Abstract
Background/Aims
The esophageal hiatus is formed by the right crus of the diaphragm in the majority of subjects. Contraction of the hiatus exerts a sphincter-like action on the lower esophageal sphincter (LES). The aim is to study the hiatal anatomy (using CT scan imaging) and function (using high-resolution manometry [HRM]), and esophageal motor function in patients with sliding and paraesophageal hiatal hernia.
Methods
We assessed normal subjects (n = 20), patients with sliding type 1 hernia (n = 18), paraesophageal type 2 hernia (n = 19), and mixed type 3 hernia (n = 19). Hernia diagnosis was confirmed on the upper gastrointestinal series. The hiatal morphology was constructed from the CT scan images. The LES pressure and relaxation, percent peristalsis, bolus pressure, and hiatal squeeze pressure were assessed by HRM.
Results
The CT images revealed that the esophageal hiatus is formed by the right crus of the diaphragm in all normal subjects and 86% of hernia patients. The hiatus is elliptical in shape with a surface area of 1037 mm2 in normal subjects. The hiatal dimensions were larger in patients compared to normal subjects. The HRM revealed impaired LES relaxation and higher bolus pressure in patients with paraesophageal compared to the sliding hernia. The hiatal pinch on HRM was recognized in significantly higher number of patients with sliding as compared to paraesophageal hernia.
Conclusions
Using a novel approach, we provide details of the esophageal hiatus in patients with various kinds of hiatal hernia. Impaired LES relaxation in paraesophageal hernia may play a role in its pathophysiology and genesis of symptoms.
Keywords: Esophageal peristalsis, Hiatal, hernia, Lower esophageal sphincter, Manometry, Tomography X-ray computed
Introduction
In normal subjects, the lower esophageal sphincter (LES) and stomach are located in the abdomen. When the stomach is located in the chest (partially or totally), unrelated to swallows, the condition is known as hiatus hernia (HH), an extremely common medical condition. In 1952 Frank Nicholson classified HH into sliding (type 1), which is the most common variety (85% cases) and paraesophageal (type 2).1–3 The major difference between type 1 and type 2 HH is that in the former the LES and a part of the stomach migrate into the chest. On the other hand, in type 2, the stomach migrates into the thorax through the diaphragmatic hiatus, alongside the LES and distal esophagus, and hence the term paraesophageal. The latter is further categorized into types 2 and 3; the difference between the two is that LES is located in a normal location, ie, intra-abdominal in type 2 but similar to type 1, the LES is intrathoracic in type 3.3
The esophageal hiatus is formed by the right and left crus of the diaphragm, However, there are many variations of how 2 crus come together to form the esophageal hiatus. A study by Collis et al4 in 64 cadavers found 15 different types of arrangements of right and left crus in the formation of the esophageal hiatus. Listerud and Harkins5 dissected 204 fresh cadavers and described 11 different types of arrangements of right and left crus in the formation of esophageal hiatus. The most common type is the one in which the right crus divides into 2 bundles to encircle the esophagus and the left crus joins the left branch of the right crus to form the left hiatal margin.6
The right and left crus of the diaphragm and esophageal hiatus can be visualized on CT scan imaging.7 We recently studied the anatomy of esophageal hiatus using CT scan imaging in normal subjects.8 The goal of our current study is to determine the hiatal anatomy in patients with different types of HH using our novel approach of constructing the 3-dimensional (3D) anatomy of the esophageal hiatus. We also describe patterns of hiatal squeeze in different types of esophageal HH using high-resolution manometry (HRM).
Materials and Methods
Subjects
It is a retrospective study of patients having undergone surgery for HH repair with a primary diagnosis of HH at the University of California San Diego (UCSD) between years 2013 to 2017. Only those patients who were operated for the first time for HH repair were included in this study. The demographics and symptoms of all patients were determined from the electronic medical records. Chief symptoms at the time of presentation, age, sex, weight (body mass index), smoking history, and any other pertinent information was noted. Twenty normal subjects were randomly chosen from the medical radiology database of UCSD. Their medical records were reviewed to assure that they did not have a HH. These subjects had CT scan done for indication other than the pathology in the area of interest. All CT scans were performed using a GE HD 750 (Integrity Medical, Fort Myers, FL, USA), 64 slice CT scanner and images were acquired at 100–120 kilovolts and 200–300 milliamps. The approval was obtained from the UCSD institutional review board for the retrospective chart review for this study (HRPP# 171538).
