Significance
To which extent gene regulatory programs are optimized by evolution is one of the fundamental biological questions. Swimming motility is one of the costliest bacterial behaviors, but expression of motility genes nevertheless increases when bacteria grow slower on poor carbon sources. Here, we show that competitive fitness benefit provided by the ability of motile bacteria to follow gradients of secondary nutrients shows similar negative dependence on the growth rate as the investment in motility. Thus, bacteria appear to pre-invest into the motile behavior in proportion to the anticipated benefit that can be provided by chemotaxis when nutrient gradients become available in their environment.
Keywords: chemotaxis, selection, fitness cost, growth, metabolism
Abstract
Microorganisms possess diverse mechanisms to regulate investment into individual cellular processes according to their environment. How these regulatory strategies reflect the inherent trade-off between the benefit and cost of resource investment remains largely unknown, particularly for many cellular functions that are not immediately related to growth. Here, we investigate regulation of motility and chemotaxis, one of the most complex and costly bacterial behaviors, as a function of bacterial growth rate. We show with experiment and theory that in poor nutritional conditions, Escherichia coli increases its investment in motility in proportion to the reproductive fitness advantage provided by the ability to follow nutrient gradients. Since this growth-rate dependent regulation of motility genes occurs even when nutrient gradients are absent, we hypothesize that it reflects an anticipatory preallocation of cellular resources. Notably, relative fitness benefit of chemotaxis could be observed not only in the presence of imposed gradients of secondary nutrients but also in initially homogeneous bacterial cultures, suggesting that bacteria can generate local gradients of carbon sources and excreted metabolites, and subsequently use chemotaxis to enhance the utilization of these compounds. This interplay between metabolite excretion and their chemotaxis-dependent reutilization is likely to play an important general role in microbial communities.
Bacterial responses to environmental changes range from adjustment of metabolism to induction of different stress protection mechanisms and tactic behavior. In most cases, there is an apparent qualitative benefit of investment into a cellular function under particular environmental conditions, e.g., uptake of a specific carbon source when it is available. However, demonstrating that a regulatory strategy exactly follows the physiological benefit of the respective cellular function remains challenging, although several recent studies suggested that allocation of metabolic resources may indeed optimize growth (1–6).
Most prokaryotes can swim by rotating flagellar filaments, powered by the proton motive force. Swimming bacteria can typically follow various chemical gradients in their environment, by modulating the frequency of cell reorientations (tumbles) dependent on their swimming direction relative to the gradient (7, 8). The core of the chemotaxis signaling pathway includes stimulus-specific chemoreceptors (Tar, Tsr, Trg, Tap, and Aer in Escherichia coli), which regulate the cytoplasmic histidine kinase CheA and thereby phosphorylation of the response regulator CheY. Phosphorylated CheY controls rotation of flagellar motor; in the case of E. coli, promoting its directional switching that induces tumbles. E. coli chemotaxis pathway further includes CheZ, the phosphatase of CheY, and the adaptation enzymes CheR and CheB.
Flagellar motility consumes several percent of total cellular protein and energy budget (9, 10), which is primarily spent for the biogenesis of flagella and powering of their rotation, respectively, and to a lesser extent for the chemotactic signaling. Consistent with this high cost of motility, expression of flagellar genes can significantly reduce growth (11). Nevertheless, E. coli motility is up-regulated in poor carbon sources, as a part of the regulon controlled by cyclic adenosine monophosphate (cAMP) and its receptor protein (CRP) (1, 12, 13). Although this investment strategy could potentially enhance chemotaxis-mediated nutrient uptake in heterogeneous environments (14), thus enabling risk-prone foraging behavior (12), under which conditions it might be advantageous remained unclear, particularly considering that selection pressure on motility and chemotaxis is environment specific (15).
In this study, we investigated the relation between costs and benefits of motility in E. coli cells grown in a number of carbon sources of different quality (Fig. 1A). Our results suggest that bacteria invest in swimming behavior in proportion to the fitness benefit provided by chemotaxis, both of which depend on the growth rate. This demand-dependent investment could be recapitulated by mathematical modeling that considers growth-dependent proteome partitioning, chemotaxis, and competition for nutrients. Tactic enhancement of nutrient utilization was observed in externally introduced or in self-generated gradients of nutrients, suggesting that it is important across a wide range of environments.
Fig. 1.
