CD74 plays a pivotal role in the correct folding and functional stability of major histocompatibility complex class II (MHC-II) molecules and in the presentation of antigenic peptides, acting as a regulatory factor in the antigen presentation process. In our study, we demonstrate a novel role of CD74 during IBDV infection, showing that chicken CD74 plays a significant role in IBDV binding to target B cells by interacting with the viral VP2 protein. This is the first report demonstrating that CD74 is involved as a novel attachment receptor in the IBDV life cycle in target B cells, thus contributing new insight into host-pathogen interactions.
KEYWORDS: IBDV, CD74, attachment receptor, virus binding
ABSTRACT
Infectious bursal disease virus (IBDV) is an important member of the Birnaviridae family, causing severe immunosuppressive disease in chickens. The major capsid protein VP2 is responsible for the binding of IBDV to the host cell and its cellular tropism. In order to find proteins that potentially interact with IBDV VP2, a liquid chromatography-mass spectrometry (LC-MS) assay was conducted, and the host chicken CD74 protein was identified. Here, we investigate the role of chicken CD74 in IBDV attachment. Coimmunoprecipitation assays indicated that the extracellular domain of CD74 interacted with the VP2 proteins of multiple IBDV strains. Knockdown and overexpression experiments showed that CD74 promotes viral infectivity. Confocal assays showed that CD74 overexpression allows the attachment of IBDV and subvirus-like particles (SVPs) to the cell surface of nonpermissive cells, and quantitative PCR (qPCR) analysis further confirmed the attachment function of CD74. Anti-CD74 antibody, soluble CD74, depletion of CD74 by small interfering RNA (siRNA), and CD74 knockdown in the IBDV-susceptible DT40 cell line significantly inhibited IBDV binding, suggesting a pivotal role of this protein in virus attachment. These findings demonstrate that CD74 is a novel important receptor for IBDV attachment to the chicken B lymphocyte cell line DT40.
IMPORTANCE CD74 plays a pivotal role in the correct folding and functional stability of major histocompatibility complex class II (MHC-II) molecules and in the presentation of antigenic peptides, acting as a regulatory factor in the antigen presentation process. In our study, we demonstrate a novel role of CD74 during IBDV infection, showing that chicken CD74 plays a significant role in IBDV binding to target B cells by interacting with the viral VP2 protein. This is the first report demonstrating that CD74 is involved as a novel attachment receptor in the IBDV life cycle in target B cells, thus contributing new insight into host-pathogen interactions.
INTRODUCTION
Infectious bursal disease virus (IBDV), first identified in 1957 (1), is a nonenveloped, double-stranded RNA, icosahedral virus belonging to the genus Avibirnavirus of the family Birnaviridae (2). IBDV targets immature B lymphoid cells and can replicate in other immune cells, such as macrophages (3), monocytes (4, 5), and natural killer (NK) cells (6). Chickens 3 to 6 weeks of age, when the bursa of Fabricius (BF) is maximally developed, are highly susceptible to this virus. IBDV causes a highly immunosuppressive and contagious disease in young chickens, manifesting itself with BF destruction and immunosuppression (1, 7) and leading to devastating economic losses in the poultry industry worldwide (1, 8). To complete its life cycle, the virus needs to rely on the host cell (9). The first step in the virus infection process is viral attachment to specific receptors (10, 11). However, the cellular receptors for virus binding have not been well characterized, especially for very virulent IBDV (vvIBDV) strains.
Thus, we tried to identify the cellular receptors involved in virus binding based on the capsid protein VP2, which determines virus-host binding and cell tropism (12–14). Previous studies demonstrated that the addition of both recombinant Hsp90 protein and anti-Hsp90 antibody in cell culture medium can inhibit infection of DF-1 cells by IBDV, proving that cHsp90α is a functional part of the IBDV receptor complex in DF-1 cells (15). Delgui et al. characterized the role of the interaction between the VP2 Ile-Asp-Ala (IDA) motif and α4β1 integrin in the binding of IBDV to susceptible cells (16). They noted the structural similarity between the IBDV VP2 projection domain (P domain) and the corresponding projection domains of reovirus capsid polypeptides, which were proven to be able to use integrins as essential entry molecules and/or binding receptors (11). The VP2 IDA motif plays a critical role in the binding of IBDV-derived subvirus-like particles (SVPs) and virus particles to IBDV-susceptible cells, and a single point mutation in this motif completely abrogates SVP cell binding and virus infectivity (16). Further results showed that IBDV activates the phosphorylation of c-Src to induce cell entry via an α4β1 integrin-mediated pathway by activating downstream phosphatidylinositol 3-kinase (PI3K)/Akt-RhoA signaling and actin cytoskeleton rearrangement (17), but more details still need to be uncovered. As a nonenveloped virus, the outermost part of the IBDV viral particle is the primary capsid VP2 protein (18, 19), playing a pivotal role in determining cell tropism (20–22) and the interactions with host cellular receptors (15, 16). Based on the crystal structures of IBDV VP2 and SVPs, VP2 can be divided into three domains: the base domain (B domain), the shell domain (S domain), and the P domain (18, 19, 23). The P domain (amino acids [aa] 206 to 350), also known as the hypervariable region, is responsible for epitope recognition by neutralizing antibodies and interacting with receptor components (24). Mundt first recognized the role of the VP2 protein in cell tropism, showing that specific mutations (Q253H/A284T) could change the cellular tropism of IBDV (25). Our previous results confirmed by reverse genetics that the dual Q253H/A284T mutation of the VP2 protein is the molecular basis for cell tropism of IBDV (22). In addition, another dual-mutation site of VP2, located at aa 279 and 284, could also lead virulent IBDV to adapt to chicken embryonic fibroblasts (26).
CD74 (27), also known as the invariant chain (Ii) of major histocompatibility complex class II (MHC-II) molecules (28), has been proven to play important roles in the functional stability and correct folding of MHC-II molecules (29) as well as in antigen peptide presentation (30). At present, two isoforms of chicken CD74 have been uncovered: Ii-1 and Ii-2 (corresponding to human p33 and p41, respectively) (31). Ii-1 has the function of enhancing the presentation of Ii-dependent MHC-II-restricted epitopes (31), while Ii-2 could act as a regulatory factor in antigen presentation epitopes (32). Moreover, CD74 is the cellular receptor of the macrophage migration-inhibitory factor (MIF), participating in the activation of downstream inflammatory pathways (33).
In this study, vvIBDV VP2 was used to search for host proteins involved in IBDV-host interactions. We found that the cellular protein CD74 is responsible for virus-host binding and specifically that CD74 is a novel IBDV attachment receptor.
RESULTS
Identification of CD74 as a putative receptor for IBDV.
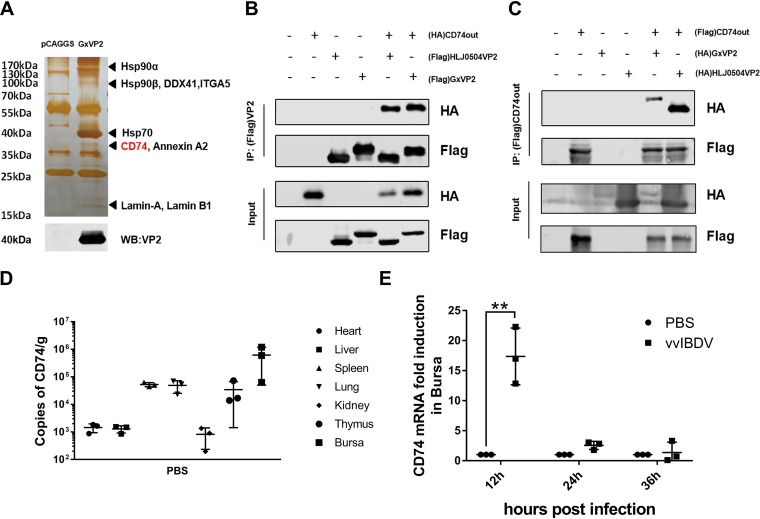
Previous studies showed that the IBDV primary capsid protein VP2 is responsible for the interaction with cellular proteins and antigenicity (34). To investigate possible receptor molecules, we performed affinity purification with an IBDV major capsid protein VP2 monoclonal antibody (mAb) in DT40 cells, which were incubated with VP2 protein (or an empty vector as a negative control) for 6 h, followed by mass spectrometry analysis. As shown in Fig. 1A, a variety of host proteins were associated with VP2, including chicken CD74. Based on the liquid chromatography-mass spectrometry (LC-MS) results, to further confirm the interaction of VP2 with the CD74 extracellular domain, we used a coimmunoprecipitation (co-IP) assay. 293T cells were transfected with Flag-CD74out (or hemagglutinin [HA]-CD74out) (a eukaryotic expression plasmid for the CD74 extracellular domain spanning aa 57 to 286) and VP2-HA (or VP2-Flag) of the Gx or HLJ0504 viral strain. The cell lysate was immunoprecipitated with anti-Flag agarose and immunoblotted with an anti-HA antibody. The results showed bands corresponding to VP2 (Fig. 1B) or the CD74 extracellular domain (Fig. 1C) in the Flag co-IP assay, indicating an interaction between the CD74 extracellular domain and Gx/HLJ0504 VP2.
