Introduction
Optical coherence tomography OCT is an integral part of contemporary eye care, providing high-resolution, cross-sectional imaging of ocular structures in the clinic. These cross-sectional images are used to reveal changes in ocular micro-anatomy and are also used for quantitative analysis and comparison.1
However, beginning with the first reports of OCT,2 there has been a literal physical divide between OCT’s use for the front of the eye3 and the back of the eye.4,5 Namely, an anterior segment OCT system in its native state cannot be used to image the retina well, and conversely, a retinal OCT system in its native state cannot be used to image the anterior segment well. Without changes to the imaging optics of the system and necessary related configuration changes, each system is limited to its particular region of the eye.
Because of this physical separation in imaging capabilities, OCT is limited in its use as a comprehensive scanner for the entire eye. A straightforward clinical example is the ocular biometry needed for cataract surgery. An anterior segment OCT system alone or a retinal OCT system alone cannot provide needed global ocular biometric values such as axial length. Similarly, for a patient with high myopia, a retinal OCT system provides information about myopic maculopathy and pathologic myopia6–9 but cannot provide the axial length of the eye – a major associated risk for posterior eye changes in myopia. In a narrow angle glaucoma patient, an anterior segment OCT system provides information about the angle configuration10–13 but cannot show the retinal nerve fiber layer thickness data needed to assess glaucoma status. In clinical research, to study ocular shape deformations, “regular” OCT systems provide the source images but cannot provide the ocular biometric parameters needed to perform spatial correction of the OCT images.14
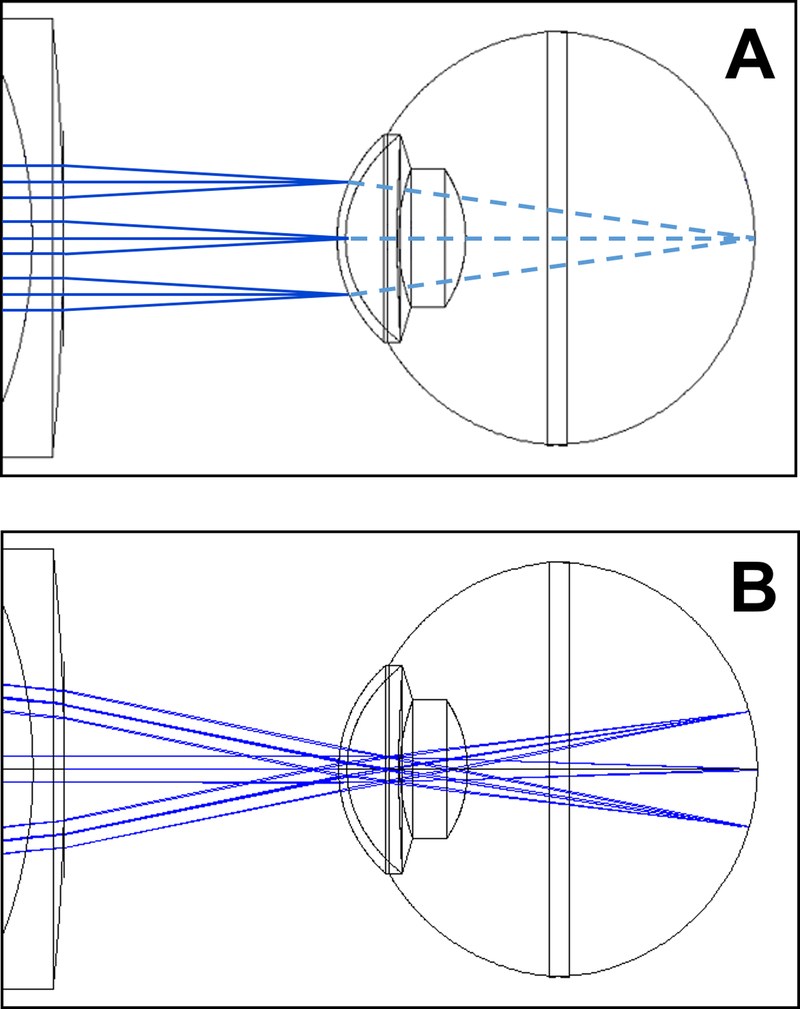
The reasons for the physical separation between anterior segment and retinal OCT systems are technical in nature. There are fundamental differences in designing an imaging system for the front and back of the eye (Fig. 1). For an anterior segment system, light exiting the system only has to pass through air and focus on the first structure it encounters – the cornea. In contrast, for retinal systems, light exiting the system has to pass through multiple, variably refractive structures such as the cornea and lens before focusing on its target structure – the retina. The scanning geometry is also different for anterior segment and retinal OCT. To image the cornea from limbus to limbus, the OCT system only needs to scan light from side to side. The same would not work for the retina because the eye is designed to focus parallel rays of light onto the fovea; a retinal system that scans light from side to side would result only in an image of the fovea (Fig. 1A, dashed lines). Instead, to see more of the macula beyond just the fovea, the retinal scanner has to pivot light through the pupil in a fan shaped configuration.
