition, and eating patterns in functional dyspepsia patients. Dig Dis Sci. 2010;55:60–65. doi: 10.1007/s10620-008-0698-8. [DOI] [PubMed] [Google Scholar]
- 153.Mullan A, Kavanagh P, O’Mahony P, Joy T, Gleeson F, Gibney MJ. Food and nutrient intakes and eating patterns in functional and organic dyspepsia. Eur J Clin Nutr. 1994;48:97–105. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.