ABSTRACT
Stem cell compartments in metazoa get regulated by systemic factors as well as local stem cell niche-derived factors. However, the mechanisms by which systemic signals integrate with local factors in maintaining tissue homeostasis remain unclear. Employing the Drosophila lymph gland, which harbors differentiated blood cells, and stem-like progenitor cells and their niche, we demonstrate how a systemic signal interacts and harmonizes with local factor/s to achieve cell type-specific tissue homeostasis. Our genetic analyses uncovered a novel function of Lar, a receptor protein tyrosine phosphatase. Niche-specific loss of Lar leads to upregulated insulin signaling, causing increased niche cell proliferation and ectopic progenitor differentiation. Insulin signaling assayed by PI3K activation is downregulated after the second instar larval stage, a time point that coincides with the appearance of Lar in the hematopoietic niche. We further demonstrate that Lar physically associates with InR and serves as a negative regulator for insulin signaling in the Drosophila larval hematopoietic niche. Whether Lar serves as a localized invariable negative regulator of systemic signals such as insulin in other stem cell niches remains to be explored.
KEY WORDS: Hematopoietic niche, Drosophila, Systemic signal, Lar, InR-Pi3K-Akt signaling
Summary: Lar limits insulin signaling to regulate the size and activity of the larval hematopoietic niche in Drosophila.
INTRODUCTION
The niche, an essential entity of stem cell biology, nourishes and protects the stem and progenitor cells within a localized environment (Scadden, 2006). In this microenvironment, stem and progenitor cells achieve a balance between signals that elicit self-renewal versus those that evoke differentiation. (Scadden, 2014; Tulina and Matunis, 2001; Xie and Spradling, 2000; Yamashita et al., 2005).
In addition to its role of maintaining stem cells, alteration in the niche results in various tumorigenic conditions and dysfunctionalities (Hoggatt et al., 2016; Morrison and Spradling, 2008). As the niche by itself impacts the stem/progenitor cell function extrinsically, it is believed to be a better ‘druggable target’ for regenerative medicine than the stem or progenitor cells themselves (Scadden, 2006; Wagers, 2012). Therefore, there is a pressing need to understand the basic biology of a stem cell niche.
Although a vast array of literature demonstrates how the niche maintains the stem/progenitor cells (Fuchs et al., 2004; Dey et al., 2016; Kiger et al., 2001; Mandal et al., 2007; Lo Celso and Scadden, 2011; Chacon-Martinez et al., 2018; Li and Xie, 2005; Losick et al., 2011; Lin, 2002), our understanding of the mechanisms by which the niche is developmentally regulated or maintained is still in its infancy.
A high degree of conservation of transcriptional regulators and signaling pathways controlling blood cell development between Drosophila and humans makes it a valuable model to investigate hematopoiesis (Evans et al., 2003; Banerjee et al., 2019). This similarity also assures that any newly identified regulation will further unravel the complex process of hematopoiesis in mammals. In order to understand the underpinning of hematopoietic niche maintenance, we initiated a UAS-Gal4-based (Brand and Perrimon, 1993) RNAi-mediated loss-of-function genetic screen employing the Drosophila larval hematopoietic organ: the lymph gland.
The lymph gland consists of a cellular niche, the posterior signaling center (PSC), lying adjacent to the medullary zone (MZ), which houses the progenitor cells. Differentiated cells arising from the progenitors populate the peripheral cortical cone (CZ) (Jung et al., 2005; Krzemień et al., 2007; Mandal et al., 2007; Banerjee et al., 2019; Sharma et al., 2019). Along with its role in progenitor maintenance, the niche is also implicated in mounting an immune response during wasp infection (Sinenko et al., 2011; Louradour et al., 2017; Crozatier et al., 2004).
A major negative regulator that stood out in our screen is Lar (Leukocyte-antigen-related-like). Lar, a well-conserved receptor protein tyrosine phosphatase (RPTP) (Streuli et al., 1989) is reported in the basal lamina of epithelial tissues, liver, muscles and adipose cells of mammals (Ahmad et al., 1995, Murphy et al., 2005, Zabolotny et al., 2001) and in the nervous system of Caenorhabditis elegans (Harrington et al., 2002), Hirudo medicinalis (Gershon et al., 1998) and Drosophila (Desai et al., 1997, Kaufmann et al., 2002). Although LAR protein was isolated in a screen of the immunoglobulin superfamily and is homologous to leukocyte common antigens (LCA) (Streuli et al., 1988), its hematopoietic function is not well understood.
Our genetic analyses establish Lar as the inhibitor of insulin signaling within the hematopoietic niche during normal development. In Drosophila, pertaining to its open circulatory system (Bodmer and Venkatesh, 1998), all the organs experience systemic signaling via the hemolymph. The current study demonstrates that the differential control over systemic insulin signaling is mandatory for the homeostasis of the lymph gland. Our expression analysis and genetic data along with the evidence that Lar is physically associated with Insulin-like receptor (InR) illustrate how a local developmental regulator (Lar) at the cellular level can integrate with the systemic signal and help the cells to respond in a way fitting with the physiological context.
We propose that Lar function empowers the hematopoietic niche to behave as an ‘interlocutor of tissue and organismal state’, a concept put forth by David Scadden (Scadden, 2014).
RESULTS
Loss of Lar from niche affected hematopoietic cell fate specification
It has been well established that the PSC, or niche, in addition to its role in the maintenance of progenitor cells through Hh signaling (Mandal et al., 2007; Sharma et al., 2019; Tokusumi et al., 2010; Baldeosingh et al., 2018; Banerjee et al., 2019), evokes PVF-PVR signaling in the CZ. This signaling impinges on MZ cells and brings quiescence to the otherwise proliferating progenitors (Mondal et al., 2011). Therefore, the niche is a robust signaling center that plays a pivotal role in lymph gland homeostasis (Fig. 1A).
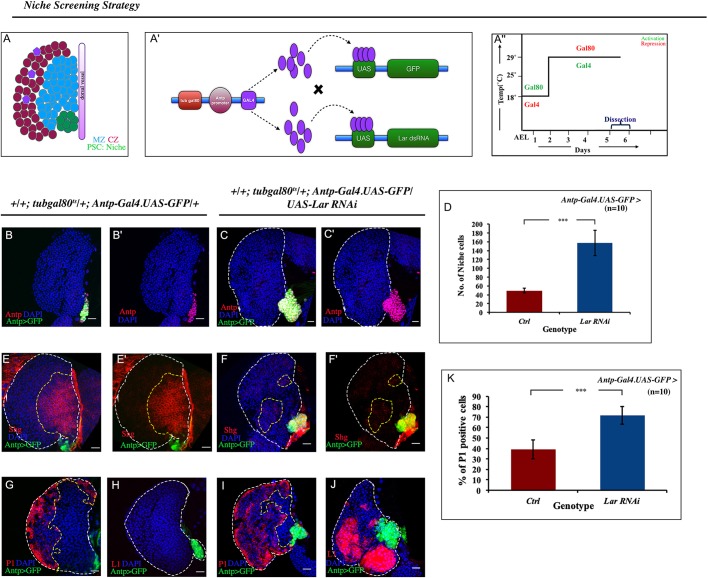
Fig. 1.
Lar plays a vital role in hematopoietic niche maintenance. (A) A third instar lymph gland showing the cortical zone (CZ, red and violet), the medullary zone (MZ, blue) and the hematopoietic niche (PSC, green). The shaded purple line indicates the dorsal vessel. (A′) Schematic showing the strategy employed for the hematopoietic niche genetic screening. (A″) Info-graph denoting the regime of the experiments. (B-C′) Downregulation of Lar using Antp-Gal4.UAS-GFP resulted in increase in the niche cell number (Antp, magenta) (C,C′) compared with control (B,B′). (D) Three- to fourfold increase in niche cell number upon Lar downregulation (n=10, P=3.429×10-10, two-tailed unpaired Student's t-test). (E-F′) The number of hemocyte progenitors [red, E-cadherin (Shg)] in the wild type (WT) (E,E′) decreases when Lar is downregulated from the niche (F,F′). (G-J) Lar downregulation from the niche resulted in an increase of the differentiated population in the lymph gland: plasmatocytes (red, P1; compare I with G) and lamellocytes (red, L1; compare H with J). (K) Quantification of P1-positive cells seen in G and I (n=10; P=1.230×10−7; two-tailed unpaired Student's t-test). White dashed lines mark the lymph gland, yellow dashed lines mark the progenitor zone. The genotype of the larvae is described in the panels. Data are mean±s.d. ***P<0.0005. Scale bars: 20 µm.
In a niche-specific RNAi-based loss-of-function genetic screen (Fig. 1A′,A″), it was observed that downregulation of Lar expression from the niche (Antp-Gal4.UAS-GFP/UAS-Lar RNAi; Fig. S1A-B′) led to a remarkable increase in the number of niche cells when compared with control (Fig. 1B-C′). Quantitative analysis revealed this increment to be three- to fourfold (Fig. 1D). Analogous results were obtained upon knocking down Lar using another independent niche-specific driver PCol85-Gal4 (Krzemień et al., 2007) (Fig. S1C,D) as well as by using another independent RNAi line (Vienna Drosophila Resource Center, line v107996; Fig. S1E). Likewise, overexpression of Lar in the niche led to a decline in niche cell numbers (Fig. S1F-G).
However, despite the increase in niche cell number observed upon loss of Lar, a drastic reduction in the progenitor pool was evident [Fig. 1E-F′, progenitors visualized using E-cadherin (also known as Shg); Jung et al., 2005]. Hh signaling plays a pivotal role in progenitor maintenance. In niches in which Lar expression is downregulated, Hh expression is not compromised (Fig. S1H-J). Interestingly, the Ci155 level in the progenitors is highly reduced (Fig. S1K-M), suggesting a problem with Hh signaling. That the progenitors were unable to maintain themselves was further endorsed by the expansion of both plasmatocytes [assayed by P1 (NimC1); compare Fig. 1G,I,K; Jung et al., 2005; Vilmos et al., 2004; Asha et al., 2003] and crystal cells (assayed by Hindsight; Benmimoun et al., 2012; Fig. S1N-P). Lar downregulation from the niche resulted in the appearance of lamellocytes, a cell-type that is absent in control lymph glands [L1 (Atilla): Fig. 1H,J; β-PS (Mys): Fig. S1Q,R]. Hetero-allelic combination of Lar (Lar13.2/Lar5.5) also resulted in an increase in niche cell number (Fig. S1S,T), increased differentiation (Fig. S1U-W) and generation of lamellocytes (Fig. S1X,Y), mimicking the phenotype of niche-specific downregulation of Lar (Fig. 1B-C′,G-J, Fig. S1C-E,U-Y). In both of these alleles, the resulting transcript encodes for a protein that is truncated in the extracellular domain. In addition, the transmembrane peptide and cytoplasmic PTP domains are not present (Krueger et al., 1996). Overexpressing full-length Lar in the niche of this hetero-allelic combination rescued the mutant phenotype (Fig. S1Z,Z′).
Thus, the above results suggest that Lar is a crucial molecule that, in addition to controlling niche cell number, ensures functionality of the niche, thereby regulating cell fate specification.
