Abstract
Objective:
This novel study sought to untangle the association between hydration state and the estrous cycle in the vocal folds, since the voice is reported to negatively change in speakers during the estrous cycle and with dehydration. We hypothesized that there would be alterations in vocal fold tissue morphology depending on hydration state and that these changes would vary with the estrous cycle.
Study Design:
Prospective, in vivo study design.
Methods:
Female Sprague Dawley rats (n=30) were used in this study. Sixteen rats were systemically dehydrated to an average of 10% reduction in body weight by withholding water(range of body weight loss: 8% to 13%). Fourteen rats were assigned to euhydrated, control condition. Estrous stage of female Sprague Dawley rats (n=30) was determined via cytological evaluation of vaginal smears. Following euthanization, larynges were prepared for histological staining with hematoxylin and eosin (HE), Masson’s trichrome and alcian blue (pH 2.5). To quantify hyaluronan, alcian blue staining was completed pre- and post-hyaluronidase incubation. The change in staining percent was quantified with image analysis algorithms and reported as the hyaluronan quantity. Relative collagen distribution (index of dehydration), hyaluronan quantity and tissue morphology were the outcome measures.
Results:
Systemic dehydration was associated with changes in hyaluronan quantity in the rat vocal fold lamina propria. Dehydration did not significantly affect the collagen distribution nor the tissue morphology. Estrous stage alone does not impact the quantity of vocal fold hyaluronan, alter tissue morphology, or change collagen distribution.
Conclusion:
Decreases in hyaluronan quantity in the lamina propria of the rat vocal fold may play a role in tissue fluid balance during systemic dehydration. Future studies will expand this work to investigate additional components of the vocal fold extracellular matrix to fully elucidate the impact of hydration state on the vocal fold.
Keywords: rats, larynx, vocal folds, dehydration, estrous stage, in vivo
1.1. Introduction
Vocal fold dehydration is believed to cause changes in voice, and avoiding periods of dehydration is often clinically recommended [1–4]. Dehydration of the vocal folds is not fully understood, but dehydration of the whole body by 10% of body weight loss also dehydrates the vocal folds [5]. Moreover, systemic dehydration may affect in vivo vocal folds by similar changes in viscoelastic properties that have been observed in excised vocal folds [6]. Vocal fold hydration homeostasis is regulated by proteins, electrolytes, and extracellular matrix macromolecules such as hyaluronan, all of which also impact the tissue’s mobility and pliability [6, 7].
Interestingly, fluid homeostasis in the body is also influenced by hormones. The human larynx is a hormone target in a variety of situations. Sexual maturation and disorders of endocrine organs such as the thyroid, pituitary and adrenal glands can all affect voice parameters [8, 9]. The human vocal fold expresses estrogen and progesterone receptors, as well as androgen receptors [10]. Estrogen and progesterone are the 2 main sex hormones influencing the reproductive cycle of females. Women report a variety of voice changes associated with their menstrual cycle including vocal fatigue, breathiness, loss of high notes, and hoarseness [11]. Vocal fold edema and erythema have also been observed in women at certain points of their menstrual cycle [11]. This study focuses specifically on systemic dehydration and it’s interaction with the ovarian reproductive cycle of females.
Estrogen and progesterone have been reported to influence systemic water homeostasis [12–14]. Estrogen increases the capillary permeability, leading to systemic fluid retention in the interstitial space [15]. The mechanism of fluid retention is unclear; however, one study points to estrogens reducing the osmotic threshold for vasopressin release as well as renal electrolyte balance [15].
In contrast to estrogen, progesterone has been shown to cause increased urinary excretion of sodium and diuresis in women by antagonizing the sodium conserving effects of the mineralocorticoid hormone, aldosterone [13], and the amiloride-sensitive epithelial sodium channel (ENaC) in rat kidneys [14]. Presumably, this water retention and diuresis due to estrogen and progesterone, respectively, could result in changes in the vocal fold biomechanics and affect voice quality in a cyclic manner associated with the menstrual cycle.
All female mammals share the same ovarian reproductive system in which regulatory hormones are released in cyclical fashion and involve the hypothalamus, pituitary gland, and ovaries. The period of the ovarian cycle in which the follicle develops is known as the follicular phase and estrogen is the driving sex hormone during this period [16]. The luteal phase of the ovarian cycle follows ovulation of the follicle and progesterone is the dominant sex hormone during this period. This hormonal cycle of estrogen-ovulation-progesterone is conserved across mammals.
A rat model was selected to examine the role of sex hormones on vocal fold dehydration. The estrous cycle of rats lasts a period of 4–5 days and estrogen and progesterone are the dominant hormones [17]. The rat estrous cycle is divided into 4 phases: proestrus, estrus, metestrus, and diestrus [17]. Proestrus and estrus correspond to the follicular phase; metestrus and diestrus correspond to the luteal phase. The follicular and luteal phases of the ovarian cycle are also observed in women. The rat was used in this study as a model of women’s menstrual cycle because rats share similarities in their sex hormone cycles, have a short estrous cycle, and estrous stage can easily be determined.