Upper Gastrointestinal Series
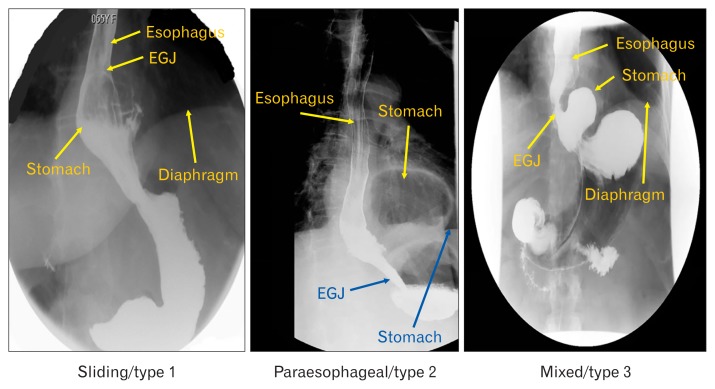
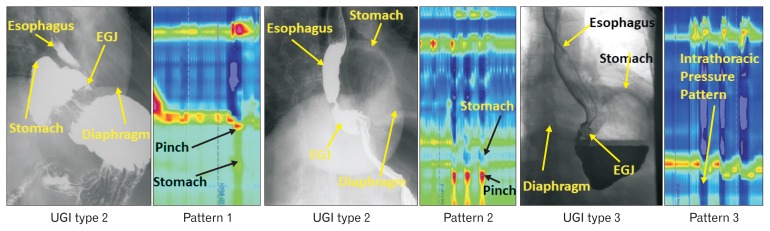
An upper gastrointestinal series, performed prior to surgery was reviewed by a senior radiologist (G.G.) to determine the type of HH present. The HH were classified into 3 types; type 1 (sliding HH), type 2 (paraesophageal HH), and type 3 (mixed: sliding and paraesophageal HH). The definition of type 1 HH was when the esophagogastric junction (EGJ) and stomach were located above the diaphragmatic hiatus and the EGJ was located above the gastric fundus. For type 2 HH, the EGJ was located at or below the level of diaphragmatic hiatus and part of the stomach alongside the esophagus (> 2 cm), above the diaphragm. For type 3 HH, the EGJ and stomach were located above the diaphragm and 2 cm or more of the fundus was located cephalad to the LES and esophagus (Fig. 1).
Figure 1.
Radiographic patterns in 3 types of hiatal hernia (HH) patients. EGJ, esophagogastric junction.
High-resolution Manometry
An HRM catheter with 36 pressure sensors (EAZ 4810; Medtronic Inc, Minneapolis, MN, USA) was used for the esophageal manometry. The results of HRM study, performed as a part of the preoperative work up in all patients was analyzed. The standard protocol for the HRM at our institution is as follows: patient in the left lateral position, landmark ID for the LES pressure measurement, 10 wet swallows, and finally 3 deep breaths to determine the diaphragmatic squeeze location and pressure. The Manoview software (3.0) (Medtronic Inc, Minneapolis, MN, USA) was used for analysis and following parameters were extracted from the generated report: basal LES pressure, percent LES relaxation with swallows, incidence of peristalsis, simultaneous pressure waves, distal contractile integral (DCI), patterns of stomach pressure, and diaphragmatic squeeze pressure with deep breaths. The LES pressure was referenced to stomach pressure when no hernia was present and to hernia pressure when the latter was present.
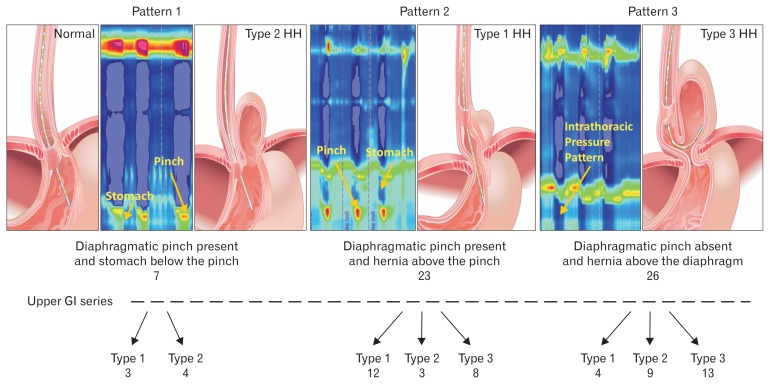
Based on the presence or absence of diaphragmatic pinch, location of diaphragmatic pinch in relationship to LES and pressure sensors below the LES showing thoracic (negative with inspiration) or abdominal waveform (positive with inspiration), the 3 HRM EGJ patterns were identified: pattern 1, diaphragmatic pinch present at the same location as the LES (expected in normal subjects and patients with type 2 HH); pattern 2, diaphragmatic pinch present distal to the LES, with at least 2 sensors below the LES showing intrathoracic and another 2 sensors below the pinch showing intra-abdominal waveform (expected in patients with type 1 and type 3 HH);2,9 and pattern 3, diaphragmatic pinch absent and all sensors below the LES showing intrathoracic pressure pattern (catheter did not enter the abdomen) (Fig. 2).
