For many viral infections, current treatment options are insufficient. Because the development of each antiviral drug is time-consuming and expensive, the prospect of finding broad-spectrum antivirals that can fight multiple, diverse viruses—well-known viruses as well as (re)emerging species—has gained attention, especially for the treatment of viral coinfections. While most known broad-spectrum agents address processes in the host cell, we found that targeting lipids of the free virus outside the host cell with the natural products labyrinthopeptin A1 and A2 is a viable strategy to inhibit the proliferation of a broad range of viruses from different families, including chikungunya virus, dengue virus, Zika virus, Kaposi’s sarcoma-associated herpesvirus, and cytomegalovirus. Labyrinthopeptins bind to viral phosphatidylethanolamine and induce virolysis without exerting cytotoxicity on host cells. This represents a novel and unusual mechanism to tackle medically relevant viral infections.
KEYWORDS: lanthipeptides, antivirals, drug discovery, mechanism of action, phosphatidylethanolamine, lipids, drug synergism, dengue virus, DENV, Zika virus, ZIKV
ABSTRACT
To counteract the serious health threat posed by known and novel viral pathogens, drugs that target a variety of viruses through a common mechanism have attracted recent attention due to their potential in treating (re)emerging infections, for which direct-acting antivirals are not available. We found that labyrinthopeptins A1 and A2, the prototype congeners of carbacyclic lanthipeptides, inhibit the proliferation of diverse enveloped viruses, including dengue virus, Zika virus, West Nile virus, hepatitis C virus, chikungunya virus, Kaposi’s sarcoma-associated herpesvirus, cytomegalovirus, and herpes simplex virus, in the low micromolar to nanomolar range. Mechanistic studies on viral particles revealed that labyrinthopeptins induce a virolytic effect through binding to the viral membrane lipid phosphatidylethanolamine (PE). These effects are enhanced by a combined equimolar application of both labyrinthopeptins, and a clear synergism was observed across a concentration range corresponding to 10% to 90% inhibitory concentrations of the compounds. Time-resolved experiments with large unilamellar vesicles (LUVs) reveal that membrane lipid raft compositions (phosphatidylcholine [PC]/PE/cholesterol/sphingomyelin at 17:10:33:40) are particularly sensitive to labyrinthopeptins in comparison to PC/PE (90:10) LUVs, even though the overall PE amount remains constant. Labyrinthopeptins exhibited low cytotoxicity and had favorable pharmacokinetic properties in mice (half-life [t1/2] = 10.0 h), which designates them promising antiviral compounds acting by an unusual viral lipid targeting mechanism.
IMPORTANCE For many viral infections, current treatment options are insufficient. Because the development of each antiviral drug is time-consuming and expensive, the prospect of finding broad-spectrum antivirals that can fight multiple, diverse viruses—well-known viruses as well as (re)emerging species—has gained attention, especially for the treatment of viral coinfections. While most known broad-spectrum agents address processes in the host cell, we found that targeting lipids of the free virus outside the host cell with the natural products labyrinthopeptin A1 and A2 is a viable strategy to inhibit the proliferation of a broad range of viruses from different families, including chikungunya virus, dengue virus, Zika virus, Kaposi’s sarcoma-associated herpesvirus, and cytomegalovirus. Labyrinthopeptins bind to viral phosphatidylethanolamine and induce virolysis without exerting cytotoxicity on host cells. This represents a novel and unusual mechanism to tackle medically relevant viral infections.
INTRODUCTION
One-third of the overall global mortality caused by infectious diseases is a consequence of viral infections (1). One decisive factor contributing to these numbers is the increased worldwide incidence of newly emerging as well as reemerging viral threats (2). Demographic changes, increased travel activities, and global warming facilitate the spread of pathogenic viruses, including severe acute respiratory syndrome (SARS) virus, Middle East respiratory syndrome (MERS) virus, human immunodeficiency virus (HIV), respiratory syncytial virus (RSV), Herpesviridae, hepatitis B virus (HBV), hepatitis C virus (HCV), and influenza viruses. Pathogens causing neglected tropical diseases like dengue virus (DENV) or chikungunya virus nowadays affect not only tropical regions but also subtropical or even temperate zones.
Regarding DENV, the WHO currently classifies 3.9 billion people in 128 countries as being at risk of infection. Bhatt et al. estimated that the global incidence of infections with one of the four DENV serotypes (DENV 1 to 4) is about 390 million per year (3). A fraction of these infections becomes life-threatening due to dengue hemorrhagic fever or dengue shock syndrome (4). More recently, Zika virus (ZIKV) has surfaced as a (re)emerging viral threat. Besides causing Zika fever, infection with ZIKV has been linked to Guillain-Barré syndrome. During a ZIKV epidemic in South America in 2015, an association between ZIKV infections and infants born with microcephaly was observed (5). Experiments with ZIKV-infected IFNAR−/− mice demonstrated that the virus impairs fetal growth, damages the brain, and leads to miscarriage (6). Furthermore, ZIKV was shown to induce fetal brain lesions in nonhuman primates (7). Flaviviruses such as DENV or ZIKV may quickly mutate to escape vaccination or drug treatment. Antivirals against both DENV and ZIKV are still lacking, although significant efforts have been made to identify small-molecule inhibitors (8).
Most current antiviral drug discovery efforts focus on direct-acting antivirals (DAAs) that target specific viral enzymes or genomes. It is common to coadminister different DAAs to counteract resistance development (“many drugs, one bug”). In contrast to this, we embarked on the discovery of broad-spectrum antiviral agents that are active against a variety of viruses through a common mechanism that ideally would be not prone to viral resistance development (“one drug, many bugs”). This approach has caught recent attention due to several potential advantages, including a reduced treatment complexity and fewer drug-drug interactions in the case of coinfections with two or more viruses (9–12). It also provides a treatment option for emerging and known infections for which specific drugs have not yet been developed. For agents directed against host cell targets, a slower development of resistance is expected, as the dependence on cellular processes is hard to overcome by single viral mutations.
In this study, we report the broad-spectrum antiviral activity of labyrinthopeptins A1 and A2 (LabyA1 and LabyA2). LabyA1 and LabyA2 are posttranslationally modified peptides isolated from the actinomycete Actinomadura namibiensis DSM 6313 (13, 14). Labyrinthopeptins are members of class III lanthipeptides, characterized by the eponymous nonproteinogenic amino acid lanthionine that forms intramolecular thioether bridges (15), and in particular by carbacycles, which are spanned by the αC-quaternary substituted amino acid labionin (Fig. 1A). Following a first report on the anti-HIV and anti-herpes simplex virus (HSV) activities of LabyA1 (16), we herein describe the broad antiviral spectrum of the compounds with a focus on the flaviviruses ZIKV and DENV. In addition, we decipher their molecular mechanism of action and provide evidence for a molecular synergism of LabyA1 and LabyA2. Mechanistic and in vivo pharmacokinetics (PK) studies further underline their potential as novel lead compounds for the treatment of viral infections.
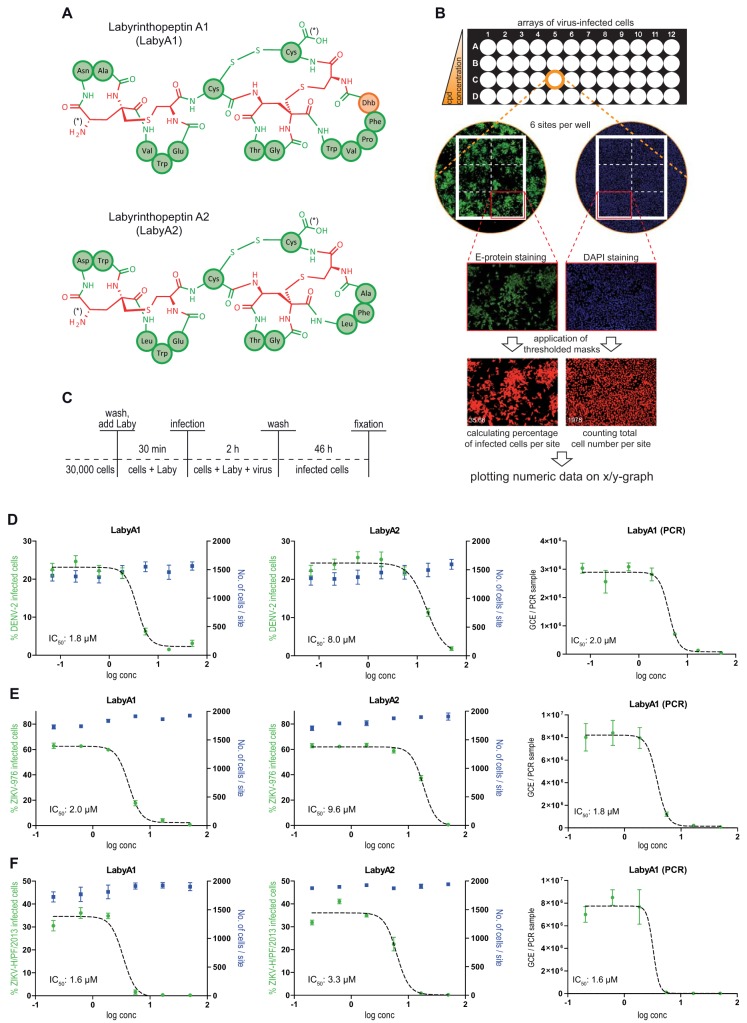
FIG 1.
Labyrinthopeptins efficiently prevent infection of Huh-7 cells with DENV and ZIKV. (A) Schematic primary structures of labyrinthopeptins A1 and A2. The chemical structure of labionin, an αC-quaternary substituted lanthionine, is highlighted in red. Proteinaceous amino acids in three-letter notation are indicated in green. Didehydrobutyrine (Dhb) is indicated in orange. N and C termini are indicated by an asterisk. For further details, see reference 14. (B) Evaluation of viral infection by high-content imaging. Cells were treated with a compound of interest (here LabyA1 and LabyA2) and infected with virus (here ZIKV-H/PF/2013) according to Materials and Methods. Fixed cells were DAPI stained and immunostained for the Zika viral epitope (Alexa Fluor 488). Accordingly processed 96-well plates were analyzed by high-content imaging using an ImageXpressMicro automated microscope (Molecular Devices). Values obtained from the six sites acquired per well were averaged and plotted onto a semilogarithmic X/Y chart. IC50 values were calculated by nonlinear regression using GraphPad Prism. (C) Assay setup for infection of Huh-7 cells with DENV or ZIKV. After fixation, arrayed cells were processed for high-content imaging to assess the number of infected cells (dose-response assay). (D to F) Left and middle panels, dose-response curves obtained for labyrinthopeptin-treated Huh-7 cells infected with DENV-2 (D), ZIKV-976 (E), or ZIKV-H/PF/2013 (F). The total cell number as well as the percentage of virus-infected cells was determined by high-content imaging. Right panels, dose-response curves obtained by RT-PCR from RNA extracted from cell culture supernatants of the same cells. LabyA1 inhibited viral infection of cells more efficiently than LabyA2. Data shown are means of 2 to 5 assays ± SEM. Nonlinear regression was performed with GraphPad Prism.
RESULTS
Labyrinthopeptins exert broad-spectrum antiviral activity.
To study the antiviral activity of LabyA1 and LabyA2, Huh-7 cells were pretreated with increasing concentrations of the compounds up to 24.1 μM and 26.0 μM, respectively. Cells were subsequently infected with either DENV-2 (New Guinea C strain), ZIKV-976 (Uganda strain), or ZIKV-H/PF/2013 (French Polynesia strain) (Fig. 1C). The total cell number as well as the number of DENV- or ZIKV-infected cells was assessed by high-content imaging after staining of nuclei and immunostaining of viral epitopes (Fig. 1B) (17, 18). LabyA1 potently inhibited cellular infections with DENV-2, ZIKV-976, and ZIKV-H/PF/2013 with 50% inhibitory concentration (IC50) values of 1.8 μM, 2.0 μM, and 1.6 μM, respectively. The results were confirmed via reverse transcription-quantitative PCR (qRT-PCR) by determining the amount of DENV or ZIKV genome copy equivalents (GCE) in the cell culture supernatant of the infected cells (Fig. 1D to F). The anti-DENV activity covered all four DENV serotypes, as shown by infection experiments with monocyte-derived dendritic cells (MDDC) (Table 1). Infection of cells by other flaviviruses such as West Nile virus (WNV) and hepatitis C virus (HCV) was also impaired (IC50s, 0.2 μM and 1.1 μM). Virus inhibition, however, was not restricted to flaviviruses but covered a variety of evolutionary unrelated viruses (Table 1). In addition to herpes simplex virus (HSV) and human immunodeficiency virus (HIV) (16), LabyA1 inhibited infection of cells with chikungunya virus (CHIKV) and CHIKVgp-bearing lentiviral pseudotypes with IC50 values of 2.2 μM and 1.8 μM, respectively. A 3′-GFP-CHIKV derived from the invertebrate cell line C6/36 was more sensitive to LabyA1 treatment (IC50 = 0.4 μM) than mammalian BHK-21-derived 3′-GFP-CHIKV (IC50 = 2.1 μM). Infection of cells with the herpesviruses Kaposi’s sarcoma-associated herpesvirus (KSHV) and human cytomegalovirus (HCMV) was also affected, with IC50 values of 2.0 μM and 1.3 μM, respectively. Nonetheless, only weak activity (IC50 = 24.1 μM) was observed against tick-borne encephalitis virus (TBEV). Infectivity of the nonenveloped coxsackievirus (CSV) and adenovirus (type 5) was affected by neither LabyA1 (IC50 values, >48 μM and >25 μM, respectively) nor LabyA2 (IC50 values, >52 μM and >50 μM, respectively). In general, LabyA2 showed IC50 values that were 2- to 15-fold higher than those of LabyA1. Cytotoxicity of labyrinthopeptins was not observed at maximal concentrations of 24 μM to 48 μM, underscoring the specificity of the broad antiviral effects.
TABLE 1.