FIGURE 1. Differences in OCT scanning between anterior segment (A) and retina (B).
In each image, there are three example paths in solid blue representing a middle and two edge A scans. For anterior segment scanning (A), the scan proceeds from side to side (up and down in this image) and is focused on the anterior segment. Continuing on (illustrated as dashed light blue lines), these paths then converge on the same spot at the retina. Because the light for each A scan was focused at the anterior segment, they will be defocused at the retina. If recorded, the resulting retinal image would be a blurry image on one local spot at the retina, usually the foveal region. In regular retinal OCT scanning (B), The paths converge at the pupil and then fan out to scan the retina from side to side. The optics are also designed to focus light at the retina. These differences between the two systems make it not straight-forward to image both the anterior segment and the retina well in a single scan.
In addition to these scanning differences, OCT is an interferometric imaging technique, meaning the reference arm and sample have to be matched in optical path length. To produce interferometric data from both the front and back of the human eye, greater than 20 mm of reference and sample arm matching is required. A typical contemporary spectral domain OCT system only generates interferometric data of sufficient signal to noise ratio within 2 mm of the reference arm, far short of what is needed to see both ends of the eye.
There have been multiple efforts in recent years to try to overcome these technical challenges and bridge the divide between anterior segment OCT and retinal OCT to create “whole eye” OCT systems. These can be broadly categorized as sequential systems (take an anterior segment image first and then a retinal image) and simultaneous systems (anterior segment and retinal image acquired at same time).
Sequential Anterior/Posterior OCT Systems
The general premise of these systems is to first take an image in one area, alter the system configuration, and then take a second image in the second area. Current commercial OCT systems can do this to some degree already. For example, the Heidelberg Spectralis, Optovue iVue, and Leica (Bioptigen) C series can all take a retinal OCT image (the usual configuration) then enable anterior segment imaging by physically replacing the terminal lens set from the system, appropriately changing the software and reference arm, and taking the anterior segment OCT image. In a less cumbersome manner, a Zeiss Cirrus can internally perform the terminal lens exchange and associated configuration changes depending on whether a corneal or retinal scan is selected. The primary disadvantage of performing whole eye imaging in this fashion is the large separation in time between the anterior segment OCT and retinal OCT images. This separation introduces ambiguities in the spatial relationship between the anterior segment and retinal images. For instance, the eye may be oriented differently during the two captures so that the two images do not share an axis and hence do not line up properly; the physical separation between the images (needed to determine axial length) can also be ambiguous without complete knowledge of the imaging system and conditions.
