Significance
All solid tissues rely on cadherin family cell–cell adhesion molecules for their cohesion and organization. Cadherin adhesive function depends on the actin cytoskeleton, but actin’s contribution to cell–cell adhesion is not fully understood. We demonstrate that actin polymerization-dependent protrusive activity operates continuously to push lateral membranes of neighboring cells together to keep cadherins in contact. Actin-dependent protrusive activity functions as a safety mechanism to quickly repair cadherin adhesive junctions whenever they fail and to prevent myosin-dependent contractile forces from tearing the junctions further apart. Since loss of cadherin adhesion is associated with a number of diseases including cancer progression, then misregulation of actin protrusive activity should be considered as a possible contributing factor to epithelial pathophysiology.
Keywords: E-cadherin, actin, epithelial, junction, adhesion
Abstract
Cadherin-mediated cell–cell adhesion is actin-dependent, but the precise role of actin in maintaining cell–cell adhesion is not fully understood. Actin polymerization-dependent protrusive activity is required to push distally separated cells close enough to initiate contact. Whether protrusive activity is required to maintain adhesion in confluent sheets of epithelial cells is not known. By electron microscopy as well as live cell imaging, we have identified a population of protruding actin microspikes that operate continuously near apical junctions of polarized Madin-Darby canine kidney (MDCK) cells. Live imaging shows that microspikes containing E-cadherin extend into gaps between E-cadherin clusters on neighboring cells, while reformation of cadherin clusters across the cell–cell boundary correlates with microspike withdrawal. We identify Arp2/3, EVL, and CRMP-1 as 3 actin assembly factors necessary for microspike formation. Depleting these factors from cells using RNA interference (RNAi) results in myosin II-dependent unzipping of cadherin adhesive bonds. Therefore, actin polymerization-dependent protrusive activity operates continuously at cadherin cell–cell junctions to keep them shut and to prevent myosin II-dependent contractility from tearing cadherin adhesive contacts apart.
Cadherin family cell–cell adhesion molecules are essential for tissue cohesion and organization throughout life. While the cadherin ectodomain mediates homophilic binding, strong adhesion requires contributions from cytosolic factors including the actin cytoskeleton (1). The apical junction of cell–cell interface is the prominent site of actin polymerization in epithelial cells even long after the junction has been established (2–4). However, we still do not fully understand either the function or the regulation of the actin at cell–cell adhesive junctions.
Cells generally organize actin into either contractile networks that use myosin to generate pulling forces or protrusive networks that use actin polymerization to generate pushing forces (5). Actomyosin contractility plays a major role in cadherin biology, especially during development when cadherin adhesive junctions propagate tensile forces across interconnected sheets of cells to drive various cell movements (6). Contractility also contributes to junction maturation and stabilization in epithelial sheets (7, 8). Junctional actin polymerization is suggested to build contractile actomyosin (9–14). Observations in cells strongly suggest that cadherin–catenin complexes couple to contractile actin networks and that the complex is under tension (8, 15, 16).
Coupling cadherins to the contractile actin cytoskeleton offers great morphogenetic power to sculpt tissues, but relying on only contractile forces to stabilize junctions in established epithelia is not fail-safe. In vitro measurements show that piconewton pulling forces stabilize the connection between the cadherin–catenin complex and F-actin (17). In addition, myosin-dependent contractility leads to adherens junction remodeling, perhaps to fortify the junction and make it resilient against tearing (7, 11, 18, 19). However, continuing to pull on a broken junction would only tend to propagate the defect, which could tear tissues apart (20–23). Cadherin-mediated adhesion is important, and cells tend to evolve safety mechanisms to ensure that important functions remain robust in the face of a perturbation or occasional failure.
We thus reasoned that actin polymerization-dependent protrusive activity might operate as a safety mechanism to keep lateral membranes of neighboring cells close to each other to promote E-cadherin binding. Protrusions are known to be important for initiating the formation of cell–cell adhesion at the leading edge of motile cells (24, 25). However, cell–cell adhesion in mature epithelial sheets (7, 8, 26–28) is intrinsically more stable than that in newly forming epithelial monolayers (24, 25). Previous work in endothelial cells has shown lamellipodium- and filopodium-like protrusions at the junctions of motile cells and newly forming monolayers (29–33). At mature endothelial junctions, lamellipodium-like protrusions crawl over the top of the neighboring cell at the sites lacking vascular endothelial (VE)–cadherin clusters, so that VE–cadherin clusters can form at the new cell–cell contact created by the lamellipodium and integrate into and strengthen the linear junction when the lamellipodium retracts (31). At unstable or remodeling endothelial junctions, bundled actin structures from 1 cell are engulfed by the neighboring cell and associated with VE–cadherin, although they are thought to enhance adhesion through filopodial retraction (19, 29, 30, 34). Above all, whether protrusive activity continues to operate in mature epithelial junctions is not known. Junctional actin assembly in epithelia depends on factors associated with protrusive actin networks (2, 3, 23, 35). Thus, we looked for protrusive activities in Madin-Darby canine kidney (MDCK) (kidney tubular epithelial) cell sheets 3–4 d postconfluency with established apical–basal polarity.