FIG 1.
Identification of CD74 as a putative receptor for IBDV. (A) Affinity purification with mAb against the IBDV major capsid protein VP2 in DT40 cells, incubated with VP2 (or the empty vector as a negative control), followed by mass spectrometry analysis. Many host proteins were associated with VP2, including chicken CD74. (B and C) Interaction between VP2 and the CD74 extracellular domain detected via a coimmunoprecipitation (co-IP) assay. (B) Western blot (WB) analysis using an antibody against the HA tag showing the bands corresponding to VP2 in the Flag co-IP assay. (C) Western blot analysis using an antibody against the HA tag showing the bands corresponding to the CD74 extracellular domain in the Flag co-IP assay. (D) To determine the distribution of CD74 in organs, the heart, liver, spleen, lung, kidney, thymus, and bursa of Fabricius of uninfected SPF chickens were collected to measure CD74 transcription levels. The most abundant CD74 expression was found in the bursa. (E) To confirm the involvement of chicken CD74 in the IBDV infection process, SPF chickens were challenged by vvIBDV or PBS. Bursas were collected for RT-qPCR analysis of CD74 transcription levels. CD74 mRNAs were significantly induced by vvIBDV at 12 h p.i. (*, P < 0.05; **, P < 0.01). The arithmetic means and standard deviations for at least three independent experiments performed in duplicate are shown.
To determine the distribution of CD74, organs (heart, liver, spleen, lung, kidney, thymus, and bursa) of uninfected specific-pathogen-free (SPF) chickens were collected for assessment of CD74 transcription levels. The results indicated that CD74 was most abundantly expressed in the bursa of Fabricius, which is the primary target organ of IBDV (Fig. 1D).
To study the role of CD74 in vvIBDV infection, SPF chickens were challenged with vvIBDV or phosphate-buffered saline (PBS). Bursas were collected for reverse transcription-quantitative PCR (RT-qPCR) analysis of CD74 transcription levels. As shown in Fig. 1E, CD74 mRNA levels were significantly induced by vvIBDV at 12 h postinfection (p.i.) (P < 0.05), compared with the PBS group.
CD74 is essential for IBDV infection.
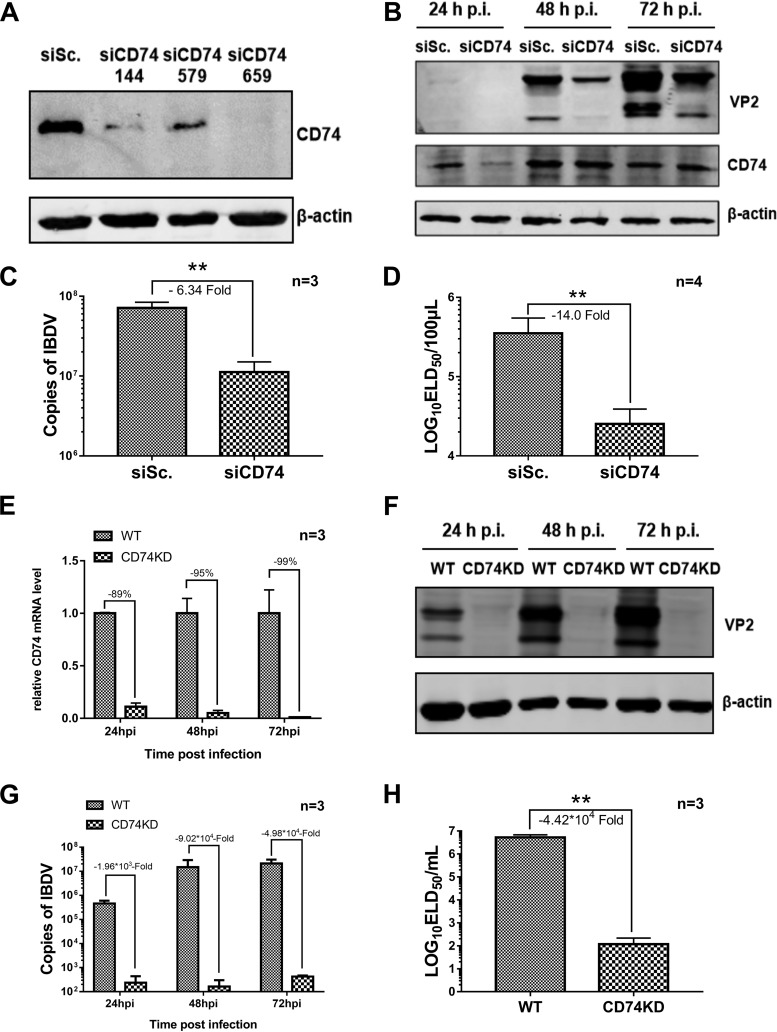
To determine whether CD74 was involved in IBDV infection, the expression level of the CD74 Ii-2 isoform was upregulated by plasmid transfection and downregulated by small interfering RNA (siRNA) interference in DT40 cells. After 24 h of siRNA interference or overexpression of CD74, the vvIBDV Gx strain was added to the cells at a multiplicity of infection (MOI) of 1, and cells and supernatants were collected for Western blot, qPCR, and 50% egg lethal dose (ELD50) analyses. The results indicated that effective downregulation of CD74 (Fig. 2A) clearly suppressed IBDV infectivity, as shown by VP2 protein expression declining at 24 to 48 h p.i. in Western blot assays (Fig. 2B), by the IBDV copy number dropping off at 48 h p.i. in the qPCR analysis (6.34-fold decrease; P < 0.05) (Fig. 2C), and by the IBDV titer being downregulated at 48 h p.i. in the ELD50 assay (14.0-fold decrease; P < 0.05) (Fig. 2D) compared with the siRNA negative control.
FIG 2.
CD74 knockdown suppresses IBDV replication. The expression of the CD74 Ii-2 isoform was downregulated by siRNA interference or knockdown by shRNA in DT40 cells. The vvIBDV Gx strain at an MOI of 1 was added to the CD74 siRNA interference groups or the CD74 KD cell line. Infected cells were washed with PBS at 4 h p.i., and DT40 complete medium was then added. Cells and supernatants were collected at 24, 48, and 72 h p.i. for Western blotting and qPCR and at 72 h p.i. for ELD50 analysis. (A) CD74 expression levels in DT40 cells determined by Western blotting, showing that CD74 downregulation was effective. siSc., scrambled control siRNA. (B) Western blot assays showing IBDV VP2 expression declining at 24 to 48 h p.i. (C) qPCR analysis showing IBDV copy numbers dropping off at 48 h p.i. (6.34-fold decrease compared with the siRNA negative control; *, P < 0.05). (D) ELD50 assay showing the IBDV titer being downregulated at 48 h p.i. (14.0-fold decrease compared with the siRNA negative control; *, P < 0.05). (E) CD74 mRNA level in DT40 cells determined by qPCR, indicating that CD74 knockdown was effective. (F) IBDV VP2 protein expression was downregulated significantly at 24 to 72 h p.i. in the CD74 KD groups. (G) The IBDV copy number was downregulated at 24 to 72 h p.i. (103- to 104-fold decrease compared with the wild-type [WT] control; P < 0.05). (F) ELD50 assay showing the IBDV titer being downregulated at 72 h p.i. (4.42 × 104-fold decrease compared with wild-type cells; P < 0.05). The arithmetic means and standard deviations for at least three independent experiments performed in duplicate are shown.