The principal focus of this study is to investigate the interaction of systemic dehydration and the female reproductive cycle on the rat vocal fold histology. Body weight change of the individual is the standard method of quantifying systemic dehydration in most studies, regardless of species [18, 19]. Body weight change is highly influenced by confounding variables, e.g. food intake, metabolism, bodily excretion. Renin expression was measured as an additional marker of systemic dehydration. Renin is an enzyme that senses changes in blood volume and is mainly produced and secreted by the kidney. Renin is the first component of renin-angiotensin-aldosterone system (RAAS) and helps the body maintain water balance [20]. Vaginal cytology of rats was used to establish estrous stage [21]. Rats were dehydrated according to established protocols in our laboratory [5]. We compared vocal fold histology in control and dehydrated rats in the follicular and luteal phases for changes in histopathology, hyaluronan quantification, and changes in collagen distribution as a measure of index of dehydration (Histology section 2.4). We hypothesized that there would be alterations in vocal fold tissue morphology with dehydration and that these changes would vary with the estrous cycle.
2.1. Materials and Methods
2.1. Animals
Thirty female Sprague-Dawley rats, 4 months old, acquired from Envigo RMS, Inc. (Indianapolis, IN) were used in this study. Animals were acclimatized for 5–7 days and kept in a temperature and humidity-controlled room with a 12h light/12h dark schedule in the animal facility at Purdue University until the end of the experiment. This study was approved by Purdue Animal Care and Use Committee (PACUC) under the protocol number 1703001551.
2.2. Estrous staging and systemic dehydration
Animals were assigned to two groups: control (euhydrated, n=14) with water ad libitum, or dehydrated (n=16) by water withholding for 72 hours. The estrous stage of each rat was determined by cytological evaluation of vaginal smears collected daily. Based on cytological evaluation, rats were assigned to follicular phase (proestrus/estrus stage; estrogen dominant) and luteal phase (diestrus; progesterone dominant) [21]. The final design had: n=14 euhydrated rats with n=8 follicular phase and n=6 luteal phase; and n=16 dehydrated rats with n=9 follicular phase and n=7 luteal phase. Body weights of all animals were recorded prior to dehydration, (after acclimation period for control animals), and right before euthanasia to assess body weight loss.
2.3. Euthanasia and tissues collection
Animals were euthanized by exposure to carbon dioxide following PACUC guidelines. Larynx of each animal was collected for histopathologic evaluation (section 2.4). For renin analysis, samples of kidney were collected from each animal and immediately transferred to a sterile tube containing RNAlater™ stabilization solution (Invitrogen™, ThermoFisher Scientific Inc., Carlsbad, CA) and transferred to −20 °C for RNA preservation until further analysis (section 2.5.1). In addition, blood was collected from the heart into heparinized tubes for plasma separation. Plasma was kept at −80 °C and used to measure the level of renin using ELISA (section 2.5.2).
2.4. Histology
Larynges were fixed in 10% neutral buffered formalin, dehydrated, and embedded in paraffin. Larynges were prepared for histological staining with hematoxylin and eosin (HE), Masson’s trichrome, and alcian blue (pH 2.5) according to standard procedures. All microscopic slides underwent histological evaluation by a veterinary pathologist who was blinded to treatment conditions (control vs dehydrated) and estrous phase (follicular vs luteal) of samples.
2.4.1. Histopathology
A semi-quantitative scoring system was adapted from previous work in our lab and assessed six morphological aspects: 1) epithelial cell death; 2) mononuclear cell infiltrate; 3) polymophornuclear cell infiltrate; 4) hemorrhage or congestion; 5) collagen distribution; and 6) presence of mucus. The infiltration of cells was graded on four levels: 0, normal; 1, increased cell number in lamina propria; 2, increased cell number in epithelium; and 3, increased cell number in muscle. The collagen distribution was graded on four levels: 0, normal; 1, mild; 2, moderate; and 3, marked. All other items in the scoring were evaluated as normal or present: 0, normal; 1, present. Total semi-quantitative scores were summed for each larynx evaluated (maximum score of 12)
2.4.2. Hyaluronan quantification
To quantify hyaluronan, alcian blue staining was completed on serially sectioned rat vocal folds that underwent pre- and post-hyaluronidase incubation (MilliporeSigma, St. Louis, MO) [22]. Alcian blue stains glycosaminoglycans such as hyaluronan [23]. Hyaluronidase incubation removes hyaluronan from the lamina propria. All alcian blue-stained slides were then digitally scanned with Leica biosystems Aperio CS2 scanner (Aperio Technologies, Vista, CA). Images of the vocal fold midmembranous region were analyzed using Aperio ImageScope software (vl2.3.2.5030). A color deconvolution algorithm was applied to virtual slides. Color deconvolution can separate the blue stain of glycosaminoglycans from surrounding tissue. Hyaluronan of the midmembranous vocal fold lamina propria was quantified by determining the difference in positive alcian blue stain in pre- and post-hyaluronidase treated slides.
2.4.3. Index of dehydration
An index of dehydration was quantified on Masson’s trichrome stained slides that were also digitally scanned with Leica biosystems Aperio CS2 scanner (Aperio Technologies, Visa, CA) and analyzed with Aperio ImageScope software (vl2.3.2.5030). We used the ratio of nonstaining area to total area as a means to compare hydration in the control and dehydrated rat vocal folds. A positive pixel count algorithm detected blue stained areas of collagen in the vocal fold midmembranous lamina propria and normalized the count to the area evaluated. Thus, the lower the index, the more water content separated the collagen fibers of the vocal fold.