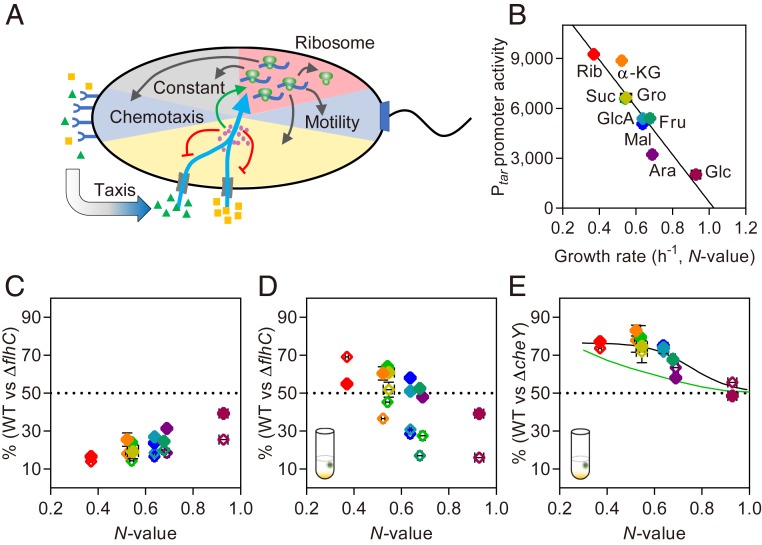
Nutrient-dependent costs and benefits of motility in the presence of introduced gradients. (A) Schematics illustrating growth-rate dependent partitioning of the cellular protein budget into different functional sectors, as well as coutilization of 2 different nutrients (triangles and squares) regulated by cAMP (small circles). Also indicated is chemotaxis-mediated enhancement of nutrient uptake. (B) Relation between growth rate, denoted as nutritional (N-) value of the carbon source and motility gene expression. Cells were cultured with shaking (180 r.p.m.) in minimal medium containing different carbon sources as indicated (Rib: 20 mM ribose, α-KG: 12 mM α-ketoglutarate, Suc: 15 mM succinate, Gro: 0.4% glycerol, GlcA: 20 mM gluconate, Fru: 20 mM fructose, Mal: 0.2% maltose, Ara: 20 mM arabinose, Glc: 0.4% glucose). Expression of Ptar-GFP reporter and growth rate were measured at log phase of growth using plate reader (see Methods and SI Appendix, Supplementary Text). Solid line shows resource allocation model with 2 fitting parameters as detailed in SI Appendix, Supplementary Text; zero promoter activity is predicted at the N-value of 1.03. (C and D) Growth competition in the coculture of motile WT cells (expressing CFP) and nonmotile ΔflhC cells (expressing YFP), with flhDC expressed either under native promoter (solid diamonds) or under Ptac (open diamonds) promoter induced with 10 µM IPTG. Shown is fraction (in %) of the WT cells in a mixed population grown for 72 h without shaking in the absence (C) or in the presence (D) of a bead containing 12% casein hydrolysate. N-values were taken from B. (E) Same as D, but for competition between WT and ΔcheY cells. Solid lines show fit to the data using simulations of 2-compartment chemotaxis model (black) and prediction of the full 2D model (green) as detailed in SI Appendix, Supplementary Text. Cartoon in D and E illustrates emergence of gradients of casein hydrolysate from an introduced bead. In all competition experiments, both strains were initially inoculated at a 1:1 ratio.
Results
Growth-Rate Dependent Investment in Motility.
We first assessed regulation of motility dependent on the growth rate, by culturing E. coli MG1655 Δflu (deleted for the major surface adhesin to minimize cell aggregation) with shaking in different carbon sources. We observed that, similar to other cAMP-CRP regulated genes (5), activity of the chemotaxis (Ptar) or the flagellin (PfliC) gene promoters showed a negative relationship with the growth rate (Fig. 1B and SI Appendix, Fig. S1A). The growth rate on a particular sole carbon source under these conditions of well-mixed culture was defined as the nutritional value of this carbon source (N-value), and it was used as a carbon source identifier throughout the manuscript. The up-regulation of motility genes at low N-value was reflected at the phenotypic level as a proportional increase of the cell swimming speed (SI Appendix, Fig. S1B) and of the chemotactic efficiency of a bacterial population (SI Appendix, Fig. S1C), which could be explained by the increased number (SI Appendix, Fig. S1D) and length (SI Appendix, Fig. S1E) of flagella.
We next used the mathematical model that is based on the previous theory of the growth-dependent protein resource allocation (5, 16) to describe how motility gene expression varies with the growth rate. This model assumes that a fixed finite size of the protein budget can either be dedicated to nutrient uptake or growth, i.e., protein synthesis, but trade-offs determine optimal growth rate (Fig. 1A, see SI Appendix, Supplementary Text for details). Indeed, the model could well reproduce the observed growth-rate dependence of motility gene expression (Fig. 1B, solid line).
Relative Fitness Cost of Motility Depends on Carbon Source.
In order to directly evaluate the costs and benefits of motility as a function of the N-value, we used pairwise growth competition between strains to assess their relative reproductive fitness under particular conditions (11, 17). To monitor the ratio of 2 strains in cocultures using flow cytometry, we respectively labeled them with either cyan or yellow fluorescent proteins, CFP or YFP (SI Appendix, Fig. S2 A and B). This differential labeling had no direct effect on the relative fitness of bacteria (SI Appendix, Fig. S2C). Unless specified otherwise, the relative numbers of the 2 competing strains were determined in the early stationary phase (at 72 h) and thus reflect cumulative differences in growth over the entire duration of coculturing.
When the wild type (WT) was cocultured with ΔflhC, a knockout of the master transcription activator required for expression of all motility genes, ΔflhC consistently outgrew the WT cells, both without (Fig. 1C and SI Appendix, Fig. S2 A and B) and with (SI Appendix, Fig. S2D) stirring of the culture. Moreover, the fraction of WT cells decreased steadily as the N-value became lower (Fig. 1C and SI Appendix, Fig. S2D), which means that the relative fitness cost of motility, i.e., growth disadvantage of WT relative to ΔflhC cells in the coculture, becomes increasingly high at lower nutrient quality. A nearly identical result was obtained for the competition of the WT with ΔfliC mutant that lacks only flagellin (SI Appendix, Fig. S2E), demonstrating that the fitness cost of motility is primarily due to the biosynthesis of flagella (11). Nevertheless, flagellar rotation is also apparently costly since the WT was outcompeted by flagellated but nonmotile ΔmotAB cells (SI Appendix, Fig. S2F).