Results
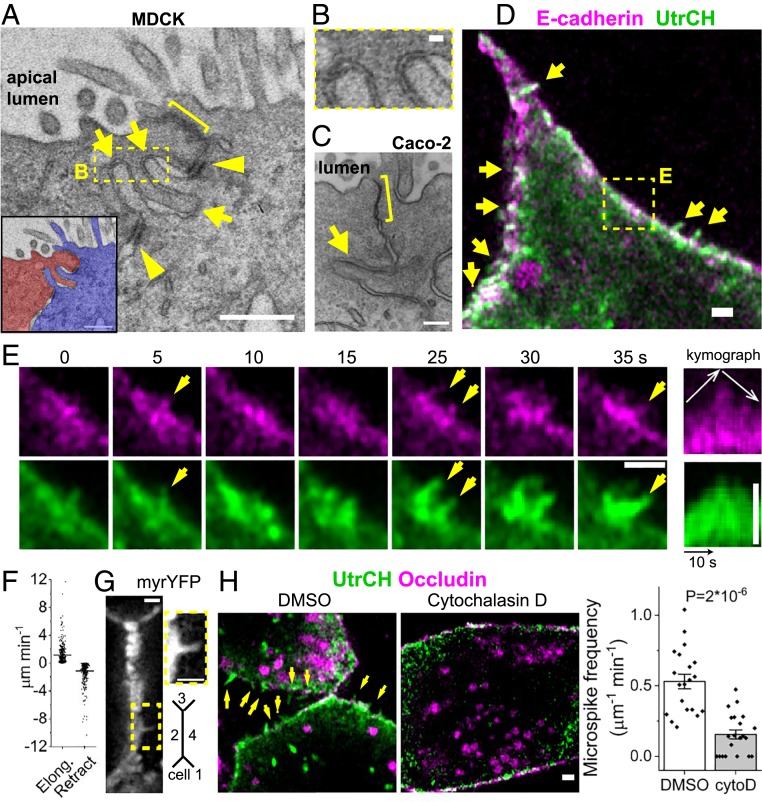
By thin-section electron microscopy we found membrane protrusions of 389 ± 21 nm in length (mean ± SEM, n = 20 microspikes) that burrowed into neighboring cells near the apical junctional complex in MDCK cells (Fig. 1 A and B and SI Appendix, Fig. S1A). Forty-one percent of junctions (n = 66) in MDCK cells have protrusions. We also examined Caco-2 intestinal epithelial cells (C2bbE1 clone) and found protrusions at 41% of junctions (n = 51) (Fig. 1C and SI Appendix, Fig. S1A). Lateral membrane protrusions have long been recognized (3, 36), but we found protrusions also prevalent near apical junctional complex which consists of tight junctions and adherens junctions (1). The intercellular distance between the tip of a protrusion and the neighboring cell’s membrane is 20–40 nm, resembling that between adherens junctions (Fig. 1B) (37–39). Using live cell imaging of F-actin marker UtrCH, we discovered similar filopodium-like microspikes (Fig. 1D) (40). By examining a single labeled cell in a cell sheet, we were able to see membrane structures that are otherwise masked by homogenous labeling, like immunofluorescence. Actin microspikes are 381 ± 18 nm long and persist for 6 ± 1 s (median ± SEM, n = 91 microspikes, SI Appendix, Fig. S1B) and undergo dynamic elongation, retraction, and pivoting (Fig. 1E, SI Appendix, Fig. S1C, and Movie S1). They elongate and retract at the average rate of 1.1 μm min−1 and −1.1 μm min−1, respectively (Fig. 1F). After falling back into the cell body, some microspikes bundle with the junctional actin belt (SI Appendix, Fig. S1C). The frequency of actin microspikes is 0.53 ± 0.05 per μm of junction length per minute (mean ± SEM, n = 20 cells). Membrane-targeted yellow fluorescent protein (YFP) also showed dynamic protrusions (Fig. 1G, SI Appendix, Fig. S1E, and Movie S2). The frequency of membrane protrusions is 0.41 ± 0.04 μm−1 min−1 (mean ± SEM, n = 21 junctions in 5 cells). Blocking actin-filament (+) end dynamics with cytochalasin D (41) eliminated microspikes, indicating their dependence on actin assembly (Fig. 1H). Simultaneous imaging of actin and E-cadherin and kymograph analysis showed E-cadherin tracks along 88% of elongating microspikes (n = 33) and 86% of retracting microspikes (n = 21), respectively (Fig. 1E and SI Appendix, Fig. S1D).
Fig. 1.