2.5. Renin as a dehydration biomarker
Plasma levels and expression (mRNA) of renin were tested as biomarkers of systemic dehydration.
2.5.1. RNA extraction and quantitative polymerase chain reaction for renin mRNA
Total RNA was extracted from kidney samples randomly selected from control (n=4) and dehydrated (n=5) rats using RNeasy®Plus Mini kit (QIAGEN Inc., Germantown, MD) following manufacturer’s instructions.
Specific quantitative polymerase chain reaction (qPCR) primers for renin (NM_012642) and beta-actin (NM_031144.3) (endogenous control) were designed and synthetized by RealTimePrimers (Real Time Primers, LLC, Elkins Park, PA, USA). Sequences of primers used are: Renin - forward 3’-GAT CAG GGA AGG TCA AAG GT-5’, reverse 3’-AGA CAG GGA TGA CTC CAT CA-5’; and Beta-actin - forward 3’-CAC ACT GTG CCC ATC TAT GA-5’, reverse 3’-CCG ATA GTG ATG ACC TGA CC-5’.
A two-step reverse transcriptase-qPCR (RT-qPCR) was performed using Superscript™ IV VILO™ Master Mix (Invitrogen™ Inc.) for cDNA synthesis, and Power SYBR® Green PCR Master Mix (Applied Biosystems® by Life Technologies™, Foster City, CA) for qPCR. The cDNA synthesis was performed with 1 μg of total RNA, and diluted cDNA (1:10) was added as 10% of the final qPCR reaction volume (2.5 μL in 25 μL). The qPCR was run in triplicate/sample following the standard protocol for AmpliTaq Gold® Polymerase (40 cycles of 95 °C for 15 sec, and 60 °C for 1 min) followed by melt curve stage, and carried out in a QuantStudio™ 3 Real-Time PCR System (Applied Biosystems® by Life Technologies™).
Relative expression of renin was calculated by ΔΔCt method [24] using beta-actin as normalizer.
2.5.2. ELISA for plasma renin levels
Plasma renin levels were measured in control and dehydrated rats using RayBio® Rat Renin 1 ELISA Kit (RayBiotech Life, Norcross, GA) following manufacturer’s protocol. Plasma from the same rats tested by RT-qPCR (section 2.5.1) was used to quantify renin levels in the blood. Briefly, whole blood collected into heparin tubes was gently mixed and centrifuged for 10 min at 5,000 g at 4 °C to isolate the plasma. The assay uses a 96-well plate coated with specific antibody for rat renin. Samples (100 μL) and standards were analyzed in duplicates. After samples and standards were incubated for 2.5 h, plate was washed, and biotinylated anti-rat renin 1 antibody was added. After incubation for 1 h and multiple washes, HPR-conjugated streptavidin was added to the wells and incubated for 45 min. Plate was washed again and incubated with TMB substrate reagent for 30 min. The intensity of color developed is proportional to the amount of renin bound. Stop solution was added to each well changing the color from blue to yellow and intensity was immediately measured at 450 nm. Plate was analyzed in a SpectraMax i3x microplate reader (Molecular Devices, LLC., San Jose, CA).
2.6. Statistical Analysis
Statistical analysis was performed using GraphPad Prism version 6.0e for Mac (GraphPad Software, La Jolla, CA). For every comparison, normality tests (D’Agostino-Pearson and Shapiro-Wilk tests) were performed prior to selecting the test statistic. A t-test was used when data passed normality tests, and Mann-Whitney nonparametric test was used for small or unbalanced sample sizes. For histopathology observations, comparisons were done between control (n=14) versus dehydrated (n=16) groups. Within the dehydration group, further comparisons were made between follicular (n=9) and luteal (n=7) phase groups. Likewise, within the control group, further comparisons were made between the follicular (n=8) and luteal (n=6) groups. The renin mRNA expression and plasma levels were compared between control (n=4) and dehydrated (n=5) rats. The alpha-value was corrected because of the multiple comparison being used. Thus, differences between groups were considered statistically significant when p≤0.03. A summary of statistical analyses is provided in Table 1.
Table 1.
Summary of data analyzed, statistical tests, and results.