Antiviral activity spectrum of LabyA1 and LabyA2
| Virus | Tested cells | Assay | IC50 (μM) |
CC50 (μM) |
||
|---|---|---|---|---|---|---|
| LabyA1 | LabyA2 | LabyA1 | LabyA2 | |||
| Enveloped viruses | ||||||
| CHIKV | 293-T | Quantification of cells infected with 3′-GFP-CHIKV | 2.2 | NDb | ND | ND |
| CHIKV (pseudotype) | 293-T | Quantification of lentiviral luciferase reporter particles pseudotyped with CHIKV glycoproteins | 1.8 | >5.0 | ND | ND |
| DENV-1 | MDDC | Determination of infected cells by flow cytometry (E protein immunostaining) | 0.8 | 12.0 | >24.1 | >26.0 |
| DENV-2 | Huh-7 | Determination of infected cells by high content imaging (E-protein immunostaining) | 1.8 | 8.0 | >24.1 | >26.0 |
| DENV-2 | Huh-7 | Quantification of viral load in cell culture supernatant by qRT-PCR | 2.0 | 7.6 | >24.1 | >26.0 |
| DENV-2 | MDDC | Determination of infected cells by flow cytometry (E protein immunostaining) | 0.4 | 5.2 | >24.1 | >26.0 |
| DENV-2 | MDDC | Quantification of viral load in cell culture supernatant by qRT-PCR | 0.3 | 4.2 | >24.1 | >26.0 |
| DENV-3 | MDDC | Determination of infected cells by flow cytometry (E protein immunostaining) | 1.1 | 9.4 | >24.1 | >26.0 |
| DENV-4 | MDDC | Determination of infected cells by flow cytometry (E protein immunostaining) | 0.3 | 4.0 | >24.1 | >26.0 |
| HCMV | NHDF | Quantification of virus-derived GFP expression in infected cells | 1.3 | 5.4 | ND | ND |
| HCV | Huh-7 | Intracellular Renilla luciferase activity of HCV JcR2a reporter construct | 1.1 | 1.7 | >50.0 | >50.0 |
| HIV (laboratory strains)a | MT-4 T cells | Determination of virus-induced cytopathicity | 1.9 | ND | 33.0 | ND |
| PBMC | Quantification of viral load via p24-Ag ELISA | 1.7 | ND | 45.0 | ND | |
| MDM | Quantification of viral load via p24-Ag ELISA | 2.4 | ND | ND | ND | |
| HIV (clinical isolates)a | PBMC | Quantification of viral load via p24-Ag ELISA | 1.0 | ND | 45.0 | ND |
| HSV-1a | HEL | Determination of virus-induced cytopathicity | 1.6 | 12.4 | >48.2 | ND |
| HSV-1 (Tk deficient)a | HEL | Determination of virus-induced cytopathicity | 1.9 | 10.7 | >48.2 | ND |
| HSV-2a | HEL | Determination of virus-induced cytopathicity | 0.4 | 5.5 | >48.2 | ND |
| HSV-2 (Tk deficient)a | HEL | Determination of virus-induced cytopathicity | 0.3 | 3.2 | >48.2 | ND |
| KSHV | HEK-293 (GFP) | Determination of virus-induced cytopathicity by counting remaining GFP fluorescent cells | 2.0 | 15.0 | ND | ND |
| TBEV | Vero | Visual plaque reduction after cells were immunostained for viral E protein | 24.1 | >26.0 | >24.1 | >26.0 |
| WNV | Huh-7 | Quantification of viral load in cell culture supernatant by microplaque assay | 0.2 | 0.7 | >48.2 | >52.0 |
| ZIKV-976 | Huh-7 | Determination of infected cells by high-content imaging (E protein immunostaining) | 2.0 | 9.6 | >24.1 | >26.0 |
| ZIKV-976 | Huh-7 | Quantification of viral load in cell culture supernatant by qRT-PCR | 1.8 | 5.7 | >24.1 | >26.0 |
| ZIKV-H/PF-2013 | Huh-7 | Determination of infected cells by high-content imaging (E protein immunostaining) | 1.6 | 3.3 | >24.1 | >26.0 |
| ZIKV-H/PF-2013 | Huh-7 | Quantification of viral load in cell culture supernatant by qRT-PCR | 1.6 | 3.4 | >24.1 | >26.0 |
| Nonenveloped viruses | ||||||
| Adenovirus 5 | A-549 | Determination of virus-induced cytopathicity by MTT assay | >25.0 | >50.0 | ND | ND |
| CSV B | Vero | Determination of virus-induced cytopathicity by MTT assay | >48.2 | >52.0 | ND | ND |
IC50 values and CC50 (cellular cytotoxicity) values for HIV, HSV-1, and HSV-2 are taken from reference 16.
ND, not determined.
Labyrinthopeptins inhibit DENV infection by interacting with viral particles.
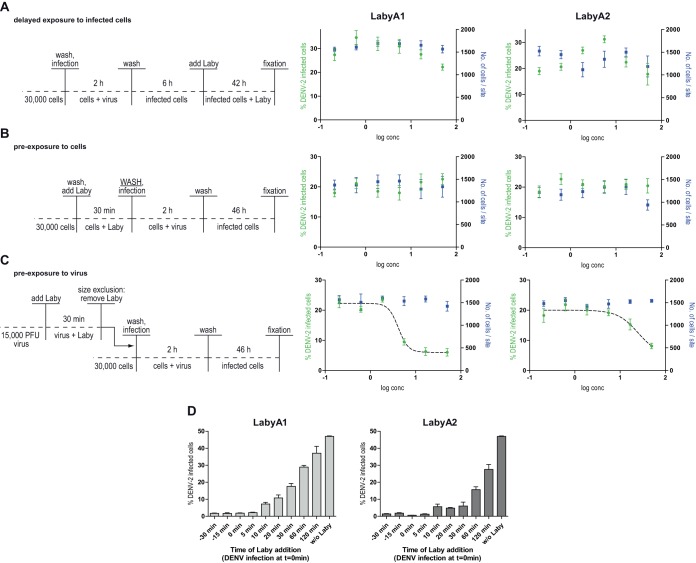
To address the antiviral mode of action of LabyA1 and LabyA2, we focused our research on the flaviviruses DENV and ZIKV. First, we performed time-of-drug-addition experiments: Huh-7 cells were infected with DENV, and LabyA1 or LabyA2 was added 6 h postinfection. An antiviral activity was not observed under these conditions (Fig. 2A), implying that the compounds do not interfere with viral RNA replication but likely earlier steps of the viral life cycle. To test whether labyrinthopeptins impaired viral entry through binding of a cellular target, Huh-7 cells were preincubated with either LabyA1 or LabyA2 for 30 min. Cells were then thoroughly washed before the addition of viral inocula to remove labyrinthopeptins from the medium. Again, no antiviral activity was observed (Fig. 2B). This finding suggested that binding of labyrinthopeptins to a cellular target is unlikely. To investigate whether labyrinthopeptins act directly on virions, we preincubated DENV particles with LabyA1 or LabyA2 for 30 min. The samples were processed by centrifugal spin filter columns (100-kDa size exclusion) to separate virions from unbound labyrinthopeptin, and retentates were used for infection of cells. Following this treatment, we observed a labyrinthopeptin-dependent inhibition of viral infection (Fig. 2C). The absolute inhibition was weaker than that in the initial experiment shown in Fig. 1D, probably due to the relatively short labyrinthopeptin exposure time of 30 min. This suggested that both labyrinthopeptins impair infection by interacting with a component of the virion. We could further show that they interfere with the initial steps of viral infection. Both LabyA1 and LabyA2 exerted their full activity when applied to cells together with the virus. However, the antiviral activity decreased when the labyrinthopeptins were applied as early as 10 min after virus addition (Fig. 2D).
FIG 2.
Labyrinthopeptins inhibit DENV infection by interacting with viral particles. For the experiments, the assay shown in Fig. 1C was modified. (A) Delayed exposure: LabyA1 or LabyA2 was given to infected cells 6 h postinfection. (B) Preexposure to cells: cells were preincubated with Laby, washed, and subsequently infected. (C) Preexposure to virus: DENV-2 particles were preincubated with LabyA1 or LabyA2. Compounds were removed via centrifugal membrane filters prior to infection. Only under these conditions could dose-response curves be determined. Data shown are means of 3 assays ± SEM. (D) Efficiency of labyrinthopeptins in blocking DENV infection is decreased when the compound is applied as early as 10 min after viral infection. LabyA1 or LabyA2 was added to the cells at the indicated time points, whereas infection with DENV was performed at time point 0 min. At 6 h postinfection, the inoculum together with the compound was replaced by fresh growth medium. The remainder of the assay was performed as described for Fig. 1C. Data shown are means of 2 assays ± SEM.
Labyrinthopeptins do not interact with viral proteins or with glycan motifs.
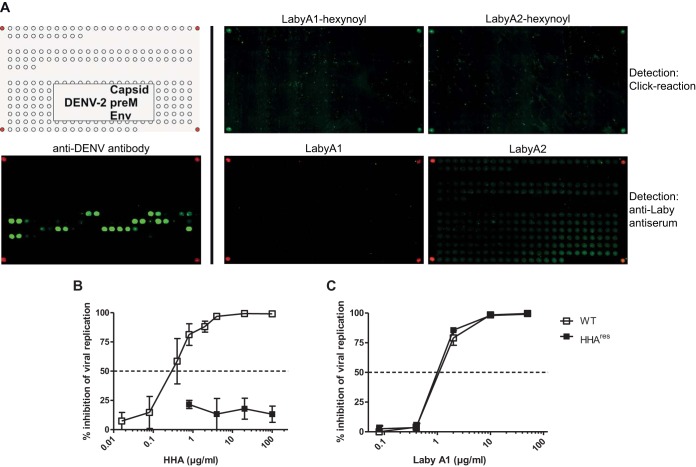
Suspecting that labyrinthopeptins bind to a viral target, we set out to identify their binding partner. Although the protein sequence homology among enveloped viruses affected by labyrinthopeptins is low, we first searched for proteinaceous binding epitopes. For this purpose, microarrays of overlapping peptides with a length of 15 amino acids and a sequence overlap of 3 amino acids were spotted on glass slides using the SC2 method (19). The peptides covered the structural proteins of DENV-2 (E, preM, and C proteins) (Fig. 3A). Slides were incubated with the labyrinthopeptins, and detection was carried out using polyclonal rabbit antibodies directed against either LabyA1 or LabyA2, followed by staining with an anti-rabbit Cy3-IgG conjugate. However, no binding of either labyrinthopeptin derivative to any peptidic sequence of the investigated proteins was detected. In an alternative detection approach, N-terminally modified LabyA1 and LabyA2 derivatives carrying a hexynoyl moiety were synthesized. These molecules were also tested in viral infection assays and fully retained their antiviral activity. Following the incubation with hexynoyl-labyrinthopeptins on the DENV-2 glass slides, an azide-modified biotin moiety was attached using click chemistry and visualized using a Cy3-conjugated anti-biotin IgG. Still, binding of neither LabyA1 nor LabyA2 to any peptide was observed. This implies that labyrinthopeptins do not bind to a linear epitope of surface proteins accessible on DENV virions.
FIG 3.
Labyrinthopeptins do not target viral peptides or glycan motifs. (A) Peptide microarrays. The DENV array covered the structural proteins of DENV 2 (E, preM, and C proteins). The top left panel shows the layout of the array, with the corner spots being biotin controls (red). The bottom left panel is the array processed with a polyclonal anti-DENV antibody (PA5-32207; Thermo) directed against the E protein (Env). Several binding epitopes were identified, exemplifying the integrity of this assay. In the upper middle and right panels, arrays were incubated with labyrinthopeptin-hexynoyl derivatives. A biotin moiety was attached using click chemistry, and staining was done afterwards using a Cy3-conjugated anti-biotin antibody. In the lower middle and right panels, arrays were incubated with native LabyA1 or LabyA2 and detection was carried out using the respective anti-LabyA1 or anti-LabyA2 polyclonal antibodies stained with an anti-rabbit Cy3 IgG. (B and C) Antiviral activity of LabyA1 against HHAres DENV-2. Huh-7 cells were infected with WT DENV or with HHAres DENV in the presence of various concentrations of the plant lectin HHA (B) or LabyA1 (C). At 4 days postinfection, the number of DENV-infected cells was quantified by flow cytometry. The mean percentage of inhibition of viral replication ± SEM of up to 4 independent experiments is shown. LabyA1 exhibited equipotent dose-dependent antiviral activities against both WT (IC50: 1.1 μg/ml or 0.53 μM) and HHAres (IC50, 1.0 μg/ml or 0.48 μM) DENV.
Next, the hypothesis that labyrinthopeptins may bind to viral glycans was tested. For this purpose, a mutant DENV-2 that is resistant to Hippeastrum hybrid lectin (HHAres DENV) was applied. HHAres DENV lacks both N-glycosylation sites Asn67 and Asn153 on the E protein (20). However, HHAres DENV was not cross-resistant to LabyA1 (Fig. 3B and C). Therefore, we concluded that LabyA1 does not interact with the N-glycans on the DENV E protein.
Labyrinthopeptins interact with the membrane lipid PE, thereby perforating membranes.
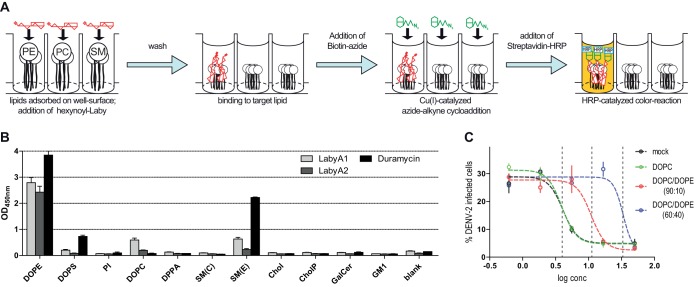
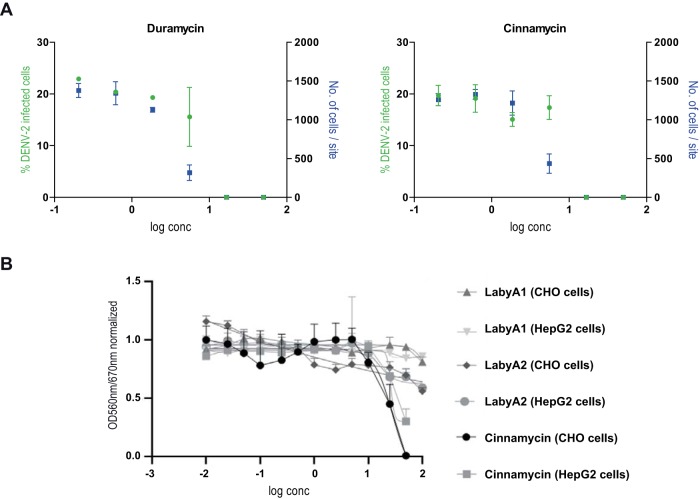
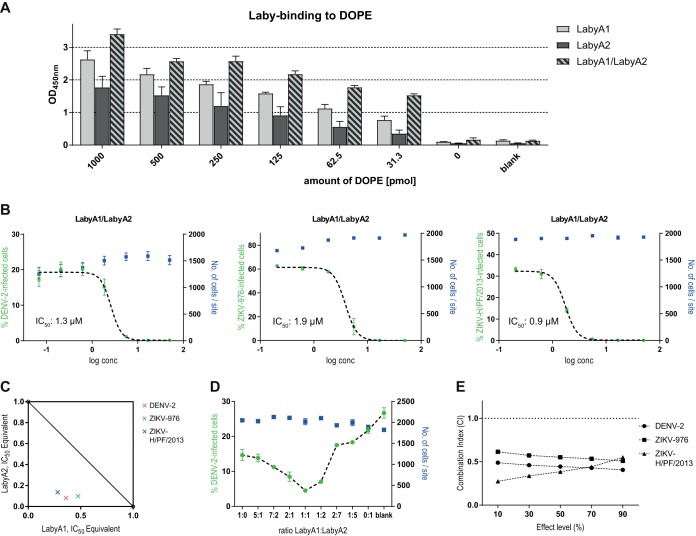
Lipids within virus particles are other potential candidate binding partners for labyrinthopeptins. To test this hypothesis, 96-well arrays with 11 species of adsorbed lipids were prepared using plates with a Polysorp surface. Following the incubation of each well with hexynoyl-labyrinthopeptins A1 and A2, again an azide-modified biotin moiety was attached, and binding events were visualized with a streptavidin-horseradish peroxidase (HRP)-catalyzed color reaction (Fig. 4A). We observed a pronounced binding of both labyrinthopeptins to phosphatidylethanolamine (PE), a weak binding to sphingomyelin with an ethanolamine head group [SM(E)] and phosphatidylcholine (PC), but hardly any binding to the other eight lipid species analyzed (Fig. 4B). Thus, there is a preference for lipids with an ethanolamine head group and for glycerophospholipids rather than sphingolipids. A PE-binding ability had already been reported for the lanthipeptides duramycin and cinnamycin (21, 22). We confirmed the specific PE binding of a duramycin-biotin conjugate in our assay (Fig. 4B). However, unlike labyrinthopeptins, neither duramycin nor cinnamycin showed specific antiviral activity but exerted pronounced toxic effects on cells when applied in the same concentration range (Fig. 5). After identifying LabyA1 and LabyA2 as PE-binding peptides, we asked whether their antiviral activity is influenced by the presence of additional PE lipids during viral infection. For this purpose, dose-response assays with DENV-infected cells were conducted in the presence of small unilamellar vesicles (SUVs) consisting of various amounts of 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) and 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE) (Fig. 4C). The SUVs were added to the cells before LabyA1 treatment and infection. While the presence of SUVs containing solely DOPC had no impact on the antiviral activity of LabyA1, SUVs that consisted of DOPC and DOPE in a 60:40 ratio led to an 8-fold decrease of LabyA1’s potency. We interpret this drop to be a consequence of the competition of viral versus SUV PE for LabyA1 interaction partners.