Actin assembly drives microspike protrusions toward apical junction. (A–C) Thin-section electron micrograph of confluent epithelial cells (sagittal view). Arrows, protrusions; brackets, apical junction complex; arrowheads, desmosomes. (A and B) Kidney MDCK cells. Box is enlarged in B to show the intercellular distance between the tip of protrusions and the neighboring cell. (C) Intestinal Caco-2 cells. (Scale bars, 500 nm [A], 40 nm [B], and 200 nm [C].) (D and E) Live images of microspikes (arrows) in confluent cell sheets sparsely transfected by Ecad–GFP and mChe–Utr. Box is tracked in E. The kymograph shows microspike elongation and retraction. (Scale bar, 1 μm.) (F) Mean ± SEM rates of microspike elongation and retraction (n = 433 and 420 events from 470 microspikes in 5 cells). (G) Membrane-targeted YFP (LynD3cpV). (Inset) A membrane protrusion. (Scale bar, 1 μm.) (H) Live images of cells treated with 200 nM cytochalasin D for 20 min. GFP–Utr and Ocln–mChe. Arrows, microspikes. Mean ± SEM microspike frequency is normalized to junction length and frame interval (n = 20 and 21 cells from 2 experiments, 2-sided Mann–Whitney U test). (Scale bar, 1 μm.)
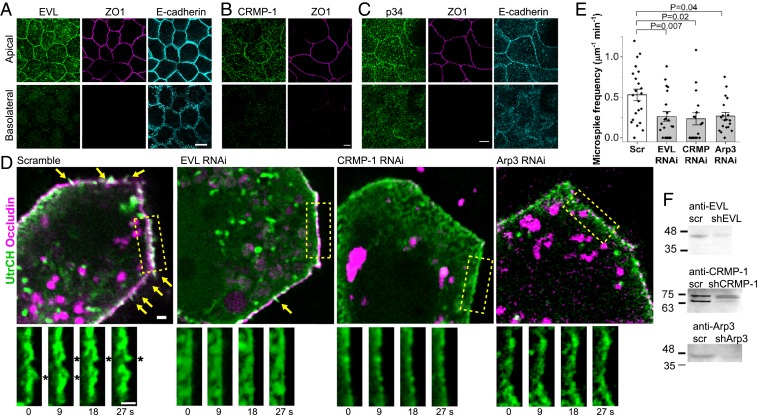
We next asked which actin assembly factors promote microspikes. Previous work identified EVL, CRMP-1, and Arp2/3 as 3 factors necessary for actin assembly at apical cell–cell junctions (2, 3, 23). Arp2/3 nucleates the formation of new actin filaments (42), while EVL and CRMP-1 form a complex that elongates the (+) ends of existing actin filaments (23). Immunofluorescence showed that all 3 factors localize to apical junctions (Fig. 2 A–C). We therefore tested the role of each factor in microspike formation using RNA interference (RNAi) knockdown. Depletion of EVL, CRMP-1, or the Arp3 subunit of Arp2/3 complex with previously validated interfering RNAs (23) reduced microspikes (Fig. 2 D and E and Movie S3). Conversely, overexpressing EVL or CRMP-1 led to more microspikes which are longer and more stable (SI Appendix, Fig. S2 E–K). Filopodia and microspikes extend by elongating parallel actin bundles from their (+) ends (40), consistent with EVL and CRMP-1’s function (43). Although Arp2/3 does not make actin bundles, the branched actin it produces serves as the base from which microspikes extend (44, 45).
Fig. 2.
EVL, CRMP-1, and Arp2/3 promote microspikes. (A–C) Immunofluorescence of EVL, CRMP-1, or the p34 subunit of Arp2/3 complex with junctional markers. Methanol fixation. (Scale bars, 5 μm.) (D) Live images of microspikes (arrows and asterisks) in cell sheets expressing short-hairpin RNA (shRNA) against EVL, CRMP-1, or the Arp3 subunit of Arp2/3 complex. Scramble/scr, control shRNA. GFP–Utr and Ocln–mChe. (Scale bars, 1 μm.) (E) Mean ± SEM (n = 22 junctions of 223 μm in total length of scramble, 21 junctions of 158 μm of EVL RNAi, 17 junctions of 152 μm of CRMP RNAi, and 19 junctions of 254 μm of Arp3 RNAi from 2 experiments; 2-sided Mann–Whitney U test). (F) Whole cell lysate Western blot of knockdown.