To further improve the efficiency of CD74 downregulation, we used a short hairpin RNA (shRNA) for CD74 to establish a CD74 knockdown (KD) DT40 cell line (screened with red fluorescence labeling). The percentage of red fluorescence reached 91.1% after fluorescence-activated cell sorting was repeated three times, and the knockdown efficiency of CD74 was determined by Western blotting (data not shown). Next, CD74 knockdown cells were infected with vvIBDV at an MOI of 1 (wild-type DT40 groups were used as controls), and cells and supernatants were collected at 24, 48, and 72 h p.i. for Western blot, qPCR, and ELD50 analyses. The qPCR results showed that CD74 expression was indeed mostly knocked down (Fig. 2E). Moreover, the CD74 knockdown cell line significantly suppressed IBDV infectivity, with VP2 protein expression declining at 24 to 72 h p.i. in Western blot assays (Fig. 2F), the IBDV copy number dropping off at 24 to 72 h p.i. in the qPCR analysis (103- to 104-fold decrease; P < 0.05) (Fig. 2G), and the IBDV titer being downregulated at 72 h p.i. in the ELD50 assay (4.42 × 104-fold decrease; P < 0.05) (Fig. 2H) compared with wild-type cells.
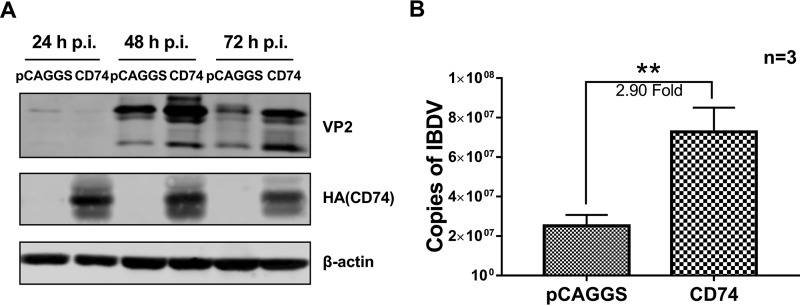
Moreover, overexpression of the CD74 Ii-2 isoform remarkably promoted IBDV infectivity: the IBDV copy number was increased in the overexpression group compared with the control group (2.90-fold increase; P < 0.05) (Fig. 3A), and IBDV VP2 protein expression was upregulated at 48 to 72 h p.i. in the overexpression group (Fig. 3B). Overall, these results show that CD74 isoform Ii-2 is responsible for IBDV infection and that cellular CD74 is essential for vvIBDV infection.
FIG 3.
CD74 overexpression promotes IBDV replication. CD74 Ii-2 isoform overexpression promotes IBDV infectivity. The expression of the CD74 Ii-2 isoform was upregulated by plasmid transfection in DT40 cells. Next, vvIBDV at an MOI of 1 was used to infected the cells as described in the text. Cells and supernatants were collected at 24, 48, and 72 h p.i. for Western blotting and at 48 h p.i. for qPCR analysis. (A) CD74 Ii-2 isoform overexpression remarkably promotes IBDV replication. The IBDV copy number was increased in the overexpression groups at 72 h p.i. compared with the nonoverexpression group (2.90-fold increase; *, P < 0.05). (B) IBDV VP2 protein expression is upregulated at 48 to 72 h p.i. in the overexpression group.
CD74 isoform Ii-2 confers attachment ability to a vvIBDV-nonpermissive cell line.
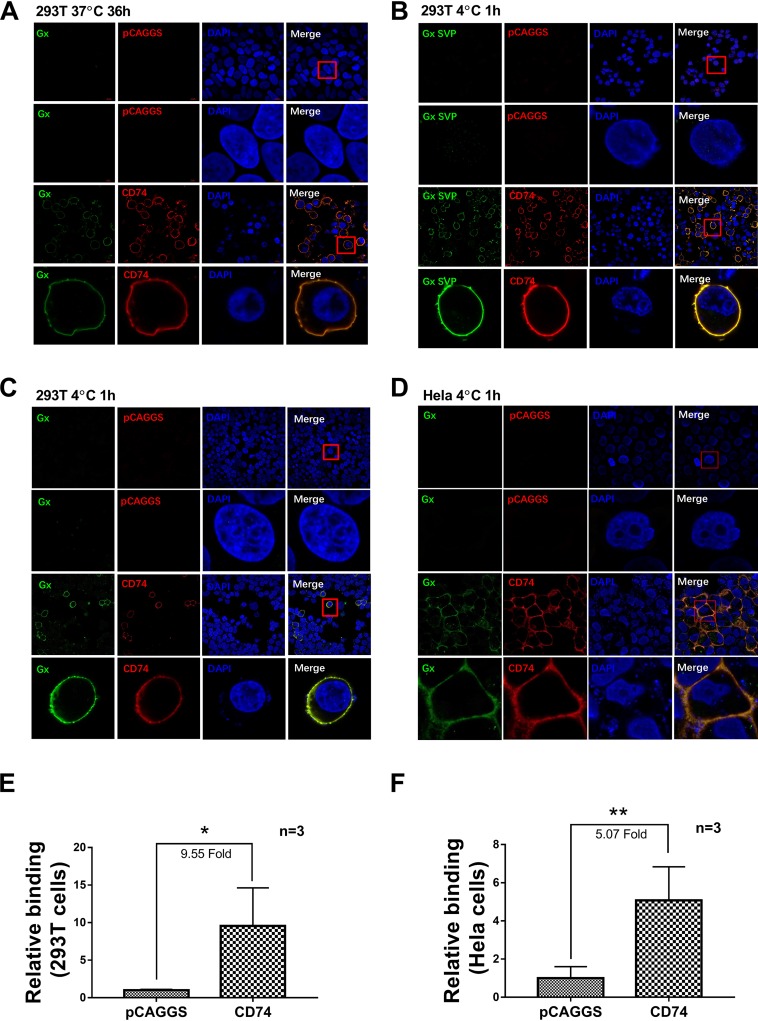
To further investigate if CD74 can confer susceptibility to IBDV infection, 293T cells (nonpermissive for vvIBDV) were transfected with a full-length chicken CD74 Ii-2 plasmid with an HA tag (or the empty vector as a negative control). Twenty-four hours after transfection, treated and control cells were incubated with vvIBDV at an MOI of 5 at 37°C for 36 h to investigate whether CD74 confers susceptibility to IBDV infection. Cells were processed for confocal analysis, using IBDV VP2 mAb and an HA tag antibody as primary antibodies. The results showed that no virus (green fluorescence) was detected in the empty vector control cells (Fig. 4A, top), while in the CD74 Ii-2 overexpression group, CD74 (red fluorescence) accumulated on the cell membrane, colocalizing with IBDV (green fluorescence) particles (Fig. 4A, bottom). No virus was observed to enter CD74-overexpressing nonpermissive cells (Fig. 4A, bottom), demonstrating that CD74 could confer attachment ability but not susceptibility to IBDV infection.
FIG 4.
CD74 isoform Ii-2 confers to vvIBDV the ability to attach to a vvIBDV-nonpermissive cell line. (A) 293T cells (nonpermissive to vvIBDV) were transfected with a full-length chicken CD74 Ii-2 plasmid with an HA tag (or the empty vector as a negative control). Twenty-four hours after transfection, cells were incubated with vvIBDV at an MOI of 5 at 37°C for 36 h to investigate whether CD74 confers susceptibility to IBDV infection. (Top) Cells were processed for confocal analysis, using IBDV VP2 mAb and an HA tag antibody as the primary antibodies. No virus (green fluorescence) was detected in the empty vector control. (Bottom) In the CD74 Ii-2 overexpression group, CD74 (red fluorescence) accumulated on the cell membrane, colocalizing with the IBDV particles (green fluorescence). No virus was observed to enter CD74-overexpressing nonpermissive cells. CD74 could confer attachment ability but not susceptibility to IBDV infection. (B) 293T cells overexpressed HA-tagged chicken CD74 Ii-2 or the empty vector for 24 h and were then incubated with 200 μg SVPs at 4°C for 1 h. After washing with PBS 5 times, cells were processed for confocal analysis as described above. SVPs (green fluorescence) were bound to the membranes of CD74-overexpressing cells (red fluorescence) and colocalized with CD74, but no binding was observed for the empty vector group. (C and D) 293T and HeLa cells (both of which are nonpermissive to vvIBDV) were transfected with a eukaryotic expression plasmid of HA-tagged chicken CD74 Ii-2 (or the empty vector as a negative control) and maintained for 24 h under normal culture conditions. The cells were then incubated with vvIBDV at an MOI of 50 at 4°C for 1 h for the binding assay and washed with PBS 5 times to remove the unbound viruses. Cells were processed for confocal analysis using the same antibodies and procedure as the ones described above. No virus (green fluorescence) was found to bind or infect 293T (C) or HeLa (D) cells transfected with the empty vector. In contrast, IBDV (green fluorescence) colocalized with overexpressed chicken CD74 (red fluorescence) on the membrane of nonpermissive 293T (C) and HeLa (D) cells. (E and F) qPCR analysis indicating that CD74 overexpression promotes IBDV binding to 293T or HeLa cells compared with the empty vector transfection group (9.55-fold increase in 293T cells [E] and 5.07-fold increase in HeLa cells [F]; both P < 0.05). The arithmetic means and standard deviations for at least three independent experiments performed in duplicate are shown.