Figure 2.
Esophagogastric junction (EGJ) patterns of hiatal hernia (HH) on manometry and relationship with the radiological diagnosis in 3 types of hernia (see text for explanation). EGJ pattern 1 expected in patients with normal subjects and patients with paraesophageal hernia type 2. High-resolution manometry (HRM) EGJ pattern 2 expected in patient with sliding HH (type 1) and pattern 3 expected in patient with type 3 HH. GI, gastrointestinal.
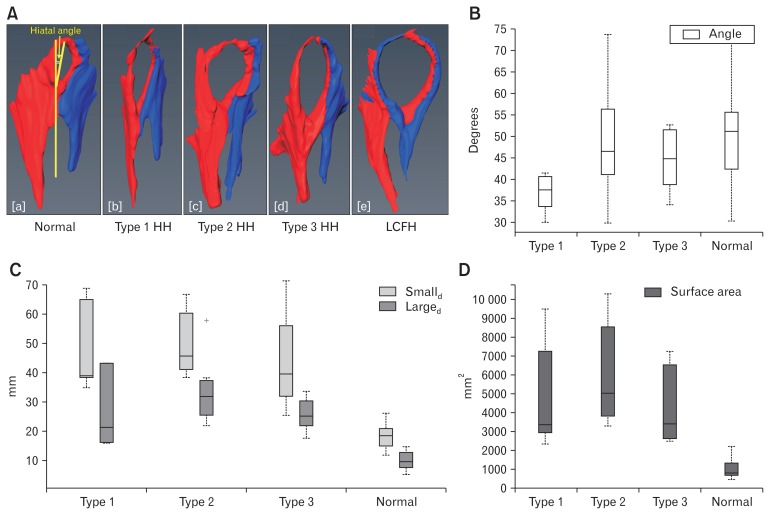
CT image analysis: the CT scan of chest and upper abdomen performed prior to surgery (when available) was analyzed to determine the anatomy of the diaphragmatic hiatus. The CT scan findings of HH patients were compared to 20 subjects without HH on the CT scan images. Coronal sections (30–40 slices), built from the axial slices of 2.0 mm to 2.5 mm thickness were uploaded into the computer software (Amira, Carlsbad, CA, USA). Following structures were identified and their margins marked in each of the CT scan image: right and left crus of diaphragm, esophagus, stomach, and vertebral bodies. Different colors were assigned to each structure and the 3D anatomy of the region was constructed. Once built, several dimensions of the hiatus were measured (long and short diameter, surface area, and angle of hiatus with vertebral column) (Fig. 3).
Figure 3.
Hiatal anatomy and hiatal dimension. (A) Anatomy of esophageal diaphragmatic hiatus in normal subjects and patients with various types of hiatal hernia (HH). The hiatus is larger in dimension in 3 types of HH compared to normal subjects. (B–D) Hiatal dimensions (angle, large diameter [Larged] and small diameter [Smalld], and surface area) in normal subjects, patients with type 1, type 2, and type 3 HH. (B) Angle of the hiatus in relationship to spine, (C) long and short dimensions of hiatus, and (D) cross sectional area of the hiatus. Normal subjects have significantly smaller surface area, large and small diameters compared to 3 types of hernia but there is no difference among 3 type of HH. Data showed in median and interquartile range. LCFH, left crus forming right hiatal margin. +Outlier value in the group.
We prospectively studied one patient diagnosed with type 3 HH on the upper gastrointestinal (UGI) series and an HRM pattern 2 seen on clinical HRM study. A manometry catheter was placed transnasally and a CT scan was performed with the catheter in place.
Statistical Methods
Quantitative data are reported as mean (± standard deviation) or median (interquartile range 25–75) when appropriate. The normality of distributions was checked using the Shapiro-Wilk test. One-way analysis of variance (ANOVA) followed by Bonferroni’s or Tukey’s post hoc test was performed for multiple comparisons, or the non-parametric one-way ANOVA with Kruskal-Wallis test with Dunn’s multiple comparison tests was used. P < 0.05 was considered significant.
Results
Demographics and Symptoms
Patient in all 3 HH types were predominantly females. Major symptoms of type 1 HH patients were heartburn, epigastric discomfort, chest pain, and sore throat. In addition to the above symptoms, patients with type 2 and type 3 HH also reported solid food dysphagia (sensation of food sticking in throat), bloating, nausea, vomiting, and regurgitation (Table 1).