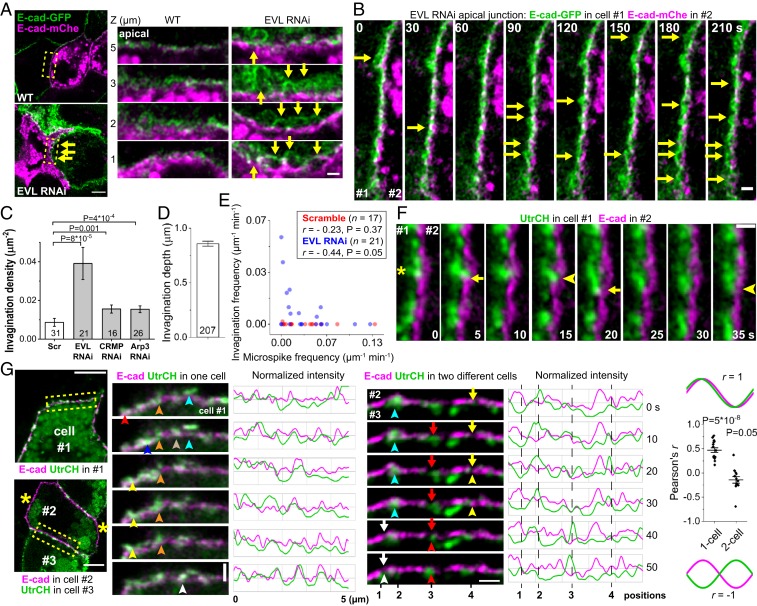
EVL, CRMP-1, or Arp2/3 depletion also leads to junction failure and cell sheet tearing (21, 23), so we examined the distribution of E-cadherin in these cells. We mixed cells expressing E-cadherin–green fluorescent protein (GFP) and E-cadherin–mChe and imaged junctions that formed between them. In control cells, adjacent membranes are in close contact through cadherin cell–cell contacts. In EVL-depleted cells, these contacts are unstable and spontaneously unzip into deep invaginations (Fig. 3 A and B, SI Appendix, Fig. S3, and Movies S4 and S5). Invaginations are hemispherical or even tubular (Fig. 3A, EVL RNAi, z = 3 μm) and can last for minutes (Fig. 3B and Movie S5). Every μm2 of cell–cell contact has 0.04 invaginations in EVL-depleted cells, compared to 0.01 invaginations in control (Fig. 3C). These invaginations can be ∼1 µm deep (Fig. 3D), much larger than clathrin-coated pits which are 70–200 nm (46). Failures in cadherin adhesive bonds cause junctions to detach and blister, which makes cell sheets prone to tearing (23).
Fig. 3.
Microspikes push near apical junctions to repair E-cadherin adhesion failure. (A and B) Live images of mixed culture of cells expressing Ecad–GFP or Ecad–mChe. Arrows, deep invaginations. (A) z = 0 is basal plane. (Scale bar, 5 μm.) Boxed regions of the junction between GFP and mChe cells are enlarged. (Scale bar, 1 μm.) (B) Scale bar, 1 μm. (C) Invaginations density-normalized to lateral membrane area (n cells from 2 experiments; 2-sided Mann–Whitney U test). (D) Maximum invagination depth in EVL RNAi cells (n invaginations from 2 experiments). (E) The frequency of invaginations and microspikes at the apical junction in each cell. Pearson’s r (n cells, 2-sided t test). (F) Ecad–GFP and actin (Utr–mChe) markers expressed separately in 2 cells. An elongating microspike (asterisk) from cell #1 indents the membrane of cell #2 (arrows); opposing membrane tracks the retracting microspike tip and flattens (arrowheads). E-cadherin channel-shifted by 0.34 µm to the right. (Scale bar, 1 μm.) (G) Ecad–GFP and Utr–mChe in 1 or 2 cells. Note that cell #2 lacks junctional actin labeling (asterisks), despite some vesicular signal. (Scale bar, 5 μm.) Boxed regions are tracked in time series. (Scale bar, 1 μm.) Junctional intensities were normalized to the maximum values in each frame. Color-coded arrowheads track different microspikes, and arrows track gaps between E-cadherin clusters. Positions 1 to 4, see text. The chart shows Pearson’s r between E-cadherin and actin intensity profiles (n = 16 movies of 7 single cells and 13 movies of 5 cell pairs from 2 experiments; 2-sided 1-sample t test comparing the mean to 0). All bar charts show mean ± SEM. Sample size n is shown on charts unless specified.
The number of microspikes is inversely related to the number of invaginations at the apical junction in the cell, suggesting that protrusive activity prevents unzipping of cadherin adhesions (Fig. 3E). To test whether microspikes can exert pushing forces on the opposing membrane of the neighboring cell to prevent junction unzipping, we simultaneously imaged microspikes in 1 cell and the membrane labeled by E-cadherin in the neighboring cell (Fig. 3F and Movie S6). Microspikes correlate with the indentations but not the bulges on the neighboring cell’s membrane, consistent with electron microscopy (Fig. 1 A–C). Furthermore, E-cadherin on the opposing membrane tracks the retracting microspike. Eighty-four percent of microspikes and opposing membranes show this type of dynamics (n = 51 microspikes in 5 cell pairs from 2 experiments) (SI Appendix, Fig. S4A). Therefore, microspikes project cadherins into neighboring cells (Fig. 1E) and indent the opposing membrane in established epithelial sheets, not just in newly forming cell–cell contacts (24, 25).
To better understand the relation between microspikes and cell–cell adhesion, we imaged E-cadherin and actin markers expressed in the same cell. The 2 markers’ intensity profiles along the junction largely overlap, indicating that E-cadherin is present in protruding microspikes (Fig. 3G and Movie S7). Furthermore, the changing pattern of intensity profiles supports that E-cadherin is concentrated into dynamic clusters that can appear or disappear over time in correlation with actin (12).