Loss of Lar resulted in hyperactivation of insulin signaling
Lar plays a crucial role in the nervous system by assisting motor axon guidance, axon migration and synapse formation (Chagnon et al., 2004; Um and Ko, 2013). It is, therefore, intriguing how this molecule that is otherwise mostly attributed to the nervous system is an essential player of Drosophila larval hematopoiesis.
Being an RPTP, we next enquired about the possible kinase/s that can be modulated by Lar. In-vitro studies in the mammalian system have revealed that Lar can inhibit insulin receptor activation (Mooney et al., 1997; Kulas et al., 1996, 1995; Tsujikawa et al., 2001; Ahmad et al., 1995). Insulin signaling was assayed by an in-vivo Pi3K reporter fly line, tGPH (Britton et al., 2002), and a membranous:cytoplasmic ratio of tGPH expression was compared between experiment and control niche cells. In the mature third instar larval control niche (green; Fig. 2A), tGPH is mainly present in the cytoplasm (Fig. 2B,B′), indicating low insulin signaling. In contrast, upon downregulation of Lar from the niche, tGPH is mostly membranous (Fig. 2C,C′), signifying the activation of Pi3K in this scenario. Quantitative analysis of the membranous:cytoplasmic ratio of tGPH revealed a 1.5-fold increase in Pi3K activity (Fig. 2D) in the Lar downregulated niche compared with the control. Incidentally, insulin signaling is essential in the hematopoietic niche. Downregulating insulin receptor function from the niche leads to a decrease in niche cell numbers (Benmimoun et al., 2012; Tokusumi et al., 2012; Fig. 2E,G), a phenotype antagonistic to Lar. Likewise, activation of the insulin receptor dramatically increased the niche cell number compared with the control (Tokusumi et al., 2012; Fig. 2F,G). These observations, along with our expression study, raised the possibility that Lar might inhibit insulin signaling in the hematopoietic niche. For validation of our hypothesis, it was essential to understand how members of the insulin signaling pathway (Fig. S2A) behave upon downregulation from the niche. Increased insulin signaling phosphorylates Akt, leading to its activation (Tsuchiya et al., 2014), which further initiates a cascade of phosphorylation events. One of the downstream target members phosphorylated by insulin signaling is eIF-4E binding protein (4EBP; EIF4EBP1) (Tettweiler et al., 2005). In comparison with control, profound upregulation of pAkt and p4EBP expression occurred in the niches in which Lar was downregulated (Fig. 2H-H″,I-I″,J,J′,K,K′,N,O). The above results indicated the occurrence of hyperactivated insulin signaling in the absence of Lar function from the niche. Furthermore, upregulating insulin signaling independently by overexpression of the positive regulators Akt (Fig. S2B) or Rheb (Saucedo et al., 2003; Fig. S2C) caused an increase in niche cell number. Likewise, downregulating the negative regulators Pten (Tokusumi et al., 2012; Fig. S2D) or Tsc1 (Tokusumi et al., 2012; Fig. S2E) from the niche increased the number of niche cells (Fig. S2F).
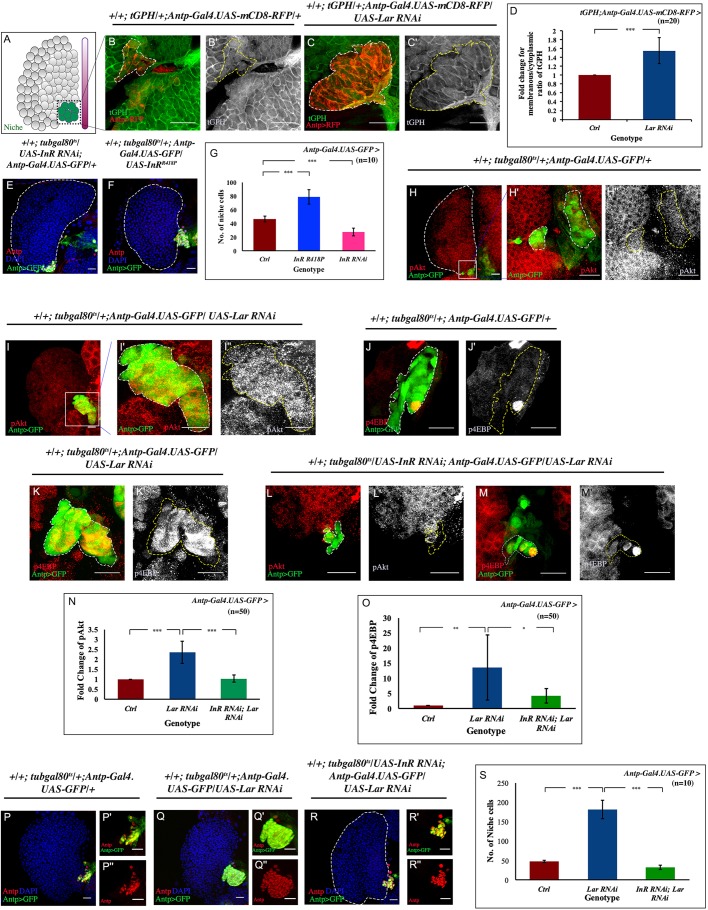
Fig. 2.
Loss of Lar from niche causes hyperactivated insulin signaling. (A) Schematic of lymph gland highlighting region of interest, the niche, which has been magnified in panels B-C′,H′,H″,I′-M′. (B-C′) The membranous:cytoplasmic ratio of tGPH (Pi3K92E reporter, green) was higher in Lar knockdown from the niche (C,C′) compared with the wild-type (WT) niche (B,B′). (D) Quantification of membranous:cytoplasmic ratio of tGPH seen in B-C′ (number of cells subjected to intensity analyses=20; P=8.724×10−8; two-tailed unpaired Student's t-test). (E) Downregulation of InR from the hematopoietic niche resulted in a decrease in the niche cell number. (F) Activated insulin led to an increase in the niche cell number. (G) Statistical analysis of the niche cell number for WT, InR knockdown and insulin active from the hematopoietic niche (n=10; P=1.176×10−7 for WT versus InR RNAi, P=1.360×10−6 for WT versus UAS InRR418P; two-tailed unpaired Student's t-test). (H-I″) Low levels of phosphorylated Akt (pAKT; red, gray) expression are seen in the WT niche (H-H″), compared with an accumulation of pAkt following loss of Lar from the hematopoietic niche (green) (I-I″). (J-K′) A basal level of p4EBP is present in WT niche (J,J′), whereas Lar downregulation resulted in elevated p4EBP levels within the niche (K,K′). (L-M′) Rescue of both pAkt and p4EBP expression in a double knockdown of InR and Lar from the niche. (N) Statistical analysis reveals elevated pAkt expression in Lar knockdown PSC (n=50; P=2.996×10−5; two-tailed unpaired Student's t-test) which was restored in double knockdown of InR and Lar (n=50; P=1.936×10−5 for Lar RNAi; InR RNAi versus Lar RNAi; two-tailed unpaired Student's t-test). (O) Quantitative analysis revealed more p4EBP-positive cells in the Lar-downregulated niche (n=50; P=0.008; two-tailed unpaired Student's t-test), whereas following double knockdown of InR and Lar, hyperactivated insulin signaling visualized using p4EBP is restored (n=50; P=0.032 for Lar RNAi; InR RNAi versus Lar RNAi; two-tailed unpaired Student's t-test). (P-R″) An increase in niche cell numbers observed upon loss of Lar from the niche (Q-Q″) is reverted to WT levels (P-P″) in a simultaneous knockdown of both InR and Lar from the niche (R-R″). (S) Statistical analysis of the data in P-R″ (n=10; P=1.995×10−8 for WT versus Lar RNAi, P=3.244×10−9 for Lar RNAi; InR RNAi versus Lar RNAi; two-tailed unpaired Student's t-test). White dashed lines in B,C,H′,I′,J,K,L,M and yellow dashed lines in B′,C′,H″,I″,J′,K′,L′,M′ outline the niche. White dashed lines in E,F,H,R mark the boundary of the lymph gland. The genotype of the larvae is described in the panels. Data are mean±s.d. *P<0.05, **P<0.005 and ***P<0.0005. Scale bars: 20 µm.
Increased cell proliferation due to hyperactivation of insulin signaling
We next examined a double knockdown genetic fly line of InR and Lar. Strikingly, upregulated tGPH, pAkt and p4EBP expression observed upon Lar downregulation (Fig. 2C,C′,I-I″,K,K′) was suppressed in this double knockdown (Fig. S2G,G′, Fig. 2L-M′,N,O). The drop in the expression of insulin pathway components resulted in a significant reduction in niche cell numbers (Fig. 2P-S). Interestingly, downregulation of two other known phosphatases (PTP69D and PTP99A; Desai et al., 1997; Sun et al., 2000) from the niche did not affect the cell number (Fig. S2H-J). These results demonstrate that Lar brings about niche-specific regulation of insulin signaling.
Next, we wanted to ascertain whether Lar regulation is at the level of functionality of insulin signaling (Pi3K/Akt) or the level of InR expression. To determine this, we used a fly line InR::V5 in which the intracellular domain of InR has been tagged with a V5 epitope (Luhur et al., 2017). This construct effectively reports InR levels as validated by the elevated expression of InR during starvation (Luhur et al., 2017; Puig and Tjian, 2005; Fig. S2L,L′). No significant change in InR expression was observed (Fig. S2M-N) in the Lar-downregulated niche compared with the control (Fig. S2K,K′,N).
These results validate that cell proliferation caused by Lar knockdown in the hematopoietic niche is due to the upregulated activity of insulin signaling (Pi3K/Akt), with no change in the expression of InR.
Lar and InR interact physically
Previous mammalian in-vitro studies have demonstrated that InR physically associates with Lar (Ahmad and Goldstein, 1997). Surface plasmon resonance (SPR) using Drosophila Lar and insulin receptor peptide also reported a similar association in Drosophila (Madan et al., 2011). The transmembrane phosphatase Lar is known to regulate signaling by dephosphorylating multiple tyrosine kinases; however, SPR demonstrated that InR is the most preferred substrate for the catalytic domain D1 (Madan et al., 2011). The genetic interaction we demonstrated between Lar and insulin signaling in the hematopoietic niche raised the possibility of a physical association between Lar and InR. To confirm this interaction in vivo, we used wild-type larval cell lysates and the antibodies directed against pInR and Lar to perform co-immunoprecipitation (co-IP). Fig. 3A shows a positive physical interaction between InR and Lar. A lower amount of Lar protein is associated with InR, reflecting a similarity between mammalian Lar-InR interactions, in which only 11.8% of Lar was associated with InR (Ahmad and Goldstein, 1997). Co-IP with rabbit GFP antibody not directed against pInR served as negative control, whereas co-IP with a known interactor of Lar, N-cadherin (Cad-N; Prakash et al., 2009) was performed as the positive control (Fig. S3A,B). This in vivo physical association between InR and Lar is further biochemical evidence of their interaction.