Table 1.
Demography and Symptoms
| Hiatus hernia type | Age (yr) | M/F | BMI (kg/m2) | Heartburn | Epigastric discomfort | Chest pain | Sore throat | Dysphagia | Bloating | Nausea | Regurgitation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Type 1 HH (n = 18) | 56 ± 12 | 33/67 | 28 ± 5 | 27 | 4 | 5 | 2 | 11 | 0 | 2 | 5 |
| Type 2 HH (n = 19) | 66 ± 11 | 26/74 | 28 ± 4 | 16 | 5 | 4 | 0 | 11 | 2 | 5 | 4 |
| Type 3 HH (n = 19) | 68 ± 9 | 0/100 | 29 ± 5 | 27 | 9 | 7 | 2 | 8 | 2 | 9 | 9 |
M, male; F, female; BMI, body mass index; HH, hiatal hernia.
Data are presented as mean ± SD or %.
Upper Gastrointestinal Series
A total of 56 patients with the diagnosis of HH and 20 normal subjects without HH were part of this study. Based on the UGI evaluation, 18 patients were classified as sliding HH (type 1), 19 as paraesophageal HH (type 2), and another 19 as mixed HH (type 3). The size of hernia as seen in the UGI series in 3 subtypes is shown in Table 2.
Table 2.
Hernia Size
| Modalities | Type 1 HH | Type 2 HH | Type 3 HH | P-valuea | P-valueb | P-valuec |
|---|---|---|---|---|---|---|
| CT scan | 4 ± 2 | 10 ± 4 | 9 ± 2 | 0.009 | 0.723 | 0.041 |
| UGI | 4 ± 2 | 5 ± 1 | 5 ± 1 | 0.088 | 0.232 | 0.999 |
P-values are different between type 1 and 2 HH.
P-values are different between type 1 and 3 HH.
P-values are different between type 2 and 3 HH.
HH, hiatal hernia; UGI, upper gastrointestinal.
Data are presented as mean ± SD.
Esophageal Hiatus in Patients With and Without Hiatal Hernia
The 3D surface anatomy of the region of interest in 20 subjects without HH revealed that the hiatus was formed by the right crus of the diaphragm in all subjects. The right crus, after originating from the right side of the lumbar vertebra (L1–L3), divided into 2 bundles and encircled the esophagus. The left crus reinforced the left bundle of the right crus to form the left margin of the esophageal hiatus (Fig. 3A[a]). The oval-shaped hiatus was placed obliquely across the spine with the anterior end located at a more cranial location as compared to the posterior end. The hiatus wall is thinner at the cranial and anterior end as compared to the right, left, and posterior edges. The long and short diameters of the hiatus in subjects with no HH were 18.0 ± 4.0 mm and 10.0 ± 3.0 mm respectively, with a cross sectional area of 810 mm2 (interquartile range, 671).
The CT scan was available in 20 patients with HH, 6 patients with type 1, 7 patients with type 2, and 7 patients with type 3. The HH size by CT scan was 4.4 ± 2.0 cm, 9.9 ± 4.0 cm, and 8.7 ± 1.9 cm, for types 1, 2, and 3, respectively (Table 2). The type of HH identified was the same between CT scan and UGI series in 6/6 patients with type 1, 5/7 for type 2 and 7/7 for type 3. Two patients diagnosed with type 2 HH on the UGI had type 3 HH on the CT scan. Similar to normal subjects, the hiatus was formed by the right crus of the diaphragm in the majority (17/20 subjects) of HH patients (Fig. 3A[b–d]). In 3 subjects, one of each, type 1, type 2, and type 3, both the left and right crus contributed to the formation of hiatus (Fig. 3A[e]). The shape of hiatus was elliptical in all types of HH, with hiatal dimensions significantly larger (3 to 4 times) in patients as compared to subjects without HH. The hiatal margin appeared thinner, especially at the anterior end in all types of hernias. The angle of the hiatus with the spine, long and short diameter, and cross-sectional area in 3 types of hernia and normal subjects are shown in Figures 3B–D. The dimensions of the hiatus was significantly smaller in type 1 HH compared to type 2 and type 3 HH (P < 0.05).
High-resolution Manometry
Esophageal length
The esophageal lengths (distance between the lower edge of UES and upper edge of LES) of type 1, type 2, and type 3 HH were 19 ± 3, 18 ± 3, and 17 ± 3 cm, respectively. These values were not statistically significant from each other.