To test whether the increased relative fitness cost of motility at low N-values is due to the elevated expression of flagellar genes, we placed the flhDC operon encoding transcriptional activator of the entire flagellar regulon under control of the synthetic Ptac promoter that is inducible by β-d-thio-galactoside (IPTG), but independent of cAMP-CRP regulation (18). Although in this background the expression of motility genes could be elevated above their native levels and largely decoupled from the growth rate (SI Appendix, Fig. S2G), the fitness cost of motility remained proportional to gene expression (SI Appendix, Fig. S2H).
Competitive Advantage of Chemotaxis in Presence of Introduced Nutrient Gradients.
To explore the potential fitness benefits of motility and chemotaxis under conditions of competitive growth, nutrient gradients were introduced into the unstirred culture by using small (40 µL) agarose beads that contain 12% casein hydrolysate, a mixture of amino acids (SI Appendix, Fig. S3A). In the presence of these gradients and in poor carbon sources, the fraction of WT cells in the coculture with ΔflhC strain was >50% (Fig. 1D and SI Appendix, Fig. S3B), suggesting that the ability to locate nutrient gradients exceeded the high fitness cost of motility. In contrast, in carbon sources with high N-value, motility remained overall disadvantageous, despite its comparatively low fitness cost.
Confirming that this advantage of motility in the presence of nutrient gradients was due to chemotaxis, the WT outcompeted the motile but nonchemotactic ΔcheY strain in the same growth-rate dependent manner (Fig. 1E). Since these 2 strains have similar investment in motility, the competitive reproductive fitness advantage of the WT relative to ΔcheY provides a direct measure for the relative fitness benefit provided by chemotaxis under our experimental conditions. Swimming itself provided no advantage but rather carried significant cost, as ΔcheY strain was outcompeted by the flagellated but nonmotile ΔmotAB strain (SI Appendix, Fig. S3C). Importantly, a similar growth-rate dependent advantage of motility and chemotaxis was observed in Ptac_flhDC background (Fig. 1 D and E), despite a very different dependence of gene expression on the carbon source (SI Appendix, Figs. S2G and S3D). This demonstrates that the increased benefit of chemotaxis in poor carbon sources is due to the increased importance of following nutrient gradients rather than to the higher investment in motility.
We next investigated how relative fitness benefit provided by chemotaxis depends on the concentration of the secondary nutrient. For E. coli grown on ribose, it exceeded the fitness cost of motility for the levels of casein hydrolysate in beads above 5% (SI Appendix, Fig. S3B). This corresponds to ∼20 mM aspartate and 10 mM serine, the 2 strongest attractants and at the same time preferentially consumed amino acids for E. coli (19). This fitness benefit of chemotaxis was nearly identical in experiments with 5% and 12% casein hydrolysate in beads over the entire range of the N-values (SI Appendix, Fig. S3E). In poor carbon sources, the trade-off between this benefit and the cost of motility was also similar for both these levels of casein hydrolysate (SI Appendix, Fig. S3G). However, at higher N-values where chemotaxis generally confers less benefit, the cost of motility apparently exceeds the benefit of chemotaxis toward lower levels of casein hydrolysate (SI Appendix, Fig. S3G). Consistently, this balance between the relative fitness cost of motility and benefit of chemotaxis was affected by up-regulation of flagellar genes, using Ptac_flhDC construct: Although the benefit of chemotaxis remained the same or even increased slightly in the Ptac_flhDC background (Fig. 1E and SI Appendix, Fig. S3F), it was apparently overweighed by the higher cost of motility for most carbon sources, particularly at the lower level of casein hydrolysate (Fig. 1D and SI Appendix, Fig. S3H).
Computational modeling was then used to rationalize how the advantage provided by chemotaxis to the secondary nutrient depends on the N-value of the primary carbon source (Fig. 1A and SI Appendix, Fig. S4 A and B and Supplementary Text). Growth was described using the previously proposed model (20), which predicts the growth rate on 2 coutilized nutrients based on the growth rates on individual substrates. The primary carbon source was initially spatially homogeneous, while the secondary nutrient formed a gradient (SI Appendix, Fig. S4A). The N-value of the secondary nutrient, 1.0 h−1, was in the range of the growth rates observed at varying concentrations of casein hydrolysate (SI Appendix, Fig. S4C). Chemotactic cells were assumed to both spread due to random swimming and follow nutrient gradients, while nonchemotactic ones only spread randomly. Both cell types grew at the same rate on given nutrients. Here we simulated 2 different models: First, we described the process of gradient formation and chemotaxis using full spatial 2-dimensional (2D) simulations based on partial differential equations (PDEs; see SI Appendix, Supplementary Text), where the second nutrient leaked at constant rate from one side of the simulation box, mimicking diffusion out of the bead (SI Appendix, Fig. S4 B and G). Since the simulated gradient of secondary nutrient was confined to the immediate proximity of its source (SI Appendix, Fig. S4G), we also used a simplified model based on ordinary differential equations (ODEs) to fit experimental data. In this latter model, space is divided into 2 compartments, with the second nutrient being confined to one compartment while cells moved between the 2 compartments. Simulations of both models could qualitatively (prediction of the PDE model) and even quantitatively (data fitting by the ODE model) reproduce the observed dependence of the benefit of chemotaxis on the N-value (Fig. 1E). In our simulations, the reproductive fitness benefit of being able to localize the regions with higher concentration of the secondary nutrient is larger at low N-values, because in poor carbon sources the secondary nutrient is comparatively more nutritionally advantageous (SI Appendix, Fig. S4D). Consistent with experimental results (Fig. 1E), this dependence on the N-value was observed even without taking into consideration dependence of motility on the carbon source.