| Data | Comparison between groups (n) | Passed normality test† | Statistical test£ | P value | T value§ | DF§ | U value¶ | Results |
|---|---|---|---|---|---|---|---|---|
| RT-qPCR Renin | Control (4) vs. Dehydrated (5) | no | Mann-Whitney | 0.0159 | N/A | N/A | 0 | * |
| ELISA Renin | Control (4) vs. Dehydrated (5) | no | Mann-Whitney | 0.0159 | N/A | N/A | 0 | * |
| Hyaluronan quantification | 1. Control (14) vs. Dehydrated (16) | yes | t-test | 0.03 | 2.287 | 28 | N/A | * |
| 2. Follicular phase: control (8) vs. dehydrated (9) | yes | t-test | 0.1854 | 1.388 | 15 | N/A | ns | |
| 3. Luteal phase: control (6) vs. dehydrated (7) | no | Mann-Whitney | 0.1375 | N/A | N/A | 10 | ns | |
| 4. Dehydrated: follicular (9) vs. luteal (7) | no | Mann-Whitney | 0.6556 | N/A | N/A | 27 | ns | |
| 5. Control: follicular (8) vs. luteal (6) | no | Mann-Whitney | 0.9291 | N/A | N/A | 23 | ns | |
| Tissue morphology - HE semi-quantitative score | 1. Control (14) vs. Dehydrated (16) | no | Mann-Whitney | 0.3751 | N/A | N/A | 91 | ns |
| 2. Follicular phase: control (8) vs. dehydrated (9) | no | Mann-Whitney | >0.9999 | N/A | N/A | 36 | ns | |
| 3. Luteal phase: control (6) vs. dehydrated (7) | no | Mann-Whitney | 0.2005 | N/A | N/A | 12 | ns | |
| 4. Dehydrated: follicular (9) vs. luteal (7) | no | Mann-Whitney | 0.8899 | N/A | N/A | 28.5 | ns | |
| 5. Control: follicular (8) vs. luteal (6) | no | Mann-Whitney | 0.2088 | N/A | N/A | 15 | ns | |
| Index of dehydration | 1. Control (14) vs. Dehydrated (16) | yes | t-test | 0.0673 | 1.904 | 28 | N/A | ns |
| 2. Follicular phase: control (8) vs. dehydrated (9) | yes | t-test | 0.0945 | 1.785 | 15 | N/A | ns | |
| 3. Luteal phase: control (6) vs. dehydrated (7) | no | Mann-Whitney | 0.5245 | N/A | N/A | 16 | ns | |
| 4. Dehydrated: follicular (9) vs. luteal (7) | no | Mann-Whitney | 0.5848 | N/A | N/A | 26 | ns | |
| 5. Control: follicular (8) vs. luteal (6) | no | Mann-Whitney | 0.1399 | N/A | N/A | 12 | ns |
D’Agostino-Pearson and Shapiro-Wilk normality tests were applied.
Statistical tests were performed using GraphPad Prism version 6.0e for Mac.
T value and degrees of freedom (DF) calculated for t-test
Mann-Whitney U value calculated for Mann-Whitney rank test. The smallest possible value of U is zero. N/A: not applicable
Results: P value ≤ 0.03; ns: non-significant
3.1. Results
3.1. Estrous stage and systemic dehydration
Control (euhydrated) rats did not show body weight loss during the course of the study, while dehydrated rats had an average body weight loss of −11.4% (range of 8% to 13%) after 72 hours of water deprivation. The expression of renin mRNA in the kidney of dehydrated rats was significantly increased by 2 fold change compared to control rats (p=0.0159).
In addition, plasma levels of renin were also increased in the dehydrated group with a median of 322.3 pg/mL in contrast to 160.4 pg/mL found in the control animals (p=0.0159). These results support that systemic dehydration was achieved, and indicate that both renin mRNA expression and plasma renin are adjunct markers of systemic dehydration in rats subjected to 72 hours of water withholding.
3.2. Histology
The quantity of hyaluronan in the rat vocal folds was significantly lower for dehydrated rats, (Figure 1). Hyaluronan quantification was based on analysis of digitized images of alcian blue (pH 2.5) stained slides pre- and post-hyaluronidase incubation. No significant differences were observed for tissue morphology or the index of dehydration between control and dehydrated groups (Figure 2 and 3). The reduction in quantity of hyaluronan following dehydration did not appear to be influenced by estrous stage. The specific estrus phase (follicular vs. luteal) did not influence other dependent measures either. Representative photomicrographs from each histological parameter are represented in Figure 4.
Figure 1. Hyaluronan quantification.
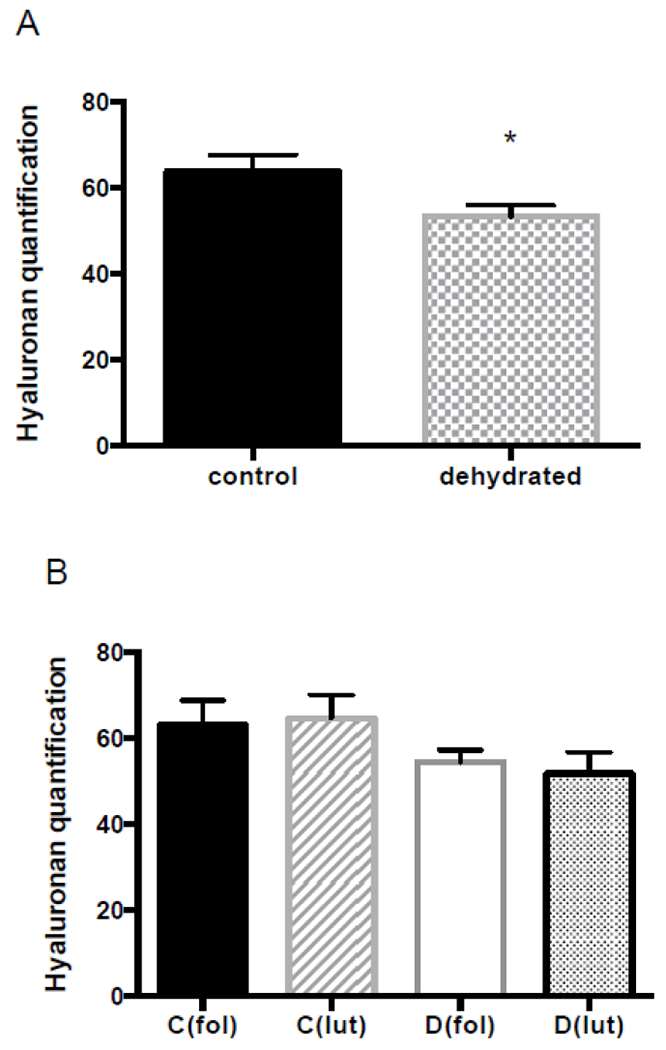
(A) Control versus dehydrated group (72h water withholding). (B) Control and dehydrated groups divided into follicular and luteal phase groups. Comparisons were performed as follows: C(fol) vs. C(lut), D(fol) vs. D(lut), C(fol) vs. D(fol), and C(lut) vs. D(lut). No significant differences were observed. Graphics show mean with standard error of the mean (SEM). *Significant difference with p≤0.03.