FIG 4.
Labyrinthopeptins bind to PE lipids. (A) Arrays of adsorbed lipids were prepared using plates with a Polysorp surface. Following the incubation of each well with hexynoyl-labyrinthopeptins, an azide-modified biotin moiety was attached via click chemistry. Binding to the respective lipids was determined by monitoring the OD450 after streptavidin-HRP-catalyzed color reaction. (B) A prominent binding of LabyA1 and LabyA2 to dioleoylphosphatidylethanolamine (DOPE) is evident. Duramycin-biotin, a known PE lipid binder, was used as a positive control. For lipid abbreviations, see Table 3 (values are means of 4 assays ± SEM). (C) PE-containing liposomes (SUVs) compete with the antiviral activity of LabyA1. Labyrinthopeptin treatment and DENV-2 infection of Huh-7 cells were carried out in the presence of SUVs with the indicated compositions. SUVs corresponding to 100 μM total lipid were added. Dose-response assays were conducted as described for Fig. 1C. PE-containing SUVs led to diminished LabyA1 activity on DENV infection. Dashed vertical lines indicate IC50 values of 1.9, 5.3, and 16 μM, respectively. Data shown are means of 3 assays ± SEM.
FIG 5.
(A) Duramycin-biotin and cinnamycin exert cytotoxic effects. Dose-response curves were obtained by high-content imaging. A decrease in cell number/site (blue symbols) at higher compound concentrations indicates cytotoxicity. This effect presumably masks any antiviral activity. Values shown are means of 2 assays ± SEM. (B) CHO and Hep G2 cell toxicity of cinnamycin, LabyA1, and LabyA2 investigated by an MTT assay. Values shown are means of 3 assays ± SEM. CC50 values for cinnamycin were 28.4 and 26.1 μg/ml on CHO and Hep G2 cells, respectively. CC50 values for LabyA1 were >100 μg/ml on both CHO and Hep G2 cells. CC50 values for LabyA2 were around 100 μg/ml on both CHO and Hep G2 cells.
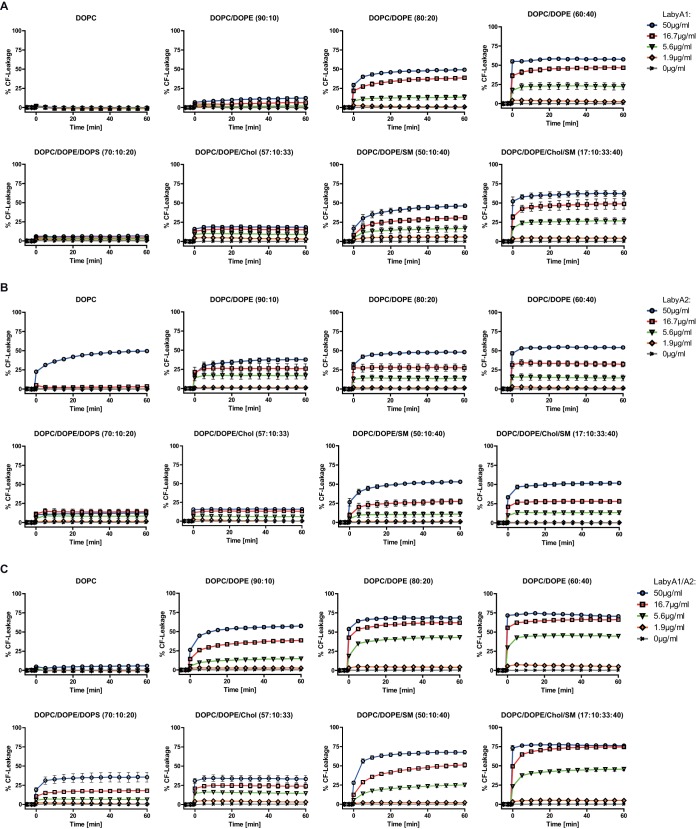
We then investigated whether the binding of labyrinthopeptins to PE caused a disruption of membranes, an effect that has been reported for PE-binding cyclotides like Kalata B1 (23, 24). To probe this, large unilamellar vesicles (LUVs) of different lipid compositions were prepared, loaded with self-quenching concentrations of carboxyfluorescein (CF), and then exposed to various concentrations of LabyA1 or LabyA2 (Fig. 6A and B). A rise in fluorescence indicated the liberation of CF due to vesicle leakage. Both labyrinthopeptin species induced leakage in a PE- and concentration-dependent manner, with LabyA2 being more efficient than LabyA1. Introduction of negatively charged phosphatidylserine (PS) lipids into the LUVs (DOPC/DOPE/DOPS [where DOPS is 1,2-dioleoyl-sn-glycero-3-phospho-l-serine] at an 80:10:10 ratio) resulted in a decrease, whereas incorporation of more rigid SM lipids (DOPC/DOPE/SM at a 50:10:40 ratio) led to a considerable increase in labyrinthopeptin-induced CF leakage. Eventually, LabyA1 and LabyA2 were most effective in releasing CF from DOPC/DOPE/cholesterol (Chol)/SM LUVs (17:10:33:40 ratio), which served as membrane raft models (25). Under these conditions, LabyA1 was more efficient in releasing CF than LabyA2. The results provide evidence that labyrinthopeptins perforate lipid membranes by interacting with PE. Although PE is the major ligand for both LabyA1 and LabyA2, we observed that the extent of their lytic activity was dependent on the composition, the charge, and the lipid phase behavior of the target membrane.
FIG 6.
CF leakage studies were carried out with LUVs of the indicated lipid compositions in the presence of various concentrations of LabyA1 (A), LabyA2 (B), or LabyA1/LabyA2 (C). Data obtained are expressed as a percentage of CF leakage relative to maximum fluorescence obtained by the addition of Triton X-100. Labyrinthopeptins induce leakage of CF in a PE-dependent manner. CF leakage from LUVs resembling membrane rafts (DOPC/DOPE/Chol/SM [17:10:33:40]) is vastly increased compared to that of DOPC/DOPE (90:10) LUVs, even though the overall PE amount remains constant. Data shown are means of 3 assays ± SEM.
Labyrinthopeptins A1 and A2 act synergistically when being applied at equimolar concentrations.
In this context, we also investigated how a combined application of equal amounts of LabyA1 and LabyA2 affects the extent of CF leakage from the liposomes. For these experiments, the molar ratio of LabyA1 to LabyA2 was kept at 1:1. Surprisingly, CF leakage from LabyA1/LabyA2-treated PE-containing liposomes was markedly increased compared to CF leakage from the corresponding samples treated with the individual compounds (Fig. 6C). Subsequent experiments showed that these observations are associated with a higher overall binding of the LabyA1/LabyA2 combination to the target lipid PE: decreasing amounts of DOPE adhered to a 96-well plate, whereas the overall amount of lipids was kept constant by adding increasing amounts of DPPA (1,2-dipalmitoyl-sn-glycero-3-phosphate). The array was again treated with either hexynoyl-LabyA1, hexynoyl-LabyA2, or both compounds in an equimolar combination (LabyA1/LabyA2). As indicated above, azide-modified biotin was used to visualize the binding events. Figure 7A illustrates the superior PE-binding efficacy of the LabyA1/LabyA2 combination compared to that of the individual compounds. The measured signals of the LabyA1/LabyA2-treated wells are consistently higher than the signals derived from LabyA1-treated wells, with differences ranging from 1.2-fold to 2-fold. These results are in line with the antiviral activity found in vitro. As shown in Fig. 7B, cellular infections with DENV-2, ZIKV-976, and ZIKV-H/PF/2013 were inhibited by a combined LabyA1/LabyA2 treatment, with IC50 values of 1.3 μM, 1.9 μM, and 0.9 μM, respectively. All of these values are lower than the IC50 values recorded for the individual compounds, arguing for a synergistic interaction of LabyA1 and LabyA2. Figure 7C shows the isobologram analysis at a 50% effect level. The concentrations of LabyA1 and LabyA2 required in combination to produce a 50% effect (i.e., the IC50) are located below the line of additivity drawn through the IC50 of LabyA1 (x axis) and the IC50 of LabyA2 (y axis), thus underscoring the previous observations. Further in vitro testing with DENV-2-infected Huh-7 cells revealed that LabyA1 and LabyA2 combined at a 1:1 ratio (0.75 μM each) indeed yielded the highest antiviral potency compared to the combination at ratios which were shifted toward either LabyA1 or LabyA2. For these, a significant decrease in activity indicated by an increasing percentage of infected cells was measured (Fig. 7D). To provide a quantitative estimation of the synergistic interaction, we computed the combination index (CI) according to a report by Chou (26), where a CI of <1 indicates synergism of the two drugs. Data sets from the DENV and ZIKV experiments shown in Fig. 1 (LabyA1 and LabyA2 individually) and Fig. 4B (LabyA1 and LabyA2 combined) were used. CIs were approximately 0.5 across several effect levels (IC10, IC30, IC50, IC70, and IC90) for all viruses tested (Fig. 7E).
FIG 7.
Labyrinthopeptins A1 and A2 act synergistically. (A) The indicated amounts of DOPE were adhered to 96-well Polysorp plates, whereas the total lipid amount in every well was adjusted to 1,000 pmol with DPPA. The blank control contained no lipid. Binding of hexynoyl-labyrinthopeptins derivatives was determined as described for Fig. 4A. (B) Dose-response curves obtained for Huh-7 cells infected with DENV-2, ZIKV-976, or ZIKV-H/PF/2013 after treatment with equimolar concentrations of LabyA1 and LabyA2 in combination. Their combined concentration is equal to the concentrations of the individual compounds used in Fig. 1D to F. (C) Isobologram analysis at the 50% effect level. The IC50 of LabyA1 is plotted on the x axis as IC50 equivalents (e.g., for DENV-2, one IC50 equivalent of LabyA1 corresponds to 1.8 μM; see Fig. 1D). IC50 equivalents of LabyA2 are plotted on the y axis. The connecting line is the line of additivity. The colored data points indicate the individual concentrations of LabyA1 and LabyA2 used in combination at the IC50 for the given viruses. (D) Huh-7 cells were infected with DENV-2 after treatment with LabyA1 and LabyA2 in combination. Their combined concentration was fixed at 1.5 μM, with the indicated LabyA1:LabyA2 ratios being tested. A ratio of “1:0” implies that only LabyA1 was used. (E) Combination index (CI) for the LabyA1/LabyA2 combination. CIs were calculated for several effect levels (IC10, IC30, IC50, IC70, and IC90) for the viruses indicated. The dashed horizontal line is at a CI of 1. All values less than 1 indicate synergism, while all values greater than 1 indicate antagonism. All values shown in panels A, B, and D are means of 3 assays ± SEM.
Labyrinthopeptins perforate viral particles and show favorable pharmacokinetics.
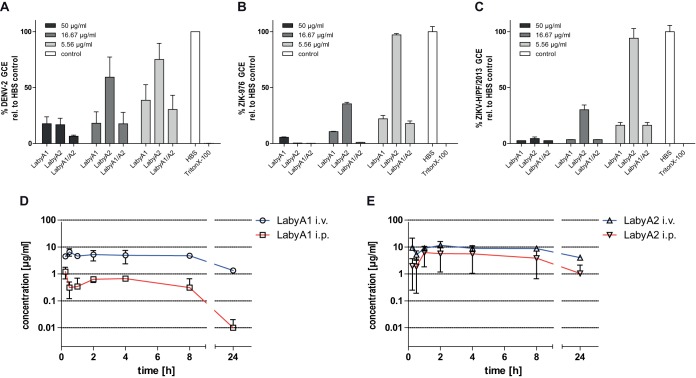
Following the observation of lytic effects in CF leakage studies, RNase protection assays with DENV or ZIKV were conducted to verify that labyrinthopeptins also perforate lipid membranes of enveloped viral particles. An intact lipid envelope protects the viral genome from RNase digestion, whereas the genome of particles with perforated envelopes is susceptible to digestion (27). Following treatment of viral particles with either LabyA1, LabyA2, or LabyA1/LabyA2 and RNase digestion, the number of genome copy equivalents (GCE) was quantified, with a decrease being indicative of viral lysis. The percentage of GCE was markedly reduced upon preincubation of DENV or ZIKV with either of the labyrinthopeptins (Fig. 8A to C). LabyA2 was less potent in releasing viral genomes than LabyA1, which correlates with their respective antiviral activities (Fig. 1C).
FIG 8.