Competitive Advantage of Chemotaxis in Absence of Introduced Gradients.
Surprisingly, we observed that chemotaxis could benefit bacteria even without imposed nutrient gradients, as revealed when coculturing the WT with ΔcheY or with similarly nonchemotactic ΔcheB strain without shaking (Fig. 2A and SI Appendix, Fig. S5A). The competitive advantage of the WT largely decreased at moderate mixing and was entirely abolished at strong mixing (Fig. 2B), indicating that it is caused by—apparently self-generated—gradients of nutrients. Equal reproductive fitness of the WT and ΔcheY cells in the stirred culture further confirms that the cost of the chemotactic signaling, incurred by the CheY phosphorylation cycle, is negligible compared to the overall cellular energy budget. In the unstirred culture, the enrichment of the WT was comparable in magnitude to the one observed in the presence of imposed gradients (Fig. 1E and SI Appendix, Fig. S3E), and it again decreased with the increasing N-value of the carbon source (Fig. 2A).
Fig. 2.
Chemotaxis provides selective advantage even without introduced gradients. (A) Growth competition between WT and ΔcheY cells, performed as in Fig. 1. Cocultures were grown without shaking and in the absence of introduced gradients. flhDC was expressed either under native promoter (solid diamonds) or under Ptac (open symbols) promoter induced with 10 µM IPTG. N-values were taken from Fig. 1B. Solid line shows fit to the data using simulations of 2-compartment chemotaxis model (SI Appendix, Supplementary Text). (B) Same as A for flhDC expressed under native promoter, but under moderate (120 r.p.m., solid diamonds) or strong (180 r.p.m., open squares) mixing. (C) Correlation between motility gene expression in different carbon sources (from SI Appendix, Fig. S1A) and the relative fitness advantage of chemotactic cells (from A). Solid line is a linear fit to the data. (D and E) Growth of WT and ΔcheY cells in the coculture on ribose as sole carbon source without shaking (D), as well as corresponding growth rates (E). ***P < 0.01 in 2-tailed t test. In D, densities of WT (red squares) and ΔcheY cells (green diamonds) were calculated based on the measured density (OD600) of the coculture (teal circles) and the ratio between WT and ΔcheY cells (Inset) determined using flow cytometry (see Methods and SI Appendix, Supplementary Text).
Nearly identical dependence of the relative fitness benefit of chemotaxis on the N-value was observed when motility genes were expressed under control of the Ptac promoter (Fig. 2A). Thus, as in the case of introduced gradients (Fig. 1E), the increased relative fitness benefit at low N-values is not the consequence of up-regulation of motility but rather due to the increased importance of chemotaxis in poor carbon sources. Despite this apparent lack of causality, we observed that the investment in motility in the WT cells showed strong positive correlation with the benefit of chemotaxis (Fig. 2C). Since this correlation was much less pronounced in the Ptac_flhDC background where flagellar expression was decoupled for the native regulation (SI Appendix, Fig, S5B), we hypothesized that bacteria naturally regulate investment in motile behavior to be proportional to the potential benefit provided by chemotaxis (see Discussion).
To further understand whether the relative fitness benefit of chemotaxis in the absence of introduced gradients is limited to a specific growth stage, we used flow cytometry to measure the composition of the coculture during growth on ribose (Fig. 2D), α-ketoglutarate (SI Appendix, Fig. S6A), gluconate (SI Appendix, Fig. S6B), or glucose (SI Appendix, Fig. S6C). The WT cell fraction increased steadily throughout the early (SI Appendix, Fig. S6 A, Inset) or entire (Fig. 2 D, Inset and SI Appendix, Fig. S6 B, Inset) growth phase, with WT cells growing almost twice as fast as ΔcheY cells on ribose (Fig. 2E). No significant difference between the WT and ΔcheY was observed during growth on glucose (SI Appendix, Fig. S6C), confirming that at high N-value chemotaxis provides no competitive advantage. Importantly, even during growth on ribose no advantage of chemotaxis could be seen when the 2 strains were incubated separately, and in that case ΔcheY cells even grew to slightly higher density in the stationary phase (SI Appendix, Fig. S6 D and E).
Gradients of Carbon Sources and Excreted Metabolites Could Explain Advantage of Chemotaxis.