Lower esophageal sphincter pressure and swallow-induced relaxation
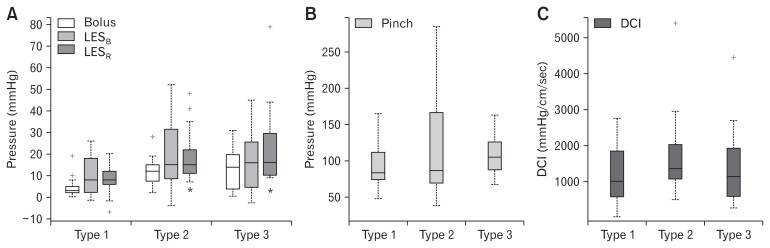
The baseline LES pressure was not different in type 1, type 2, and type 3 HH (11 ± 9, 19 ± 16, and 16 ± 14 mmHg, respectively). The swallow induced LES relaxation (median integrated relaxation pressure) was significantly different between type 1 compared to type 2 and type 3 HH, with residual LES pressure higher and percent relaxation lower in type 2 as compared to type 1 (Fig. 4A). There was no difference in the above parameters between types 2 and 3 patients. The intrabolus pressure was also higher in patients with types 2 and 3 as compared to type 1 HH (Fig. 4A).
Figure 4.
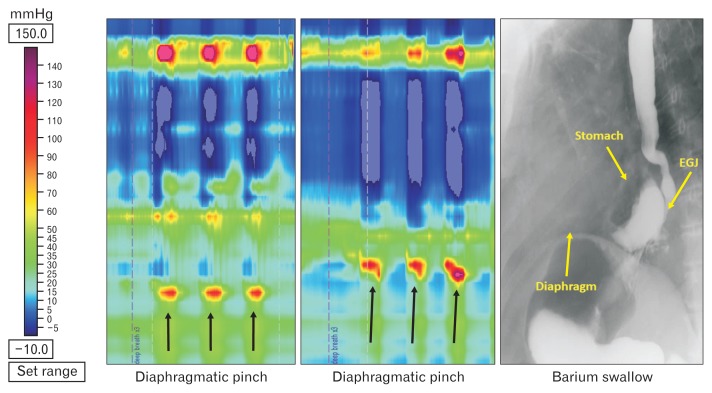
Manometry data (median and interquartile range) in 3 types of hiatal hernia (HH) patients: (A) lower esophageal sphincter basal pressure (LESB), residual pressure (LESR) with swallow and bolus pressure, (B) diaphragmatic pinch pressure, and (C) distal contractile integral (DCI). +Sign corresponds to outliers, *P < 0.05 compared to type 1 HH.
Diaphragmatic pinch pressure
The mean hiatal squeeze pressure was 91 ± 31 mmHg (n = 16) in type 1 HH, with an inverse (though not statistically significant) relationship between the size of hernia and diaphragmatic pinch pressure (r = −0.5, P = 0.051). The mean hiatal squeeze pressure was 129 ± 85 mmHg in type 2 HH (n = 7), and 109 ± 33 mmHg in type 3 HH (n = 7), with no difference as compared to type 1 HH (Fig. 4B).
Peristalsis and contraction integral
The esophageal peristalsis was normal and there was no difference in the peak contraction amplitude and DCI in the 3 types of HH (Fig. 4C).
Relationship Between Types of Hiatal Hernia on Manometry, Upper Gastrointestinal Series, and Computed Tomography Scan Images
The diaphragmatic pinch was identified on HRM more often in type 1 HH (16/18) as compared to type 2 HH (7/19) and type 3 HH (7/19) patients. The prevalence of 3 EGJ manometry patterns and types of HH observed on UGI series is shown in the flow diagram (Fig. 2). The majority of patients with type 1 HH (12) revealed HRM EGJ pattern 2. On the other hand, 50% of patients with types 2 and 3 HH revealed HRM EGJ pattern 3, ie, the catheter did not enter the abdomen; it was coiled in the herniated portion of the stomach.
In 7 patients with type 2 HH on UGI series with an identifiable diaphragmatic pinch on the HRM, 4 patients revealed HRM EGJ pattern 1 (expected for 2 HH) but 3 patients revealed HRM EGJ pattern 2, which would be expected in either type 1 HH or type 3 HH on the UGI series (Fig. 5). In one patient with a diagnosis of type 3 HH on UGI series, the HRM pattern changed from HRM EGJ pattern 2 to HRM EGJ pattern 1, only a few minutes later (Fig. 6), suggesting that similar to type 1 HH, the LES in paraesophageal hernia can also slide in and out of the hiatus.
Figure 5.
Manometry patterns in 3 different patients with type 2 paraesophageal hiatal hernia (HH) identified on the upper gastrointestinal (UGI) series. EGJ, esophagogastric junction.