Abbreviations: C, control group; D, dehydrated group; fol, follicular phase; and lut, luteal phase.
Figure 2. Tissue morphology score.
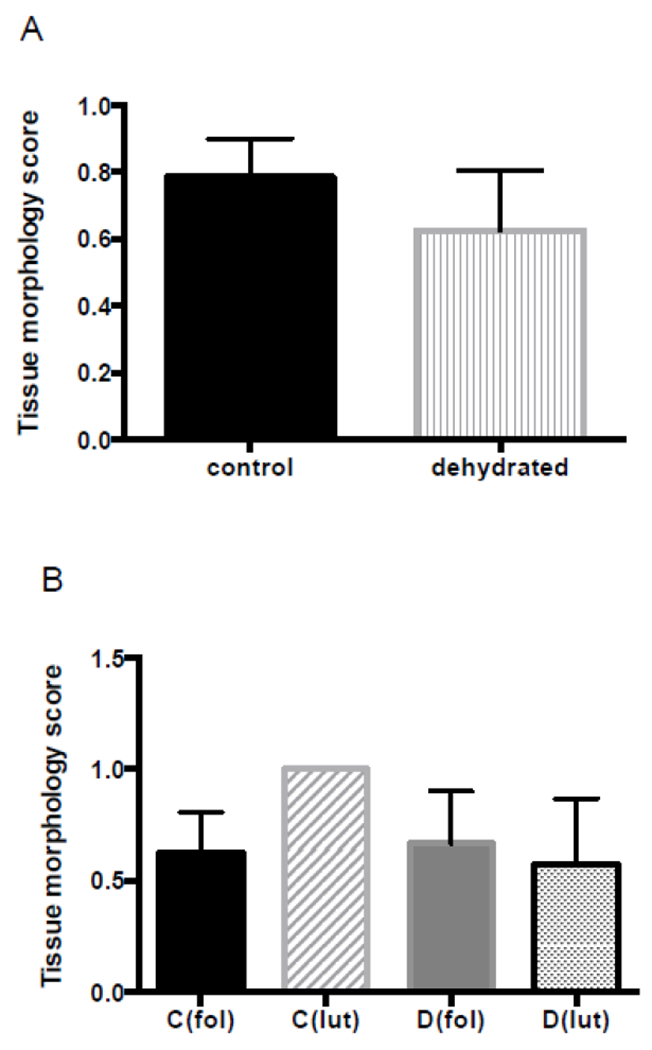
(A) Control versus dehydrated group (72h water withholding). (B) Control and dehydrated groups divided into follicular and luteal phase groups. Comparisons were performed as follows: C(fol) vs. C(lut), D(fol) vs. D(lut), C(fol) vs. D(fol), and C(lut) vs. D(lut). No significant differences were observed. Graphics show mean with SEM.
Abbreviations: C, control group; D, dehydrated group; fol, follicular phase; and lut, luteal phase.
Figure 3. Index of dehydration (relative collagen distribution).