Microspikes always correlate with the E-cadherin in the same cell (Fig. 3G). However, previous work showed that it is possible for E-cadherin clusters in a trans adhesion to dissolve on only 1 side of the junction (47). Therefore, we wished to know the dynamic relationship between microspikes in 1 cell and cadherin clusters in a neighboring cell. To examine this, we labeled E-cadherin in 1 cell and actin in the neighboring cell. In contrast to the coordinated activities between E-cadherin and actin in the same cell, actin intensity in 1 cell anticorrelates with E-cadherin intensity in the neighboring cell (Fig. 3G, SI Appendix, Fig. S4A, and Movie S7). This suggests that microspikes extend into the gaps between E-cadherin clusters. Their dynamical relation is most easily seen from the intensity profiles which track the behavior of 4 microspikes over time: microspikes at positions 1 and 3 initiated and advanced, while microspikes at positions 2 and 4 were extant and subsequently dissolved (Fig. 3G). Comparing actin intensity to E-cadherin intensity shows that a microspike initiated within ∼20 s of the disappearance of E-cadherin (position 1 at 40–50 s, position 3 at 10–40 s, and position 4 at 0–20 s). In contrast, microspikes disappeared within ∼20 s after filling the gap in E-cadherin intensity (position 2 at 0–30 s and position 4 at 30–40 s). The gap is not filled by merging adjacent E-cadherin clusters as observed when junction is just formed (24) since the clusters remain in their positions (Fig. 3G) (12, 48), but rather by E-cadherin projected by microspikes (Fig. 1E) (25). Therefore, microspikes rise in those sites where cadherin–cadherin homophilic binding interactions are absent or failing, while restoration of cadherin clusters is followed by microspike withdrawal (SI Appendix, Fig. S4B and Movie S8).
Unregulated myosin pulling forces can tear epithelial tissues apart (20, 22); we then tested if contractility causes junction unzipping. Inhibiting myosin activity by blocking myosin light-chain kinase (MLCK) activity with 1-(5-Iodonaphthalene-1-sulfonyl)-1H-hexahydro-1,4-diazepine (ML-7) or myosin II-specific inhibitor blebbistatin rescued membrane detachment and invaginations in EVL-depleted cells that cannot assemble actin protrusions (Fig. 4 A and B and SI Appendix, Fig. S5A). Furthermore, junctional E-cadherin that was down-regulated in EVL-depleted cells can be functionally rescued by blebbistatin (Fig. 4C and SI Appendix, Fig. S5 B and C). Remarkably, blebbistatin also increased junctional E-cadherin in a wild-type background (Fig. 4D). Therefore, myosin II-mediated tension weakens E-cadherin homophilic adhesion, causing junction unzipping (49).
Fig. 4.
Inhibiting myosin II rescues weakened E-cadherin adhesion. (A) Immunofluorescence of membrane unzipping (arrowheads) treated with 25 µM ML-7 for 30 min. Focused at 1.5 µm below apical junction. (Insets) Contrast was rescaled to reveal details. (Scale bar, 5 μm.) (B) n = 120 junctions in each condition; 2-sided Mann–Whitney U test. (C) Apical junctional E-cadherin staining intensity treated with 50 µM (−) blebbistatin for 1 h (n = 44, 75, 36, and 51 junctions; 2-sided Mann–Whitney U test). (D) Apical junctional E-cadherin staining intensity in wild-type cells treated with 50 µM (−) blebbistatin for 1 h, normalized to dimethylsulfoxide (DMSO) (n = 4 experiments, each shown with the mean of ≥30 junctions; 2-sided paired t test). Formaldehyde fix. (Scale bar, 5 μm.) (E) Live images of microspikes (arrows) at the apical junction in wild-type cells treated with 10 µM ML-7 for 22 h (n = 11 junctions of 109 μm in total length for DMSO and 20 junctions of 281 μm for ML-7; 2-sided Mann–Whitney U test). mChe–Utr. (Scale bar, 1 μm.) All charts show mean ± SEM.
Inhibiting EVL-mediated actin protrusions led to higher contractility that unzipped junctions, suggesting that actin protrusions negatively regulate contractility (Fig. 4A). We asked if contractility also negatively regulates microspikes. MLCK inhibition for 30 min did not induce more frequent microspike formation (SI Appendix, Fig. S5D). However, prolonged treatment with the inhibitor ML-7 led to increased microspike formation (Fig. 4E). Cellular actin morphology also changed from the diffusive cortical actin network to the enriched junctional actin after prolonged myosin inhibition, phenocopying the actin morphology in EVL and CRMP-1 overexpressing cells (Fig. 4E and SI Appendix, Fig. S2 E and K). This suggests that 1) once myosin tearing force is inhibited, junction unzipping stops immediately; and 2) contractile actomyosin and protrusive actin regulate each other through competition on a longer time scale at the cellular level.
Discussion
We have identified protruding actin microspikes near apical cell–cell junctions and have shown that cadherin adhesive bonds in cells require continuous actin polymerization. We think the protrusive activity serves as a repair mechanism to quickly repair failing adhesive junctions, and we propose a model for how it might work (Fig. 5). Dissolution of a cadherin cluster in 1 cell triggers the formation of an actin microspike in the neighboring cell. The elongating microspike, which contains E-cadherin, extends toward and indents the plasma membrane of the neighboring cell, providing an opportunity for reengagement of cadherin homophilic bonds. Once adhesion is reestablished, the microspike withdraws, pulling the actin and the cadherins back into the junctional actin belt to strengthen the junction (30, 50). This explains why E-cadherin is correlated with microspikes in the same cell but anticorrelated with microspikes in the adjacent cell. Our model provides a role for continuous actin polymerization in maintaining mature adherens junctions in epithelial cells in a manner analogous to that proposed in endothelial cells (32).