We hypothesized that chemotaxis enables WT bacteria to follow beneficial (most likely nutrient) gradients that transiently emerge in unstirred bacterial cultures. Oxygen could be excluded as a possible gradient source, since sealing the cocultures with heavy mineral oil to prevent aeration, or growing them under anaerobic conditions, had no significant effect on the relative fitness benefit of chemotaxis (Fig. 3A). Consistently, disruption of the aerotaxis receptor Aer yielded no competitive disadvantage in the coculture with the WT cells (SI Appendix, Fig. S7A). Furthermore, no advantage was observed for the WT cells competed against strains individually deleted for genes that encode chemotaxis receptors Tap (SI Appendix, Fig. S7B), Tsr (SI Appendix, Fig. S7C), or Trg (Fig. 3B), in any of the used carbon sources including ribose, the established ligand of Trg. In contrast, cells lacking Tar had clear competitive disadvantage relative to the WT in several carbon sources, which was comparable to the disadvantage of the general chemotaxis mutant ΔcheY (Figs. 2A and 3C).
Fig. 3.
Gradients of carbon sources and extracellular metabolites explain fitness advantage of chemotaxis. (A) Growth competition between WT and ΔcheY cells performed as in Fig. 2A, but in medium sealed with heavy mineral oil (solid diamonds) or under anaerobic conditions (open squares). (B and C) Growth competition between WT and Δtrg (B), Δtar (C), or ΔtrgΔtar (D) cells, performed as in Fig. 2A. N-values were taken from Fig. 1B. (E and F) Chemotactic response of WT (E) or Δtrg (F) strains toward supernatant of WT or ΔcheY cultures measured by FRET (SI Appendix, Supplementary Text and Fig. S8 A–D).
Although the WT and Δtar strains grew similarly when cocultured on ribose (Fig. 3 B and C), the WT had clear advantage during growth on these carbon sources when competed against the strain lacking both Tar and Trg (Fig. 3D). These results indicate emergence of 2 types of transient gradients within the culture: those of carbon sources and of excreted extracellular metabolites. The importance of both types of gradients could explain why during growth on ribose, either the ribose-specific receptor Trg or Tar that does not detect ribose but presumably responds to excreted metabolites, could individually mediate the advantage of chemotaxis.
We speculated that the ensuing higher efficiency of resource exploitation provides competitive advantage to the chemotactic bacteria in the coculture, enabling them to actively withdraw nutrient resources from the nonchemotactic cells, and that this fitness benefit provided by chemotaxis increases with the extent of nutrient limitation. In the absence of competition, however, nonchemotactic cells can eventually consume these nutrients and thus achieve similar growth. To test whether the lack of chemotaxis might indeed result in a less efficient consumption of the carbon source, we used a previously described assay of the pathway activity based on the Förster (fluorescence) resonance energy transfer (FRET) (21) to compare chemotactic response of the WT cells to supernatant derived from either WT or ΔcheY cultures. The WT cells, but not Δtrg cells, responded much more sensitively to the ΔcheY supernatant than to the WT supernatant (Fig. 3 E and F and SI Appendix, Fig. S8 A–D), indicating that the former indeed contains significantly higher levels of a Trg ligand—most likely less efficiently consumed ribose. Chemotaxis toward the carbon source gradients could also at least partly account for the Tar-dependent competitive advantage during growth on succinate, α-ketoglutarate, and glycerol (Fig. 3C), since Tar mediates attractant responses to all of these compounds (SI Appendix, Fig. S8 E–G). In contrast, Tsr apparently mediates repellent response to succinate and α-ketoglutarate (SI Appendix, Fig. S8 H–J), which might explain why during growth on these 2 carbon sources the deletion of tsr even increases the advantage of chemotaxis (SI Appendix, Fig. S7C).
Succinate Is a Major Excreted Chemoattractant.
Following gradients of extracellular metabolites excreted by bacteria themselves appears to be comparably important for the observed fitness benefit. Such chemotaxis toward extracellular metabolites—mediated by Tar—could explain why Δtrg cells compete equally with the WT during growth on ribose (Fig. 3B), despite clearly lacking response to this sugar (SI Appendix, Fig. S8 K–M). It is also likely to explain the advantage of chemotaxis during growth on carbon sources that do not themselves elicit a chemotactic response, such as gluconate (SI Appendix, Fig. S8 M and N).
Consistent with this hypothesis, we detected comparatively high (10–100 µM) levels of several potential chemoattractants and nutrients in the culture supernatant of WT cells growing on ribose, α-ketoglutarate, gluconate, or glucose (Fig. 4A and SI Appendix, Fig. S9A and Datasets S1 and S2), and culture supernatant could both elicit a chemotactic response (Fig. 3E) and enhance growth of an E. coli culture (SI Appendix, Fig. S9B) at dilutions of up to 10−3 to 10−4. In particular, high levels of succinate, which is sensed by E. coli as a chemoattractant at concentrations above 1 µM (SI Appendix, Fig. S8F), were excreted by ribose-, gluconate- or glucose-fed cells (Fig. 4A and SI Appendix, Fig. S9A). Secretion of succinate indicates that, under our conditions, these carbon sources are at least partly catabolized via mixed acid fermentation (22), presumably due to low levels of oxygen in the unstirred culture. Addition of 5 mM succinate was observed to promote culture growth, dependent of the N-value of the primary carbon source (SI Appendix, Fig. S9C). It further seems that succinate and several other extracellular metabolites are secreted into the culture medium and not released from lysing cells, since levels of these compounds were similar in the supernatant and in the sample from the whole culture broth (SI Appendix, Fig. S9D). Levels of external succinate were lower in the α-ketoglutarate-fed cultures, but high levels of another potent Tar-specific chemoattractant, l-glutamate, were detected instead (SI Appendix, Fig. S9A).