To confirm that IBDV attaches to CD74 on the cell membrane via its VP2 protein, VP2 SVPs of the vvIBDV Gx strain expressed in Pichia pastoris were used, as follows (35): HA-tagged chicken CD74 Ii-2 or the empty vector was overexpressed in 293T cells for 24 h, and the cells were then incubated with 200 μg SVPs at 4°C for 1 h. Confocal analysis indicated that SVPs (green fluorescence) were bound to CD74-overexpressing cell membranes (red fluorescence) and colocalized with CD74 but were not bound to the membranes of empty-vector-transfected cells (Fig. 4B). These results indicated that IBDV VP2 alone was able to bind CD74-overexpressing nonpermissive cells.
To further investigate whether CD74 can confer attachment ability in IBDV infection, two kinds of nonpermissive cells were used. 293T and HeLa cells (both of which are nonpermissive to vvIBDV) were transfected with a eukaryotic expression plasmid of HA-tagged chicken CD74 Ii-2 (or the empty vector as a negative control) and maintained for 24 h under normal culture conditions. Cells were incubated with vvIBDV at an MOI of at 4°C for 1 h for binding assays and washed with PBS 5 times to remove the unbound virus. No virus (green fluorescence) was detected by confocal analysis to bind or infect 293T cells (Fig. 4C, top) or HeLa cells (Fig. 4D, top) transfected with the empty vector. As expected, however, IBDV (green fluorescence) could colocalize with overexpressed chicken CD74 (red fluorescence) on the membrane of nonpermissive cells, both 293T cells (Fig. 4C, bottom) and HeLa cells (Fig. 4D, bottom). qPCR analysis indicated that CD74 overexpression promoted IBDV binding, with a 9.55-fold increase in 293T cells (Fig. 4E) and a 5.07-fold increase in HeLa cells (Fig. 4F) (both P < 0.05), compared with cells transfected with the empty vector.
These results demonstrate that CD74 isoform Ii-2 is able to confer the ability of virus to attach to vvIBDV-nonpermissive cell lines.
A soluble form of CD74 and CD74 antibody impair IBDV attachment.
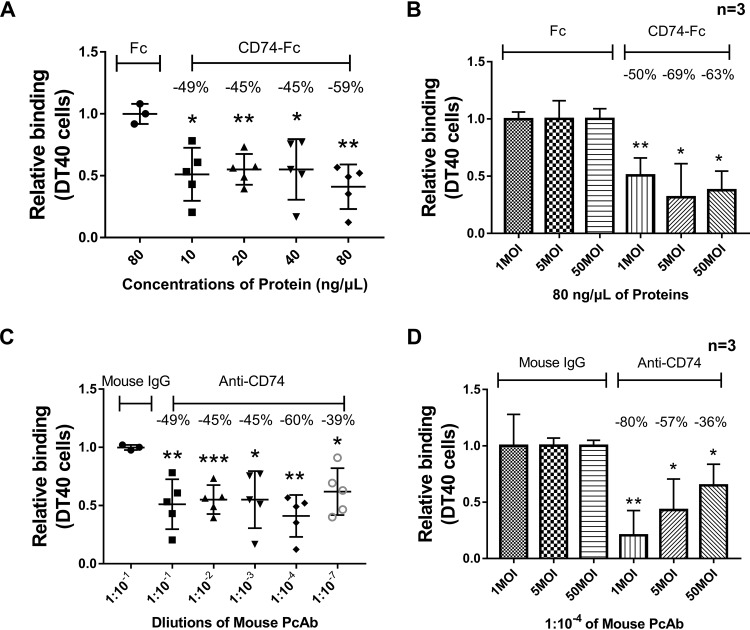
To further confirm that CD74 played an essential role in virus binding, a blocking assay with soluble CD74 protein or anti-CD74 antibody was performed in the following experiments. The viability of DT40 cells was not affected by the determined concentrations in the blockade assay (data not shown). In the protein blocking assay, Fc-tagged chicken CD74 Ii-2 protein (deleted transmembrane domain) and Fc protein (as a negative control) were expressed and purified. A total of 10, 20, 40, or 80 ng/μl of CD74-Fc or Fc protein was incubated with vvIBDV at an MOI of 50 at 4°C for 1 h. Next, the mixture was added to DT40 cells (prechilled at 4°C) and allowed to bind at 4°C for another 1 h. After washing, cells were collected for IBDV copy number determination, using 28S rRNA as the reference, with the value for the negative control being normalized to 1 to calculate the relative binding ability for IBDV. The results demonstrated that at all concentrations, IBDV binding to DT40 cells was markedly blocked, with the blocking percentage ranging from 45% to 59% (Fig. 5A) (P < 0.05). In another experiment, 80 ng/μl (i.e., the determined optimum blocking concentration) of CD74-Fc or Fc protein was incubated with vvIBDV at an MOI of 1, 5, or 50 at 4°C for 1 h. Cells were processed as described above to determine their relative ability to bind to DT40 cells. The results showed that all viral doses could be blocked by 80 ng/μl CD74-Fc protein (P < 0.05), compared with the Fc protein group (Fig. 5B).
FIG 5.
A soluble form of CD74 and CD74 antibody impair IBDV attachment. (A) In the protein blocking assay, 10, 20, 40, or 80 ng/μl of CD74-Fc or Fc protein was incubated with vvIBDV at an MOI of 50 at 4°C for 1 h, and the mixture was then added to DT40 cells (prechilled at 4°C) and bound at 4°C for another hour. The cells were then washed 5 times with PBS and collected to measure IBDV copy numbers using 28S rRNA as the reference. The value for the negative control was normalized to 1 to calculate the relative binding ability for IBDV. All concentrations of protein were able to markedly block IBDV binding to DT40 cells, with the blocking percentages ranging from 45% to 59% (P < 0.05). (B) A total of 80 ng/μl of CD74-Fc or Fc protein was incubated with vvIBDV at an MOI of 1, 5, or 50 at 4°C for 1 h. Cells were processed as described above to determine the relative IBDV binding ability. All viral doses could be blocked by 80 ng/μl CD74-Fc protein, compared with the Fc protein group (P < 0.05). (C) In the antibody blocking assay, different dilutions (1:10−1, 1:10−2, 1:10−3, 1:10−4, and 1:10−7) of CD74 mouse polyclonal antibody or mouse IgG were incubated with DT40 cells, which were maintained at 4°C for 1 h. After washing twice with PBS, vvIBDV at an MOI of 50 was added to the cells for binding at 4°C for another hour, and the cells were then washed 5 times to dispose of the unbound virus. Cells were then processed as described above to determine the relative binding ability. The CD74 polyclonal antibody (PcAb) was able to block the attachment ability of IBDV at all dilutions (blocking percentages ranging from 39% to 60%), and the optimum blocking dilution was 1:10−4 (60% of the binding capacity blocked). (D) A 1:10−4 dilution of CD74 antibody or mouse IgG was incubated with vvIBDV at an MOI of 1, 5, or 50 at 4°C for 1 h. Cells were processed as described above to assess their relative binding ability. Virus at all doses was significantly blocked by the CD74 antibody compared with the mouse IgG group (all P < 0.05).
In the antibody blocking assay, different dilutions (1:10−1, 1:10−2, 1:10−3, 1:10−4, and 1:10−7) of CD74 mouse polyclonal antibody or mouse IgG were incubated with DT40 cells, and vvIBDV at an MOI of 50 was added to the cells. After washing with PBS to dispose of the unbound virus, cells were processed as described above to determine their relative binding ability. The results are summarized in Fig. 5C. The CD74 polyclonal antibody was able to block the attachment ability of IBDV at all dilutions (blocking percentage of 39% to 60%), and the optimum blocking dilution was 1:10−4 (60% of the binding capacity blocked; P < 0.05).
In another experiment, a 1:10−4 dilution (i.e., the determined optimum blocking concentration) of CD74 antibody or mouse IgG was incubated with vvIBDV at an MOI of 1, 5, or 50 at 4°C for 1 h. Cells were processed as described above to determine the relative ability of the virus to bind to DT40 cells. Notably, all virus doses were blocked by the CD74 antibody, compared with the mouse IgG group (all P < 0.05) (Fig. 5D).