Fig. 5.
A cell–cell adhesion repair model by actin protrusions. Both (i) spontaneous cadherin adhesive unbinding and (ii) myosin-mediated contractility can cause an adhesion failure. Actin microspikes are triggered by signals mediated by either (iii) unbound cadherins or (iv) unknown factors (question mark). Microspikes push cadherin clusters into proximity to promote readhesion.
Inhibiting contractility did not disrupt mature junctions in normal cells. To the contrary, inhibiting contractility rescued failing junctions in cells lacking continuous actin polymerization. We thus propose in our model myosin II-contractile forces as another cause of failing cadherin adhesive bonds. Interestingly, then, cadherin homophilic binding in cells does not really require the actin cytoskeleton. Rather, actin polymerization is necessary to constantly push lateral membranes together because contractile forces are always pulling adhesions apart. Once myosin II is inactivated, then protrusive activity is no longer required.
Protrusive activity at cell–cell borders helps explain the otherwise perplexing biochemistry of junctional actin assembly. Contractile actin networks are usually assembled through formin-mediated actin polymerization reactions, and some formins contribute to junctional actin assembly (9, 13, 14, 27, 51). However, other studies also implicated Arp2/3 as a key actin assembly factor at cadherin cell–cell contacts (2, 3, 35). But Arp2/3 is more closely associated with the assembly of protrusive actin networks, not contractile networks (5). Recent work also identified the actin-filament elongation factors, EVL and CRMP-1, as important for actin assembly near cadherin cell–cell contacts (23). Again, these factors are most closely identified with protrusive actin (23, 52). Some of the Arp2/3-dependent actin assembly at junctions is linked to membrane trafficking (53). Here we show that these factors continue to drive protrusive activity at cell–cell boundaries long after the cells made contact and long after whole cell motility ceased.
Imaging cadherins and actin in living cells shows that cadherin-mediated adhesion is a far more dynamic and active process than previously understood (12, 47). The physiological necessity of mechanically stable cell–cell contacts motivated searches for molecular mechanisms that would stabilize cadherin-mediated adhesion. The cadherin ectodomain alone was sufficient for homophilic binding (38, 54), while experimentally clustering ectodomains in cells could substitute for the cadherin cytoplasmic tail and catenins to mediate strong adhesion (55). The primary function, then, of the actin cytoskeleton was, presumably, to promote cadherin clustering (56), either via a direct linkage to the cadherin–catenin complex (57) or through a corralling mechanism (58). Adhesion was never considered to be static, but the earlier mechanistic work did not predict the fast cadherin–catenin–actin dynamics that operates normally and continuously at cell–cell contacts. A key challenge for the future of adhesion research is to understand the purpose of all these fast actin and cadherin turnover dynamics where actin pulls and pushes on cadherin clusters that are themselves constantly assembling, dissolving, and trafficking to and from the cell surface. Like the actin cytoskeleton itself, cadherin adhesive contacts might be under constant construction. While constant construction requires energy, it would allow cells to rapidly modulate cell–cell adhesion response to signals.
Methods
Live Cell Imaging.
MDCK II cells were transiently transfected with UtrCH to avoid its F-actin stabilizing effect on microspikes (SI Appendix, Fig. S1B). For microspikes, cell sheets were transfected at 90% confluency and imaged in 2–3 d at 1- to 2-s frame intervals on a Zeiss LSM 880 confocal microscope with an Airyscan detector and a 63×, 1.4 numerical aperture objective. UtrCH images were threshold-segmented and processed with the CellGeo software for microspike detection (59). For unzipping, stable E-cadherin–GFP and –mChe transfected cells were imaged 4 d postconfluency at 30-s frame intervals. See SI Appendix, Supplementary Methods.
Supplementary Material
Acknowledgments
We thank J. Kemp, S. McMaster, A. Nadkarni, and H. Yu-Kemp for reagents; D. Barry, A. Belmont, K. Cavanaugh, J. Chen, J. Devany, Z. Fu, S. Hilgenfeldt, D. Leckband, T. Saif, W. Sun, and D. Tsygankov for discussion; A. Cyphersmith, G. Fried, J. Kim, and M. Sivaguru for assistance on imaging; and the National Institutes of Health for funding: R01-GM106106 (to W.M.B.) and R01-DK098398 (to V.W.T.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1908654117/-/DCSupplemental.