Fig. 4.
Metabolic profile of the culture medium and emergence of gradients. (A) Extracellular metabolites secreted in the culture medium by WT cells grown without shaking with ribose as the sole carbon source for 60 h. Metabolites were analyzed by liquid chromatography–mass spectrometry (SI Appendix, Supplementary Text). (B) Time dependence of densities (OD600) of WT (solid symbols) or ΔcheY (open symbols) cells grown individually without shaking, with ribose as the sole carbon source, in the top (circles), middle (squares), and bottom (triangles) layers of the liquid culture. **P < 0.05, ***P < 0.01 in 2-tailed t test. (C) Proportion of WT cells in different layers of the cocultures with ΔcheY cells grown for 72 h without shaking with indicated carbon sources. (D) Full 2D simulations of competition between the WT and ΔcheY cells cocultured on different primary nutrients and excreting secondary nutrients as detailed in SI Appendix, Supplementary Text. Shown are simulations for chemotactic response to only primary nutrient (black open diamonds), to both primary and secondary nutrients (blue open triangles), and to only secondary nutrient (green open circles), with chemotactic sensitivity varying dependent on the carbon source as in SI Appendix, Fig. S1C. Also shown is the simulation with chemotactic response to both primary and secondary nutrients but with constant high sensitivity of chemotaxis, simulating motility induction under Ptac promoter (red open squares). (E) Spatial maps of WT cell density (Top, in units of OD) and of concentration of secondary nutrient s (Bottom, in millimolar), for indicated time points (t in hours) and for N-value of the primary nutrient 0.3 h−1.
Emergence of Nutrient and Cell Gradients in the Culture.
How gradients of carbon sources and extracellular metabolites emerge in the culture? One possible origin of transient gradients, predicted by a previous theoretical study (14), could be local fluctuations in the nutrient consumption by bacteria. Another likely origin is gradual sedimentation of bacteria, which is common in aquatic habitats and could establish a nonuniform profile of cell density and therefore of nutrient consumption in the unstirred culture. Consistent with this second scenario, an increasing fraction of bacteria could be found in the sediment at the later stages of culture growth (Fig. 4B). This fraction was significantly smaller in the WT than in the ΔcheY culture, indicating that chemotaxis in emergent gradients reduces sedimentation of the WT cells. This difference was even more pronounced in the coculture, where the fraction of the WT was markedly higher in the upper layers of the culture than near the sediment (Fig. 4C).
To better understand this interplay between emergent gradients of secreted and consumed metabolites and chemotaxis, we modified our numerical models to include excretion of the secondary nutrient as a byproduct of the carbon source consumption (SI Appendix, Fig. S10 and Supplementary Text). This secondary nutrient is then coutilized along the primary nutrient for growth. In the full 2D simulations, cell sedimentation due to gravity was also explicitly considered. We observed that chemotaxis toward the secondary nutrient was alone sufficient to gain an N-value dependent competitive growth advantage (Fig. 4D). The 2-compartment ODE model that considered only chemotaxis toward the secondary nutrient and assumed constant chemotactic efficiency could then be used to quantitively reproduce experimental data (Fig. 2A). Notably, nearly identical advantage of chemotaxis was observed when the efficiency of motility and chemotaxis was assumed to depend on the N-value (Fig. 4D), consistent with experiments (Fig. 2A).
The 2D simulations provided further insights into spatial dynamics that might be at play during our experiments (Fig. 4E and SI Appendix, Fig. S10G). We observed that sedimentation favored the initial nucleation of dense patches of chemotactic cells, formed at the bottom of the simulation box via chemotaxis toward self-generated gradients of excreted metabolites. These patches later merged and eventually traveled as a band from the bottom to the top of the box, leaving behind a partially depleted medium. Although chemotaxis toward the primary carbon source had itself little importance in our simulations because its gradients were relatively weak (Fig. 4D and SI Appendix, Fig. S10G), chemotaxis toward the secondary nutrient nevertheless increased availability of the primary carbon source to the WT cells since at the later time points both gradients were coincident. Chemotactic response to the primary carbon source became more important when cell sedimentation was assumed to moderately increase in our simulations, which resulted in much steeper carbon source gradients (SI Appendix, Supplementary Text). Thus, the combination of feeding on higher levels of secondary nutrients and reaching higher levels of the primary carbon source in the upper layer of culture gives the WT cells a growth advantage over the nonchemotactic cells, which were denser at the bottom (SI Appendix, Fig. S10G).