CD74 isoform Ii-2 is responsible for IBDV binding to DT40 cells.
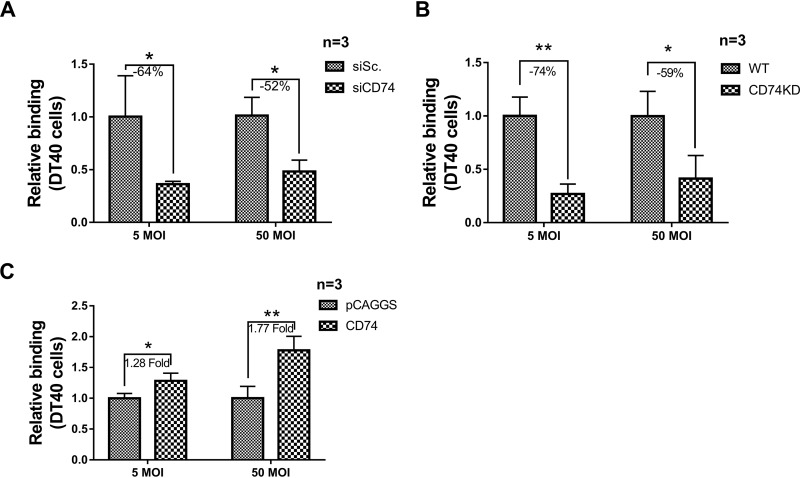
To determined the ability of IBDV to bind to DT40 cells, CD74 was downregulated by siRNA interference. After DT40 was transfected with CD74 siRNA for 24 h, cells were processed by the binding assay described above to determine their binding ability. The results demonstrated that the IBDV binding capacity was downregulated by CD74 siRNA interference, with binding suppressed by 64% for vvIBDV at an MOI of 5 and by 52% at an MOI of 50, compared with the control siRNA group (both P < 0.05) (Fig. 6A).
FIG 6.
CD74 isoform Ii-2 is responsible for IBDV binding to DT40 cells. (A) CD74 was downregulated by siRNA interference to assess binding to DT40 cells. After DT40 cells were transfected with CD74 siRNA or CD74 Ii-2 plasmids for 24 h, cells were processed as described in the text to assess their binding ability. The IBDV binding capacity was downregulated by CD74 siRNA interference, with binding reduced by 64% for vvIBDV at an MOI of 5 and by 52% at an MOI of 50 compared with the control group (both P < 0.05). (B) CD74 shRNA was used to establish a CD74 knockdown DT40 cell line (screened with red fluorescence labeling). A binding assay (same as the one described above) was applied to investigate the capacity of IBDV to bind CD74 knockdown cells (with wild-type DT40 cells as negative controls). Less IBDV binding was observed in CD74 knockdown cells than in wild-type cells, with a 74% decrease for vvIBDV at an MOI of 5 and a 59% decrease at an MOI of 50 compared with wild-type DT40 cells (both P < 0.05). (C) CD74 was overexpressed by transfecting CD74 plasmids, to assess the capacity for attachment to DT40 cells. After DT40 cells were transfected with CD74 Ii-2 plasmids for 24 h, cells were processed as described above to assess their binding ability. The IBDV binding capacity was induced by CD74 overexpression, with a 1.28-fold increase for vvIBDV at an MOI of 5 and a 1.77-fold increase at an MOI of 50, compared with the pCAGGS empty vector group (both P < 0.05). The arithmetic means and standard deviations for at least three independent experiments performed in duplicate are shown.
The same binding assay as the one described above was then applied to investigate the capacity of IBDV to bind to the CD74 knockdown cell line (with wild-type DT40 cells as a negative control). The results showed fewer IBDV particles binding CD74 knockdown cells than wild-type DT40 cells, with reductions of 74% for vvIBDV at an MOI of 5and 59% at an MOI of 50, compared with the empty vector transfection group (both P < 0.05) (Fig. 6B).
In another experiment, CD74 was overexpressed by transfecting CD74 plasmids to detect the ability of the virus to bind to DT40 cells. After DT40 cells were transfected with CD74 Ii-2 plasmids for 24 h, cells were processed as described above for the binding assay. As expected, IBDV binding was induced by CD74 overexpression, being increased 1.28-fold for vvIBDV at an MOI of 5 and 1.77-fold at an MOI of 50 (both P < 0.05), compared to the pCAGGS empty vector groups (Fig. 6C). Taken together, these results demonstrate that CD74 isoform Ii-2 is responsible for IBDV binding to DT40 cells.
DISCUSSION
In this study, we demonstrate that CD74 is involved in the process of IBDV infection and plays a pivotal role in IBDV attachment to the chicken B lymphocyte line DT40. It has been reported that IBDV could activate the phosphorylation of c-Src to promote viral entry (17). CD74, as one of the cellular receptors for MIF, could also induce c-Src phosphorylation by forming a complex (33). Therefore, we investigated whether CD74 might be involved in the early stages of IBDV infection. The interaction of the CD74 extracellular domain with the capsid protein VP2 was confirmed by a coimmunoprecipitation assay. Overexpression and RNA interference (RNAi) assays demonstrated that CD74 promoted IBDV infectivity. Moreover, vvIBDV replication significantly dropped off in the CD74 KD cells compared to wild-type DT40 cells. These results indicated that CD74 is an important host factor involved in IBDV infection. Our results furthermore show that CD74 overexpression on the surface of nonpermissive cells could allow the virus to bind the membrane but not to enter the cell. The binding capacity was significantly inhibited by CD74 RNA interference, antibody, protein blockade assays, and CD74 knockdown, further proving that CD74 is involved in IBDV attachment, the earliest stage of IBDV infection. Based on all these results, the identification of CD74 as a novel receptor for IBDV attachment to DT40 cells provides fresh insight to help us understand the process of virus replication and pathogenicity and could contribute to drug target discovery and disease prevention and control.
As a multifunctional protein, CD74 takes part in a variety of immune-related processes (29, 36) and in particular plays an important role in regulating the function and expression of MHC-II molecules (28). Moreover, previous studies showed that CD74 is involved as a signaling molecule in B cell maturation (37) and MHC-I expression on the cell surface (38). Chicken CD74 (MHC-II Ii) exists in two isoforms (Ii-1 and Ii-2), resulting from alternative splicing, and is strongly expressed in the major immune organs (31, 32). Northern blot results in 6-week-old chickens revealed high levels of Ii-1 and Ii-2 mRNA expression in the bursa and spleen and low levels of Ii-1 in the heart, liver, and thymus, while Ii-2 was not expressed in these tissues (31). Consistently with these results, we found the highest mRNA levels of CD74 Ii-2 in the bursa and the spleen of 3-week-old chickens. Previous studies indicated that Ii-1 was more extensively distributed than Ii-2 and that Ii-2 was expressed only in organs important for immune function related to class II antigen presentation (31). Pathogenic IBDV mainly targets immature B cells in the bursa; therefore, we focused on the CD74 Ii-2 isoform to explore whether CD74 is involved in IBDV infection. Interactions between CD74 and viruses have rarely been reported, but previous research showed that the HIV Vpu-CD74 interaction has multiple downstream consequences for the immune system (39). However, CD74 has not been reported to be a cellular receptor of any virus. The chicken CD74 amino acid sequence is quite different from those of mammals, with 47.19% identity to human CD74, 51.7% to monkey, 50.0% to wolf, and 46.6% to mouse, indicating that chicken CD74 might have some functions that are from those of mammalian CD74. Our results revealed that CD74 interacts with the outermost IBDV VP2 protein and acts as the attachment receptor for IBDV infection, putting forward a novel role of CD74 in the viral infection process.
VP2, involved in cell tropism (22, 34), has been identified as an important cellular receptor binding protein (15, 16). IBDV SVPs are formed by self-assembling VP2 protein upon its expression, and their structure has been solved by X-ray crystallography (19, 23). Currently, SVPs are extensively applied in IBDV receptor studies (15, 17): previous studies demonstrated that SVPs could compete with IBDV by directly blocking virus attachment (15, 17). Therefore, we used SVPs derived by expression in yeast (35) to investigate the IBDV attachment receptor and found that SVPs could bind CD74-overexpressing 293T cells (cells that are nonpermissive to vvIBDV), further proving the binding capacity of SVPs and the interaction between IBDV VP2 and CD74.