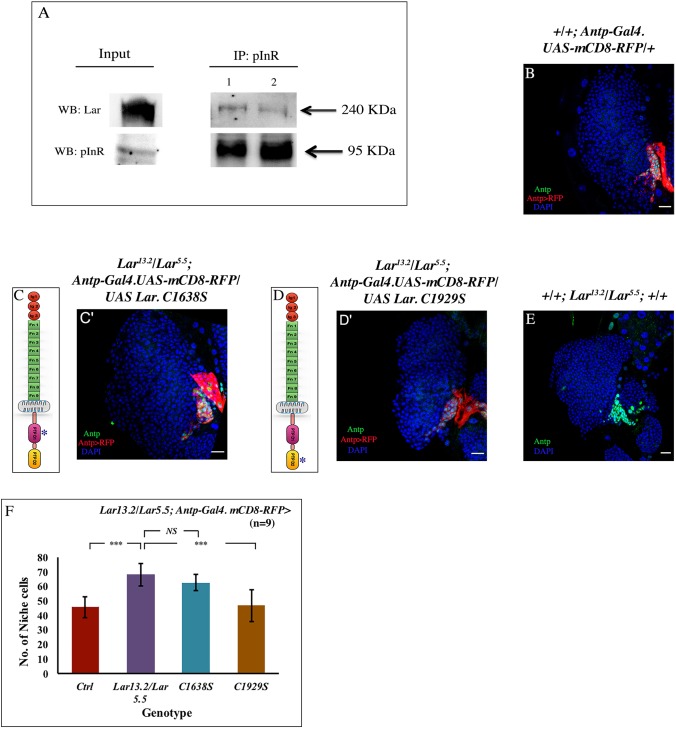
Fig. 3.
Lar physically and genetically interacts with InR. (A) pInR, immunoprecipitated from third instar larval lysate, shows an association with Lar, demonstrating a physical interaction between InR and Lar. (B-E) No rescue of niche cell number occurred upon overexpressing mutated PTPD1 (C1638S) domain in a Lar13.2/Lar5.5 background (C,C′), when compared with Lar13.2/Lar5.5 (E), whereas overexpressing mutated PTPD2 (C1929S) domain in Lar13.2/Lar5.5 (D,D′) resulted in a niche cell number (Antp, green) that was comparable with the control (B). (C,D) Schematic representation of the Lar protein (an RPTP), which has three Ig (immunoglobulin) domains (red circles), nine Fn-III (fibronectin type III) domains (green) and a transmembrane domain (gray); the pink indicates the PTP D1 (catalytic) domain and the yellow is the PTPD2 domain. The constructs used are mutated at PTPD1 (C1638S) and PTPD2 (C1929S) domains (denoted by an asterisk), respectively. (F) Quantification of the niche cell number in the above genotypes (n=9, P=8.458×10−6 for WT versus Lar13.2/Lar5.5, P=0.103 for Lar13.2/Lar5.5 versus UAS C1638S, P=0.0003 for Lar13.2/Lar5.5 versus UAS C1929S; two-tailed unpaired Student's t-test). The genotype of the larvae is described in the panels. The niche is marked with Antp antibody (green) in B,C′,D′,E and red represents Antp-Gal4.UASmcD8 RFP in B,C′,D′. Data are mean±s.d. ***P<0.0005. NS, not significant. Scale bars: 20 µm.
The maximum phosphatase/catalytic activity of Lar resides in its cytoplasmic PTPD1 domain. The cytoplasmic PTPD2 domain inhibits the catalytic function of the PTPD1 domain (Krueger et al., 2003). We investigated whether the genetic interaction of Lar with InR observed in our study is at the level of the PTPD1 domain. Lar protein with mutated PTPD1 (C1638S) and mutated PTPD2 (C1929S) domains were independently overexpressed in a Lar mutant (Lar13.2/5.5), and the niche cell number was assayed. Niche-specific overexpression of UAS C1638S in the Lar mutant did not rescue the phenotype (Fig. 3C,C′). However, overexpressing UAS C1929S (Fig. 3D,D′) in the same genetic background resulted in a significant drop in niche cell numbers, implying the involvement of the catalytic PTPD1 domain in this process (Fig. 3B-F).
Together, the domain-specific genetic interaction further reveals that the phosphatase activity of Lar is essential to regulate InR function, which is crucial for controlling the niche cell number.
ROS-mediated EGFR/ERK pathway enhances differentiation upon loss of Lar from the niche
Lar downregulation from the niche resulted in the generation of lamellocytes (Fig. 1H,J, Fig. S1Q,R), a cell type only evoked during an immune response (Rizki and Rizki, 1992; Sorrentino et al., 2002; Crozatier et al., 2004). Previous studies have demonstrated that reactive oxygen species (ROS) levels increase in the niche during wasp infection, which is essential to mount a proper immune response (Louradour et al., 2017; Sinenko et al., 2011; Banerjee et al., 2019). As lamellocytes were generated upon Lar downregulation, we investigated whether ROS levels were altered in the hematopoietic niche (Fig. 4A) using two different reporters: the redox-sensitive dye DHE (Owusu-Ansah et al., 2008) and Glutathione S-transferase D1-GFP (gstD-GFP), a ROS-inducible GST promoter GFP (Sykiotis and Bohmann, 2008). In control lymph glands, although the progenitors have elevated level of ROS, as visualized by DHE (Banerjee et al., 2019; Owusu-Ansah and Banerjee, 2009), a basal level of ROS can be detected in the hematopoietic niche (Sinenko et al., 2011). Upon Lar loss from the niche, twofold upregulation of the intensity of DHE was evident (Fig. S4A-C). As a genetic correlate, we employed a fly line that expresses gstD-GFP. Analogous to DHE labeling, the gstD-GFP signal was extremely low in the wild type; however, upon knockdown of Lar, an eightfold upregulation of the gstD-GFP signal was evident (Fig. 4B-D). Thus, both reporter analyses revealed that loss of Lar from the hematopoietic niche induces high ROS.
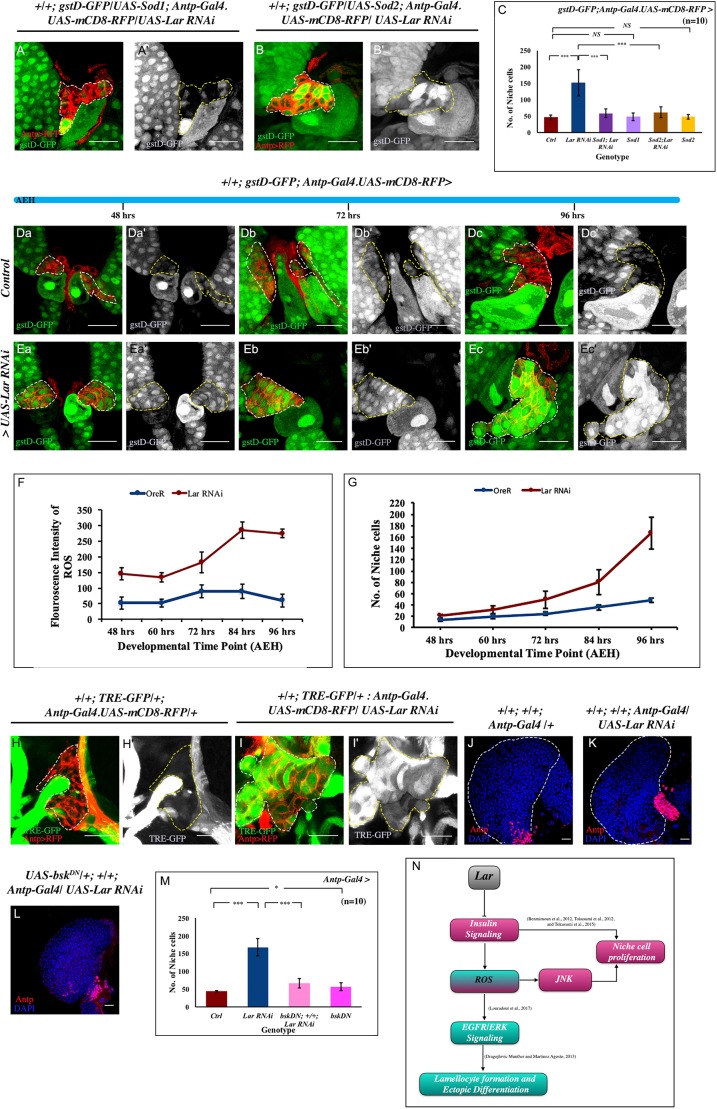
Fig. 4.
Hyperactivated insulin leads to upregulated ROS levels in the hematopoietic niche causing ectopic differentiation via the EGFR/ERK pathway. (A) Schematic of lymph gland highlighting region of interest, the niche, which has been magnified in panels B-C′,O,O′,Q,Q′. (B-C′) ROS levels (gstD-GFP, green) increase following Lar knockdown (C,C′) compared with control (B,B′). (D) Quantification shows an eightfold increase of mean fluorescence intensity in gstD-GFP in Lar knockdown niches (n=50; P=6.866×10−5; two-tailed unpaired Student's t-test). (E,E′) At 72 h AEH, a basal level of dpERK (red) expression can be detected in the control MZ. (F,F′) Lar downregulation from the niche led to a robust increase in dpERK. (G) Quantification shows a twofold increase of dpERK in progenitors following Lar downregulation from the niche (n=50; P=4.932×10−5; two-tailed unpaired Student's t-test). (H-J) Loss of Lar from the niche generated lamellocytes (β-PS, green; I), not seen in the control (H); however, scavenging ROS in the background of Lar knockdown (J) suppressed lamellocyte formation. (K-N) The plasmatocyte population (P1) increased upon Lar downregulation from the niche (L) compared with the control (K). Niche-specific overexpression of Sod1 alone does not affect differentiation (M), whereas scavenging ROS by Sod1 upon Lar downregulation rescues the plasmatocyte population (N). (O-P) Downregulating Pten led to high ROS and lamellocyte generation. (Q-R) Double knockdown of Lar and InR from the niche rescued the high ROS level and ectopic lamellocyte formation. (S) Quantification shows a significant increase in ROS intensity following Pten and Tsc1 knockdown from the niche, whereas a rescue is observed in double knockdown of InR and Lar (n= 50; P=8.021×10−5 for WT versus Pten RNAi, P=0.0004 for WT versus Tsc1 RNAi, P=7.294×10−6 for WT versus InR RNAi; Lar RNAi, P=0.235 for WT versus InR RNAi; two-tailed unpaired Student's t-test). (T) Scheme based on our study: hyperactivated insulin signaling resulted in upregulated ROS in the niche, which responded in a manner similar to the immune response. The aberrant ERK activation resulted in ectopic differentiation along with lamellocyte production. White dashed lines in B,C,O,Q and yellow lines in B′,C′,O′,Q′ outline the niche. White dashed lines in H-N,P,R mark the boundary of the lymph gland. Yellow dashed lines in K-N mark the progenitor zone. The genotype of the larvae is described in the panels. Data are mean±s.d. ***P<0.0005. Scale bars: 20 µm.
ROS-induced lamellocyte differentiation and release from the lymph gland observed during wasp infection is attributed to an increase in NF-κB signaling in the hematopoietic niche and the activated epidermal growth factor receptor (EGFR) pathway in the progenitors (Louradour et al., 2017). However, there was no significant alteration in the expression of D4 lacZ, a genetic readout of NF-κB signaling in Lar knockdown niches, when compared with control (Fig. S4D-F). In addition, NF-κB signaling is mostly involved in lymph gland dispersal and the release of lamellocytes in circulation (Louradour et al., 2017); however, no such phenomenon was observed in lymph glands with Lar knockdown niches. Thus, the lamellocytes generated upon Lar loss from the niche do not involve the NF-κB pathway.