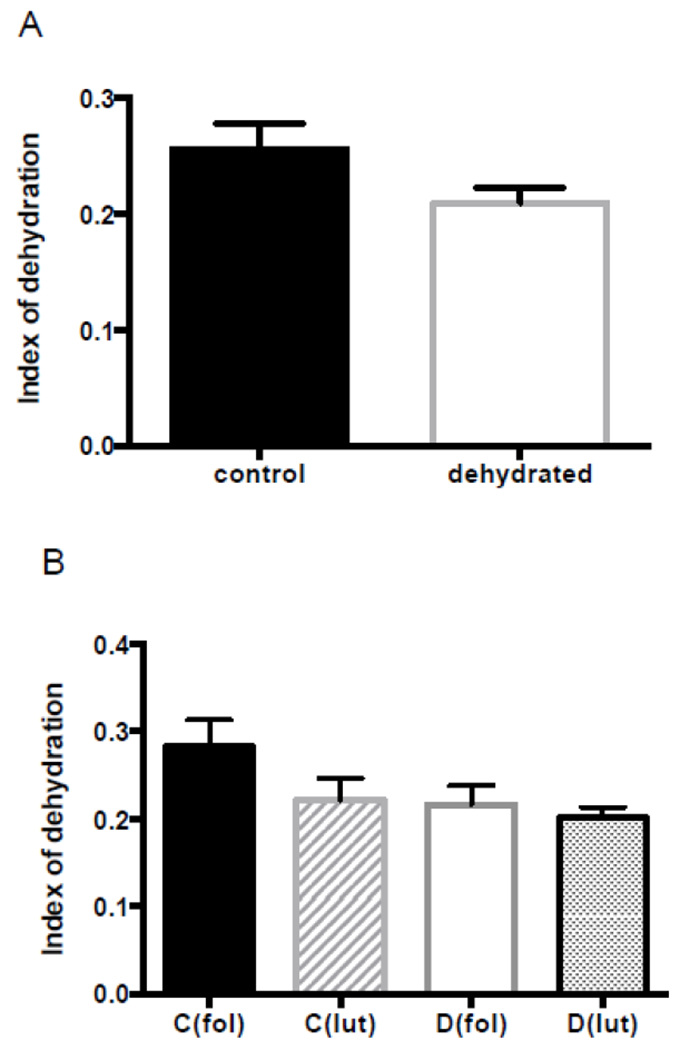
(A) Control versus dehydrated group (72h water withholding). (B) Control and dehydrated groups divided into follicular and luteal phase groups. Comparisons were performed as follows: C(fol) vs. C(lut), D(fol) vs. D(lut), C(fol) vs. D(fol), and C(lut) vs. D(lut). No significant differences were observed. Graphics show mean with SEM.
Abbreviations: C, control group; D, dehydrated group; fol, follicular phase; and lut, luteal phase.
Figure 4. Representative images of vocal fold histology.
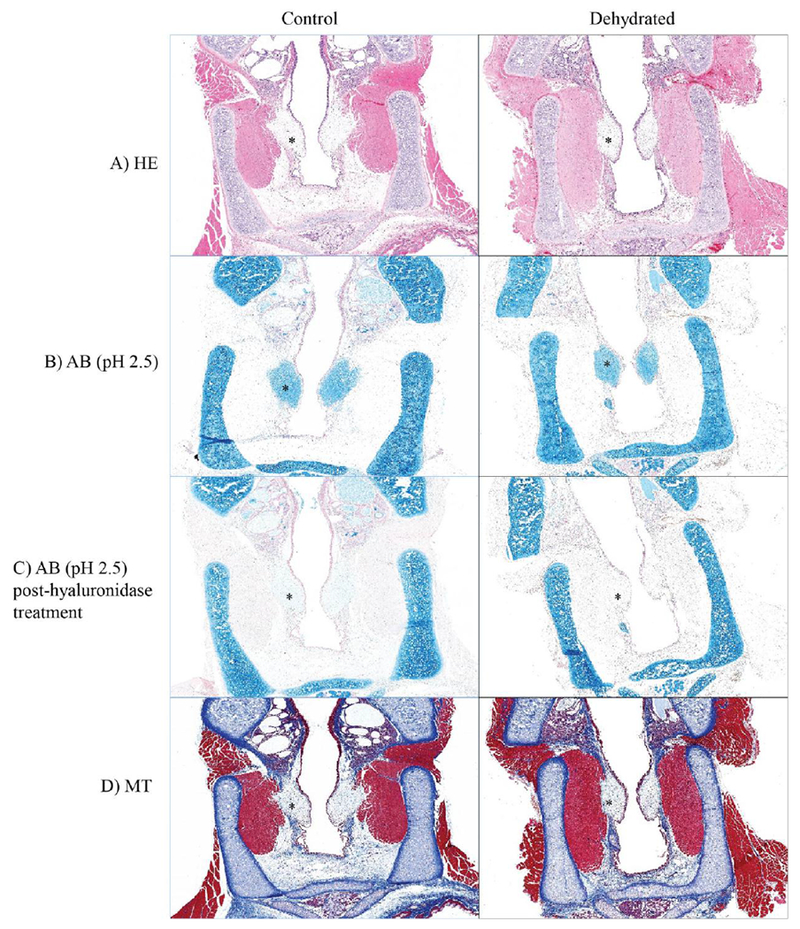
Left: vocal folds in the control group. Right: vocal folds in the dehydrated group. (A) Hematoxylin and eosin-stained photomicrograph of vocal folds. (B) Alcian blue (pH 2.5) staining. (C) Alcian blue (pH 2.5) staining post-hyaluronidase treatment. (D) Masson’s trichrome staining demonstrating collagen distribution in the vocal folds. *Vocal fold.
4.1. Discussion
This study investigated the interaction of acute systemic dehydration and female sex hormones on the rat vocal fold. The novelty of this study is imparted by the use of an animal model in which the female reproductive stage could accurately be determined in collaboration with study conditions inducing systemic dehydration. The knowledge that voice is altered by hydration status as well as hormonal status, especially in women, invoked a hypothesis driven study to determine the interplay of the 2 conditions (dehydration and estrous stage). Ultimately, we found that the effects of dehydration impacted hyaluronan content in the rat vocal fold lamina propria; and that the estrous stage alone did not influence the hyaluronan content or observable morphologic changes in the vocal folds of rats.