In previous IBDV receptor studies, the chicken fibroblast line DF-1 (15, 16) and the chicken bursa-derived pre-B cell line DT40 (40) have been used. We chose DT40 cells to investigate the IBDV receptor due to their susceptibility to both pathogenic and attenuated IBDV, while DF-1 cells might not be permissive to all the current pathogenic IBDV strains, including the vvIBDV Gx and HLJ0504 strains in this study (40). Taking into account the potential side effects of antibodies on cells, as proposed previously by Luo et al., we used a CD74 knockdown cell line to investigate the relationship between CD74 and IBDV binding capacity (40). The binding capacity was significantly reduced, but the reduction still provides evidence that CD74 is involved in IBDV attachment to pre-B cells. Moreover, IBDV replication was significantly inhibited in CD74 knockdown cells compared to the wild-type groups, indicating that CD74 is important for IBDV infection. Combined with the binding results, this led us to suppose that the effect of CD74 might be not only on binding but also on other steps of IBDV replication.
Hypotheses about the IBDV receptor complex have been formulated in some previous studies, and in particular, Hsp90 has been confirmed to be a putative component of the receptor complex (15). Moreover, IBDV could hijack α4β1-dependent c-Src signaling to allow initial virus entry (17). In our study, we demonstrate that IBDV CD74 is also a candidate component of the receptor complex. The complex formed by human CD74 and its ligand can activate c-Src (33). Therefore, we hypothesize that CD74 (or a complex containing CD74) might have an effect similar to that of α4β1 in activating c-Src, with IBDV hijacking the pathway to entry into target cells (17). More details about the downstream mechanism of CD74 in IBDV replication still need to be explored.
In conclusion, our study demonstrates that CD74 is a novel attachment receptor for IBDV and presumably one of the components of the IBDV receptor complex. The initial infection process of IBDV still needs to be explored in further detail.
MATERIALS AND METHODS
Cell cultures, viruses, and antibodies.
293T and HeLa cells were grown in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum (FBS) (Gibco) under an atmosphere of 5% CO2 at 37°C. DT40 cells (a chicken lymphoma cell line) were a generous gift from Venugopal Nair of the Pirbright Institute. Cells were cultured at 37°C in a 5% CO2 atmosphere in RPMI 1640 medium (Gibco, USA) supplemented with 50 μM β-mercaptoethanol, 1% l-glutamine (Gibco), 2% chicken serum (Sigma), and 10% FBS.
The vvIBDV strain Gx had been identified and preserved in our laboratory (41). The viral loads of the Gx strain were determined by performing 50% egg lethal dose (ELD50) assays. SVPs of the Gx strain were expressed and preserved by our laboratory (35).
The SVPs of vvIBDV Gx VP2 (full length, aa 1 to 441 in the polyprotein) with an N-terminal 6×His amino acid tag were expressed in P. pastoris strain SMD1168 (Invitrogen) based on the protocol of our laboratory (35). Briefly, recombinant yeasts were cultured until the optical density at 600 nm (OD600) reached 2 to 6 and then resuspended at an OD600 of 1 with 0.5% (vol/vol) methanol added every 12 h. Cells were harvested at 120 h and disrupted, and the supernatant contained the SVPs. The SVPs were detected by SDS-PAGE, Western blotting, and electronic microscopy.
Anti-HA tag, -Flag tag, and -β-actin antibodies were purchased from Sigma. The custom chicken CD74 polyclonal antibody was obtained from the Biodragon Immunotechnologies Company (Beijing). The IBDV VP2 mAbs were produced and preserved by our laboratory.
Animals.
All animal experiments were approved by the Committee on the Ethics of Animal Experiments at the Harbin Veterinary Research Institute (Harbin, China), Chinese Academy of Agricultural Sciences, and performed in accordance with the guidelines for experimental animals of the Ministry of Science and Technology (Beijing, China). All chickens and chicken embryos were cared for in accordance with humane procedures. To establish a chicken model of vvIBDV infection, 18 3-week-old SPF chickens were infected with a total volume of 200 μl vvIBDV (1 × 102 ELD50) or PBS (pH 7.0) per chicken. PBS was used for the viral dilutions. Samples (including heart, liver, spleen, lung, kidney, thymus, and bursa) were collected for subsequent experiments.
Protein precipitation and mass spectrometry.
DT40 cells were washed twice with PBS (pH 7.0) and then lysed with IP lysis buffer for 30 min. The cell lysate was incubated with VP2 protein expressed in 293T cells for 10 h with protein A/G-agarose and anti-VP2 antibody. Precipitates were washed four times in IP lysis buffer and once in PBS. Bound proteins were separated by SDS-PAGE and silver staining (Thermo Fisher Scientific). Differential bands between infected and mock groups were excised from the gel and subjected to mass spectrometry.
RNA extraction and quantitative PCR.
Specific primers and TaqMan probes for chicken 28S rRNA (42), CD74 (catalog no. 4351372; Thermo Fisher Scientific), and IBDV loads (43) were synthesized or purchased from Invitrogen (China). Total RNA was extracted from tissues or cells using the RNeasy minikit (Qiagen, Germany), and 1 μg RNA was reverse transcribed to cDNA using the ReverTra Ace qPCR RT master mix with a genomic DNA (gDNA) remover (Toyobo, Japan) in a 20-μl reaction mixture. The cDNA was analyzed by qPCR performed using Premix Ex Taq (probe qPCR) (TaKaRa, Japan). Quantitative PCR was performed under the following conditions: 95°C for 30 s for initial denaturation, followed by 45 cycles for 5 s at 95°C for denaturation and for 20 s at 60°C and collection of the PCR product. All standards, controls, and infected samples were examined in triplicate on the same plate. The cDNA copies were normalized to 28S cDNA copies measured from the same samples. The method was used for the relative quantification of CD74. IBDV loads and CD74 levels were measured quantitatively by the absolute analysis method.
ELD50.
vvIBDV-infected DT40 supernatants were diluted with PBS, and 100-μl samples were then inoculated on the chorioallantoic membrane of 9-day-old SPF eggs (purchased from Harbin Veterinary Research Institute). The mortality of eggs was observed daily for 7 days after infection. All the chicken embryos were cared for in accordance with humane procedures.
Eukaryotic expression of recombinant proteins and purification.
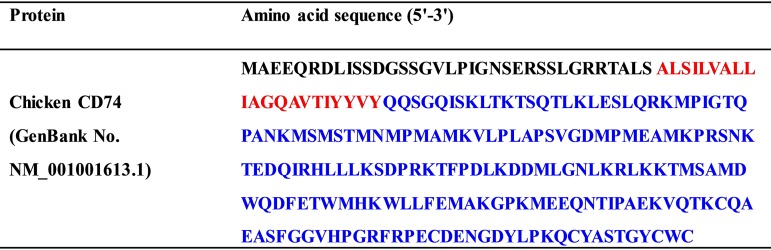
The full-length CD74 gene (Table 1) with a deleted transmembrane domain (aa 33 to 56) was amplified and fused with the N-terminal signal peptide and the C-terminal Fc tag by overlapping PCR. The CD74-Fc gene was constructed in the CAG vector (Addgene, MA). Next, chicken CD74-Fc and Fc proteins were expressed by transient transfection of Expi293F cells (Thermo Fisher Scientific) using Polyfectine (Sigma). Cell culture supernatants were harvested at 4 days posttransfection and centrifuged at 12,000 × g to remove cell debris. The supernatants were sterile filtered, and proteins were purified by using protein A resin (GenScript).
TABLE 1.
Amino acid sequence for full-length chicken CD74a
Red type represents the transmembrane domain, and blue type represents the extracellular domain (GenBank accession no. NM_001001613.1).
RNA interference and overexpression assays.
To downregulate chicken CD74, we used negative-control sense siRNA 5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense siRNA 5′-ACGUGACACGUUCGGAGAATT-3′, CD74-gga-144 sense siRNA 5′-GGCUGUCACCAUCUACUAUTT-3′ and antisense siRNA 5′-AUAGUAGAUGGUGACAGCCTT-3′, CD74-gga-579 sense siRNA 5′-GCACAAGUGGCUGCUCUUUTT-3′ and antisense siRNA 5′-AAAGAGCAGCCACUUGUGCTT-3′, and CD74-gga-659 sense siRNA 5′-GCGAUGAGAACGGUGACUATT-3′ and antisense siRNA 5′-UAGUCACCGUUCUCAUCGCTT-3′, all synthesized by GenePharma. Five million cells were electrotransfected using Amaxa cell line Nucleofector kit T (Lonza) according to the manufacturer’s protocol. The efficiency of RNA silencing was verified 48 h after transfection by Western blotting. To overexpress chicken CD74, DT40 cells were electrotransfected with pCAGGS-CD74 using the same protocol as the one described above.