Employing loss- and gain-of-function of Spitz, a ligand for EGFR, it is evident that high ROS in the niche is capable of activating Spitz, which in turn triggers the EGFR/ERK pathway, both in the circulation (Sinenko et al., 2011) and in lymph gland progenitors (Louradour et al., 2017). During wasp infection, ectopic secretion of Spitz from the niche is also implicated for lamellocyte generation in the circulation (Sinenko et al., 2011). We wondered whether this circuit was initiated by ROS to generate lamellocytes upon Lar loss from the niche. Interestingly, compared with the control, expression of the reporter of activated EGFR (dpERK) was twofold higher in the MZ upon Lar downregulation from the niche (Fig. 4E-G). Scavenging excess ROS by overexpression of superoxide dismutase (Sod1 and Sod2) in conjunction with Lar downregulation resulted in the suppression of lamellocytes (Fig. 4H-J). Previous studies illustrate that Drosophila MAPK activation in lymph gland progenitors expands the populations of differentiated hemocytes, both plasmatocytes and crystal cells, throughout the lymph gland along with lamellocyte induction (Dragojlovic-Munther and Martinez-Agosto, 2013; Zettervall et al., 2004). Strikingly, labeling tissues with a differentiation marker (P1) demonstrated rescue of proper zonation in the lymph glands, in which Lar downregulation from the niche was in concordance with ROS rescue (Fig. 4K-N). Rescue of the precocious differentiation upon scavenging ROS further endorsed that high ROS in the niche caused EGFR/ERK activation, which resulted in ectopic differentiation and lamellocyte generation. Hence, loss of Lar from the hematopoietic niche interferes with its normal function of progenitor maintenance owing to the upregulation of ROS.
Increased ROS levels upon Lar knockdown in niche caused by hyperactivated insulin signaling
Lar in the hematopoietic niche is a negative regulator of the insulin pathway. We inquired whether similar to Lar loss, downregulation of other negative regulators of insulin signaling (Pten or Tsc1) generate ROS. Interestingly, downregulating Pten or Tsc1 from the niche resulted in high ROS response, as visualized by gstD-GFP expression (Fig. 4O,O′,S, Fig. S4G,G′). Moreover, the increased ROS seen following loss of Pten from the niche is sufficient to generate lamellocytes, analogous to the immune response (Fig. 4P).
Based on the above results, we rationalized that the increased ROS and lamellocyte induction observed in Lar loss was a consequence of hyperactivated insulin signaling. To validate this, we analyzed the niche of a genotype in which both insulin receptor function and Lar were simultaneously downregulated. Indeed, in this genotype, both expression of ROS and the ectopic lamellocyte induction were rescued (Fig. 4Q-S, Fig. S4H,H′), thereby endorsing that generation of ROS and lamellocyte induction in Lar loss was an outcome of excessive insulin signaling (Fig. 4T).
Accumulation of ROS upon Lar knockdown in the niche induced proliferation
The increased ROS levels following loss of Lar from the niche also led to the partial rescue of the number of niche cells (Fig. 5A-C, Fig. S5A-C). Temporal analyses of the expression of gstD-GFP demonstrated that the robust increase in ROS detected upon Lar attenuation in the late third instar was a gradual increase of ROS throughout larval development (compare Fig. 5Da-Dc′ with Fig. 5Ea-Ec′,F). A thorough investigation of the developmental timeline illustrated that ROS accumulation magnified after 72 h after egg hatching (AEH), a time point that corresponds with the maximum response in cell proliferation in Lar knockdown compared with control niches (Fig. S5D-E″″, Fig. 5G). Although the increased ROS alone in the niche cells does not cause proliferation (Sinenko et al., 2011), we were interested to understand how elevated ROS upon Lar downregulation contributes towards proliferation.
Fig. 5.
Gradual increase in ROS over time is associated with increased niche cell proliferation. (A-B′) Scavenging ROS by overexpressing both Sod1 and Sod2 in conjunction with loss of Lar from the niche resulted in rescue in both ROS (gstD-GFP, green) and a partial rescue in niche cell number (Antp-Gal4.UAS-RFP, red). (C) Quantification of niche cell number in A-B′ (n=10; P=1.321×10−5 for WT versus Lar RNAi, P=2.493×10−5 for Lar RNAi versus UAS Sod1; Lar RNAi, P=0.708 for WT versus UAS Sod1, P=2.952×10−5 for Lar RNAi versus UAS Sod2; Lar RNAi, P=0.551 for WT versus UAS Sod2; two-tailed unpaired Student's t-test). (Da- Ec′) Temporal analysis for ROS was carried out both in control and in Lar loss from the niche. In the developmental timeline from 48 h to 96 h AEH, a basal level of ROS was detected in the wild-type (WT) niche (Da-Dc′). A gradual accumulation of ROS, which maximized after 72 h AEH, was seen upon Lar abrogation from the niche (Ea-Ec′). (F,G) Graphical representation of ROS (F) and niche cell number (G) in control and experiment. (H-I′) Upregulation of JNK signaling visualized by its reporter TRE-GFP in Lar knockdown (I,I′) compared with WT niche (H,H′). (J-L) Increased niche number (Antp, red) observed on Lar loss from the niche (K), compared with control (J), is partially rescued by downregulating JNK (bskDN) in conjunction with Lar downregulation (L). (M) Quantification of J-L and Fig. S5F (n=10; P=9.474×10−6 for WT versus Lar RNAi, P=3.249×10−6 for Lar RNAi versus bskDN; +; Lar RNAi, P=0.019 for WT versus bskDN; two-tailed unpaired Student's t-test). (N) Lar regulates insulin signaling, thereby controlling niche cell number. The absence of Lar function leads to increased insulin signaling, resulting in upregulated ROS that contributes to cell proliferation by JNK activation. White dashed lines in A,B,Da,Db,Dc,Ea,Eb,Ec,H,I and yellow dashed lines in A′,B′,Da′,Db′,Dc′,Ea′,Eb′,Ec′,H′,I′ outline the niche. White dashed lines in J,K mark the boundary of the lymph gland. The genotype of the larvae is described in the panels. Data are mean±s.d. *P<0.05, ***P<0.0005. NS, not significant. Scale bars: 20 µm.
Jun N-terminal kinase (JNK) is a proliferative signal acting downstream of ROS (Ohsawa et al., 2012; Shen and Liu, 2006). In the wild type, JNK activation is not reported in the hematopoietic niche (Sinenko et al., 2011). However, in Lar knockdown niches, a robust increase in the level of TRE-GFP, a reporter for activated JNK (Chatterjee and Bohmann, 2012), indicated that ROS, in this case, has caused JNK activation (Fig. 5H-I′). That the activated JNK contributed towards proliferation was evident when a rescue in cell number was achieved upon simultaneous downregulation of JNK, by dominant negative Drosophila JNK (bskDN), and Lar from the niche (Fig. 5J-M, Fig. S5F). The consequence of the upregulated JNK is limited to proliferation, which becomes evident when overexpressing JNK using UAS-Hep-act in the niche does not induce lamellocytes (Fig. S5G).
These results established that the increase in cell proliferation observed in the mid-third instar stages upon Lar downregulation was an outcome of a gradual accumulation of ROS. The elevated ROS elicited JNK signaling, which worked in conjunction with hyperactivated insulin signaling (Fig. 5N), which is known to activate Myc in the hematopoietic niche (Lam et al., 2014; Tokusumi et al., 2015).
Lar fine-tunes insulin signaling within the niche
Our results suggest that control over insulin signaling is essential in the niche; however, several studies have shown that insulin is essential for niche cell proliferation (Benmimoun et al., 2012; Tokusumi et al., 2015, 2012). We rationalized that Lar expression in the niche must be temporally restricted in order to fine-tune insulin signaling. Although Lar is expressed robustly in the niche of the third instar lymph gland (Fig. 6A-A″), time kinetics of both Lar and Pi3K expression was carried out, using Lar immunolabeling on the tGPH reporter fly line (Fig. 6B-D″) to test out the above idea. Our results showed that Lar expresses throughout the niche, but its expression was first detectable at 48 h AEH (Fig. 6C-C″). Interestingly, Lar expression was enriched wherever tGPH was less membranous, portraying the antagonistic relationship of Lar and insulin signaling in the niche (Fig. 6E-Eb″). These observations further implicated that the inhibition of Lar on insulin signaling in the niche happens post-second instar. The expression studies, in conjunction with our genetic findings, endorsed that there was progressive deregulation of insulin signaling in the absence of Lar function in the hematopoietic niche (Fig. 6F).
Fig. 6.
Lar and insulin have an antagonistic relationship with each other. (A) Expression of Lar in third instar lymph gland visualized by Lar antibody (red). (A′-A″) Magnification of boxed area in A representing the niche: a robust Lar expression (red) can be detected in the hematopoietic niche (green). (B-B″) At 36 h AEH, Lar expression level was hardly detectable in the niche. (C-C″) Lar expression was detectable by 48 h AEH. (D-D″) Robust Lar expression was seen in the niche at 96 h AEH. (E-Eb″) Co-immunostaining of Lar and insulin reporter (tGPH) indicated their antagonistic relationship. At 60 h AEH, Lar expression was enriched in the niche. (Ea-Ea″) Magnified area 1 of E reveals that regions with high tGPH (green) expression were coupled with low Lar (magenta) expression. (Eb-Eb″) Magnified area 2 of E represents the niche with low tGPH (green) but high Lar (magenta) expression. Arrow 1 indicates peripheral hemocytes of the lymph gland; arrow 2 indicates the niche. (F) Schematic based on our expression analysis proposing the antagonistic relationship of insulin and Lar. The peach bar is the dorsal vessel, and pale green pink-outlined cells are the inner core cells of the lymph gland, which are low in membranous tGPH but high in Lar expression. In the niche, the highest level of Lar expression is seen with a concomitant decrease in the membranous tGPH expression. (G-J′) Micrograph (left) and schematic (right) showing the effect of insulin signaling on niche cell proliferation. (G-H′) Niche cell number declined upon blocking insulin signaling by removal of ligand through starvation (H,H′) as compared with control (G,G′). (I-J′) On starvation (J-J′), the increased niche cell number (red, Antp) seen upon Lar loss (I-I′) reverted to control. (K) Statistical analysis of niche cell number in G-J′. White dashed lines outline the niche. The genotype of the larvae is described in the panels. Data are mean±s.d. *P<0.05, ***P<0.0005. Scale bars: 20 µm.
Temporal analysis of Lar and insulin signaling depicted an inverse correlation between Lar expression and Pi3K activation (tGPH expression) (Fig. 6Ea-Eb″). However, compared with the dynamicity of tGPH, the InR expression remains almost constant during the developmental timeline (Fig. S6A-E′). The above observations suggested that Lar modulates insulin signaling by affecting the functionality of InR and not its expression during development.
The activation of Pi3K, an integral component of the insulin signaling cascade, is regulated through the binding of Drosophila insulin-like peptides (DILPS) to insulin receptors present on the cell surface (Geminard et al., 2009). The level of various DILPS depends on the nutritional condition of the animal and, during starvation, their level goes down in the system, leading to decreased insulin signaling (Shim et al., 2012). A previous study reported that hematopoietic niche cell number declines upon starvation (Tokusumi et al., 2012; Fig. 6H). As Lar downregulation in the niche resulted in hyperactivated insulin signaling leading to overproliferation of the niche, we wondered whether lowering levels of the insulin ligand via starvation could rescue the observed hyperproliferation.