References
- 1.Takeichi M., Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 15, 397–410 (2014). [DOI] [PubMed] [Google Scholar]
- 2.Verma S., et al. , Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J. Biol. Chem. 279, 34062–34070 (2004). [DOI] [PubMed] [Google Scholar]
- 3.Tang V. W., Brieher W. M., α-Actinin-4/FSGS1 is required for Arp2/3-dependent actin assembly at the adherens junction. J. Cell Biol. 196, 115–130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coravos J. S., Martin A. C., Apical sarcomere-like actomyosin contracts nonmuscle Drosophila epithelial cells. Dev. Cell 39, 346–358 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blanchoin L., Boujemaa-Paterski R., Sykes C., Plastino J., Actin dynamics, architecture, and mechanics in cell motility. Physiol. Rev. 94, 235–263 (2014). [DOI] [PubMed] [Google Scholar]
- 6.Wickström S. A., Niessen C. M., Cell adhesion and mechanics as drivers of tissue organization and differentiation: Local cues for large scale organization. Curr. Opin. Cell Biol. 54, 89–97 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Kannan N., Tang V. W., Synaptopodin couples epithelial contractility to α-actinin-4-dependent junction maturation. J. Cell Biol. 211, 407–434 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kannan N., Tang V. W., Myosin-1c promotes E-cadherin tension and force-dependent recruitment of α-actinin to the epithelial cell junction. J. Cell Sci. 131, jcs211334 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carramusa L., Ballestrem C., Zilberman Y., Bershadsky A. D., Mammalian diaphanous-related formin Dia1 controls the organization of E-cadherin-mediated cell-cell junctions. J. Cell Sci. 120, 3870–3882 (2007). [DOI] [PubMed] [Google Scholar]
- 10.Verma S., et al. , A WAVE2-Arp2/3 actin nucleator apparatus supports junctional tension at the epithelial zonula adherens. Mol. Biol. Cell 23, 4601–4610 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leerberg J. M., et al. , Tension-sensitive actin assembly supports contractility at the epithelial zonula adherens. Curr. Biol. 24, 1689–1699 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu S. K., et al. , Cortical F-actin stabilization generates apical-lateral patterns of junctional contractility that integrate cells into epithelia. Nat. Cell Biol. 16, 167–178 (2014). [DOI] [PubMed] [Google Scholar]
- 13.Rao M. V., Zaidel-Bar R., Formin-mediated actin polymerization at cell-cell junctions stabilizes E-cadherin and maintains monolayer integrity during wound repair. Mol. Biol. Cell 27, 2844–2856 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acharya B. R., et al. , Mammalian diaphanous 1 mediates a pathway for E-cadherin to stabilize epithelial barriers through junctional contractility. Cell Rep. 18, 2854–2867 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Yonemura S., Wada Y., Watanabe T., Nagafuchi A., Shibata M., alpha-Catenin as a tension transducer that induces adherens junction development. Nat. Cell Biol. 12, 533–542 (2010). [DOI] [PubMed] [Google Scholar]
- 16.Borghi N., et al. , E-cadherin is under constitutive actomyosin-generated tension that is increased at cell-cell contacts upon externally applied stretch. Proc. Natl. Acad. Sci. U.S.A. 109, 12568–12573 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buckley C. D., et al. , Cell adhesion. The minimal cadherin-catenin complex binds to actin filaments under force. Science 346, 1254211 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.le Duc Q., et al. , Vinculin potentiates E-cadherin mechanosensing and is recruited to actin-anchored sites within adherens junctions in a myosin II-dependent manner. J. Cell Biol. 189, 1107–1115 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huveneers S., et al. , Vinculin associates with endothelial VE-cadherin junctions to control force-dependent remodeling. J. Cell Biol. 196, 641–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin A. C., Gelbart M., Fernandez-Gonzalez R., Kaschube M., Wieschaus E. F., Integration of contractile forces during tissue invagination. J. Cell Biol. 188, 735–749 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang V. W., Brieher W. M., FSGS3/CD2AP is a barbed-end capping protein that stabilizes actin and strengthens adherens junctions. J. Cell Biol. 203, 815–833 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jodoin J. N., et al. , Stable force balance between epithelial cells arises from f-actin turnover. Dev. Cell 35, 685–697 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu-Kemp H.-C., Kemp J. P. Jr, Brieher W. M., CRMP-1 enhances EVL-mediated actin elongation to build lamellipodia and the actin cortex. J. Cell Biol. 216, 2463–2479 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams C. L., Chen Y. T., Smith S. J., Nelson W. J., Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J. Cell Biol. 142, 1105–1119 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasioukhin V., Bauer C., Yin M., Fuchs E., Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 100, 209–219 (2000). [DOI] [PubMed] [Google Scholar]
- 26.Smutny M., et al. , Multicomponent analysis of junctional movements regulated by myosin II isoforms at the epithelial zonula adherens. PLoS One 6, e22458 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishimura T., et al. , DAAM1 stabilizes epithelial junctions by restraining WAVE complex-dependent lateral membrane motility. J. Cell Biol. 215, 559–573 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Erami Z., et al. , Intravital frap imaging using an E-cadherin-GFP mouse reveals disease- and drug-dependent dynamic regulation of cell-cell junctions in live tissue. Cell Rep. 14, 152–167 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brevier J., Montero D., Svitkina T., Riveline D., The asymmetric self-assembly mechanism of adherens junctions: A cellular push-pull unit. Phys. Biol. 5, 016005 (2008). [DOI] [PubMed] [Google Scholar]