Twenty-four hours after transfection, CD74-downregulated or -overexpressing DT40 cells were incubated with vvIBDV at an MOI of 1 at 37°C for 4 h and washed three times with PBS (pH 7.0), and DT40 complete medium was added. Cells were collected at 24, 48, and 72 h p.i. for Western blot assays and at 48 or 72 h p.i. for qPCR and ELD50 analyses to determine IBDV replication and titers, respectively.
In another experiment, CD74-downregulated or -overexpressing DT40 cells were chilled at 4°C for 20 min and then incubated with vvIBDV at an MOI of 50 at 4°C for 1 h. Cells were washed three times with PBS (pH 7.0) and collected for qPCR analysis to detect IBDV attachment.
Coimmunoprecipitation analysis.
293T cells were cotransfected with the bait and prey plasmids. Thirty-six hours after transfection, total protein was isolated from 293T cells using IP lysis buffer. Co-IP analysis was performed by using anti-Flag M2 affinity gel (Sigma) according to the manufacturer’s instructions. Immunoblot analysis of the proteins was subsequently performed using mAbs against HA and Flag (Sigma).
Blocking assay.
To check for inhibition of IBDV attachment by soluble CD74-Fc protein, IBDV at an MOI of 50 was incubated with different dilutions of CD74-Fc protein or Fc protein (negative control) for 1 h at 4°C. The mixture was added to the chilled DT40 cells for 1 h at 4°C, and the cells were then washed 4 times with PBS and collected for RT-qPCR to detect IBDV binding.
In another experiment, to check the inhibition of IBDV attachment by CD74 antibodies, cells were incubated with different dilutions of the specific mouse polyclonal antibodies or mouse IgG (negative control) for 1 h at 4°C, followed by two washes with chilled PBS. IBDV (MOI of 50) was added to the cells for 1 h at 4°C. The cells were then washed 4 times with PBS and collected to detect IBDV attachment.
Confocal imaging.
293T and HeLa cells were plated onto cell imaging dishes (Eppendorf) and transfected with pCAGGS-HA-CD74. After 24 h, the cells were adsorbed with the vvIBDV Gx strain at an MOI of 50 or 200 μg Gx SVPs and bound at 4°C for 1 h or incubated at 37°C for 36 h. The cells were washed four times with PBS (PH 7.0); fixed with 4% formaldehyde for 30 min, followed by further washing with PBS; treated with 1% Triton X-100 for 5 min; washed twice with PBS; incubated with IBDV VP2 mAb (1:200) and HA tag antibody in PBS for 1 h; and washed three times. The cells were then stained with goat anti-mouse IgG(H+L) cross-adsorbed Alexa Fluor 488 secondary antibody (Invitrogen) (1:200) to visualize IBDV and SVPs, goat anti-rabbit IgG(H+L) cross-adsorbed Alexa Fluor 546 secondary antibody (Invitrogen) (1:200) to visualize HA-tagged CD74, and 4′,6-diamidino-2-phenylindole (DAPI) to visualize the cell nuclei. The cells were incubated for 1 h with secondary antibodies in PBS and washed 5 times with PBS. Images were captured on a Zeiss LSM880 confocal laser scanning microscope with Fast Airyscan using ZEN2 software.
CD74 knockdown by shRNAs.
Lentivirus packaging was performed by GenePharma (Shanghai, China). The shRNA sequences were sense sequence 5′-GCGAUGAGAACGGUGACUATT-3′ and antisense sequence 5′-UAGUCACCGUUCUCAUCGCTT-3′. DT40 cells were infected by lentivirus, and the infected cells were screened by red fluorescent protein (RFP) fluorescence-activated cell sorting (MoFlo XDP). CD74 knockdown-positive cells were detected by using a fluorescence microscope (Evos FL; AMG) and a flow cytometer (BD Accuri C6) to measure the percentage of RFP-positive cells and assessed by Western blotting and qPCR determine the efficiency of knockdown of CD74.
Statistical analysis.
Statistical analysis was performed with the unpaired t test. P values of less than 0.05 were considered statistically significant. Data are reported as means ± standard deviations.
ACKNOWLEDGMENTS
This work was supported by the Major Project of the National Natural Science Foundation of China (grant no. 31430087), the Heilongjiang Provincial Natural Science Foundation of China (grant no. YQ2019C029), the National Key Research and Development Program of China (grant no. 2016YFE0203200), and China’s Agricultural Research System (grant no. CARS-41-G15).
We declare no competing financial interests.
REFERENCES
- 1.Lasher HN, Davis VS. 1997. History of infectious bursal disease in the U.S.A.—the first two decades. Avian Dis 41:11–19. doi: 10.2307/1592439. [DOI] [PubMed] [Google Scholar]
- 2.Azad AA, Barrett SA, Fahey KJ. 1985. The characterization and molecular cloning of the double-stranded RNA genome of an Australian strain of infectious bursal disease virus. Virology 143:35–44. doi: 10.1016/0042-6822(85)90094-7. [DOI] [PubMed] [Google Scholar]
- 3.Khatri M, Palmquist JM, Cha RM, Sharma JM. 2005. Infection and activation of bursal macrophages by virulent infectious bursal disease virus. Virus Res 113:44–50. doi: 10.1016/j.virusres.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt E, Muller H. 1987. Susceptibility of chicken blood lymphoblasts and monocytes to infectious bursal disease virus (IBDV). Arch Virol 94:297–303. doi: 10.1007/bf01310722. [DOI] [PubMed] [Google Scholar]
- 5.Inoue M, Yamamoto H, Matuo K, Hihara H. 1992. Susceptibility of chicken monocytic cell lines to infectious bursal disease virus. J Vet Med Sci 54:575–577. doi: 10.1292/jvms.54.575. [DOI] [PubMed] [Google Scholar]
- 6.Jahromi MZ, Bello MB, Abdolmaleki M, Yeap SK, Hair-Bejo M, Omar AR. 2018. Differential activation of intraepithelial lymphocyte-natural killer cells in chickens infected with very virulent and vaccine strains of infectious bursal disease virus. Dev Comp Immunol 87:116–123. doi: 10.1016/j.dci.2018.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Hirai K, Shimakura S. 1974. Structure of infectious bursal disease virus. J Virol 14:957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van den Berg TP, Eterradossi N, Toquin D, Meulemans G. 2000. Infectious bursal disease (Gumboro disease). Rev Sci Tech 19:509–543. doi: 10.20506/rst.19.2.1227. [DOI] [PubMed] [Google Scholar]
- 9.Smith AE, Helenius A. 2004. How viruses enter animal cells. Science 304:237–242. doi: 10.1126/science.1094823. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, He WR, Shen L, Dong H, Yu J, Wang X, Yu S, Li Y, Li S, Luo Y, Sun Y, Qiu HJ. 2015. The laminin receptor is a cellular attachment receptor for classical swine fever virus. J Virol 89:4894–4906. doi: 10.1128/JVI.00019-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez S, Arias CF. 2004. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol 12:271–278. doi: 10.1016/j.tim.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 12.Guerrero CA, Mendez E, Zarate S, Isa P, Lopez S, Arias CF. 2000. Integrin alpha(v)beta(3) mediates rotavirus cell entry. Proc Natl Acad Sci U S A 97:14644–14649. doi: 10.1073/pnas.250299897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Haywood AM. 1994. Virus receptors: binding, adhesion strengthening, and changes in viral structure. J Virol 68:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zárate S, Cuadras MA, Espinosa R, Romero P, Juárez KO, Camacho-Nuez M, Arias CF, López S. 2003. Interaction of rotaviruses with Hsc70 during cell entry is mediated by VP5. J Virol 77:7254–7260. doi: 10.1128/jvi.77.13.7254-7260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin TW, Lo CW, Lai SY, Fan RJ, Lo CJ, Chou YM, Thiruvengadam R, Wang AH, Wang MY. 2007. Chicken heat shock protein 90 is a component of the putative cellular receptor complex of infectious bursal disease virus. J Virol 81:8730–8741. doi: 10.1128/JVI.00332-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delgui L, Ona A, Gutierrez S, Luque D, Navarro A, Caston JR, Rodriguez JF. 2009. The capsid protein of infectious bursal disease virus contains a functional alpha 4 beta 1 integrin ligand motif. Virology 386:360–372. doi: 10.1016/j.virol.