A strategy was designed wherein, via starvation, the ligand for insulin was blocked; thereby, the insulin signaling was abrogated (Shim et al., 2012). Larvae of both control and Lar knockdown were starved beyond 48 h AEH (Fig. S6F), a timeline that corresponds with the appearance of Lar in the niche. On analysis of these larvae in the late third instar, it was evident that, although the size was significantly small (Fig. S6G), there was no delay in development (assayed by mouth hooks) (Fig. S6H-J). The niche cell number in the late third instar of such starved larvae showed a rescue of hyperproliferation, in contrast to Lar loss (Fig. 6G-K).
These results attest that the increased niche cell number seen upon Lar loss-of-function is the outcome of hyperactivated insulin signaling. Therefore, Lar in the niche appears to be responsible for fine-tuning the systemic signaling encountered by the niche during development.
DISCUSSION
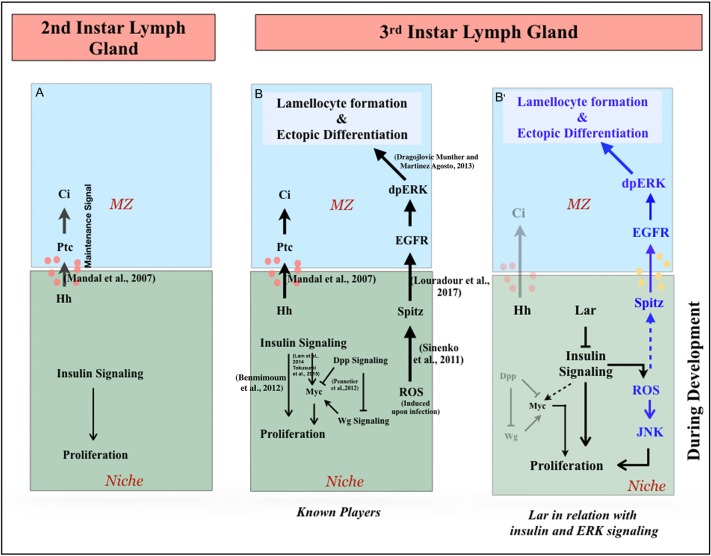
Our effort to understand the maintenance of the hematopoietic niche led us to discover the role of Lar in regulating insulin signaling in the niche, which is crucial for lymph gland homeostasis. Lar in the hematopoietic niche acts as a rheostat, restricting excessive insulin signaling to limit proliferation in later developmental stages. A physiological consequence of insulinemia in the niche is upregulated ROS. As a result, the ROS/Spitz/EGFR/ERK circuit that is evoked during an immune response (Louradour et al., 2017; Sinenko et al., 2011) gets activated during normal development. Lar abrogation from the niche also activates JNK to bolster niche cell proliferation (Fig. 7A-B′). In addition to Dpp (Pennetier et al., 2012), insulin signaling is also known to stimulate cell proliferation via Myc in the hematopoietic niche (Lam et al., 2014; Tokusumi et al., 2015). It is, therefore, also possible that the InR/Pi3K activation and immense proliferation observed upon Lar loss from the hematopoietic niche might also involve Myc activation.
Fig. 7.
Representation of the role of Lar in the niche to maintain lymph gland homeostasis. (A) In the early instar lymph gland, absence of Lar in the niche ensures insulin signaling-mediated proliferation. (B) Known players: insulin signaling and Dpp-Wg signaling activate the Myc (Dmyc) circuit relevant for niche cell proliferation and niche function. The signaling pathway is upregulated as a result of the niche-mediated immune response during wasp infection. (B′) Post second instar, the appearance and subsequent increase in Lar expression curtails insulin signaling to restrict proliferation. Thus, the increase in insulin signaling due to Lar downregulation led to proliferation in the niche cell number along with an accumulation of ROS. The high levels of ROS in the niche ectopically induce JNK, bolstering the proliferation. High ROS also feeds into the known pathway that is activated only during infection. Here, Spitz from the niche upregulates phosphorylated ERK in MZ, which results in ectopic differentiation along with lamellocyte production. Loss of Lar engages this pathway developmentally in the absence of infection.
Lar is a transmembrane type IIA receptor protein tyrosine phosphatase, which has two intracellular phosphatase domains (D1 and D2) and extracellular immunoglobulin (Ig) and fibronectin type III (FNIII) domains (Chagnon et al., 2004). The different domains of Lar provide this single molecule the ability to carry out diverse functions. A major interactor of Lar in Drosophila is the actin cytoskeleton. This interaction is often encountered in the developing nervous system, in which it plays a significant role in axonal migration and synapse morphogenesis (Lanier and Gertler, 2000).
In addition, in oocytes, Lar is implicated in follicle cell development and patterning through actin organization (Bateman et al., 2001; Frydman and Spradling, 2001). A study in Drosophila germline stem cells (GSCs) has demonstrated that Lar can act as a cell adhesion molecule by localizing E-cadherin at the GSC-hub interface, thereby maintaining the attachment between male GSCs and hub cells (Srinivasan et al., 2012). Moreover, evidence of the physical interaction of Lar with N-cadherin in the Drosophila embryo further endorses the interaction of Lar with cell adhesion molecules (Prakash et al., 2009).
Many in-vitro studies in the mammalian system have revealed that Lar interacts with various tyrosine kinases (Kulas et al., 1996; Mooney et al., 1997), thereby modulating different signaling pathways. In vitro studies using mammalian cell lines demonstrated that InR physically associates with Lar (Ahmad and Goldstein, 1997). SPR has shown that the most preferred substrate for Drosophila Lar is InR (Madan et al., 2011), but the evidence for physical interaction remained to be demonstrated. Our current study provides the first in vivo physical association of Lar with InR in Drosophila.
Besides the evidence of physical interaction, our genetic data demonstrate that loss of Lar in the hematopoietic niche results in hyperactivated insulin signaling. Upregulated PI3K expression (assayed using tGPH expression) suggests that Lar directly acts on InR and not on any other regulators of InR signaling such as Pten or Tsc1/2 in the hematopoietic niche. No alteration in InR expression upon Lar loss from the niche further confirms that Lar-InR interaction impinges on PI3K-Akt-insulin signaling and not on InR expression. Furthermore, our results show that the catalytic function of Lar protein that resides in the PTPD1 domain is crucial for Lar-InR interaction.
LAR can modulate multiple tyrosine kinases; it appears that the spatial distribution of LAR gives it specificity for its cell-type tyrosine kinase receptor (Kulas et al., 1996). Equivalent evidence comes from our current in vivo study, in which we have successfully demonstrated that, in hematopoietic tissue, wherever there is a high membranous tGPH (reporting insulin signaling), Lar expression is low, and vice versa. This reciprocal expression, coupled with the genetic analyses, projects a mechanism underpinning the activation of receptor tyrosine kinases by RPTPs in a cell-type-specific manner.
Insulin signaling helps to coordinate nutritional status with systemic growth control both in invertebrates and in the mammalian system (Hietakangas and Cohen, 2009; Johnson et al., 2013). Through its receptor, insulin is now known to have a much broader pleiotropic role, controlling several physiological processes. Therefore, inappropriate activation of insulin signaling has been linked with various aberrant scenarios such as infection, cancer and diabetes (Brännmark et al., 2013; Fröjdö et al., 2009; Ray et al., 2014; Roth et al., 2018; Poloz and Stambolic, 2015).
The hematopoietic niche cells can sense the systemic insulin level, which is essential for their proliferation. Overactivation of InR increases the niche cell number, whereas downregulation causes it to decline (Tokusumi et al., 2012). The systemic insulin level is also directly sensed by the hemocyte progenitors. Knockdown of insulin signaling in the progenitors results in their precocious differentiation (Shim et al., 2012; Benmimoun et al., 2012), demonstrating that physiological levels of insulin signaling are essential for their maintenance. Our expression data and genetic analyses unravel a differential requirement of insulin signaling in the niche compared with progenitor cells.
We further illustrate how the pleiotropic effect of excessive insulin signaling in the niche affects the homeostasis of the organ. The hyperactivated insulin signaling in the niche generates excessive ROS. This high level of ROS in the niche has a two-prong effect in two different cell types of the developing lymph gland. First, within the niche, it evokes JNK to provide a thrust to the ongoing proliferation of the niche cells. Previous literature has demonstrated that oxidative stress leads to the activation of JNK, which is known to have both pro- (Ohsawa et al., 2012) and anti-proliferative functions (Owusu-Ansah et al., 2008). Thus, by the stimuli, strength and duration of the JNK activation, diverse responses ranging from apoptosis, survival and altered proliferation can be evoked (Wagner and Nebreda, 2009). Our current study demonstrates that the ectopic activation of JNK due to the gradual accumulation of ROS collaborates with hyperactivated insulin signaling to boost cell proliferation. The second effect of the elevated ROS in the niche is the activation of ERK in the hemocyte progenitors. Activation of the Spitz/EGFR pathway by ectopic ROS in the niche is known to activate ERK in the progenitors (Louradour et al., 2017). The activated ERK causes ectopic differentiation and lamellocyte generation.
The cumulative effect of deregulated signals disturbs the homeostasis of the organ
Interestingly, hyperactivation or hypoactivation of insulin signaling is also associated with deregulated hematopoiesis in the vertebrate system. For example, altered insulin signaling in diabetic mice affects the composite microenvironment of the bone marrow leading to compromised function of the hematopoietic niche (Ferraro et al., 2011). Our work provides a strong genetic link between Lar and Insulin signaling, which should be tested in vertebrates. Although LAR is expressed in T-cell lineages in vertebrates, it remains to be seen whether Lar is also present in vertebrate hematopoietic niche/s and functions similarly. It is also intriguing to observe that, similar to the vertebrate system (Ferraro et al., 2011; Eliasson and Jönsson, 2010), a low level of ROS is present in the Drosophila hematopoietic niche (Louradour et al., 2017; Sinenko et al., 2011). It will be fascinating to see whether the hyperactivation of insulin signaling also generates ROS in the vertebrate niche and affects cell fate specification via the same mechanism that is elucidated in this study.
Our study unravels a check on insulin signaling by Lar that authorizes the hematopoietic niche to act as the ‘interlocutor’, evaluating the physiological state of an organism and thereby relaying it to the hemocyte progenitors for their homeostasis.
MATERIALS AND METHODS
Fly stocks
In this study, the following Drosophila strains were used: Antp-Gal4 (S. Cohen, University of Copenhagen, Denmark), PCol85-Gal4 (M. Crozatier, Université de Toulouse, France), InR.V5/TM6B, Hu (N. Sokol, Indiana University, USA), Lar5.5/ CyO (V. Vactor, Harvard Medical School, USA), PCol85-Gal4;D4 lacZ (A. Sharma, NITTE University, India), gstD-GFP (D. Bohmann, University of Rochester, USA). Hml-GAL4.Δ (S. Sinenko, Russian Academy of Sciences, Moscow); UAS-Lar RNAi (II) and UAS-Tsc1 RNAi were from the Vienna Drosophila Resource Center. The following stocks were procured from Bloomington Drosophila Stock Center: OreR, UAS-Lar RNAi, UAS-InR RNAi, UAS-InRR418P, tGPH, UAS-Sod1, UAS-Sod2, UAS-Pten RNAi, TRE-GFP, UAS-bskDN, UAS-mCD8-RFP, UAS-Rheb, UAS-Akt, UAS-Dicer, UAS-Hep-act, UAS-Lar.C1638S, UAS-Lar.C1929S, UAS-Lar.K and Lar13.2/CyO. Detailed genotype of the fly lines used for the current work is listed in Table S1.