Figure 6.
Changing manometry patterns of hiatal hernia (HH) in a patient with type 3 HH on the upper gastrointestinal series. EGJ, esophagogastric junction.
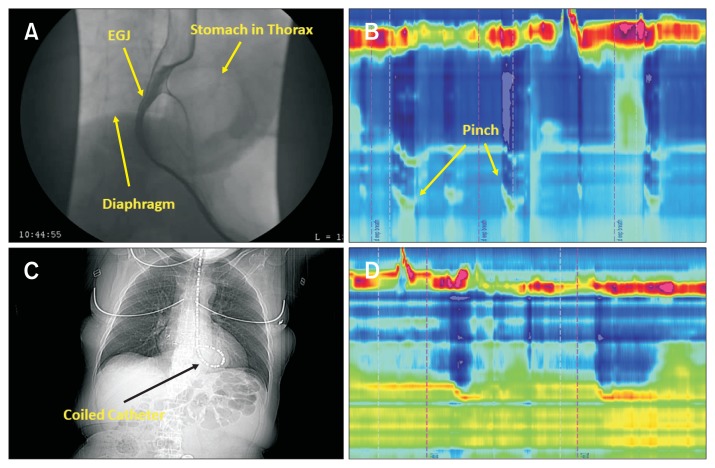
In one patient in whom we performed CT scan with the HRM catheter in place, the catheter was coiled above the diaphragm, in the hernia sac. The HRM revealed HH pattern 3, confirming our assumption of the catheter coiling in the hernia sac in patients with manometry pattern 3 (Fig. 7).
Figure 7.
CT scan finding with manometry catheter in place. (A) Upper gastrointestinal (UGI) series show a patient with type 3 hiatal hernia (HH). (B) High-resolution manometry (HRM) esophagogastric junction (EGJ). Pattern 2 is consistent with the presence of stomach above the diaphragm and the tip of catheter in the abdomen. (C) On a separate day, the manometry catheter could not be advanced into the abdomen as shown in the coronal image. (D) HRM pattern is consistent with HRM EGJ pattern 3.
With regards to the size of HH, concordance between HRM and UGI series was present in only 5/30 patients; in the remainder it was larger on HRM than UGI series.10 With regards to the HH size on CT scan and HRM, again there was poor concordance in patients with type 1 HH. The number of patients with types 2 and type 3 HH with the available CT scans, in whom the diaphragmatic pinch was present, were small to make an adequate comparison.
Discussion
The anatomy of esophageal hiatus was of considerable interest in 1940s and 50s to the surgeons because the understanding at that time was that it played a major role in the anti-reflux barrier function. Surgical dissection studies in cadavers revealed that in the majority of subjects, the right crus splits into two bundles to surround the esophagus. The CT scan images clearly show splitting of the right crus into right and left bundles before the left bundle of right crus joins left crus to form the left margin of the hiatus. Our findings are in agreement with a more recent study in which 80% of the subjects were found to have what we described.6 We are the first to describe the hiatal anatomy using CT scan segmentation. We had only 6–7 subjects in each type of HH in whom CT scan was available for segmentation and did not find significant differences in terms of the anatomical arrangement of esophageal hiatus. In all cases the right crus was dividing into 2 bundles and forming the esophageal hiatus. An important aspect of the crural diaphragm anatomy that is not revealed by the CT imaging is the crossing of the fibers of 2 bundles of the right crus in a scissor-like fashion that we found in a recent study11 and was reported earlier by Marchand.12 The hiatal dimensions were larger in patients with HH, which is expected, but there was no significant difference in various hiatal measurements among the 3 HH groups.
The manometry catheter did not enter into the abdomen in a significantly greater number of patients with paraesophageal HH, types 2 and type 3, as compared to sliding or type 1 HH. When the HRM catheter tip did enter into the abdomen, the diaphragmatic squeeze pressure with deep inspiration was not different among 3 types of HH. Difficulty with the catheter crossing the hiatus to enter into the abdomen during routine manometry is most likely related to a tightly stuffed/packed hiatus with esophagus and stomach, at least in type 2 hernia. It is likely that in patients with type 3 HH, a greater mass of stomach herniates through the diaphragmatic hiatus.