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 17.Ye C, Han X, Yu Z, Zhang E, Wang L, Liu H. 2017. Infectious bursal disease virus activates c-Src to promote alpha4beta1 integrin-dependent viral entry by modulating the downstream Akt-RhoA GTPase-actin rearrangement cascade. J Virol 91:e01891-16. doi: 10.1128/JVI.01891-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coulibaly F, Chevalier C, Gutsche I, Pous J, Navaza J, Bressanelli S, Delmas B, Rey FA. 2005. The birnavirus crystal structure reveals structural relationships among icosahedral viruses. Cell 120:761–772. doi: 10.1016/j.cell.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Lee CC, Ko TP, Chou CC, Yoshimura M, Doong SR, Wang MY, Wang AH. 2006. Crystal structure of infectious bursal disease virus VP2 subviral particle at 2.6A resolution: implications in virion assembly and immunogenicity. J Struct Biol 155:74–86. doi: 10.1016/j.jsb.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Ren X, Zhang L, Gao Y, Gao H, Wang Y, Liu C, Cui H, Zhang Y, Jiang L, Qi X, Wang X. 2015. Binding chicken Anx2 is beneficial for infection with infectious bursal disease virus. Virus Res 210:232–240. doi: 10.1016/j.virusres.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 21.van Loon AA, de Haas N, Zeyda I, Mundt E. 2002. Alteration of amino acids in VP2 of very virulent infectious bursal disease virus results in tissue culture adaptation and attenuation in chickens. J Gen Virol 83:121–129. doi: 10.1099/0022-1317-83-1-121. [DOI] [PubMed] [Google Scholar]
- 22.Qi X, Gao H, Gao Y, Qin L, Wang Y, Gao L, Wang X. 2009. Naturally occurring mutations at residues 253 and 284 in VP2 contribute to the cell tropism and virulence of very virulent infectious bursal disease virus. Antiviral Res 84:225–233. doi: 10.1016/j.antiviral.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Garriga D, Querol-Audí J, Abaitua F, Saugar I, Pous J, Verdaguer N, Castón JR, Rodriguez JF. 2006. The 2.6-angstrom structure of infectious bursal disease virus-derived T=1 particles reveals new stabilizing elements of the virus capsid. J Virol 80:6895–6905. doi: 10.1128/JVI.00368-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fahey KJ, Erny K, Crooks J. 1989. A conformational immunogen on VP-2 of infectious bursal disease virus that induces virus-neutralizing antibodies that passively protect chickens. J Gen Virol 70(Part 6):1473–1481. doi: 10.1099/0022-1317-70-6-1473. [DOI] [PubMed] [Google Scholar]
- 25.Mundt E. 1999. Tissue culture infectivity of different strains of infectious bursal disease virus is determined by distinct amino acids in VP2. J Gen Virol 80(Part 8):2067–2076. doi: 10.1099/0022-1317-80-8-2067. [DOI] [PubMed] [Google Scholar]
- 26.Lim BL, Cao Y, Yu T, Mo CW. 1999. Adaptation of very virulent infectious bursal disease virus to chicken embryonic fibroblasts by site-directed mutagenesis of residues 279 and 284 of viral coat protein VP2. J Virol 73:2854–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones PP, Murphy DB, Hewgill D, McDevitt HO. 1979. Detection of a common polypeptide chain in I-A and I-E sub-region immunoprecipitates. Mol Immunol 16:51–60. doi: 10.1016/0161-5890(79)90027-0. [DOI] [PubMed] [Google Scholar]
- 28.Anderson MS, Miller J. 1992. Invariant chain can function as a chaperone protein for class II major histocompatibility complex molecules. Proc Natl Acad Sci U S A 89:2282–2286. doi: 10.1073/pnas.89.6.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kropshofer H, Vogt AB, Hammerling GJ. 1995. Structural features of the invariant chain fragment CLIP controlling rapid release from HLA-DR molecules and inhibition of peptide binding. Proc Natl Acad Sci U S A 92:8313–8317. doi: 10.1073/pnas.92.18.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.German RN, Castellino F, Han R, Reis e Sousa C, Romagnoli P, Sadegh-Nasseri S, Zhong GM. 1996. Processing and presentation of endocytically acquired protein antigens by MHC class II and class I molecules. Immunol Rev 151:5–30. doi: 10.1111/j.1600-065x.1996.tb00701.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhong D, Yu W, Liu Y, Liu J, Li J. 2004. Molecular cloning and expression of two chicken invariant chain isoforms produced by alternative splicing. Immunogenetics 56:650–656. doi: 10.1007/s00251-004-0726-6. [DOI] [PubMed] [Google Scholar]
- 32.Chen F, Wu C, Pan L, Xu F, Liu X, Yu W. 2013. Cross-species association of quail invariant chain with chicken and mouse MHC II molecules. Dev Comp Immunol 40:20–27. doi: 10.1016/j.dci.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Shi X, Leng L, Wang T, Wang W, Du X, Li J, McDonald C, Chen Z, Murphy JW, Lolis E, Noble P, Knudson W, Bucala R. 2006. CD44 is the signaling component of the macrophage migration inhibitory factor-CD74 receptor complex. Immunity 25:595–606. doi: 10.1016/j.immuni.2006.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brandt M, Yao K, Liu M, Heckert RA, Vakharia VN. 2001. Molecular determinants of virulence, cell tropism, and pathogenic phenotype of infectious bursal disease virus. J Virol 75:11974–11982. doi: 10.1128/JVI.75.24.11974-11982.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Pan Q, Lu Z, Li K, Gao H, Qi X, Gao Y, Wang X. 2016. An optimized, highly efficient, self-assembled, subvirus-like particle of infectious bursal disease virus (IBDV). Vaccine 34:3508–3514. doi: 10.1016/j.vaccine.2016.02.072. [DOI] [PubMed] [Google Scholar]
- 36.Vera PL, Wang X, Meyer-Siegler KL. 2008. Upregulation of macrophage migration inhibitory factor (MIF) and CD74, receptor for MIF, in rat bladder during persistent cyclophosphamide-induced inflammation. Exp Biol Med (Maywood) 233:620–626. doi: 10.3181/0709-RM-240. [DOI] [PubMed] [Google Scholar]
- 37.Matza D, Kerem A, Medvedovsky H, Lantner F, Shachar I. 2002. Invariant chain-induced B cell differentiation requires intramembrane proteolytic release of the cytosolic domain. Immunity 17:549–560. doi: 10.1016/S1074-7613(02)00455-7. [DOI] [PubMed] [Google Scholar]
- 38.Vigna JL, Smith KD, Lutz CT. 1996. Invariant chain association with MHC class I: preference for HLA class I/beta 2-microglobulin heterodimers, specificity, and influence of the MHC peptide-binding groove. J Immunol 157:4503–4510. [PubMed] [Google Scholar]
- 39.Le Noury DA, Mosebi S, Papathanasopoulos MA, Hewer R. 2015. Functional roles of HIV-1 Vpu and CD74: details and implications of the Vpu-CD74 interaction. Cell Immunol 298:25–32. doi: 10.1016/j.cellimm.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 40.Luo J, Zhang H, Teng M, Fan JM, You LM, Xiao ZJ, Yi ML, Zhi YB, Li XW, Zhang GP. 2010. Surface IgM on DT40 cells may be a component of the putative receptor complex responsible for the binding of infectious bursal disease virus. Avian Pathol 39:359–365. doi: 10.1080/03079457.2010.506211. [DOI] [PubMed] [Google Scholar]
- 41.Wang X, Zhang H, Gao H, Fu C, Gao Y, Ju Y. 2007. Changes in VP3 and VP5 genes during the attenuation of the very virulent infectious bursal disease virus strain Gx isolated in China. Virus Genes 34:67–73. doi: 10.1007/s11262-006-0002-y. [DOI] [PubMed] [Google Scholar]
- 42.Ragland WL, Novak R, El-Attrache J, Savić V, Ester K. 2002. Chicken anemia virus and infectious bursal disease virus interfere with transcription of chicken IFN-alpha and IFN-gamma mRNA. J Interferon Cytokine Res 22:437–441. doi: 10.1089/10799900252952226. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y, Qi X, Gao H, Gao Y, Lin H, Song X, Pei L, Wang X. 2009. Comparative study of the replication of infectious bursal disease virus in DF-1 cell line and chicken embryo fibroblasts evaluated by a new real-time RT-PCR. J Virol Methods 157:205–210. doi: 10.1016/j.jviromet.2009.01.001. [DOI] [PubMed] [Google Scholar]