All stocks were maintained at 25°C on standard media. For gal80ts experiments, crosses were initially maintained at 18°C (permissive temperature) for 2 days after egg laying, and then shifted to 29°C until dissection (Fig. 1A′).
For developmental time series experiments, larvae were synchronized. Flies were allowed to lay eggs for 2 h. Newly hatched larvae within a 1 h interval were collected and transferred onto fresh food plates and aged for specified time periods at 29°C.
Immunohistochemistry
Lymph gland dissection and immunostaining was performed using previously described protocols (Mandal et al., 2007; Jung et al., 2005). The following primary antibodies were used: mouse anti-Lar [1:4, 9D82B3, Developmental Studies Hybridoma Bank (DSHB)], mouse anti-Antp (1:10, 8C11, DSHB), mouse anti-P1 (1:40, gift from I. Ando, Hungarian Academy of Sciences, Szeged, Hungary; Kurucz et al., 2007), mouse anti-L1 (1:50, gift from I. Ando), rabbit anti-Hh (1:500, gift from P. Ingham, Nanyang Technology University Singapore; Forbes et al., 1993), rat anti-Ci (1:2, 2A1, DSHB), mouse anti-Hindsight (1:5, 1G9, DSHB), rat anti-E-cadherin (1:50, DCAD2, DHSB), mouse anti-β-PS (1:5, CF.6G11, DSHB), rabbit anti-pAkt (1:100, 4054, Cell Signaling Technology), rabbit anti-p4EBP (1:100, 2855, Cell Signaling Technology), mouse anti-dpERK (1:100, M8159, Sigma-Aldrich), rabbit anti-β-galactosidase (1:100, A11132, Molecular Probes) and mouse anti-V5 (1:250, ab27671, Abcam). Secondary antibodies used in this study were: mouse Cy3 (115-165-166), mouse FITC (315-097-003), mouse Dylight (115-605-003), rabbit Cy3 (711-165-152) and rat Cy3 (712-165-153) (all 1:500), from Jackson ImmunoResearch Laboratories.
Tissues were mounted in Vectashield (Vector Laboratories) then followed by Confocal Microscopy (Zeiss LSM, 780). For p4EBP, pAkt and dpERK, fixation of tissues was carried out using 4% paraformaldehyde overnight at 4°C and blocking was carried out using 5% normal donkey serum (Jackson ImmunoResearch Laboratories, 017-000-121).
Detection of Lar expression in lymph gland
For Lar antibody staining, the lymph glands were incubated in mouse anti-Lar antibody (1:4 in PBS) before fixation (Langevin et al., 2005) for 2 h at 4°C. Tissues were then fixed in 4% paraformaldehyde prepared in ice cold 1× PBS (pH 7.2) for 5 h at 4°C. The primary antibody was washed three times for 10 min each using 0.3% PBT. Incubation in secondary antibody, washes and mounting was performed using the standard protocol (Mandal et al., 2007; Jung et al., 2005).
Detection of E-cadherin expression in lymph gland
For detection of E-cadherin, the lymph glands were incubated in rat anti-E-cadherin antibody (1:50 in PBS) before fixation (Sharma et al., 2019) for 1 h at 4°C. Tissues were then fixed in 4% paraformaldehyde prepared in ice cold 1× PBS (pH 7.2) for 5 h at 4°C. The primary antibody was washed three times for 10 min each using 0.3% PBT. Incubation in secondary antibody, washes and mounting was performed using the standard protocol.
Co-IP
Third instar wild-type larvae were used to immunoprecipitate pInR (1:50) using a previously described protocol (Prakash et al., 2009). We homogenized 30-40 larvae in cell lysis buffer [50 mM Tris (pH 7.5), 5% glycerol, 0.7% NP-40, 1.5 mM MgCl2, 125 mM NaCl, 25 mM NaF, 1 mM Na3VO2] supplemented with protease inhibitors (complete mini tablets, Roche). Lysate (200 µl) was precleared using 25 µl of Protein G beads (New England Biolabs, S1430S) for 30 min. The precleared lysate was then incubated with pInR antibody (Cell Signaling Technology, 3021, 1:40) for 4 h. For the negative control, cell lysate was prepared using Hml-GAL4.Δ. UAS-GFP larvae and precleared lysate were incubated with Rb-GFP antibody (1:100, A-11122, Thermo Fisher Scientific). For the positive control, lysate was prepared from 0-15 h dechorionated embryos; the precleared lysate was then incubated with N-cadherin antibody (1:5, DN Ex#8, DSHB). Then 30 µl of washed Protein G beads were added to the lysate antibody mixture and incubated overnight. All the incubations were carried out at 4°C on a rotospin.
The samples were washed with ice cold 1× PBS and boiled at 70°C for 5 min in 30 µl of Laemmli buffer and 0.4 µl of β-mercaptoethanol. Samples were then loaded on an 8% SDS-PAGE gel which was transferred to a nitrocellulose membrane. For the negative control, samples were loaded in a gradient gel of 15% and 8%. The blots were blocked using 5% milk prepared in TBST (Tris buffered saline with 0.1% Tween 20). The membrane was incubated overnight in primary Lar antibody (1:40) prepared in 5% bovine serum albumin (BSA, HiMedia, MB083). All three blots (experimental, negative control and positive control) were then washed with 0.1% TBST for 40 min and incubated in mHRP (mouse horseradish peroxidase; Genescript, A00160) prepared in 5% milk (1:5000) for 45 min. The membrane was again washed with 0.1% TBST for 1 h and visualized using ECL (enhanced luminol-based chemiluminescent, Bio-Rad, 170-5061) in Image Quant LAS.
Membranes were stripped using stripping buffer (for 1 l: 15 g glycine, 1 g SDS, 10 ml Tween 20, pH 2.2) for 20 min and again blocked with 5% milk after washing with TBST. The experimental blot was incubated in pInR antibody (1:750). The blot for positive control was incubated in N-Cad antibody (1:50), and the blot that served as negative control was incubated in Rb-GFP antibody (1:1000). These were then visualized the following day after incubating with goat anti-rabbit HRP (GenScript, A00098,1:5000), goat anti-rat HRP (GenScript, A00167, 1:5000) and goat anti-rabbit HRP (1:5000), respectively.
Imaging and statistical analyses
All images were captured as z-sections using a Zeiss LSM780 confocal microscope. The same settings were used for each set of experiments. All the experiments were repeated at least thrice to ensure reproducibility, with 10 lymph glands analyzed per genotype for quantification analysis in most experiments.
Quantitative analysis of cell types in lymph gland
PSC cell counting
Antp-positive cells were counted using the spot function in Imaris software (Sharma et al., 2019; http://www.bitplane.com/download/manuals/QuickStartTutorials5_7_0.pdf). Data are expressed as mean±s.d. of values from three sets of independent experiments. All statistical analyses were performed using the two-tailed Student's t-test.
Quantification of intensity analysis
Intensity analysis of pAkt, p4EBP, ROS (DHE and gstD-GFP), InRV5, D4 lacZ, dpERK, Hh and Ci155 in different genotypes was carried out using the protocol mentioned in Shim et al. (2012) (http://sciencetechblog.files.wordpress.com/2011/05/measuring-cell-fluorescence-using-imagej.pdf).
For each genotype, ∼10 biological samples and at least five regions of interest (ROIs) were quantified. Data are expressed as mean±s.d., and reflect independent experiments and not technical replicates. All statistical analyses were performed using the two-tailed Student's t-test.
Quantification of intensity analysis of tGPH
Membranous intensity of tGPH was measured using line function in ImageJ/Fiji and cytoplasmic intensity was measured with a defined ROI inside the cell cytoplasm. Mean intensity was measured as previously described (Shim et al., 2012). The ratio of membranous to cytoplasmic mean intensity was then plotted.
*P<0.05, **P<0.005 and ***P<0.0005 were considered statistically significant. All statistical analyses were performed using the two-tailed Student's t-test. Unless otherwise indicated, larvae were 96 h AEH.
Supplementary Material
Acknowledgements
We thank I. Ando, Stephen Cohen, U. Banerjee, P. Ingham, M. Crozatier, Anurag Sharma, Jishy Vargeshe, Nick Sokol, Van Vactor and D. Bohmann for reagents. We thank all members of the two laboratories for their valuable input. Special thanks to Parvathy Ramesh and Satish Tiwari for their constant help and Ashutosh Tiwari for his assistance in mutant studies. We thank IISER Mohali's Confocal Facility, the Bloomington Drosophila Stock Center at Indiana University, the Vienna Drosophila Resource Center and the Developmental Studies Hybridoma Bank at the University of Iowa for flies and antibodies.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: H.K., L.M.; Methodology: H.K., S.K.S., L.M.; Validation: H.K., S.K.S.; Formal analysis: H.K., S.K.S., S.M., L.M.; Investigation: H.K., S.K.S., L.M.; Resources: H.K.; Data curation: H.K.; Writing - original draft: H.K., L.M.; Writing - review & editing: H.K., S.M., L.M.; Visualization: H.K., S.K.S., L.M.; Supervision: L.M.; Project administration: L.M.; Funding acquisition: L.M.
Funding
The current study was funded by a Department of Science and Technology (DST), Government of India Inspire Fellowship to H.K., Council of Scientific and Industrial Research (CSIR) funding to S.K.S., institutional support to S.M. and a Wellcome Trust DBT India Alliance Senior Fellowship [IA/S/17/1/503100] to L.M. Deposited in PMC for release after 6 months.