One of the major reasons for the development of HH is a weak or defective phrenoesophageal ligament, which is expected to be present all around the circumference of the esophagus in patients with type 1 HH. On the other hand, in patients with type 2 HH, the fundus of the stomach herniates into the chest anterior to the esophagus with a normally located LES. The latter implies that the defect in the phrenoesophageal ligament is not present around the entire circumference. In 4 of 9 patients with type 2 HH on UGI series, we found an unexpected manometry pattern of type 2 HH. The latter implies that the LES was located below the hiatus on the day of upper gastrointestinal exam but was in thorax on the day of manometry. The above observations imply that similar to type 1 HH, which is well known to slide in and out of the chest, type 2 HH also has sliding features. To the best of our knowledge, we have not seen any reports that type 2 HH may also slide in and out of the chest. We observed lack of concordance in the hernia size between manometry, UGI series, and CT scan in all 3 types of HH. Since these 3 exams were done on 3 separate days, it is likely that similar to HH type 1, types 2 and 3 can also slide in and out of the chest, at least partially if not completely. We observed changing hernia size in a patient with type 3 HH during 10 minutes of HRM recording, which makes us believe that the 3 types of HH hernia are not totally separate or distinct entities.
With each swallow, the LES is pulled into the chest (approximately 2 cm) due to axial shortening of the esophagus during peristalsis.13 The phrenoesophageal ligament is stretched in an oral direction with each swallow. With transient LES relaxation, the LES is pulled 4 cm or more in the cranial direction.14 Given the large degrees of stress and strain on the phrenoesophageal ligament, it is not surprising that the prevalence of HH is high and increases with age. Some investigators have described alteration in the elastin and collagen content of the phrenoesophageal ligament,15 whether the latter is a primary abnormality remains unknown. Repeated acid reflux into the esophagus causes esophagitis that has also been shown to induce sustained contraction of the longitudinal muscle of the esophagus, which may also be important in the HH formation.16–19
Esophageal peristalsis function was normal in all types of HHs with no difference in the contraction amplitude and DCI. The above is surprising because sliding HH is commonly associated with reflux disease and hypotensive peristalsis. Since ours was a retrospective study, it is possible that surgeons excluded patients with abnormal peristalsis for surgical correction of HH. There was a difference in the LES relaxation between types of HH; normal in sliding or type 1 HH but impaired in patients with types 2 and 3 HH. One may argue that the latter could be due to compression of the LES and distal esophagus by the stomach, since the latter is located along the side of the esophagus in paraesophageal HH. However, we did not observe increase in the esophageal pressure (in between swallows) in these patients which would argue against stomach compression of the esophagus as the reason. Impaired LES relaxation resulted in high residual LES pressure during swallows and higher intrabolus pressure. It is interesting that dysphagia is more prevalent in types 2 and 3 HH patients compared to type 1 HH and it is possible that impaired LES relaxation plays a role in the dysphagia symptom. In a recent study, other authors also found increased intrabolus pressure with swallows in patients with paraesophageal hernia.20
In conclusion, our study is a comprehensive, cross sectional study of patients with paraesophageal and sliding HH. Our intent was to determine if there are unique features in the anatomy of the diaphragmatic hiatus, crural diaphragm function, LES, and esophageal motor function in these patients. The esophagus hiatus is enlarged in all types of hernia as compared to normal subjects, however, there is no difference among the 3 hernia subtypes. To our surprise, we found that the LES in paraesophageal HH type 2 may also slide in and out of the hiatus. The LES relaxation of patients with paraesophageal HH is impaired, resulting in an increased intrabolus pressure. The latter is seen in association with impaired LES relaxation, an entity termed as EGJ outflow obstruction in the Chicago classification. Dysphagia is an important symptom in patients with outflow obstruction, which may be the reason for dysphagia in these patients. There are several limitations of our study to keep in mind: (1) our study was a retrospective study and therefore does not have a perfect study design to answer all the questions we raised, (2) number of subjects that have CT scans available for the analysis were relatively small, and (3) HH size was not measured at the time of surgery, which many considered to be more accurate. In spite of the above, we feel that our study does provide some novel information worthy of further prospective inquiries.
Footnotes
Financial support: This work was supported by a NIH Grant (DK060733).
Conflicts of interest: None.
Author contributions: Ravinder K Mittal: conceived the project, analyzed data, and wrote manuscript; Dushyant Kumar: data analysis, figure preparation, and manuscript editing; Ali Zifan: data analysis and figure preparation; Gary Ghahremani: read radiological studies; David C Kunkel: manuscript writing; and Santiago Horgan: data acquisition.