Discussion
Although microorganisms evolved various regulatory strategies to reallocate their resources dependent on the environment (23), quantitative comparison of costs and benefits of a particular investment strategy for microbial fitness is rarely available. It thus typically remains unclear whether the amount of resources allocated to a particular function follows a specific evolutionary logic of optimization (6). One notable exception here is the regulation of bacterial metabolism, where relative investment into catabolism versus biosynthesis, coordinated by cAMP-CRP signaling, appears to be balanced to maximize growth (1–3, 5). Similarly, at least in some cases, the uptake of a carbon source has been apparently optimized for maximal growth (3). However, bacterial growth on many carbon sources is clearly suboptimal (24), indicating that in those cases the regulation of resource investment did not evolve to maximize growth but rather toward other evolutionary objectives (6). These might include preallocation of resources into biosynthetic or metabolic machinery that may become beneficial when bacteria become suddenly exposed to nutrients, or investment into other functions that are not apparently related to growth (25, 26). Particularly for these latter types of investment, conditions under which they provide fitness benefit and the quantitative relation between the extent and cost of investment and its benefit are unknown.
Here, we investigated costs and benefits of resource allocation into motile behavior, the major cAMP-CRP-dependent cellular function that is not immediately related to E. coli growth. Similarly to other cAMP-regulated genes, the expression of motility genes increases linearly with the decrease of growth rate on poorer carbon sources (1, 5). This results in a proportional increase of the length and number of flagellar filaments, and consequently of cell swimming velocity and chemotaxis. The relative fitness cost of motility, i.e., growth disadvantage of motile cells under conditions where motility provides little or no benefit, was also proportionally higher at lower growth rates. This increasing cost was primarily a consequence of higher investment into the biosynthesis of flagella, whereas the cost of swimming was also measurable but lower and only weakly dependent on the growth rate, and the cost the chemotactic signaling was negligible. Thus, linearly increased investment in motility at lower growth rates results in a proportionally linear increase of both the functionality and the cost of motility.
The same negative dependence on the N-value of the carbon source was observed for the relative fitness benefit that is provided by chemotaxis, when chemotactic and nonchemotactic cells are cocultured in the presence of nutrient gradients. This dependence could also be seen when expression of chemotaxis and flagellar genes was largely decoupled from the growth rate, implying that increased benefit of chemotaxis at low N-values is not the consequence of increased investment but rather due to greater importance of locating additional nutrients when the primary carbon source is poor. The growth-rate dependent regulation of motility genes thus apparently ensures that investment in motility, including chemotaxis, is proportional to the fitness benefit that chemotaxis could provide when nutrient gradients are encountered in a particular carbon source. However, since motility gene regulation was also observed in a shaking culture where gradients cannot form, we conclude that the correlation between potential benefit and investment in motility and chemotaxis is predictive (anticipatory) rather than causal (23). We hypothesize that in the case of chemotaxis such predictive investment might have emerged because chemotaxis operates on a much shorter timescale than gene expression (7) and microscale gradients of nutrients in many natural environments are highly variable and short-lived (27), making direct regulatory coupling of gene expression to the benefit provided by chemotaxis unreliable. Preallocation of increasing amounts of resources in motility and chemotaxis at low growth rates–when the payoffs are likely to be larger—might be a superior strategy, which could explain why motility genes are regulated by cAMP-CRP. The trade-off between the relative fitness benefit and the cost of motility is ultimately dependent on the abundance and quality of encountered nutrient gradients.
The growth-rate dependent fitness benefit of chemotaxis could be observed not only when gradients of nutrients were introduced into bacterial culture, but also in self-generated gradients of the excreted secondary metabolites and of the primary carbon source. Bacterial sedimentation was the likely initial source of nonuniform distribution of bacteria in a culture under our experimental conditions, followed by chemotaxis-dependent redistribution of bacteria toward higher levels of both primary and secondary nutrients. Similar interplay between sedimentation and motility may play an important role in natural (e.g., aquatic) habitats, along with numerous other sources of micro- to mesoscale heterogeneity (28). Excretion of metabolites, including amino acids, sugars, and organic acids into the environment is also a common phenomenon in bacteria and other microorganisms (29). It might serve to optimize proteome allocation during growth (2), but excreted metabolites have also been shown to mediate (mutualistic) metabolic interactions (30) or signaling (31) in various microbial communities, as well as chemotactic self-concentration of bacteria (32–34). Our work thus suggests that chemotaxis may provide a general ecological advantage in competition for reutilization of excreted metabolites, even in communities consisting of single species as long as spatial heterogeneity is allowed to emerge. Such competition for local sources of metabolites is likely to be even more important in structured natural communities (27, 28), and we hypothesize that the regulation of E. coli motility has evolved under this selection.
Methods
Detailed description of experimental methods and mathematical modeling can be found in SI Appendix.
Data Availability.
Metabolomics data and codes used for ODE and PDE simulations are available as Datasets S1–S4.
Supplementary Material
Acknowledgments
We thank Thomas Heimerl, Seigo Shima, and Gangfeng Huang for their help with experiments. This research was supported by the Max Planck Society, European Research Council grant 294761-MicRobE to V.S. and Biotechnology and Biological Sciences Research Council grant BB/N00065X/1 to R.G.E.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1910849117/-/DCSupplemental.