Labyrinthopeptins perforate viral membranes and have favorable pharmacokinetic properties. (A to C) The genome of viral particles with perforated lipid envelopes is susceptible to RNase digestion. The levels of genome copy equivalents (GCE) of labyrinthopeptin-treated DENV-2 (A), ZIKV-976 (B), or ZIKV-H/PF/2013 (C) particles were significantly diminished after RNase exposure, as measured by qRT-PCR, giving proof of the virolytic effect of labyrinthopeptins (values are means of 5 assays ± SEM). (D and E) Time-dependent plasma concentrations of LabyA1 (D) and LabyA2 (E) after respective i.v. and i.p. administration into male CD-1 mice at a dose of 10 mg/kg. Values are means of 3 assays ± SEM.
As labyrinthopeptins showed no cytotoxicity in antiviral test systems and in standard MTT [3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide]-based assays with Hep G2 and CHO cells (Fig. 5), the two compounds were injected intravenously (i.v.) and intraperitoneally (i.p.) into male CD-1 mice at a dose of 10 mg/kg to determine their pharmacokinetic parameters. Because the compounds followed nonlinear kinetics, pharmacokinetic parameters were determined using a noncompartmental analysis (Fig. 8D and E and Table 2). LabyA1 exhibited a low clearance (CL) (1.55 ml/kg/min) and a high volume of distribution (V) and had a maximal concentration (Cmax) of 7.35 μg/ml, an area under the curve (AUC) of 88.05 μg/ml · h, and a half-life (t1/2) of 10 h following i.v. administration. Intraperitoneal administration of LabyA1 resulted in a bioavailability of 7.8% compared to that by i.v. administration. Thus, in contrast to common assumptions of peptide properties, labyrinthopeptins turned out to be highly stable under in vivo conditions. We assume that the intramolecular labionin bridges render labyrinthopeptins insensitive toward proteolytic degradation. The pharmacokinetic parameters for LabyA2 were even more favorable. The experiments clearly show that the exposure levels reached with labyrinthopeptins exceed the antiviral IC50s for several hours, time frames that are compatible with once-a-day (QD) or twice-a-day (BID) dosing.
TABLE 2.
Pharmacokinetic parameters of LabyA1 and LabyA2 after administration of 10 mg/kg i.v. or i.p. into male CD1 mice
| Parametera | Value for: |
|||
|---|---|---|---|---|
| LabyA1 |
LabyA2 |
|||
| i.v. | i.p | i.v. | i.p. | |
| t1/2 (h) | 9.96 ± 0.8 | 3.18 ± 1.6 | 17.71 ± 7.2 | 7.13 ± 3.1 |
| Tmax (h) | 0.25 ± 0.0 | 2.5 ± 2 | ||
| Cmax (μg/ml) | 7.35 ± 0.9 | 1.21 ± 0.6 | 14.57 ± 7.7 | 6.23 ± 3.6 |
| AUC (μg/ml · h) | 88.05 ± 2.9 | 6.86 ± 3.8 | 177.73 ± 22.9 | 77.68 ± 66.3 |
| V (liters/kg) | 1.34 ± 0.1 | 8.80 ± 8.1 | 0.88 ± 0.2 | 1.57 ± 1.0 |
| CL (ml/kg/min) | 1.55 ± 0.04 | 28.10 ± 15.0 | 0.59 ± 0.1 | 3.17 ± 2.9 |
Tmax, time to maximum concentration; Cmax, maximum concentration.
DISCUSSION
The present study investigates the antiviral activity of the two lanthipeptides LabyA1 and LabyA2 against a variety of enveloped viruses, including the flaviviruses DENV, ZIKV, WNV, and HCV. We show that LabyA1 and LabyA2 exert their antiviral function by disrupting viral envelopes (virolysis). A prerequisite for labyrinthopeptin-induced lysis is the presence of membranous PE lipids, which are specifically bound by both analogs. In general, PE constitutes about 15 to 25% of the total phospholipids in mammalian cells. Several mechanisms ensure that PE is enriched in the inner (luminal) leaflet of the plasma membrane and the Golgi network. These include ATP-dependent selective aminophospholipid transporters (e.g., the P4 subfamily of P-type ATPases) and membrane-bound lipid exporters (scramblases) (21, 28). Hence, the absence of PE in the exoplasmic leaflet might be the reason why labyrinthopeptins exert no cytotoxicity on eukaryotic cells, since their primary ligand is essentially excluded from the cell surface. Maturing flaviviruses, on the other hand, bud from endoplasmic reticulum (ER) membranes in which PE lipids are symmetrically distributed between the inner and outer leaflets. Thus, flaviviral envelopes do not exhibit the mentioned PE asymmetry, which may explain their susceptibility toward labyrinthopeptins (29, 30). PE lipid asymmetry of viruses budding from the plasma membrane also dissipates, as shown for HIV, whose envelope’s outer leaflet displays aminophospholipids (31). In future studies, we plan to probe the ability of labyrinthopeptins to limit the spread of secreted virions in the culture after replication, e.g., by a variation of multiplicity of infection (MOI) using adapted experimental protocols.
Regarding efficacy, LabyA1 is a more potent inhibitor of viral infection than LabyA2. Our CF leakage studies indicate that this might be attributed to the higher potency of LabyA1 on membranes containing rafts. Membrane rafts represent lipid microdomains in the liquid ordered phase within a liquid-crystalline environment. It is assumed that membrane rafts are enriched in cholesterol and sphingolipid, and that lipid-lipid, protein-lipid, and protein-protein interactions contribute to their stability (28).
We further show that LabyA1 and LabyA2 exert a synergistic antiviral effect. The synergism was most pronounced when the two compounds were applied at equimolar concentrations. Notably, the two compounds are expressed in a 1:1 ratio from a single operon by their producer organism, Actinomadura namibiensis, implying that the biological advantage for the host gained from these secondary metabolites is also optimal under 1:1 expression conditions. The molecular interactions with PE that induce synergism remain to be deciphered on an atomic level. Only a few other lantibiotics are known to have synergistic properties. The two components of lacticin 3147 (LtnA1 and LtnA2) have weak individual activity but synergistically display strong antibacterial action, presumably through binding of bacterial cell wall precursors (15). Another two-component lantibiotic termed thusin exhibits antimicrobial activity at a 1:1 ratio and shows efficacy against Gram-positive bacteria (32). However, none of these have been shown either to bind PE or to have an antiviral activity.
The PE-binding property of labyrinthopeptins is shared by a number of peptides, among which the fellow lanthipeptides cinnamycin and duramycin may be the most prominent (33, 34). More recently, aegerolysins, lanthipeptides isolated from the fungus Agrocybe aegerita, have also been shown to bind PE with high affinity (21, 22). Eventually, membrane interactions of the plant-derived cyclotides could also be credited to a pronounced PE binding (23). Cinnamycin and duramycin have been long known for their cytotoxicity. It is known that membrane curvature and physical properties affect their binding to PE, which leads to membrane disruption (23, 35, 36). Distinct cyclotides confer cytoprotective effects against HIV infection in vitro (24). However, to our knowledge, LabyA1 and LabyA2 are so far the only known PE-binding peptides that exhibit a broad antiviral activity without a pronounced cytotoxicity, in clear distinction from the aforementioned peptides. This demonstrates that in spite of the ubiquitous occurrence of PE in viruses and host cells, the unique sequence and structure of labyrinthopeptins can indeed confer a specific antiviral activity.
CHIKV particles derived from C6/36 mosquito cells were approximately 5-fold more susceptible to LabyA1 than BHK-21 cell-derived CHIKV. Of the total phospholipid content, PE species reportedly represent 46 to 58% in C6/36 cells, whereas this fraction is only 15 to 25% for mammalian cell lines (37). Since we also observe that liposome leakage increases with higher PE content (Fig. 6), we hypothesize that the sensitivity to LabyA1 depends on the PE content of the virus, and we plan to investigate this link by future viral lipidomics studies. A precise lipid composition is available only for a limited number of viral envelopes. For the HIV envelope, it has been demonstrated that PE species represent 25 to 35% of all analyzed phospholipids. HIV envelopes harbor membrane rafts that are crucial for fusion and entry of HIV into target cells (31, 38, 39). In the case of HCMV envelopes, it was found that PE constitutes nearly 50% of all glycerophospholipids (40), whereas in HCV strain Jc1E2, 2.64% of all lipids are PE (41). Unfortunately, lipidome data are available for neither DENV nor ZIKV. Studies with the related WNV revealed that approximately 7% of the total lipid portion are PE species, but the presence of membrane rafts in these virions has not been investigated (65). Interestingly, TBEV was the only flavivirus that showed very low susceptibility toward labyrinthopeptins. As there is also no lipidome data available for TBEV virions, we can only speculate that the low susceptibility may be attributed to a very small amount of PE in TBEV membranes and/or a rather rigid lipid composition devoid of lipid rafts.
While the membrane-disruptive effects of labyrinthopeptins have been demonstrated with protein-free LUVs, we cannot exclude an impact of transmembrane proteins on labyrinthopeptin efficacy. Such interspersed proteins may influence lipid phase behaviors or membrane fluidity and thus might alter the susceptibility of different viruses toward labyrinthopeptins.
The pivotal role of lipids in viral life cycles has long been appreciated (42–44). Cellular lipids can act as receptors for viral fusion and protein-mediated cell entry (45) and are prerequisites for the formation of the viral replication machinery (46, 47). To enable entry, fusion, replication, and eventually their assembly, viruses modify lipid synthesis, metabolism, and compartmentalization of their respective host cells. Membrane remodeling induced by DENV, for instance, is directly linked to a shift in the host lipid repertoire upon infection (48). Especially cholesterol and membrane rafts play a central role during the entry and assembly of many viruses (49), while the synthesis of fatty acids is also required for viral maturation (44, 50). Thus, targeting the lipid metabolism of the host cell evolved as a promising approach for antiviral therapies. Targeting cholesterol metabolism by blocking 3-hydroxy-3-methyl-glutaryl-coenzyme A (HMG-CoA) reductase with statins proved to be successful for preventing infections with HIV, HCV, and influenza virus. Similarly, inhibiting fatty acid synthase by targeting acetyl-CoA carboxylase with 5-tetradecyloxy-2-furoic acid or soraphen A blocked replication of HCMV, influenza A virus (50), HIV, and HCV (51). Whereas the aforementioned approaches act on host cell lipid metabolism, compounds acting on the viral lipid membrane have also been reported: a rhodamine-derived small molecule named LJ001 was shown to intercalate into viral membranes and inactivate virions, thereby inhibiting entry for a broad spectrum of viruses (52). The therapeutic window of LJ001 was rationalized with the higher reparative capacity of biogenic cellular membranes compared to that of static viral membranes. Lipidomimetic compounds were found to alter the raft-containing viral membrane structure of HIV, thereby inhibiting virus infectivity (53). Also, protoporphyrin IX congeners were reported to exert broad-spectrum antiviral activities by targeting the viral envelope. In all three examples, a molecular target was not identified (54). A major advantage of directly targeting PE or other components of viral envelopes is that lipids are less susceptible to mutability and rapid evolutionary changes than protein or glycan motifs. Variations in viral proteins can easily occur through replication errors of the viral genome and thus do at times hamper the potency of directly acting antivirals or vaccines. However, the lipid composition of viral envelopes is solely dependent on the lipid repertoire of the membrane from which they are derived. Hence, to escape the virolytic effect of labyrinthopeptins, viral particles need to diminish the PE amounts in their envelopes significantly. This, in turn, would require a profound reprogramming of infected host cells. Another advantage of the lipid-targeting strategy is its broad application spectrum. As highlighted herein, we addressed a variety of enveloped viruses with one compound class. This may be beneficial in clinical settings when treating patients suffering from multiple viral infections. In addition, LabyA1 could be deployed as a broad-spectrum agent for antagonizing outbreaks of emerging viruses or for treating infections with pathogenic viruses for which no medical treatment is yet available (11). Because labyrinthopeptins are readily available in good yields by fermentation and are well soluble, stable, and well tolerated, we consider them promising novel lead structures against viral infections and engage in a systematic development of drug formulations for animal models of infection and in deciphering interactions of LabyA1 and LabyA2 with PE at an atomic level.
MATERIALS AND METHODS
Test agents.
LabyA1 and LabyA2 were isolated and purified as described earlier (14). Cinnamycin and duramycin-biotin were purchased from TEBU-Bio and Molecular Targeting Technologies, respectively. The lipids listed in Table 3 were purchased from Avanti Polar Lipids and dissolved as 10 mM stocks in 67% chloroform–33% methanol. Davids Biotechnologie GmbH (Regensburg, Germany) was commissioned to prepare rabbit polyclonal antibodies directed against native labyrinthopeptins.
TABLE 3.
Lipids used in this study
| Abbreviation | Designation |
|---|---|
| DOPE | 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine |
| DOPS | 1,2-Dioleoyl-sn-glycero-3-phospho-l-serine |
| PI | l-α-Phosphatidylinositol (from soy) |
| DOPC | 1,2-Dioleoyl-sn-glycero-3-phosphocholine |
| DPPA | 1,2-Dipalmitoyl-sn-glycero-3-phosphate |
| SM(C) | Ceramide phosphorylcholine (from chicken egg) |
| SM(E) | Ceramide phosphorylethanolamine |
| Chol | Cholesterol |
| CholP | Cholesteryl palmitate |
| GalCer | Galactocerebrosides (from bovine brain) |
| GM1 | Monosialoganglioside GM1 (from bovine brain) |
Cell cultures.
All cell cultures were maintained at 37°C in a humidified, CO2-controlled atmosphere, except for C6/36 mosquito cells (isolated from Aedes albopictus; ATCC CRL-1660), which were maintained at 28°C in the absence of CO2. C6/36 cells were grown in minimum Eagle’s medium (MEM) (Invitrogen, Merelbeke, Belgium) supplemented with 10% fetal bovine serum (FBS) (HyClone, Perbio Science, Aalst, Belgium), 0.01 M HEPES buffer (Invitrogen), nonessential amino acids (Invitrogen), 2 mM l-glutamine (Invitrogen), 100 U/ml penicillin, and 100 U/ml streptomycin (Invitrogen). African green monkey kidney cells (Vero cells; ATCC CCL-18) were grown in MEM (Invitrogen) supplemented with 10% FBS, 2 mM l-glutamine, and 0.075% sodium bicarbonate (Invitrogen). Baby hamster kidney cells (BHK-21, ATCC CCL-10) and human hepatoma cells (Huh-7) were kindly provided by Ralf Bartenschlager (University of Heidelberg, Heidelberg, Germany) and were grown in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% FBS and 0.01 M HEPES buffer. Huh-7.5 cells were grown in DMEM GlutaMAX (Gibco) supplemented with 10% FBS. HEK293T cells were grown in DMEM supplemented with 10% FBS, 2 mM l-glutamine (Invitrogen), 100 U/ml penicillin, and 100 U/ml streptomycin (Invitrogen).