References
- 1.Nicholson F. Diphragmatic hernia. Ann Surg. 1952;136:174–182. doi: 10.1097/00000658-195207000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones FA. Diagnosis of hiatus hernia. Proc R Soc Med. 1952;45:277–279. doi: 10.1177/003591575204500508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kahrilas PJ, Kim HC, Pandolfino JE. Approaches to the diagnosis and grading of hiatal hernia. Best Pract Res Clin Gastroenterol. 2008;22:601–616. doi: 10.1016/j.bpg.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Collis JL, Kelly TD, Willey AM. Anatomy of the crura of the diaphragm and the surgery of hiatus hernia. Thorax. 1954;9:175–189. doi: 10.1136/thx.9.3.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Listerud MB, Harkins HN. Variations in the muscular anatomy of the esophageal hiatus: based on dissections of two hundred and four fresh cadavers. West J Surg Obstet Gynecol. 1959;67:110–112. discussion 112–113. [PubMed] [Google Scholar]
- 6.Costa MM, Pires-Neto MA. Anatomical investigation of the esophageal and aortic hiatuses: physiologic, clinical and surgical considerations. Anat Sci Int. 2004;79:21–31. doi: 10.1111/j.1447-073x.2004.00060.x. [DOI] [PubMed] [Google Scholar]
- 7.Callen PW, Filly RA, Korobkin M. Computed tomographic evaluation of the diaphragmatic crura. Radiology. 1978;126:413–416. doi: 10.1148/126.2.413. [DOI] [PubMed] [Google Scholar]
- 8.Mittal RK, Zifan A, Kumar D, Ledgerwood-Lee M, Ruppert E, Ghahremani G. Functional morphology of the lower esophageal sphincter and crural diaphragm determined by three-dimensional high-resolution esophagogastric junction pressure profile and CT imaging. Am J Physiol Gastrointest Liver Physiol. 2017;313:G212–G219. doi: 10.1152/ajpgi.00130.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahrilas PJ, Peters JH. Evaluation of the esophagogastric junction using high resolution manometry and esophageal pressure topography. Neurogastroenterol Motil. 2012;24(suppl 1):11–19. doi: 10.1111/j.1365-2982.2011.01829.x. [DOI] [PubMed] [Google Scholar]
- 10.Weitzendorfer M, Köhler G, Antoniou SA, et al. Preoperative diagnosis of hiatal hernia: barium swallow X-ray, high-resolution manometry, or endoscopy? Eur Surg. 2017;49:210–217. doi: 10.1007/s10353-017-0492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zifan A, Kumar D, Cheng LK, Mittal RK. Three-dimensional myoarchitecture of the lower esophageal phincter and esophageal hiatus using optical sectioning microscopy. Sci Rep. 2017;7:13188. doi: 10.1038/s41598-017-13342-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marchand P. The anatomy of esophageal hiatus of the diaphragm and the pathogenesis of hiatus herniation. J Thorac Surg. 1959;37:81–92. [PubMed] [Google Scholar]
- 13.Edmundowicz SA, Clouse RE. Shortening of the esophagus in response to swallowing. Am J Physiol. 1991;260(3 Pt 1):G512–G516. doi: 10.1152/ajpgi.1991.260.3.G512. [DOI] [PubMed] [Google Scholar]
- 14.Pandolfino JE, Zhang QG, Ghosh SK, Han A, Boniquit C, Kahrilas PJ. Transient lower esophageal sphincter relaxations and reflux: mechanistic analysis using concurrent fluoroscopy and high-resolution manometry. Gastroenterology. 2006;131:1725–1733. doi: 10.1053/j.gastro.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Weber C, Davis CS, Shankaran V, Fisichella PM. Hiatal hernias: a review of the pathophysiologic theories and implication for research. Surg Endosc. 2011;25:3149–3153. doi: 10.1007/s00464-011-1725-y. [DOI] [PubMed] [Google Scholar]
- 16.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–932. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- 17.Paterson WG, Miller DV, Dilworth N, Assini JB, Lourenssen S, Blennerhassett MG. Intraluminal acid induces oesophageal shortening via capsaicin-sensitive neurokinin neurons. Gut. 2007;56:1347–1352. doi: 10.1136/gut.2006.115881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunne DP, Paterson WG. Acid-induced esophageal shortening in humans: a cause of hiatus hernia? Can J Gastroenterol. 2000;14:847–850. doi: 10.1155/2000/438981. [DOI] [PubMed] [Google Scholar]
- 19.Kääriäinen M. Diagnosis of reflux esophagitis. With special reference to double contrast radiography. Ann Clin Res. 1985;17(suppl 4):1–43. [PubMed] [Google Scholar]
- 20.Rengarajan A, Arguero MJ, Kadirkamanathan SS, et al. Impact of para-esophageal hernia on esophageal and esophagogastric junction (EGJ) motor physiology. Gastroenterology. 2017;152:S705–S706. doi: 10.1016/S0016-5085(17)32460-5. [DOI] [Google Scholar]