Supplementary information
Supplementary information available online at http://dev.biologists.org/lookup/doi/10.1242/dev.178202.supplemental
References
- Ahmad F., Considine R. V. and Goldstein B. J. (1995). Increased abundance of the receptor-type protein-tyrosine phosphatase LAR accounts for the elevated insulin receptor dephosphorylating activity in adipose tissue of obese human subjects. J. Clin. Invest. 95, 2806-2812. 10.1172/JCI117985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad F. and Goldstein B. J. (1997). Functional association between the insulin receptor and the transmembrane protein-tyrosine phosphatase LAR in intact cells. J. Biol. Chem. 272, 448-457. 10.1074/jbc.272.1.448 [DOI] [PubMed] [Google Scholar]
- Asha H., Nagy I., Kovacs G., Stetson D., Ando I. and Dearolf C. R. (2003). Analysis of Ras-induced overproliferation in Drosophila hemocytes. Genetics 163, 203-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldeosingh R., Gao H., Wu X. and Fossett N. (2018). Hedgehog signaling from the posterior signaling center maintains U-shaped expression and a prohemocyte population in Drosophila. Dev. Biol. 441, 132-145. 10.1016/j.ydbio.2018.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee U., Girard J. R., Goins L. M. and Spratford C. M. (2019). Drosophila as a genetic model for hematopoiesis. Genetics 211, 367-417. 10.1534/genetics.118.300223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman J., Reddy R. S., Saito H. and Van Vactor D. (2001). The receptor tyrosine phosphatase Dlar and integrins organize actin filaments in the Drosophila follicular epithelium. Curr. Biol. 11, 1317-1327. 10.1016/S0960-9822(01)00420-1 [DOI] [PubMed] [Google Scholar]
- Benmimoun B., Polesello C., Waltzer L. and Haenlin M. (2012). Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development 139, 1713-1717. 10.1242/dev.080259 [DOI] [PubMed] [Google Scholar]
- Bodmer R. and Venkatesh T. V. (1998). Heart development in Drosophila and vertebrates: conservation of molecular mechanisms. Dev. Genet. 22, 181-186. [DOI] [PubMed] [Google Scholar]
- Brand A. H. and Perrimon N. (1993). Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118, 401-415. [DOI] [PubMed] [Google Scholar]
- Brännmark C., Nyman E., Fagerholm S., Bergenholm L., Ekstrand E. M., Cedersund G. and Stralfors P. (2013). Insulin signaling in type 2 diabetes: experimental and modeling analyses reveal mechanisms of insulin resistance in human adipocytes. J. Biol. Chem. 288, 9867-9880. 10.1074/jbc.M112.432062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton J. S., Lockwood W. K., Li L., Cohen S. M. and Edgar B. A. (2002). Drosophila's insulin/PI3-kinase pathway coordinates cellular metabolism with nutritional conditions. Dev. Cell 2, 239-249. 10.1016/S1534-5807(02)00117-X [DOI] [PubMed] [Google Scholar]
- Chacon-Martinez C. A., Koester J. and Wickstrom S. A. (2018). Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development 145, dev165399 10.1242/dev.165399 [DOI] [PubMed] [Google Scholar]
- Chagnon M. J., Uetani N. and Tremblay M. L. (2004). Functional significance of the LAR receptor protein tyrosine phosphatase family in development and diseases. Biochem. Cell Biol. 82, 664-675. 10.1139/o04-120 [DOI] [PubMed] [Google Scholar]
- Chatterjee N. and Bohmann D. (2012). A versatile PhiC31 based reporter system for measuring AP-1 and Nrf2 signaling in Drosophila and in tissue culture. PLoS ONE 7, e34063 10.1371/journal.pone.0034063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crozatier M., Ubeda J.-M., Vincent A. and Meister M. (2004). Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2, E196 10.1371/journal.pbio.0020196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai C. J., Krueger N. X., Saito H. and Zinn K. (1997). Competition and cooperation among receptor tyrosine phosphatases control motoneuron growth cone guidance in Drosophila. Development 124, 1941-1952. [DOI] [PubMed] [Google Scholar]
- Dey N. S., Ramesh P., Chugh M., Mandal S. and Mandal L. (2016). Dpp dependent Hematopoietic stem cells give rise to Hh dependent blood progenitors in larval lymph gland of Drosophila. Elife 5, e18295 10.7554/eLife.18295.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragojlovic-Munther M. and Martinez-Agosto J. A. (2013). Extracellular matrix-modulated Heartless signaling in Drosophila blood progenitors regulates their differentiation via a Ras/ETS/FOG pathway and target of rapamycin function. Dev. Biol. 384, 313-330. 10.1016/j.ydbio.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson P. and Jönsson J. I. (2010). The hematopoietic stem cell niche: low in oxygen but a nice place to be. J. Cell. Physiol. 222, 17-22. 10.1002/jcp.21908 [DOI] [PubMed] [Google Scholar]
- Evans C. J., Hartenstein V. and Banerjee U. (2003). Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev. Cell 5, 673-690. 10.1016/S1534-5807(03)00335-6 [DOI] [PubMed] [Google Scholar]
- Ferraro F., Lymperi S., Mendez-Ferrer S., Saez B., Spencer J. A., Yeap B. Y., Masselli E., Graiani G., Prezioso L., Rizzini E. L. et al. (2011). Diabetes impairs hematopoietic stem cell mobilization by altering niche function. Sci. Transl. Med. 3, 104ra101 10.1126/scitranslmed.3002191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes A. J., Nakano Y., Taylor A. M. and Ingham P. W. (1993). Genetic analysis of hedgehog signalling in the Drosophila embryo. Dev. Suppl. 115-124. [PubMed] [Google Scholar]
- Fröjdö S., Vidal H. and Pirola L. (2009). Alterations of insulin signaling in type 2 diabetes: a review of the current evidence from humans. Biochim. Biophys. Acta 1792, 83-92. 10.1016/j.bbadis.2008.10.019 [DOI] [PubMed] [Google Scholar]
- Frydman H. M. and Spradling A. C. (2001). The receptor-like tyrosine phosphatase lar is required for epithelial planar polarity and for axis determination within Drosophila ovarian follicles. Development 128, 3209-3220. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Tumbar T. and Guasch G. (2004). Socializing with the neighbors: stem cells and their niche. Cell 116, 769-778. 10.1016/S0092-8674(04)00255-7 [DOI] [PubMed] [Google Scholar]
- Geminard C., Rulifson E. J. and Léopold P. (2009). Remote control of insulin secretion by fat cells in Drosophila. Cell Metab. 10, 199-207. 10.1016/j.cmet.2009.08.002 [DOI] [PubMed] [Google Scholar]
- Gershon T. R., Baker M. W., Nitabach M. and Macagno E. R. (1998). The leech receptor protein tyrosine phosphatase HmLAR2 is concentrated in growth cones and is involved in process outgrowth. Development 125, 1183-1190. [DOI] [PubMed] [Google Scholar]
- Harrington R. J., Gutch M. J., Hengartner M. O., Tonks N. K. and Chisholm A. D. (2002). The C. elegans LAR-like receptor tyrosine phosphatase PTP-3 and the VAB-1 Eph receptor tyrosine kinase have partly redundant functions in morphogenesis. Development 129, 2141-2153. 10.3410/f.1005928.72555 [DOI] [PubMed] [Google Scholar]
- Hietakangas V. and Cohen S. M. (2009). Regulation of tissue growth through nutrient sensing. Annu. Rev. Genet. 43, 389-410. 10.1146/annurev-genet-102108-134815 [DOI] [PubMed] [Google Scholar]
- Hoggatt J., Kfoury Y. and Scadden D. T. (2016). Hematopoietic stem cell niche in health and disease. Annu. Rev. Pathol. 11, 555-581. 10.1146/annurev-pathol-012615-044414 [DOI] [PubMed] [Google Scholar]
- Johnson S. C., Rabinovitch P. S. and Kaeberlein M. (2013). mTOR is a key modulator of ageing and age-related disease. Nature 493, 338-345. 10.1038/nature11861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S. H., Evans C. J., Uemura C. and Banerjee U. (2005). The Drosophila lymph gland as a developmental model of hematopoiesis. Development 132, 2521-2533. 10.1242/dev.01837 [DOI] [PubMed] [Google Scholar]
- Kaufmann N., DeProto J., Ranjan R., Wan H. and Van Vactor D. (2002). Drosophila liprin-alpha and the receptor phosphatase Dlar control synapse morphogenesis. Neuron 34, 27-38. 10.1016/s0896-6273(02)00643-8 [DOI] [PubMed] [Google Scholar]
- Kiger A. A., Jones D. L., Schulz C., Rogers M. B. and Fuller M. T. (2001). Stem cell self-renewal specified by JAK-STAT activation in response to a support cell cue. Science 294, 2542-2545. 10.1126/science.1066707 [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Van Vactor D., Wan H. I., Gelbart W. M., Goodman C. S. and Saito H. (1996). The transmembrane tyrosine phosphatase DLAR controls motor axon guidance in Drosophila. Cell 84, 611-622. 10.1016/S0092-8674(00)81036-3 [DOI] [PubMed] [Google Scholar]
- Krueger N. X., Reddy R. S., Johnson K., Bateman J., Kaufmann N., Scalice D., Van Vactor D. and Saito H. (2003). Functions of the ectodomain and cytoplasmic tyrosine phosphatase domains of receptor protein tyrosine phosphatase Dlar in vivo. Mol. Cell. Biol. 23, 6909-6921. 10.1128/MCB.23.19.6909-6921.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzemień J., Dubois L., Makki R., Meister M., Vincent A. and Crozatier M. (2007). Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature 446, 325-328. 10.1038/nature05650 [DOI] [PubMed] [Google Scholar]
- Kulas D. T., Zhang W. R., Goldstein B. J., Furlanetto R. W. and Mooney R. A. (1995). Insulin receptor signaling is augmented by antisense inhibition of the protein tyrosine phosphatase LAR. J. Biol. Chem. 270, 2435-2438. 10.1074/jbc.270.6.2435 [DOI] [PubMed] [Google Scholar]
- Kulas D. T., Goldstein B. J. and Mooney R. A. (1996). The transmembrane protein-tyrosine phosphatase LAR modulates signaling by multiple receptor tyrosine kinases. J. Biol. Chem. 271, 748-754. 10.1074/jbc.271.2.748 [DOI] [PubMed] [Google Scholar]
- Kurucz E., Márkus R., Zsámboki J., Folkl-Medzihradszky K., Darula Z., Vilmos P., Udvardy A., Krausz I., Lukacsovich Y., Gateff E. et al. (2007). Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 17, 649-654. 10.1016/j.cub.2007.02.041 [DOI] [PubMed] [Google Scholar]
- Lam V., Tokusumi T., Tokusumi Y. and Schulz R. A. (2014). bantam miRNA is important for Drosophila blood cell homeostasis and a regulator of proliferation in the hematopoietic progenitor niche. Biochem. Biophys. Res. Commun. 453, 467-472. 10.1016/j.bbrc.2014.09.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin J., Morgan M. J., Rossé C., Racine V., Sibarita J.-B., Aresta S., Murthy M., Schwarz T., Camonis J. and Bellaïche Y. (2005). Drosophila exocyst components Sec5, Sec6, and Sec15 regulate DE-Cadherin trafficking from recycling endosomes to the plasma membrane. Dev. Cell 9, 365-376. 10.1016/j.devcel.2005.07.013 [DOI] [PubMed] [Google Scholar]
- Lanier L. M. and Gertler F. B. (2000). From Abl to actin: Abl tyrosine kinase and associated proteins in growth cone motility. Curr. Opin. Neurobiol. 10, 80-87. 10.1016/S0959-4388(99)00058-6 [DOI] [PubMed] [Google Scholar]
- Li L. and Xie T. (2005). Stem cell niche: structure and function. Annu. Rev. Cell Dev. Biol. 21, 605-631. 10.1146/annurev.cellbio.21.012704.131525 [DOI] [PubMed] [Google Scholar]
- Lin H. (2002). The stem-cell niche theory: lessons from flies. Nat. Rev. Genet. 3, 931-940. 10.1038/nrg952 [DOI] [PubMed] [Google Scholar]