Monocyte-derived dendritic cells (MDDC) were isolated as described previously (55). Briefly, buffy coat preparations from healthy donors were obtained from the Blood Bank in Leuven, Belgium. Human peripheral blood mononuclear cells (PBMCs) were first isolated by density gradient centrifugation over Lymphoprep (Nycomed, Oslo, Norway). PBMCs were gently rotated at 4°C to form aggregates of monocytes. After sedimentation of the monocytes, the pellet was grown in RPMI culture medium supplemented with 25 ng/ml interleukin 4 (IL-4) and 50 ng/ml GM-CSF (granulocyte-macrophage colony-stimulating factor) (Peprotech, London, United Kingdom) or in medium left unsupplemented. After 5 days, IL-4 and GM-CSF differentiated monocytes into immature MDDC, as analyzed by various cellular markers by flow cytometry.
Dengue, Zika, and West Nile viruses.
Zika viruses (ZIKV) were obtained via the European Virus Archive (EVA). ZIKV-976 Uganda (GenBank accession no. LC002520.1) was obtained from Tatjana Avšič Županc (University of Lubljana), and ZIKV-H/PF/2013 (GenBank accession no. KJ776791.2) was obtained from Xavier de Lamballerie (Université de la Méditerranée, Marseille, France). Both ZIKV strains were propagated in Vero cells.
Dengue virus (DENV) serotype 2 laboratory-adapted New Guinea C (NGC) wild-type (WT) strain (GenBank accession no. KM204118.1) was kindly provided by V. Deubel (Institut Pasteur, Paris, France). HHA-resistant (HHAres) DENV-2 was generated by passaging a 1:20 dilution of the supernatant of HHA-exposed DENV-infected C6/36 mosquito cells (2 × 106 cells) every 3 to 4 days to fresh uninfected cells in the presence of gradually increasing concentrations of HHA. After 14 passages (∼7 weeks of cell culture), virus was recovered that could replicate in the presence of 400 nM HHA, a concentration that is ∼10-fold higher than the 50% effective concentration (EC50) for inhibition of DENV-2 replication in C6/36 cells. Low-passage clinical isolate DENV serotype 1 Djibouti strain D1/H/IMTSSA/98/606 (GenBank accession no. AF298808), laboratory-adapted DENV serotype 3 strain H87 (prototype) (GenBank accession no. M93130), and low-passage clinical isolate DENV serotype 4 strain Dak HD 34 460 (no complete sequence available, only partial unpublished sequences) were kindly provided by Xavier de Lamballerie (Université de la Méditerranée, Marseille, France). All four DENV serotypes were propagated in C6/36 cells. Supernatant-containing virus was harvested 5 days postinfection and stored at −80°C. Titers of DENV-2 and ZIKV strains were determined in a plaque assay in BHK-21 or Vero cells (expressed in PFU). Titers of DENV-1, -3, and -4 were determined in Vero cells by validation of cytopathic effects to obtain the cell culture infective dose infecting 50% of the cells (CCID50)/ml value.
West Nile virus (WNV) strain New York-99 was obtained from the National Collection of Pathogenic Viruses (NCPV), United Kingdom (NCPV 0209291v, batch no. 988). WNV was grown in Vero cells, and viral titers were determined in a plaque assay in BHK-21 or Vero cells (expressed in PFU). For antiviral assays, titers of WNV virus stocks were determined in Huh-7 cells to estimate the infection efficiency of the virus in those cells.
Monitoring effects of labyrinthopeptins on DENV and ZIKV infection by high-content imaging.
Huh-7.5 cells (3 × 104 per well) were seeded in black 96-well optical-bottom plates (MicroWell 96-well optical-bottom plate [Nunc, 165305]) in full growth medium 1 day prior to infection. After washing with phosphate-buffered saline (PBS), 40 μl assay medium (5% FBS) was added to cells containing LabyA1, LabyA2, hexynoyl-LabyA1, hexynoyl-LabyA2, cinnamycin, or duramycin-biotin. Assay concentrations were 50, 16.7, 5.56, 1.85, 0.62, 0.21, and 0.069 μg/ml. In the case of liposome competition assays, medium was supplemented with SUVs (see “Generation of liposomes” below) corresponding to 100 μM total lipid. Treatments were run in double replicates. PBS served as a control. After 30 min of incubation, cells were infected with 1.5 × 104 PFU of DENV-2, ZIKV-976, or ZIKV-H/PF/2013 to give a final volume of 60 μl/well (MOI = 0.5; assay medium was added for uninfected controls). After 2 h of incubation at room temperature (RT), cells were washed with PBS and 100 μl of assay medium was added per well. Infected cells were incubated for another 48 h at 37°C. Here, media were removed from the wells, and cells were fixed with 4% paraformaldehyde (PFA). Fixed cells were washed extensively with PBS and permeabilized with 0.25% Triton X-100 for 5 min. After blocking with 5% FBS in PBS, the primary antibody was applied for 2 h. For DENV, this was anti-dengue virus E glycoprotein antibody (ab41349, DE1; Abcam], and for ZIKV, it was Ab00779-2.0 anti-envelope protein (ZKA64, Absolute Antibody), both diluted 1:100 in 5% FBS in PBS. After washing, the secondary antibody [Alexa Fluor 488 goat anti-mouse IgG(H+L) (Life Technologies); 1:1,000 diluted in 5% FBS in PBS] was applied for 1 h. Finally, cells were stained with DAPI (4′,6-diamidino-2-phenylindole) (500 ng/ml in PBS) for 5 min. Please note that dead cells are not assessed by nuclear staining, because they detach from the surface before fixation. Fluorescent cells were analyzed by high-content imaging using the automated microscope ImageXpressMicro (Molecular Devices). The excitation wavelengths were set to 360 nm (DAPI) and 485 nm (Alexa Fluor 488), and the emission wavelengths were 460 nm (DAPI) and 516 nm (Alexa Fluor 488). Images of six sites/well were acquired (2 columns, 89-μm spacing; 3 rows, 67-μm spacing). High-content imaging was performed with the Multi Wavelength Cell Scoring module of the MetaXpress software (Molecular Devices). First, identification of DAPI-stained nuclei was conducted: objects in the DAPI channel whose average fluorescence was more than 2.5 standard deviations (SD) above the fluorescence of the local background and whose size was within 8 to 20 μm were counted as single nuclei. The numeric data obtained represent the total number of cells per site. Here, the percentage of virus-positive cells per site was calculated by evaluating the number of Alexa Fluor 488-positive cells. The following criteria had to be met to classify a cell as virus positive: (i) the average fluorescence of an object surrounding a nucleus had to be more than 2.5 standard deviations above the average fluorescence measured in uninfected wells, and (ii) the fluorescence of the object had to cover a minimum area of 100 μm2. The numeric data obtained represent the number of virus-positive cells per site, which was then expressed in relation to the total cell number per site (% virus positive). Values obtained from the six sites acquired per well were averaged and plotted onto a semilogarithmic X/Y chart. IC50 values were calculated by nonlinear regression using Graph Pad Prism.
Monitoring effects of labyrinthopeptins on DENV infection by flow cytometry.
Cells were seeded in flat-bottom polystyrene plates (Iwaki, International Medical Products, Belgium) and infected with an inoculum of DENV (∼100 CCID50/ml of each DENV serotype). MDDC (1 × 106 cells/well) were infected with the four different DENV serotypes in the absence or presence of the peptides for 4 h at 37°C. The cells were washed twice with medium to remove excessive virus and antiviral agents and were further incubated at 37°C in fresh culture medium. MDDC were used at day 2 postinfection to detect DENV antigen by flow cytometry or RT-qPCR. At the end of the culture period, the 50% cell cytotoxicity concentration (CC50) was determined in MDDC by light microscopic evaluation for morphological changes. After collection of the cells, the cells were washed with PBS, fixed, and permeabilized using a Cytofix/Cytoperm kit (BD Biosciences) according to the manufacturer’s instructions. Briefly, cells were fixed and permeabilized with Cytofix/Cytoperm buffer at 4°C for 20 min. After washing the cells with perm/wash buffer, the permeabilized cells were incubated with 5 μg/ml anti-DENV Ab (clone 3H5 for serotype 2 or clone 2H2 for the other serotypes) for 30 min at 4°C. Following a washing step, the secondary phycoerythrin-conjugated goat F(ab′)2 anti-mouse Ab (Caltag Invitrogen) was added and incubated at 4°C. As a control for unspecific background staining, cells were stained in parallel with secondary antibody only. The stained cells were washed and analyzed by flow cytometry with a FACSCalibur (BD Biosciences, San Jose, CA). Data were acquired and analyzed with CellQuest software (BD Biosciences). The mean fluorescence intensity (MFI) of the background staining was subtracted from the MFI of each sample to obtain the number of DENV-infected cells.
Effects of labyrinthopeptins on transduction by lentiviral CHIKVgp pseudoparticles.
HEK293T cells were seeded in poly-l-lysine coated 96-well plates (104 cells/well). On the following day, cell culture medium containing lentiviral, firefly luciferase-encoding particles decorated with CHIKV glycoproteins (CHIKVgp; derived from the S27 strain [see reference 56]) was incubated with various concentrations of LabyA1 or LabyA2 (100-μl total volume) for 5 min at RT. Each reaction mixture contained 1% (vol/vol) of the solvent dimethyl sulfoxide (DMSO). The cell culture medium of the cells was removed and exchanged with the pseudoparticle-drug mixture. After incubation for 48 h at 37°C, the supernatant was discarded and the cells were washed once with 1× PBS. The cells were subsequently lysed in 45 μl luciferase buffer (Promega) for 5 min at RT. A 20-μl volume of lysate was mixed with 10 μl luciferase substrate, and relative light units were measured using a Berthold plate luminometer.
Effect of labyrinthopeptin A1 on CHIKV infection.
HEK293T cells were seeded in poly-l-lysine-coated 96-well plates (104 cells/well). On the following day, CHIKV of the West African strain 37997 (equivalent to an MOI of 1) expressing a green fluorescent protein (GFP) reporter 5′ of the open reading frame (ORF) encoding the structural proteins (3′-GFP-CHIKV [see reference 57]) and produced separately in BHK-21 and C6/36 cells was incubated with various concentrations of LabyA1 (100 μl of total volume) for 5 min at RT. Each reaction mixture contained 1% (vol/vol) of the solvent DMSO. The cell culture medium of the cells was removed and exchanged with the virus-drug mixture. After incubation for 24 h at 37°C, the supernatant was discarded and the cells were washed once with 1× PBS. The cells were detached using 50 μl trypsin-EDTA for 5 min at 37°C and centrifuged at 500 × g for 5 min at 4°C. After removal of the supernatant, the cells were fixed in 50 μl 4% PFA for 90 min at RT. The percentage of GFP-positive cells was determined by flow cytometry using the BD Accuri flow cytometer.
Effects of labyrinthopeptins on HCV infection.
Huh-7.5 firefly luciferase-expressing cells (Huh-7.5 Fluc), grown in Dulbecco’s modified Eagle’s medium (DMEM) (Life Technologies) supplemented with 10% fetal bovine serum, 2 mM l-glutamine (Life Technologies), 100 U/ml penicillin (Life Technologies), and 100 μg/ml streptomycin (Life Technologies), were seeded in 96-well plates (104 cells/well) and incubated overnight at 37˚C with a 5% CO2 supply. The following day, labyrinthopeptin, at various concentrations, was added to Eppendorf tubes containing JcR2a HCV derived from cell culture (HCVcc) (in DMEM). The virus-compound preparations were inoculated into the cells in duplicate and incubated with the cells for 4 h at 37˚C with a 5% CO2 supply. After 4 h, medium containing the virus-compound mixture was aspirated from the cells. Cells were then washed once with sterile PBS to remove residual virus-compound and replenished with fresh medium. Cells were incubated for 48 h at 37˚C with a 5% CO2 supply. After 48 h, cells were washed twice with PBS and then lysed with 1× passive lysis buffer. The lysate was analyzed on a Berthold plate luminometer for luciferase activity.
Effects of labyrinthopeptins on HCMV infection.
The antiviral assay is based on the inhibition of HCMV-driven GFP expression in normal human dermal fibroblasts (NHDFs). Briefly, NHDF cells (1.6 × 104 per well) were seeded in 96-well plates 1 day prior to infection. Various concentrations of LabyA1 (final concentrations, 10, 5, 2, 1, and 0.5 μM) and LabyA2 (final concentrations, 15, 10, 5, 2, and 1 μM) were dispensed into the cells to a total volume of 200 μl/well in triplicate. Phosphonoacetic acid (PAA; 180 μM) was used a positive control for inhibition of viral replication. DMSO was added to either infected cells or uninfected cells at the highest concentration as done with the substances added to cells, as a control for substances which were diluted in DMSO. After 1 h of incubation, the GFP-expressing HCMV strain pHG-1 (here called HT8-GFP [see reference 58]), which is based on the HCMV laboratory strain AD169, was added to cells at an MOI of 0.5 PFU/cell and cells were incubated for another 4 days. At 4 days postinfection (p.i.), the media were removed from the wells and the cells were fixed with 3% PFA and washed with PBS before measurement. GFP expression of the cells was measured using a BioTek Synergy 2 microplate reader; the protocol for excitation wavelength was set to 485 nm, and the emission wavelength was 516 nm. Time-of-drug-addition (TOA) experiments were performed using 1.6 × 104 NHDF cells per well in 96-well plates. LabyA1 (3 μM), LabyA2 (7 μM), DMSO (0.1%), or PAA (180 μM) was added to the cultures at −1, 0, 1, 3, 6, 24, 48, and 72 h of addition of the virus. Cells were infected with HT8-GFP at an MOI of 0.5 at time point 0. At 96 h p.i., GFP expression of cells was measured as described above.
Effects of labyrinthopeptins on KSHV infection.
A total of 3 × 104 HEK293 cells were seeded in growth medium (DMEM; 10% FBS) onto a 96-well plate and incubated for 24 h at 37°C and 5% CO2. After removal of the medium, 180 μl growth medium together with 20 μl of a solution of KSHV (produced from cell line BJAB-rKSHV [59]) at an MOI of 0.01 was applied to each well. Simultaneously, LabyA1 or LabyA2 was added to the wells at a concentration of 50 μM with serial 2-fold dilutions down to 1.56 μM. After 48 h of incubation, the GFP-expressing HEK293 cells were counted under a fluorescence microscope. The data points given in the figures are mean results of triplicate assays.
Effects of labyrinthopeptins on TBEV infection.
A total of 1.5 × 104 Vero cells were seeded in growth medium (DMEM; 10% FBS) onto a 96-well plate and incubated for 24 h at 37°C and 5% CO2. After removal of the medium, cells were preincubated with LabyA1 or LabyA2 for 30 min. Concentrations of 50 μg/ml with serial 3-fold dilutions down to 0.07 μg/ml were applied. Cells were then infected with TBEV (Torö isolate) at an MOI of 0.01 for 1 h. After removal of the inoculum, infected cells were cultivated for 3 days in a mixture of Avicel and DMEM. Cells were fixed with 6% formaldehyde. After permeabilization with Triton X, TBEV envelope protein was detected by the respective primary and HRP-linked secondary antibodies. The enzymatic reaction was performed using TrueBlue peroxidase substrate. Pictures of cell culture plates were taken with the ChemiDoc imaging system (Bio-Rad). IC50 values were estimated by visual inspection of the wells.