- 30.Hoelzle M. K., Svitkina T., The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol. Biol. Cell 23, 310–323 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abu Taha A., Taha M., Seebach J., Schnittler H.-J., ARP2/3-mediated junction-associated lamellipodia control VE-cadherin-based cell junction dynamics and maintain monolayer integrity. Mol. Biol. Cell 25, 245–256 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Efimova N., Svitkina T. M., Branched actin networks push against each other at adherens junctions to maintain cell-cell adhesion. J. Cell Biol. 217, 1827–1845 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Paatero I., et al. , Junction-based lamellipodia drive endothelial cell rearrangements in vivo via a VE-cadherin-F-actin based oscillatory cell-cell interaction. Nat. Commun. 9, 3545 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayer A., et al. , Engulfed cadherin fingers are polarized junctional structures between collectively migrating endothelial cells. Nat. Cell Biol. 18, 1311–1323 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kovacs E. M., Goodwin M., Ali R. G., Paterson A. D., Yap A. S., Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 12, 379–382 (2002). [DOI] [PubMed] [Google Scholar]
- 36.Farquhar M. G., Palade G. E., Cell junctions in amphibian skin. J. Cell Biol. 26, 263–291 (1965). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Farquhar M. G., Palade G. E., Junctional complexes in various epithelia. J. Cell Biol. 17, 375–412 (1963). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sivasankar S., Brieher W., Lavrik N., Gumbiner B., Leckband D., Direct molecular force measurements of multiple adhesive interactions between cadherin ectodomains. Proc. Natl. Acad. Sci. U.S.A. 96, 11820–11824 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boggon T. J., et al. , C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296, 1308–1313 (2002). [DOI] [PubMed] [Google Scholar]
- 40.Mattila P. K., Lappalainen P., Filopodia: Molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454 (2008). [DOI] [PubMed] [Google Scholar]
- 41.Bear J. E., et al. , Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Mullins R. D., Heuser J. A., Pollard T. D., The interaction of Arp2/3 complex with actin: Nucleation, high affinity pointed end capping, and formation of branching networks of filaments. Proc. Natl. Acad. Sci. U.S.A. 95, 6181–6186 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanier L. M., et al. , Mena is required for neurulation and commissure formation. Neuron 22, 313–325 (1999). [DOI] [PubMed] [Google Scholar]
- 44.Svitkina T. M., et al. , Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160, 409–421 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee K., Gallop J. L., Rambani K., Kirschner M. W., Self-assembly of filopodia-like structures on supported lipid bilayers. Science 329, 1341–1345 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McMahon H. T., Boucrot E., Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 12, 517–533 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Indra I., et al. , Spatial and temporal organization of cadherin in punctate adherens junctions. Proc. Natl. Acad. Sci. U.S.A. 115, E4406–E4415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cavey M., Rauzi M., Lenne P.-F., Lecuit T., A two-tiered mechanism for stabilization and immobilization of E-cadherin. Nature 453, 751–756 (2008). [DOI] [PubMed] [Google Scholar]
- 49.Sahai E., Marshall C. J., ROCK and Dia have opposing effects on adherens junctions downstream of Rho. Nat. Cell Biol. 4, 408–415 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Biswas K. H., et al. , E-cadherin junction formation involves an active kinetic nucleation process. Proc. Natl. Acad. Sci. U.S.A. 112, 10932–10937 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kobielak A., Pasolli H. A., Fuchs E., Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 6, 21–30 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu-Kemp H.-C., Brieher W. M., Collapsin response mediator protein-1 regulates Arp2/3-dependent actin assembly. J. Biol. Chem. 291, 658–664 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bernadskaya Y. Y., Patel F. B., Hsu H.-T., Soto M. C., Arp2/3 promotes junction formation and maintenance in the Caenorhabditis elegans intestine by regulating membrane association of apical proteins. Mol. Biol. Cell 22, 2886–2899 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brieher W. M., Yap A. S., Gumbiner B. M., Lateral dimerization is required for the homophilic binding activity of C-cadherin. J. Cell Biol. 135, 487–496 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yap A. S., Brieher W. M., Pruschy M., Gumbiner B. M., Lateral clustering of the adhesive ectodomain: A fundamental determinant of cadherin function. Curr. Biol. 7, 308–315 (1997). [DOI] [PubMed] [Google Scholar]
- 56.Hong S., Troyanovsky R. B., Troyanovsky S. M., Binding to F-actin guides cadherin cluster assembly, stability, and movement. J. Cell Biol. 201, 131–143 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rimm D. L., Koslov E. R., Kebriaei P., Cianci C. D., Morrow J. S., Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. U.S.A. 92, 8813–8817 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y., Kanchanawong P., Zaidel-Bar R., Actin-delimited adhesion-independent clustering of E-cadherin forms the nanoscale building blocks of adherens junctions. Dev. Cell 32, 139–154 (2015). [DOI] [PubMed] [Google Scholar]
- 59.Tsygankov D., et al. , CellGeo: A computational platform for the analysis of shape changes in cells with complex geometries. J. Cell Biol. 204, 443–460 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.