- Lo Celso C. and Scadden D. T. (2011). The haematopoietic stem cell niche at a glance. J. Cell Sci. 124, 3529-3535. 10.1242/jcs.074112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick V. P., Morris L. X., Fox D. T. and Spradling A. (2011). Drosophila stem cell niches: a decade of discovery suggests a unified view of stem cell regulation. Dev. Cell 21, 159-171. 10.1016/j.devcel.2011.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louradour I., Sharma A., Morin-Poulard I., Letourneau M., Vincent A., Crozatier M. and Vanzo N. (2017). Reactive oxygen species-dependent Toll/NF-kappaB activation in the Drosophila hematopoietic niche confers resistance to wasp parasitism. Elife 6, e25496 10.7554/eLife.25496.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luhur A., Buddika K., Ariyapala I. S., Chen S. and Sokol N. S. (2017). Opposing post-transcriptional control of InR by FMRP and LIN-28 adjusts stem cell-based tissue growth. Cell Rep. 21, 2671-2677. 10.1016/j.celrep.2017.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madan L. L., Veeranna S., Shameer K., Reddy C. C., Sowdhamini R. and Gopal B. (2011). Modulation of catalytic activity in multi-domain protein tyrosine phosphatases. PLoS ONE 6, e24766 10.1371/journal.pone.0024766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandal L., Martinez-Agosto J. A., Evans C. J., Hartenstein V. and Banerjee U. (2007). A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature 446, 320-324. 10.1038/nature05585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal B. C., Mukherjee T., Mandal L., Evans C. J., Sinenko S. A., Martinez-Agosto J. A. and Banerjee U. (2011). Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell 147, 1589-1600. 10.1016/j.cell.2011.11.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney R. A., Kulas D. T., Bleyle L. A. and Novak J. S. (1997). The protein tyrosine phosphatase LAR has a major impact on insulin receptor dephosphorylation. Biochem. Biophys. Res. Commun. 235, 709-712. 10.1006/bbrc.1997.6889 [DOI] [PubMed] [Google Scholar]
- Morrison S. J. and Spradling A. C. (2008). Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell 132, 598-611. 10.1016/j.cell.2008.01.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A. M., Sheils O. M., McDonald G. S. and Kelleher D. P. (2005). Detection of a tyrosine phosphatase LAR on intestinal epithelial cells and intraepithelial lymphocytes in the human duodenum. Mediators Inflamm. 2005, 23-30. 10.1155/MI.2005.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa S., Sato Y., Enomoto M., Nakamura M., Betsumiya A. and Igaki T. (2012). Mitochondrial defect drives non-autonomous tumour progression through Hippo signalling in Drosophila. Nature 490, 547-551. 10.1038/nature11452 [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E. and Banerjee U. (2009). Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature 461, 537-541. 10.1038/nature08313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah E., Yavari A., Mandal S. and Banerjee U. (2008). Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 40, 356-361. 10.1038/ng.2007.50 [DOI] [PubMed] [Google Scholar]
- Pennetier D., Oyallon J., Morin-Poulard I., Dejean S., Vincent A. and Crozatier M. (2012). Size control of the Drosophila hematopoietic niche by bone morphogenetic protein signaling reveals parallels with mammals. Proc. Natl. Acad. Sci. USA 109, 3389-3394. 10.1073/pnas.1109407109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poloz Y. and Stambolic V. (2015). Obesity and cancer, a case for insulin signaling. Cell Death Dis. 6, e2037 10.1038/cddis.2015.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash S., Mclendon H. M., Dubreuil C. I., Ghose A., Hwa J., Dennehy K. A., Tomalty K. M., Clark K. L., Van Vactor D. and Clandinin T. R. (2009). Complex interactions amongst N-cadherin, DLAR, and Liprin-alpha regulate Drosophila photoreceptor axon targeting. Dev. Biol. 336, 10-19. 10.1016/j.ydbio.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig O. and Tjian R. (2005). Transcriptional feedback control of insulin receptor by dFOXO/FOXO1. Genes Dev. 19, 2435-2446. 10.1101/gad.1340505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray A., Alalem M. and Ray B. K. (2014). Insulin signaling network in cancer. Indian J. Biochem. Biophys. 51, 493-498. [PubMed] [Google Scholar]
- Rizki T. M. and Rizki R. M. (1992). Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev. Comp. Immunol. 16, 103-110. 10.1016/0145-305X(92)90011-Z [DOI] [PubMed] [Google Scholar]
- Roth S. W., Bitterman M. D., Birnbaum M. J. and Bland M. L. (2018). Innate immune signaling in Drosophila blocks insulin signaling by uncoupling PI(3,4,5)P3 production and Akt activation. Cell Rep. 22, 2550-2556. 10.1016/j.celrep.2018.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo L. J., Gao X., Chiarelli D. A., Li L., Pan D. and Edgar B. A. (2003). Rheb promotes cell growth as a component of the insulin/TOR signalling network. Nat. Cell Biol. 5, 566-571. 10.1038/ncb996 [DOI] [PubMed] [Google Scholar]
- Scadden D. T. (2006). The stem-cell niche as an entity of action. Nature 441, 1075-1079. 10.1038/nature04957 [DOI] [PubMed] [Google Scholar]
- Scadden D. T. (2014). Nice neighborhood: emerging concepts of the stem cell niche. Cell 157, 41-50. 10.1016/j.cell.2014.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S. K., Ghosh S., Geetha A. R., Mandal S. and Mandal L. (2019). Cell adhesion-mediated actomyosin assembly regulates the activity of cubitus interruptus for hematopoietic progenitor maintenance in Drosophila. Genetics 212, 1279-1300. 10.1534/genetics.119.302209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H. M. and Liu Z.-G. (2006). JNK signaling pathway is a key modulator in cell death mediated by reactive oxygen and nitrogen species. Free Radic. Biol. Med. 40, 928-939. 10.1016/j.freeradbiomed.2005.10.056 [DOI] [PubMed] [Google Scholar]
- Shim J., Mukherjee T. and Banerjee U. (2012). Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat. Cell Biol. 14, 394-400. 10.1038/ncb2453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinenko S. A., Shim J. and Banerjee U. (2011). Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 13, 83-89. 10.1038/embor.2011.223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrentino R. P., Carton Y. and Govind S. (2002). Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev. Biol. 243, 65-80. 10.1006/dbio.2001.0542 [DOI] [PubMed] [Google Scholar]
- Srinivasan S., Mahowald A. P. and Fuller M. T. (2012). The receptor tyrosine phosphatase Lar regulates adhesion between Drosophila male germline stem cells and the niche. Development 139, 1381-1390. 10.1242/dev.070052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Hall L. R., Schlossman S. F. and Saito H. (1988). A new member of the immunoglobulin superfamily that has a cytoplasmic region homologous to the leukocyte common antigen. J. Exp. Med. 168, 1523-1530. 10.1084/jem.168.5.1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Tsai A. Y. and Saito H. (1989). A family of receptor-linked protein tyrosine phosphatases in humans and Drosophila. Proc. Natl. Acad. Sci. USA 86, 8698-8702. 10.1073/pnas.86.22.8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Bahri S., Schmid A., Chia W. and Zinn K. (2000). Receptor tyrosine phosphatases regulate axon guidance across the midline of the Drosophila embryo. Development 127, 801-812. [DOI] [PubMed] [Google Scholar]
- Sykiotis G. P. and Bohmann D. (2008). Keap1/Nrf2 signaling regulates oxidative stress tolerance and lifespan in Drosophila. Dev. Cell 14, 76-85. 10.1016/j.devcel.2007.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tettweiler G., Miron M., Jenkins M., Sonenberg N. and Lasko P. F. (2005). Starvation and oxidative stress resistance in Drosophila are mediated through the eIF4E-binding protein, d4E-BP. Genes Dev. 19, 1840-1843. 10.1101/gad.1311805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi Y., Tokusumi T., Stoller-Conrad J. and Schulz R. A. (2010). Serpent, suppressor of hairless and U-shaped are crucial regulators of hedgehog niche expression and prohemocyte maintenance during Drosophila larval hematopoiesis. Development 137, 3561-3568. 10.1242/dev.053728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi Y., Tokusumi T., Shoue D. A. and Schulz R. A. (2012). Gene regulatory networks controlling hematopoietic progenitor niche cell production and differentiation in the Drosophila lymph gland. PLoS ONE 7, e41604 10.1371/journal.pone.0041604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokusumi T., Tokusumi Y., Hopkins D. W. and Schulz R. A. (2015). Bag of Marbles controls the size and organization of the Drosophila hematopoietic niche through interactions with the Insulin-like growth factor pathway and Retinoblastoma-family protein. Development 142, 2261-2267. 10.1242/dev.121798 [DOI] [PubMed] [Google Scholar]
- Tsuchiya A., Kanno T. and Nishizaki T. (2014). PI3 kinase directly phosphorylates Akt1/2 at Ser473/474 in the insulin signal transduction pathway. J. Endocrinol. 220, 49-59. 10.1530/JOE-13-0172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujikawa K., Kawakami N., Uchino Y., Ichijo T., Furukawa T., Saito H. and Yamamoto H. (2001). Distinct functions of the two protein tyrosine phosphatase domains of LAR (leukocyte common antigen-related) on tyrosine dephosphorylation of insulin receptor. Mol. Endocrinol. 15, 271-280. 10.1210/mend.15.2.0592 [DOI] [PubMed] [Google Scholar]
- Tulina N. and Matunis E. (2001). Control of stem cell self-renewal in Drosophila spermatogenesis by JAK-STAT signaling. Science 294, 2546-2549. 10.1126/science.1066700 [DOI] [PubMed] [Google Scholar]
- Um J. W. and Ko J. (2013). LAR-RPTPs: synaptic adhesion molecules that shape synapse development. Trends Cell Biol. 23, 465-475. 10.1016/j.tcb.2013.07.004 [DOI] [PubMed] [Google Scholar]
- Vilmos P., Nagy I., Kurucz E., Hultmark D., Gateff E. and Ando I. (2004). A rapid rosetting method for separation of hemocyte sub-populations of Drosophila melanogaster. Dev. Comp. Immunol. 28, 555-563. 10.1016/j.dci.2003.10.001 [DOI] [PubMed] [Google Scholar]
- Wagers A. J. (2012). The stem cell niche in regenerative medicine. Cell Stem Cell 10, 362-369. 10.1016/j.stem.2012.02.018 [DOI] [PubMed] [Google Scholar]
- Wagner E. F. and Nebreda A. R. (2009). Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer 9, 537-549. 10.1038/nrc2694 [DOI] [PubMed] [Google Scholar]
- Xie T. and Spradling A. C. (2000). A niche maintaining germ line stem cells in the Drosophila ovary. Science 290, 328-330. 10.1126/science.290.5490.328 [DOI] [PubMed] [Google Scholar]
- Yamashita Y. M., Fuller M. T. and Jones D. L. (2005). Signaling in stem cell niches: lessons from the Drosophila germline. J. Cell Sci. 118, 665-672. 10.1242/jcs.01680 [DOI] [PubMed] [Google Scholar]
- Zabolotny J. M., Kim Y. B., Peroni O. D., Kim J. K., Pani M. A., Boss O., Klaman L. D., Kamatkar S., Shulman G. I., Kahn B. B., et al. (2001). Overexpression of the LAR (leukocyte antigen-related) protein-tyrosine phosphatase in muscle causes insulin resistance. Proc. Natl. Acad. Sci. USA 98, 5187-5192. 10.1073/pnas.071050398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zettervall C. J., Anderl I., Williams M. J., Palmer R., Kurucz E., Ando I. and Hultmark D. (2004). A directed screen for genes involved in Drosophila blood cell activation. Proc. Natl. Acad. Sci. USA 101, 14192-14197. 10.1073/pnas.0403789101 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.