Effects of labyrinthopeptins on WNV infection.
Huh-7 cells were seeded in 96-well plates (104 cells/well) and incubated overnight (approximately 18 h) at 37˚C with a 5% CO2 supply. The following day, cells were preincubated for 1 h with 2-fold serial dilutions of labyrinthopeptins (LabyA1 or LabyA2) starting at a 50 μM final concentration or with the DMSO control. Each concentration was tested in triplicate. One hour after incubation with the compounds, culture medium was washed from the wells and WNV (MOI = 0.1 or 0.01) was inoculated in 100 μl medium/well together with fresh compounds for 1.5 h. Viral inoculum was washed and replaced with fresh culture medium containing drugs. Cells were incubated for 48 h (MOI = 0.1) or 72 h (MOI = 0.01) at 37˚C with a 5% CO2 supply. After the indicated times, virus-containing supernatants from treated and untreated cells were clarified by centrifugation, filtered, and stored at –80°C. To estimate the resulting titers, we performed a microplaque assay in 96-well plates. Briefly, clarified supernatants from previously labyrinthopeptin-treated cells were serially diluted (10−1 to 10−10) and inoculated onto Huh-7/Scr cells in 96-well plates (5,000 cells/well) in quadruplicate. Cultures were incubated for 5 to 7 days until the appearance of virus-mediated cytopathic effects (CPE). When CPE was evident (as estimated by cell monolayer destruction using a positive control), culture medium was discarded and cells were fixed in an aqueous solution containing 4% PFA, 10% methanol (MeOH), and 1% crystal violet (CV) for 30 min. After fixation, the cells were washed three times with PBS and the CPE was analyzed under light microscopy. Well dilutions in which no CPE was evident (compared to cell monolayers in negative and positive controls) were considered negative for WNV. Fifty percent tissue culture infective dose (TCID50)/ml and IC50 values were calculated using a standard Reed and Muench formula and GraphPad functions, respectively. Cell viability was estimated in both infected and uninfected cells using the CellTiter Glo toxicity kit from Promega.
Effect of labyrinthopeptins on CVB infection.
The experiment is based on measuring the cytopathic effect (CPE) of coxsackievirus B (CVB) infection by using an MTT assay. Briefly, Vero cells (1 × 104 cells per well) were seeded in a 96-well plate 1 day prior to infection. Prior to infection of Vero cells, coxsackievirus B3 was preincubated for 15 min with LabyA1 or LabyA2 at a concentration of 50 μM with serial 2-fold dilutions down to 0.4 μM. DMSO was used as a vehicle control. At 4 h after infection of cells, the virus/compound-containing inoculum was removed and 200 μl of fresh medium was added to the cells. At 24 h postinfection, the medium was removed and 50 μl/well MTT solution (Sigma, Darmstadt, Germany; final concentration, 0.5 mg/ml medium) was added to the cells. Cells were incubated for another 30 min at 37°C prior to removal of the MTT solution and lysis of cells in 50 μl DMSO. Samples were measured at 570 nm and 630 nm (reference wavelength) using a BioTek Synergy 2 microplate reader. Data were normalized to those for uninfected cells. Mean values and SD of eight independent experiments are given. Interferon 2A (IFN-2A) was included as a positive control (PBL Assay Science, Piscataway, NJ, USA). Cells were preincubated with IFN-2A 8 h before virus infection. The means and SD of the results of four independent experiments are depicted.
Effect of labyrinthopeptins on adenovirus infection.
A total of 1 × 104 A549 cells were seeded in 100 μl growth medium (Ham’s F-12K [Kaighn’s], 10% FCS, 100 U/ml penicillin, 100 μg/ml streptavidin) onto a 96-well plate and incubated for 24 h at 37°C and 5% CO2. After discarding the supernatant, various concentrations of labyrinthopeptin A1 (0.2 μM, 1 μM, 5 μM, 25 μM) and labyrinthopeptin A2 (0.4 μM, 2 μM, 10 μM, 50 μM) were added to the cells in a total volume of 100 μl medium containing adenovirus type 5 (AdV5 ATCC2-p) at an MOI of 0.0008. As a positive control, the nucleoside analogue ribavirin was used at a concentration of 250 μM. Each of these reaction mixtures was done in triplicate and contained 1% (vol/vol) of the solvent DMSO. The uninfected and infected controls were inoculated with 100 μl medium with or without AdV5 and 1% DMSO in quadruplicate. After 72 h of incubation at 37°C and 5% CO2, the supernatant of each well was aspirated and the cells were replenished for 45 min at 37°C with 50 μl of preheated medium containing 10% MTT solution (stock diluted in PBS at 5 mg/ml). Medium was removed, and cells were lysed in 50 μl DMSO and incubated for 15 min at RT. In a Cytation 3 plate reader (BioTek), the absorbance of the samples was measured at 570 nm, which was subsequently normalized to the absorbance measured at 630 nm. The normalized absorption was expressed relative to that of uninfected cells to calculate the percent cell viability.
Synthesis of 5-hexynoyl-LabyA1.
To a solution of 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT, 1 mg, 0.006 mmol) in dimethylformamide (DMF, 1 ml), N-methylmorpholine (NMM, 2 μl, 0.015 mmol) was added at RT. After 1 h with stirring at RT, 5-hexynoic acid (0.5 μl, 0.004 mmol) was added. After 30 min of stirring, labyrinthopeptin A1 (6 mg, 0.003 mmol) was added to the reaction mixture and allowed to stir for 16 h at RT. The reaction mixture was purified by reversed-phase high-performance liquid chromatography (HPLC) using a Gemini 5-μm, C18 column (dimensions, 250 mm by 20 mm) with 10 to 90% acetonitrile in water (containing 0.1% HCOOH) as eluent. Peaks were fractionated based on the UV detection at 220 nm. The collected desired compound was lyophilized to yield 2 mg (31.7%). The product was characterized by high-resolution mass spectrometry (HRMS).
HRMS (quadrupole time-of-flight [Q-TOF] MS): calculated for C98H126N23O26S4 [M + H]+ 2,168.8127 Da, found 2,168.7821 Da.
Synthesis of 5-hexynoyl-LabyA2.
To a solution of 2-chloro-4,6-dimethoxy-1,3,5-triazine (CDMT; 1 mg, 0.006 mmol) in dimethylformamide (DMF; 1 ml), N-methylmorpholine (NMM; 2 μl, 0.015 mmol) was added at RT. After 1 h at RT, 5-hexynoic acid (0.5 μl, 0.004 mmol) was added. After 30 min of stirring, LabyA2 (6 mg, 0.003 mmol) was added to the reaction mixture and allowed to stir for 16 h at RT. The reaction mixture was purified by reversed-phase HPLC using a Gemini 5-μm, C18 column (dimensions, 250 mm by 20 mm) with 10 to 90% acetonitrile in water (containing 0.1% HCOOH) as eluent. Peaks were fractionated based on the UV detection at 220 nm. The collected desired compound was lyophilized to yield 1.5 mg (23.8%). The product was characterized by HRMS.
HRMS (Q-TOF): calculated for C91H117N20O25S4 [M + H]+ 2,017.7381 Da, found 2,017.7376 Da.
Cytotoxicity assay (MTT).
The epithelial cell line Hep G2 (ATCC HB-8065) was cultivated in Dulbecco’s modified Eagle’s medium (DMEM) with 10% heat-inactivated fetal calf serum (FCS) at 37°C and 5% CO2. CHO cells (ATCC CCL-61) were cultivated in Ham’s F-12K medium with 10% heat-inactivated fetal calf serum (FCS) at 37°C and 5% CO2. Cells were seeded into a 96-well plate (Nunc, Roskilde, Denmark) and grown to 75% confluence. LabyA1, LabyA2, and cinnamycin were tested in the assay. Every compound was dissolved in DMSO and diluted in PBS (final DMSO concentration in the cell assay, 0.1%). Cells were incubated with the respective compound at concentrations ranging from 1 to 50 μg/ml for cinnamycin and 1 to 100 μg/ml for LabyA1 and LabyA2 for 24 h at 37°C and 5% CO2. Cells treated with vehicle only (DMSO diluted in PBS; final DMSO concentration in the cell assay, 0.1%) served as a negative control. Furthermore, pure medium (DMEM plus 10% FCS for Hep G2 cells and Ham’s F-12K plus 10% FCS for CHO cells) and completely damaged cells served as positive controls. Cells were treated with 0.5% Triton X-100 for 1 h prior to the addition of MTT (Sigma) to cause damage. After 24 h, the cells were washed twice with DMEM plus 10% FCS (for Hep G2-cells) or Ham’s F-12K plus 10% FCS (for CHO cells). MTT diluted in PBS (stock solution, 5 mg/ml) was added to the wells at a final concentration of 1 mg/ml. The cells were incubated for 3 h at 37°C and 5% CO2. Medium was removed, and 0.04 M HCl in 2-propanol was added. The cells were incubated at RT for 15 min. The supernatant was then transferred to a 96-well plate. The samples were measured at 560 nm and at 670 nm as a reference wavelength on a Tecan Sunrise ELISA reader using Magellan software. Data were normalized using the following formula: (A − B)/(C − B), with A being the respective data point, B being the value for the Triton X-100-treated control, and C being the value for the vehicle control. The experiment was repeated at least three times. The error bars indicate the standard deviations. CC50 values were calculated using GraphPad Prism software.
qRT-PCR.
Viral RNA from cell-free solutions was isolated using the NucleoSpin virus kit (Macherey-Nagel) according to the vendor’s manual. Twenty micrograms of poly(A) RNA was added per sample before purification. All purified RNA was quantified by measuring the absorbance at 260 nm.
A 2.5-μg amount of purified RNA was reverse transcribed via RevertAid reverse transcriptase (Thermo) according to the vendor’s manual with reverse primers specific for DENV-2 New Guinea C, ZIKV-976, or ZIKV-H/PF/2013 (Table 4). After the reaction, the volume was adjusted to obtain a concentration of 20 ng/μl reverse-transcribed RNA. For quantitative PCR, 5 μl of reverse-transcribed RNA (equal to 100 ng) or standard plasmid was mixed with 10 μl LightCycler 480 probes master (Roche), 500 nM forward primer, 500 nM reverse primer, and 100 nM hydrolysis probe to give a final reaction volume of 20 μl. PCR was performed as follows: initial denaturation at 95°C for 5 min, followed by 45 cycles of denaturation at 95°C for 10 s, annealing at 60°C for 20 s, and extension at 72°C for 1 s. For dose-response experiments, the absolute number of viral genome copy equivalents (GCE) was determined via linear regression, with standard curves generated from plasmids with known concentrations carrying the respective amplified fragment of the viral genome.
TABLE 4.
qRT-PCR primers and corresponding hydrolysis probes used in this studya
| Target | Forward primer (5′–3′) | Hydrolysis probe (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|---|
| DENV2 New Guinea C | TCGGAGCCGGAGTTTACAAA | 6FAM-ATTCCATACAATGTGGCA-MGB | TCTTAACGTCCGCCCATGAT |
| ZIKV976 | AAATACACATACCAAAACAAAGTGG | 6FAM-CTCAGACCAGCTGAAG-MGB | TCCACTCCCTCTCTGGTCTTG |
| ZIKVH/PF/2013 | AAGTACACATACCAAAACAAAGTGG | 6FAM-CTTAGACCAGCTGAAA-MGB | TCCGCTCCCCCTTTGGTCTTG |
MGB, TaqMan minor groove binder (quencher); OQA, Onyx Quencher A; 6FAM, 6-carboxyfluorescein.
RNase protection assay.
The RNase protection assay was performed as described elsewhere (27). A total of 1.5 × 104 PFU of DENV-2 (NGC) in assay medium (5% FBS) was mixed with labyrinthopeptins (assay concentration, 50, 16.7, or 5.56 μg/ml), HEPES-buffered saline (HBS), or 1% Triton X-100. After 2 h of incubation at RT, samples were subjected to micrococcal nuclease (New England Biolabs) according to the vendor’s protocol. Samples were incubated for 1 h at 37°C. Eventually, RNA was extracted, and the number of GCE was determined by qRT-PCR.
Peptide arrays.
Microarrays of overlapping peptides with a length of 15 amino acids and a sequence overlap of 3 amino acids were synthesized on cellulose support and then spotted on glass slides using the SC2 method (19). The peptides covered the full surface proteome of DENV-2 (E, preM, and C proteins; accession no. NP_056776), the Env GP120 sequence of HIV (variants NL4.3 and Ada, gi 395783487 and gi 37962994), and the E1 and E2 sequences of HCV H77 (GT1a) and J6CF (GT2a) (accession no. AF177036 and AF009606). To visualize the binding of labyrinthopeptins to the immobilized peptides, N-terminally modified LabyA1 and LabyA2 derivatives carrying a hexynoyl moiety that fully retained their antiviral activity were synthesized. For the incubation of the microarrays with hexynoyl-labyrinthopeptins (60 μl/slide, 1 μg/ml labyrinthopeptin-hexynoyl), an azide-modified biotin moiety was attached using click chemistry and staining was done afterwards using a Cy3-conjugated anti-biotin antibody. Alternatively, native labyrinthopeptins (60 μl/slide, 1 μg/ml) were incubated with the slides, and detection was carried out using polyclonal rabbit anti-LabyA1 or -LabyA2 antisera (100 μg/ml) stained with an anti-rabbit Cy3-IgG conjugate.
Generation of liposomes.
Lipids were purchased from Avanti Polar Lipids and dissolved as 10 mM stocks in 67% chloroform–33% methanol. Small or large unilamellar vesicles (SUVs and LUVs, respectively) were prepared from lipid films as previously described (60). The lipid solutions were dispensed into 5-ml capped glass vials according to the molar ratios indicated in Fig. 6. Solvent was evaporated in a rotary evaporator under an N2 atmosphere, and the obtained lipid film was further dried in a vacuum desiccator. Lipid films were rehydrated by the addition of 1 ml HBS (5 mM HEPES, 150 mM NaCl, pH 7.4) to give suspensions corresponding to 1 mM total lipid. For CF leakage studies, HBS was supplemented with 50 mM 5(6)-carboxyfluorescein (CF). Rehydration was performed at temperatures above the gel-liquid crystal transition temperature (Tc) of the lipid with the highest Tc. Here, suspensions were frozen and thawed 3 times and then extruded 21 times through two stacked polycarbonate membranes with 50-nm (SUV) or 100-nm (LUV) pores by using a mini-extruder kit (Avanti Polar Lipids) at a proper temperature. CF-loaded liposomes (CF-LUVs) were passed over two consecutive Sephadex-25 size exclusion columns (PD MidiTrap G-25; GE Healthcare) to remove unincorporated CF. Liposome suspensions were kept at 4°C or RT, and measurements were performed within 4 weeks.