Hyaluronan is a high molecular weight glycosaminoglycan thought to play a critical role in regulating hydration changes related to viscosity and lubrication [25]. Hyaluronan also resists the flow of water through connective tissue [26]. The dual role of hyaluronan hydration homeostasis and viscoelastic properties of the vocal folds suggest it may have a major role in the change in biomechanics identified with dehydration.
This study identified a decrease of hyaluronan in the dehydrated rat vocal fold compared to control. The role of hyaluronan in resisting water flow is likely at work in the dehydrated group. Research has shown that hyaluronan content of the rat renal medulla is decreased during dehydration [27–29]. The authors’ suggest that the reduction in hyaluronan concentration increases the diffusion of water into the tissue, an important physiological response to regulate tissue hydration during systemic dehydration. The rat vocal fold may be similarly responding to the systemic dehydration by promoting water diffusion into the lamina propria by decreasing the hyaluronan concentration. It should be noted that the magnitude of systemic dehydration induced in this study (>10% reduction in body weight loss) was a marked reduction in water. This high level of whole body dehydration has been shown to dehydrate the vocal fold tissue on proton-density weighted MRI analysis [5]. However, everyday levels of dehydration that speakers may be routinely exposed to arise from a 1–2% reduction in body weight. The effects of a lower level of systemic dehydration on vocal fold hyaluronan quantification awaits further study.
It is noteworthy that neither the tissue morphology based on the semi-quantitative scoring, nor the index of dehydration based on the quantification of collagen distribution changed after systemic dehydration. Dehydration is likely affecting viscoelastic properties of the vocal fold tissue; however, not necessarily by altering the quantity of specific tissue components. In other words, the lack of water alone affects the tissue’s optimal function. Additionally, vocal fold hydration involves systemic, local, and surface mechanisms that may impact the effects of hydration fluctuation.
Establishing evidence of direct effect of sex hormone cycles on the vocal folds requires histologic investigation. In this study, the phase of ovarian cycle did not alter rat vocal fold histology. Moreover, we did not identify an interaction of dehydration and ovarian cycle. Estrogen is known to influence vascular permeability, and thus water retention in interstitial fluid [15]; while progesterone promotes sodium excretion and diuresis [13, 14]. The lack of histologic changes due to estrous cycle alone, as well as no changes identified with the interaction of dehydration and estrous cycle, suggest alternative mechanisms of hydration homeostasis. As mentioned above, vocal fold hydration involves systemic, local, and surface mechanisms that effect hydration status. Continued work is necessary to determine if hormonal cycle impacts function, regardless of lack of histologic changes.
An important component of this study is the validation of an additional dehydration marker. Body weight loss is considered the gold standard of dehydration. In this study we demonstrated that renin expression increased after dehydration. Renin is an enzyme mainly produced and secreted by the kidney and is the first component of renin-angiotensin-aldosterone system (RAAS), a vital system of human and animal bodies that regulates blood pressure, electrolyte homeostasis, and water balance [30]. The use of renin as a biomarker of dehydration therefore has potential as an adjunct confirmation that dehydration has been achieved.
5.1. Conclusion
This study utilized a variety of histological approaches to investigate pathological changes to the vocal folds after systemic dehydration. Moreover, we examined the interaction of the female estrous cycle in driving the vocal fold changes after systemic dehydration. Our findings support the role of hyaluronan in water homeostasis of the rat vocal fold, and conclude that estrous stage is not a contributing factor in this outcome.
Acknowledgements
We would like to thank Jessica Engen and Christopher Maps for their expertise in rat handling and data collection, and members of the Histology Research Laboratory. Details of the impact estrous cycle and dehydration have on the rat larynx were presented in a scientific presentation at The Voice Foundation Annual Symposium in Philadelphia, PA in May 2019. This study was funded by R01DC015545 (National Institutes of Health/National Institute on Deafness and other Communication Disorders).