References
- 1.Hui S., et al. , Quantitative proteomic analysis reveals a simple strategy of global resource allocation in bacteria. Mol. Syst. Biol. 11, 784 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basan M., et al. , Overflow metabolism in Escherichia coli results from efficient proteome allocation. Nature 528, 99–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dekel E., Alon U., Optimality and evolutionary tuning of the expression level of a protein. Nature 436, 588–592 (2005). [DOI] [PubMed] [Google Scholar]
- 4.Schuetz R., Zamboni N., Zampieri M., Heinemann M., Sauer U., Multidimensional optimality of microbial metabolism. Science 336, 601–604 (2012). [DOI] [PubMed] [Google Scholar]
- 5.You C., et al. , Coordination of bacterial proteome with metabolism by cyclic AMP signalling. Nature 500, 301–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Basan M., Resource allocation and metabolism: The search for governing principles. Curr. Opin. Microbiol. 45, 77–83 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Berg H. C., Brown D. A., Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature 239, 500–504 (1972). [DOI] [PubMed] [Google Scholar]
- 8.Bi S., Sourjik V., Stimulus sensing and signal processing in bacterial chemotaxis. Curr. Opin. Microbiol. 45, 22–29 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Colin R., Sourjik V., Emergent properties of bacterial chemotaxis pathway. Curr. Opin. Microbiol. 39, 24–33 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Milo R., Jorgensen P., Moran U., Weber G., Springer M., BioNumbers–The database of key numbers in molecular and cell biology. Nucleic Acids Res. 38, D750–D753 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ni B., et al. , Evolutionary remodeling of bacterial motility checkpoint control. Cell Rep. 18, 866–877 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu M., et al. , Global transcriptional programs reveal a carbon source foraging strategy by Escherichia coli. J. Biol. Chem. 280, 15921–15927 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Adler J., Templeton B., The effect of environmental conditions on the motility of Escherichia coli. J. Gen. Microbiol. 46, 175–184 (1967). [DOI] [PubMed] [Google Scholar]
- 14.Celani A., Vergassola M., Bacterial strategies for chemotaxis response. Proc. Natl. Acad. Sci. U.S.A. 107, 1391–1396 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frankel N. W., et al. , Adaptability of non-genetic diversity in bacterial chemotaxis. eLife 3, e03526 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scott M., Gunderson C. W., Mateescu E. M., Zhang Z., Hwa T., Interdependence of cell growth and gene expression: Origins and consequences. Science 330, 1099–1102 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Elena S. F., Lenski R. E., Evolution experiments with microorganisms: The dynamics and genetic bases of adaptation. Nat. Rev. Genet. 4, 457–469 (2003). [DOI] [PubMed] [Google Scholar]
- 18.de Boer H. A., Comstock L. J., Vasser M., The tac promoter: A functional hybrid derived from the trp and lac promoters. Proc. Natl. Acad. Sci. U.S.A. 80, 21–25 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Y., et al. , Relation between chemotaxis and consumption of amino acids in bacteria. Mol. Microbiol. 96, 1272–1282 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermsen R., Okano H., You C., Werner N., Hwa T., A growth-rate composition formula for the growth of E.coli on co-utilized carbon substrates. Mol. Syst. Biol. 11, 801 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sourjik V., Vaknin A., Shimizu T. S., Berg H. C., In vivo measurement by FRET of pathway activity in bacterial chemotaxis. Methods Enzymol. 423, 365–391 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Clark D. P., The fermentation pathways of Escherichia coli. FEMS Microbiol. Rev. 5, 223–234 (1989). [DOI] [PubMed] [Google Scholar]
- 23.Perkins T. J., Swain P. S., Strategies for cellular decision-making. Mol. Syst. Biol. 5, 326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Towbin B. D., et al. , Optimality and sub-optimality in a bacterial growth law. Nat. Commun. 8, 14123 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Brien E. J., Utrilla J., Palsson B. O., Quantification and classification of E. coli proteome utilization and unused protein costs across environments. PLoS Comput. Biol. 12, e1004998 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori M., Schink S., Erickson D. W., Gerland U., Hwa T., Quantifying the benefit of a proteome reserve in fluctuating environments. Nat. Commun. 8, 1225 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stocker R., Seymour J. R., Ecology and physics of bacterial chemotaxis in the ocean. Microbiol. Mol. Biol. Rev. 76, 792–812 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cordero O. X., Datta M. S., Microbial interactions and community assembly at microscales. Curr. Opin. Microbiol. 31, 227–234 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paczia N., et al. , Extensive exometabolome analysis reveals extended overflow metabolism in various microorganisms. Microb. Cell Fact. 11, 122 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ponomarova O., et al. , Yeast creates a niche for symbiotic lactic acid bacteria through nitrogen overflow. Cell Syst. 5, 345–357.e6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pereira C. S., Thompson J. A., Xavier K. B., AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 37, 156–181 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Budrene E. O., Berg H. C., Dynamics of formation of symmetrical patterns by chemotactic bacteria. Nature 376, 49–53 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Park S., et al. , Motion to form a quorum. Science 301, 188 (2003). [DOI] [PubMed] [Google Scholar]
- 34.Laganenka L., Colin R., Sourjik V., Chemotaxis towards autoinducer 2 mediates autoaggregation in Escherichia coli. Nat. Commun. 7, 12984 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Metabolomics data and codes used for ODE and PDE simulations are available as Datasets S1–S4.