Lipid-binding studies.
To determine binding of labyrinthopeptins to various lipids, we used an ELISA format as previously described (61). Fifty microliters of solution (20 μM in ethanol) of the respective lipid (as listed in Table 3) was evaporated on the surface of a Nunc Polysorp 96-well plate. Wells were blocked with 30 mg/ml BSA (biotin-free) in PBS overnight at 4°C. Wells were washed three times with PBS plus 0.05% Tween 20, and 100 μl of a solution of either 10 μg/ml duramycin-biotin, 5 μM hexynoyl-LabyA1, 5 μM hexynoyl-LabyA2, or 2.5 μM each LabyA1 and LabyA2 (all dissolved in 10 mg/ml BSA in PBS) was added for 2 h at RT. After washing six times, 100 μl of click reaction mix was applied (2 mM CuSO4, 5 mM sodium ascorbate, 100 μM azide-PEG-biotin [Sigma], 10 mg/ml BSA in PBS) for 2 h. The plate was washed six times, and 100 μl of streptavidin-peroxidase conjugate (250 ng/ml streptavidin-HRP [Thermo], 10 mg/ml BSA in PBS) was added. After 1 h, the plate was again washed six times, and 100 μl color reagent (TMB ELISA substrate solution [eBioscience]) was added. After 15 min, the reaction was stopped by the addition of 50 μl H3PO4 (1 M), and the optical density at 450 nm (OD450) was determined in a plate reader (PowerWave XS2 [BioTek]).
CF leakage studies.
CF leakage studies were performed as described previously (62). CF-LUV suspensions were diluted 1:100 in HBS, and 100 μl was dispensed in black 96-well plates (Nunc 655900). The fluorescence was determined with a Synergy 4 reader (BioTek) using a fluorescence filter set with top optics (excitation, 485 nm; emission, 510 nm). Sensitivity was automatically determined so that a CF-LUV dilution made from DOPC gave arbitrary fluorescence units (AFU) of 1,000. The fluorescence baseline was determined over the course of 5 min with 1 read every 150 s. Then, 25 μl of labyrinthopeptin solution (assay concentration, 50, 16.67, 5.56, or 1.85 μg/ml) or HBS was added per well using the CyBi-SELMA pipetting robot. Fluorescence was further monitored for 1 h with one read every 5 min. Finally, complete vesicle leakage was induced by the addition of 14 μl Triton X-100 (1%) to obtain the maximum fluorescence. The percentage of CF leakage (L) at time t was determined according to:
where F(t) is the measured fluorescence at time t, F0 is the fluorescence baseline before the addition of compound, and Fx is the maximum fluorescence after the addition of Triton X-100. Values were blank corrected for each LUV composition by subtraction of the respective L(t) obtained from HBS-treated wells.
Pharmacokinetics (PK) study.
LabyA1 was dissolved in 20% DMSO, 10% ethanol, 20% N-methyl-2-pyrrolidone, and 50% H2O. LabyA2 was dissolved in 20% DMSO, 15% glycerol, and 65% PBS. Mice were administered LabyA1 and LabyA2 intravenously or intraperitoneally at 10 mg/kg (3 per experiment). About 20 μl of whole blood was collected serially from the lateral tail vein of each mouse at time points 0.25, 0.5, 1, 2, 4, and 8 h postadministration. After 24 h, the mice were sacrificed and blood was collected from the heart. Whole blood was collected into Eppendorf tubes coated with 0.5 M EDTA and immediately spun down at 13,000 rpm for 10 min at 4°C. The plasma was transferred into a new Eppendorf tube and then stored at −80°C until analysis.
PK sample preparation and analysis.
All PK plasma samples were analyzed via HPLC-tandem mass spectrometry (HPLC-MS/MS) using an Agilent 1290 HPLC system equipped with a diode array UV detector and coupled to an AB Sciex QTrap 6500 mass spectrometer. First, a calibration curve was prepared by spiking different concentrations of LabyA1 or LabyA2 into mouse plasma. The lower limits of quantification were 130 ng/ml for LabyA1 and 50 ng/ml for LabyA2. The upper limits of quantification were 40 μg/ml for LabyA1 and 2,000 ng/ml for LabyA2. The lower limits of qualification were 50 ng/ml for LabyA1 and 25 ng/ml for LabyA2. Caffeine was used as an internal standard. In addition, quality control samples (QCs) were prepared at 1, 2.5, 10, and 25 μg/ml for LabyA1 and at 200 and 2,000 ng/ml for LabyA2. A 7.5-μl volume of a plasma sample (calibration samples, QCs, or PK samples) was extracted with 37.5 μl of acetonitrile containing 12.5 ng/ml of caffeine as an internal standard for 10 min at 2,000 rpm on an Eppendorf MixMate vortex mixer. The samples were then spun down at 13,000 rpm for 5 min. Supernatants were transferred to standard HPLC glass vials. HPLC conditions were as follows: column, Agilent Zorbax Eclipse Plus C18, 50 by 2.1 mm, 1.8 μm; temperature, 30°C; injection volume, 10 μl; flow rate, 700 μl/min; solvent A, water plus 0.1% formic acid; solvent B, acetonitrile plus 0.1% formic acid; gradient for LabyA1 and LabyA2: 99% A at 0 min, 99% to 0% A from 0.1 min to 5.50 min, 0% A until 6.00 min, 0% to 99% A from 6.00 min to 6.20 min, 99% A until 8.00 min; UV detection, 190 to 400 nm. Mass spectrometric conditions were as follows: scan type, multiple-reaction monitoring (MRM), positive mode; quadrupole 1 (Q1) and quadrupole 3 (Q3) masses for caffeine, LabyA1, and LabyA2 are given in Table 5. Peak areas of each sample and of the corresponding internal standard were analyzed using MultiQuant 3.0 software (AB Sciex). For quantification, peak areas of the respective quantifier peaks (indicated in Table 5) were used and normalized to the internal standard (caffeine). PK samples were quantified using a calibration curve generated by spiking defined amounts of analyte to analyte-free matrix samples. The accuracy of the calibration curve was determined using QCs independently prepared on different days (accuracy, 87.46 to 106.80% for LabyA1 and 97.90 to 114.35% for LabyA2). PK parameters were determined using a noncompartmental analysis with PKSolver (63).
TABLE 5.
Mass spectrometric parameters for multiple-reaction monitoring of caffeine, LabyA1, and LabyA2a
| ID | Q1 m/z (Da) | Q3 m/z (Da) | Time (ms) | CE (V) | CXP (V) | DP (V) |
|---|---|---|---|---|---|---|
| LabyA1 | ||||||
| Quantifier | 692.5 | 682.8 | 150 | 17 | 16 | 71 |
| Qualifier | 692.5 | 69.9 | 150 | 129 | 8 | 71 |
| LabyA2 | ||||||
| Quantifier | 962.6 | 953.2 | 150 | 33 | 54 | 66 |
| Qualifier | 962.6 | 948.2 | 150 | 25 | 22 | 66 |
| Caffeine | ||||||
| Quantifier | 195.1 | 138.1 | 50 | 27 | 10 | 80 |
| Qualifier | 195.1 | 110.1 | 50 | 31 | 6 | 80 |
CE, collision energy; CXP, collision cell exit potential; DP, declustering potential; Q1, quadrupole 1; Q3, quadrupole 3.
Animal studies and ethics statement.
For PK experiments, 4-week-old outbred male CD-1 mice (Charles River, Netherlands) were used. All animal experiments were performed in compliance with the German animal welfare law (TierSchG BGBl. S. 1105; 25.05.1998). The mice were housed and handled in accordance with good animal practice as defined by the Federation of Laboratory Animal Science Associations (FELASA). All animal experiments were approved by the Lower Saxony State Office of Consumer Protection and Food Safety under permit no. 33.19–42502-04-15/1857 and AZ 33.9–42502-04-15/1895.
ACKNOWLEDGMENTS
Thomas Pietschmann was supported by funds from the Helmholtz Alberta initiative of infectious disease research (HAI-IDR). Andreas Meyerhans and Javier P. Martinez were supported by a grant from the Spanish Ministry of Economy, Industry and Competitiveness and FEDER grant no. SAF2016-75505-R (AEI/MINEICO/FEDER, UE). Mark Brönstrup, Andreas Meyerhans, and Javier P. Martinez acknowledge a networking contribution from the COST Action CM1407 “Challenging organic syntheses inspired by nature—from natural products chemistry to drug discovery.” Martin Messerle and Thomas F. Schulz were supported by funding from DZIF (project 07.802 TTU IICH). Christine Goffinet, Thomas Pietschmann, and Mark Brönstrup were supported by a grant provided by Innovationsfonds der Helmholtz-Zentren. Christine Goffinet was supported by a DFG grant within German-African Cooperation Projects in Infectiology (GO2153/3-1) and by funding from the Helmholtz Center for Infection Research (HZI) and the Berlin Institute of Health (BIH). Sergej Franz was supported by the Infection Biology international Ph.D. program of Hannover Biomedical Research School.
H.P. performed experiments with DENV, ZIKV, qRT-PCRs, lipid-related studies, and ELISAs, analyzed the data, and wrote the paper. K.R. performed pharmacokinetic (PK) experiments, analyzed PK data, prepared the formulation for LabyA1 and LabyA2, and wrote the paper. J.S. performed cytotoxicity assays and PK experiments. S.-K.H. performed peptide array experiments. J.W. and K.M. cultivated the labyrinthopeptin producer strain. N.V.S.B. synthesized hexynoyl-labyrinthopeptin derivatives and purified labyrinthopeptins. P.K. purified labyrinthopeptins. L.W. performed mouse experiments. S. Blockus performed coxsackievirus infection experiments. M.O. performed experiments with DENV. D.H.B. conducted HCV infection experiments. S.H. performed adenovirus infection experiments. S.F. and C.G. designed and performed CHIKV infection experiments and analyzed the data. J.P.M. performed the antiviral tests with WNV. J.P.M. and A.M. designed the experiments with WNV, analyzed the data, and contributed to the writing of the paper. T.P. designed the coxsackievirus, HCV, and adenovirus experiments and analyzed the data. A.K., R.D.S., A.M., M.M., T.F.S., and D.S. designed the research and interpreted the data. M.B. designed the research, interpreted the data, and wrote the paper.
The data sets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
C.G., H.P., M.B., M.M., S. Birudukota, S. Blockus, D.B., S.F., S.H., T.P., and T.F.S. are coinventors of a patent application on the antiviral properties of labyrinthopeptins (64).
TWINCORE is a joint venture between the Helmholtz Centre for Infection Research, Braunschweig, Germany, and the Hannover Medical School, Hannover, Germany.