Funding:
Funding was provided from R01DC015545 (National Institutes of Health/National Institute on Deafness and other Communication Disorders).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None
References
- 1.Sivasankar M and Leydon C, The role of hydration in vocal fold physiology. Current opinion in otolaryngology & head and neck surgery, 2010. 18(3): p. 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verdolini-Marston K, Titze IR, and Druker DG, Changes in phonation threshold pressure with induced conditions of hydration. Journal of voice, 1990. 4(2): p. 142–151. [Google Scholar]
- 3.Verdolini-Marston K, Sandage M, and Titze IR, Effect of hydration treatments on laryngeal nodules and polyps and related voice measures. Journal of Voice, 1994. 8(1): p. 30–47. [DOI] [PubMed] [Google Scholar]
- 4.Hartley NA and Thibeault SL, Systemic hydration: relating science to clinical practice in vocal health. Journal of Voice, 2014. 28(5): p. 652 el-652. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oleson S, et al. , Proton density-weighted laryngeal magnetic resonance imaging in systemically dehydrated rats. The Laryngoscope: p. E222–E227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan RW and Tayama N, Biomechanical effects of hydration in vocal fold tissues. Otolaryngology-Head and Neck Surgery, 2002. 126(5): p. 528–537. [DOI] [PubMed] [Google Scholar]
- 7.Chan RW, et al. , Relative contributions of collagen and elastin to elasticity of the vocal fold under tension. Annals of biomedical engineering, 2007. 35(8): p. 1471–1483. [DOI] [PubMed] [Google Scholar]
- 8.Brodnitz FS, Hormones and the human voice. Bulletin of the New York Academy of Medicine, 1971. 47(2): p. 183. [PMC free article] [PubMed] [Google Scholar]
- 9.Nygren U, et al. , Voice problems due to virilization in adult women with congenital adrenal hyperplasia due to 21 - hydroxylase deficiency. Clinical endocrinology, 2013. 79(6): p. 859–866. [DOI] [PubMed] [Google Scholar]
- 10.Kirgezen T, et al. , Sex hormone receptor expression in the human vocal fold subunits. Journal of Voice, 2017. 31(4): p. 476–482. [DOI] [PubMed] [Google Scholar]
- 11.Abitbol J, et al. , Does a hormonal vocal cord cycle exist in women? Study of vocal premenstrual syndrome in voice performers by videostroboscopy-glottography and cytology on 38 women. Journal of Voice, 1989. 3(2): p. 157–162. [Google Scholar]
- 12.Stachenfeld NS and Taylor HS, Effects of estrogen and progesterone administration on extracellular fluid. Journal of Applied Physiology, 2004. 96(3): p. 1011–1018. [DOI] [PubMed] [Google Scholar]
- 13.Landau RL and Lugibihl K, Inhibition of the sodium-retaining influence of aldosterone by progesterone. The Journal of Clinical Endocrinology & Metabolism, 1958. 18(11): p. 1237–1245. [DOI] [PubMed] [Google Scholar]
- 14.Gambling L, et al. , Estrogen and progesterone regulate α, β, and yENaC subunit mRNA levels in female rat kidney. Kidney international, 2004. 65(5): p. 1774–1781. [DOI] [PubMed] [Google Scholar]
- 15.Stachenfeld NS, et al. , Estrogen influences osmotic secretion of AVP and body water balance in postmenopausal women. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 1998. 274(1): p. R187–R195. [DOI] [PubMed] [Google Scholar]
- 16.Schillo KK, Reproductive physiology of mammals: from farm to field and beyond. 2009: Delmar Publishers. [Google Scholar]
- 17.Sato J, Nasu M, and Tsuchitani M, Comparative histopathology of the estrous or menstrual cycle in laboratory animals. Journal of toxicologic pathology, 2016. 29(3): p. 155–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armstrong LE, Assessing hydration status: the elusive gold standard. Journal of the American College of Nutrition, 2007. 26(sup5): p. 575S–584S. [DOI] [PubMed] [Google Scholar]
- 19.Bekkevold CM, et al. , Dehydration parameters and standards for laboratory mice. Journal of the American Association for Laboratory Animal Science, 2013. 52(3): p. 233–239. [PMC free article] [PubMed] [Google Scholar]
- 20.Peters J, Secretory and cytosolic (pro) renin in kidney, heart, and adrenal gland. Journal of Molecular Medicine, 2008. 86(6): p. 711–714. [DOI] [PubMed] [Google Scholar]
- 21.Cora MC, Kooistra L, and Travlos G, Vaginal cytology of the laboratory rat and mouse: review and criteria for the staging of the estrous cycle using stained vaginal smears. Toxicologic pathology, 2015. 43(6): p. 776–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray SD, et al. , Vocal fold proteoglycans and their influence on biomechanics. The Laryngoscope, 1999. 109(6): p. 845–854. [DOI] [PubMed] [Google Scholar]
- 23.Carson FL and Hladik C, Histotechnology: a self-instructional text. Vol. 96 1997: Ascp press Chicago:. [Google Scholar]
- 24.Livak KJ and Schmittgen TD, Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method, methods, 2001. 25(4): p. 402–408. [DOI] [PubMed] [Google Scholar]
- 25.Laurent TC, Laurent U, and Fraser J, Functions of hyaluronan. Annals of the rheumatic diseases, 1995. 54(5): p. 429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Comper WD and Laurent TC, Physiological function of connective tissue polysaccharides. Physiological reviews, 1978. 58(1): p. 255–315. [DOI] [PubMed] [Google Scholar]
- 27.Göransson V, et al. , Renomedullary and intestinal hyaluronan content during body water excess: a study in rats and gerbils. The Journal of physiology, 2002. 542(1): p. 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hansell P, et al. , Hyaluronan content in the kidney in different states of body hydration. Kidney international, 2000. 58(5): p. 2061–2068. [DOI] [PubMed] [Google Scholar]
- 29.Rügheimer L, et al. , Hyaluronan synthases and hyaluronidases in the kidney during changes in hydration status. Matrix Biology, 2009. 28(7): p. 390–395. [DOI] [PubMed] [Google Scholar]
- 30.Brown MJ, Renin: friend or foe? Heart, 2007. 93(9): p. 1026–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]