REFERENCES
- 1.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marston HD, Folkers GK, Morens DM, Fauci AS. 2014. Emerging viral diseases: confronting threats with new technologies. Sci Transl Med 6:253ps10. doi: 10.1126/scitranslmed.3009872. [DOI] [PubMed] [Google Scholar]
- 3.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, Drake JM, Brownstein JS, Hoen AG, Sankoh O, Myers MF, George DB, Jaenisch T, Wint GR, Simmons CP, Scott TW, Farrar JJ, Hay SI. 2013. The global distribution and burden of dengue. Nature 496:504–507. doi: 10.1038/nature12060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodenhuis-Zybert IA, Wilschut J, Smit JM. 2010. Dengue virus life cycle: viral and host factors modulating infectivity. Cell Mol Life Sci 67:2773–2786. doi: 10.1007/s00018-010-0357-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. 2016. Zika virus and birth defects—reviewing the evidence for causality. N Engl J Med 374:1981–1987. doi: 10.1056/NEJMsr1604338. [DOI] [PubMed] [Google Scholar]
- 6.Miner JJ, Cao B, Govero J, Smith AM, Fernandez E, Cabrera OH, Garber C, Noll M, Klein RS, Noguchi KK, Mysorekar IU, Diamond MS. 2016. Zika virus infection during pregnancy in mice causes placental damage and fetal demise. Cell 165:1081–1091. doi: 10.1016/j.cell.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adams Waldorf KM, Stencel-Baerenwald JE, Kapur RP, Studholme C, Boldenow E, Vornhagen J, Baldessari A, Dighe MK, Thiel J, Merillat S, Armistead B, Tisoncik-Go J, Green RR, Davis MA, Dewey EC, Fairgrieve MR, Gatenby JC, Richards T, Garden GA, Diamond MS, Juul SE, Grant RF, Kuller L, Shaw DW, Ogle J, Gough GM, Lee W, English C, Hevner RF, Dobyns WB, Gale M Jr, Rajagopal L. 2016. Fetal brain lesions after subcutaneous inoculation of Zika virus in a pregnant nonhuman primate. Nat Med 22:1256–1259. doi: 10.1038/nm.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim SP, Wang QY, Noble CG, Chen YL, Dong H, Zou B, Yokokawa F, Nilar S, Smith P, Beer D, Lescar J, Shi PY. 2013. Ten years of dengue drug discovery: progress and prospects. Antiviral Res 100:500–519. doi: 10.1016/j.antiviral.2013.09.013. [DOI] [PubMed] [Google Scholar]
- 9.Bekerman E, Einav S. 2015. Infectious disease. Combating emerging viral threats. Science 348:282–283. doi: 10.1126/science.aaa3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldescu V, Behnam MAM, Vasilakis N, Klein CD. 2017. Broad-spectrum agents for flaviviral infections: dengue, Zika and beyond. Nat Rev Drug Discov 16:565–586. doi: 10.1038/nrd.2017.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martinez JP, Sasse F, Brönstrup M, Diez J, Meyerhans A. 2015. Antiviral drug discovery: broad-spectrum drugs from nature. Nat Prod Rep 32:29–48. doi: 10.1039/c4np00085d. [DOI] [PubMed] [Google Scholar]
- 12.Warren TK, Wells J, Panchal RG, Stuthman KS, Garza NL, Van Tongeren SA, Dong L, Retterer CJ, Eaton BP, Pegoraro G, Honnold S, Bantia S, Kotian P, Chen X, Taubenheim BR, Welch LS, Minning DM, Babu YS, Sheridan WP, Bavari S. 2014. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508:402–405. doi: 10.1038/nature13027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Müller WM, Schmiederer T, Ensle P, Süssmuth RD. 2010. In vitro biosynthesis of the prepeptide of type-III lantibiotic labyrinthopeptin A2 including formation of a C-C bond as a post-translational modification. Angew Chem Int Ed Engl 49:2436–2440. doi: 10.1002/anie.200905909. [DOI] [PubMed] [Google Scholar]
- 14.Meindl K, Schmiederer T, Schneider K, Reicke A, Butz D, Keller S, Guhring H, Vertesy L, Wink J, Hoffmann H, Bronstrup M, Sheldrick GM, Sussmuth RD. 2010. Labyrinthopeptins: a new class of carbacyclic lantibiotics. Angew Chem Int Ed Engl 49:1151–1154. doi: 10.1002/anie.200905773. [DOI] [PubMed] [Google Scholar]
- 15.Chatterjee C, Paul M, Xie L, van der Donk WA. 2005. Biosynthesis and mode of action of lantibiotics. Chem Rev 105:633–684. doi: 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 16.Ferir G, Petrova MI, Andrei G, Huskens D, Hoorelbeke B, Snoeck R, Vanderleyden J, Balzarini J, Bartoschek S, Bronstrup M, Sussmuth RD, Schols D. 2013. The lantibiotic peptide labyrinthopeptin A1 demonstrates broad anti-HIV and anti-HSV activity with potential for microbicidal applications. PLoS One 8:e64010. doi: 10.1371/journal.pone.0064010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shum D, Smith JL, Hirsch AJ, Bhinder B, Radu C, Stein DA, Nelson JA, Fruh K, Djaballah H. 2010. High-content assay to identify inhibitors of dengue virus infection. Assay Drug Dev Technol 8:553–570. doi: 10.1089/adt.2010.0321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pascoalino BS, Courtemanche G, Cordeiro MT, Gil LH, Freitas-Junior L. 2016. Zika antiviral chemotherapy: identification of drugs and promising starting points for drug discovery from an FDA-approved library. F1000Res 5:2523. doi: 10.12688/f1000research.9648.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dikmans A, Beutling U, Schmeisser E, Thiele S, Frank R. 2006. SC2: a novel process for manufacturing multipurpose high-density chemical microarrays. QSAR Comb Sci 25:1069–1080. doi: 10.1002/qsar.200640130. [DOI] [Google Scholar]
- 20.Alen MM, Dallmeier K, Balzarini J, Neyts J, Schols D. 2012. Crucial role of the N-glycans on the viral E-envelope glycoprotein in DC-SIGN-mediated dengue virus infection. Antiviral Res 96:280–287. doi: 10.1016/j.antiviral.2012.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Hullin-Matsuda F, Makino A, Murate M, Kobayashi T. 2016. Probing phosphoethanolamine-containing lipids in membranes with duramycin/cinnamycin and aegerolysin proteins. Biochimie 130:81–90. doi: 10.1016/j.biochi.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Richard AS, Zhang A, Park SJ, Farzan M, Zong M, Choe H. 2015. Virion-associated phosphatidylethanolamine promotes TIM1-mediated infection by Ebola, dengue, and West Nile viruses. Proc Natl Acad Sci U S A 112:14682–14687. doi: 10.1073/pnas.1508095112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Henriques ST, Huang YH, Castanho MA, Bagatolli LA, Sonza S, Tachedjian G, Daly NL, Craik DJ. 2012. Phosphatidylethanolamine binding is a conserved feature of cyclotide-membrane interactions. J Biol Chem 287:33629–33643. doi: 10.1074/jbc.M112.372011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ireland DC, Wang CK, Wilson JA, Gustafson KR, Craik DJ. 2008. Cyclotides as natural anti-HIV agents. Biopolymers 90:51–60. doi: 10.1002/bip.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Petruzielo RS, Heberle FA, Drazba P, Katsaras J, Feigenson GW. 2013. Phase behavior and domain size in sphingomyelin-containing lipid bilayers. Biochim Biophys Acta 1828:1302–1313. doi: 10.1016/j.bbamem.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chou TC. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 27.Lok SM, Costin JM, Hrobowski YM, Hoffmann AR, Rowe DK, Kukkaro P, Holdaway H, Chipman P, Fontaine KA, Holbrook MR, Garry RF, Kostyuchenko V, Wimley WC, Isern S, Rossmann MG, Michael SF. 2012. Release of dengue virus genome induced by a peptide inhibitor. PLoS One 7:e50995. doi: 10.1371/journal.pone.0050995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Meer G, Voelker DR, Feigenson GW. 2008. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol 9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerold G, Bruening J, Weigel B, Pietschmann T. 2017. Protein interactions during the flavivirus and hepacivirus life cycle. Mol Cell Proteomics 16:S75–S91. doi: 10.1074/mcp.R116.065649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Meer G. 2011. Dynamic transbilayer lipid asymmetry. Cold Spring Harb Perspect Biol 3:a004671. doi: 10.1101/cshperspect.a004671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huarte N, Carravilla P, Cruz A, Lorizate M, Nieto-Garai JA, Krausslich HG, Perez-Gil J, Requejo-Isidro J, Nieva JL. 2016. Functional organization of the HIV lipid envelope. Sci Rep 6:34190. doi: 10.1038/srep34190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xin B, Zheng J, Liu H, Li J, Ruan L, Peng D, Sajid M, Sun M. 2016. Thusin, a novel two-component lantibiotic with potent antimicrobial activity against several Gram-positive pathogens. Front Microbiol 7:1115. doi: 10.3389/fmicb.2016.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao M. 2011. Lantibiotics as probes for phosphatidylethanolamine. Amino Acids 41:1071–1079. doi: 10.1007/s00726-009-0386-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huo L, Okesli A, Zhao M, van der Donk WA. 2017. Insights into the biosynthesis of duramycin. Appl Environ Microbiol 83:e02698-16. doi: 10.1128/AEM.02698-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Svangard E, Burman R, Gunasekera S, Lovborg H, Gullbo J, Goransson U. 2007. Mechanism of action of cytotoxic cyclotides: cycloviolacin O2 disrupts lipid membranes. J Nat Prod 70:643–647. doi: 10.1021/np070007v. [DOI] [PubMed] [Google Scholar]
- 36.Lindholm P, Goransson U, Johansson S, Claeson P, Gullbo J, Larsson R, Bohlin L, Backlund A. 2002. Cyclotides: a novel type of cytotoxic agents. Mol Cancer Ther 1:365–369. [PubMed] [Google Scholar]
- 37.Luukkonen A, Brummer-Korvenkontio M, Renkonen O. 1973. Lipids of cultured mosquito cells (Aedes albopictus). Comparison with cultured mammalian fibroblasts (BHK 21 cells). Biochim Biophys Acta 326:256–261. doi: 10.1016/0005-2760(73)90251-8. [DOI] [PubMed] [Google Scholar]
- 38.Brugger B, Glass B, Haberkant P, Leibrecht I, Wieland FT, Krausslich HG. 2006. The HIV lipidome: a raft with an unusual composition. Proc Natl Acad Sci U S A 103:2641–2646. doi: 10.1073/pnas.0511136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aloia RC, Tian H, Jensen FC. 1993. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci U S A 90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu ST, Sharon-Friling R, Ivanova P, Milne SB, Myers DS, Rabinowitz JD, Brown HA, Shenk T. 2011. Synaptic vesicle-like lipidome of human cytomegalovirus virions reveals a role for SNARE machinery in virion egress. Proc Natl Acad Sci U S A 108:12869–12874. doi: 10.1073/pnas.1109796108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Merz A, Long G, Hiet MS, Brugger B, Chlanda P, Andre P, Wieland F, Krijnse-Locker J, Bartenschlager R. 2011. Biochemical and morphological properties of hepatitis C virus particles and determination of their lipidome. J Biol Chem 286:3018–3032. doi: 10.1074/jbc.M110.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heaton NS, Randall G. 2011. Multifaceted roles for lipids in viral infection. Trends Microbiol 19:368–375. doi: 10.1016/j.tim.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lorizate M, Krausslich HG. 2011. Role of lipids in virus replication. Cold Spring Harb Perspect Biol 3:a004820. doi: 10.1101/cshperspect.a004820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tongluan N, Ramphan S, Wintachai P, Jaresitthikunchai J, Khongwichit S, Wikan N, Rajakam S, Yoksan S, Wongsiriroj N, Roytrakul S, Smith DR. 2017. Involvement of fatty acid synthase in dengue virus infection. Virol J 14:28. doi: 10.1186/s12985-017-0685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teissier E, Pecheur EI. 2007. Lipids as modulators of membrane fusion mediated by viral fusion proteins. Eur Biophys J 36:887–899. doi: 10.1007/s00249-007-0201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Villareal VA, Rodgers MA, Costello DA, Yang PL. 2015. Targeting host lipid synthesis and metabolism to inhibit dengue and hepatitis C viruses. Antiviral Res 124:110–121. doi: 10.1016/j.antiviral.2015.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Heaton NS, Randall G. 2010. Dengue virus-induced autophagy regulates lipid metabolism. Cell Host Microbe 8:422–432. doi: 10.1016/j.chom.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perera R, Riley C, Isaac G, Hopf-Jannasch AS, Moore RJ, Weitz KW, Pasa-Tolic L, Metz TO, Adamec J, Kuhn RJ. 2012. Dengue virus infection perturbs lipid homeostasis in infected mosquito cells. PLoS Pathog 8:e1002584. doi: 10.1371/journal.ppat.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chazal N, Gerlier D. 2003. Virus entry, assembly, budding, and membrane rafts. Microbiol Mol Biol Rev 67:226–237, table of contents. doi: 10.1128/mmbr.67.2.226-237.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Munger J, Bennett BD, Parikh A, Feng XJ, McArdle J, Rabitz HA, Shenk T, Rabinowitz JD. 2008. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat Biotechnol 26:1179–1186. doi: 10.1038/nbt.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koutsoudakis G, Romero-Brey I, Berger C, Pérez-Vilaró G, Monteiro Perin P, Vondran FWR, Kalesse M, Harmrolfs K, Müller R, Martinez JP, Pietschmann T, Bartenschlager R, Brönstrup M, Meyerhans A, Díez J. 2015. Soraphen A: a broad-spectrum antiviral natural product with potent anti-hepatitis C virus activity. J Hepatol 63:813–821. doi: 10.1016/j.jhep.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 52.Wolf MC, Freiberg AN, Zhang T, Akyol-Ataman Z, Grock A, Hong PW, Li J, Watson NF, Fang AQ, Aguilar HC, Porotto M, Honko AN, Damoiseaux R, Miller JP, Woodson SE, Chantasirivisal S, Fontanes V, Negrete OA, Krogstad P, Dasgupta A, Moscona A, Hensley LE, Whelan SP, Faull KF, Holbrook MR, Jung ME, Lee B. 2010. A broad-spectrum antiviral targeting entry of enveloped viruses. Proc Natl Acad Sci U S A 107:3157–3162. doi: 10.1073/pnas.0909587107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieto-Garai JA, Glass B, Bunn C, Giese M, Jennings G, Brankatschk B, Agarwal S, Börner K, Contreras FX, Knölker H-J, Zankl C, Simons K, Schroeder C, Lorizate M, Kräusslich H-G. 2018. Lipidomimetic compounds act as HIV-1 entry inhibitors by altering viral membrane structure. Front Immunol 9:1983. doi: 10.3389/fimmu.2018.01983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Neris RLS, Figueiredo CM, Higa LM, Araujo DF, Carvalho CAM, Verçoza BRF, Silva MOL, Carneiro FA, Tanuri A, Gomes AMO, Bozza MT, Da Poian AT, Cruz-Oliveira C, Assunção-Miranda I. 2018. Co-protoporphyrin IX and Sn-protoporphyrin IX inactivate Zika, Chikungunya and other arboviruses by targeting the viral envelope. Sci Rep 8:9805. doi: 10.1038/s41598-018-27855-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alen MM, De Burghgraeve T, Kaptein SJ, Balzarini J, Neyts J, Schols D. 2011. Broad antiviral activity of carbohydrate-binding agents against the four serotypes of dengue virus in monocyte-derived dendritic cells. PLoS One 6:e21658. doi: 10.1371/journal.pone.0021658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weber C, Konig R, Niedrig M, Emmerich P, Schnierle BS. 2014. A neutralization assay for chikungunya virus infections in a multiplex format. J Virol Methods 201:7–12. doi: 10.1016/j.jviromet.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsetsarkin K, Higgs S, McGee CE, De Lamballerie X, Charrel RN, Vanlandingham DL. 2006. Infectious clones of Chikungunya virus (La Reunion isolate) for vector competence studies. Vector Borne Zoonotic Dis 6:325–337. doi: 10.1089/vbz.2006.6.325. [DOI] [PubMed] [Google Scholar]
- 58.Borst EM, Messerle M. 2005. Analysis of human cytomegalovirus oriLyt sequence requirements in the context of the viral genome. J Virol 79:3615–3626. doi: 10.1128/JVI.79.6.3615-3626.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kati S, Hage E, Mynarek M, Ganzenmueller T, Indenbirken D, Grundhoff A, Schulz TF. 2015. Generation of high-titre virus stocks using BrK.219, a B-cell line infected stably with recombinant Kaposi’s sarcoma-associated herpesvirus. J Virol Methods 217:79–86. doi: 10.1016/j.jviromet.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Henriques ST, Huang YH, Rosengren KJ, Franquelim HG, Carvalho FA, Johnson A, Sonza S, Tachedjian G, Castanho MA, Daly NL, Craik DJ. 2011. Decoding the membrane activity of the cyclotide kalata B1: the importance of phosphatidylethanolamine phospholipids and lipid organization on hemolytic and anti-HIV activities. J Biol Chem 286:24231–24241. doi: 10.1074/jbc.M111.253393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Makino A, Baba T, Fujimoto K, Iwamoto K, Yano Y, Terada N, Ohno S, Sato SB, Ohta A, Umeda M, Matsuzaki K, Kobayashi T. 2003. Cinnamycin (Ro 09-0198) promotes cell binding and toxicity by inducing transbilayer lipid movement. J Biol Chem 278:3204–3209. doi: 10.1074/jbc.M210347200. [DOI] [PubMed] [Google Scholar]
- 62.Huang YH, Colgrave ML, Daly NL, Keleshian A, Martinac B, Craik DJ. 2009. The biological activity of the prototypic cyclotide kalata b1 is modulated by the formation of multimeric pores. J Biol Chem 284:20699–20707. doi: 10.1074/jbc.M109.003384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhang Y, Huo M, Zhou J, Xie S. 2010. PKSolver: an add-in program for pharmacokinetic and pharmacodynamic data analysis in Microsoft Excel. Comput Methods Programs Biomed 99:306–314. doi: 10.1016/j.cmpb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 64.Brönstrup M, Prochnow H-P, Birudukota NVS, Schulz T, Messerle M, Pietschmann T, Haid S, Blockus S, Laqmani-Goffinet C, Franz S, Banda DH. 18 November 2016, filing date. Labyrinthopeptins as anti-viral agents. Patent application WO2017085257A1.
- 65.Martin-Acebes MA, Merino-Ramos T, Blazquez AB, Casas J, Escribano-Romero E, Sobrino F, Saiz JC. 2014. The composition of West Nile virus lipid envelope unveils a role of sphingolipid metabolism in flavivirus biogenesis. J Virol 88:12041–12054. doi: 10.1128/JVI.02061-14. [DOI] [PMC free article] [PubMed] [Google Scholar]