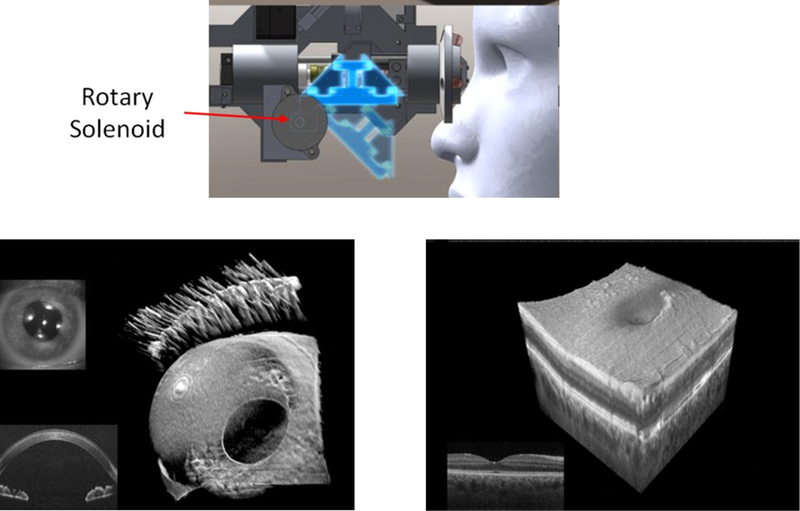
Faster research versions of this sequential imaging have been described where upon scanning, the system first acquires an anterior segment image or volume and can then rapidly switch itself to a retinal configuration to take the retinal image or volume (Fig. 2). An early version used an optical switch to transition between three reference arm lengths appropriate for anterior chamber, posterior chamber, and retinal distances. However, no imaging optics were changed, and hence only the anterior segment image was in focus with only a small, unfocused area of the retina imaged.15 Subsequent versions incorporated appropriate matching of imaging optics as well with the physical switching either involving automatic addition of a different set of optics16 or using a tunable lens to change the area of focus from the anterior segment to the retina.17 Interlaced versions which can alternate frames of the volume between the anterior segment and retina have also been described.18,19 The rapid interlacing is accomplished by having combined anterior segment and retinal OCT sample and reference arms and using polarization to switch between the two paths (described in more detail in the simultaneous sections below); a single spectrometer is used to detect the rapidly switched signals. These faster sequential versions mitigate some of the mismatches that may occur with asynchronous acquisition of the anterior segment and retinal OCT images. However, to truly ensure the relationship between the anterior segment and retinal OCT images or to try to capture dynamic processes involving both parts of the eye, simultaneous whole eye OCT systems are needed.
FIGURE 2. Example of a sequential anterior/posterior OCT system.16.
This research system mechanically switches between either an anterior segment optical path (dark blue position) or a posterior segment optical path (lighter blue position) using a rotary solenoid. The switch in optical paths engages different optics to produce either an anterior segment volume (bottom left) or posterior segment volume (bottom right).
Whole Eye (Simultaneous Anterior/Posterior) OCT Systems
Whole eye OCT systems simultaneously take images of both the anterior segment and retina at the same time to produce a true comprehensive eye OCT scanner. These whole eye OCT systems range from the straightforward – literally combining two separate anterior and posterior segment OCT systems together – to more sophisticated single systems capable of imaging the whole eye at once. Below, these systems are detailed along with their particular strengths and weaknesses.
Two System Combinations
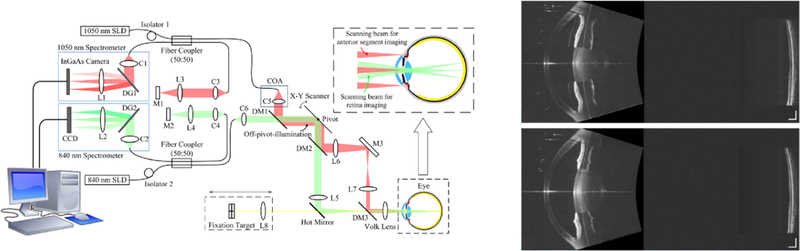
One way to OCT scan both the anterior segment and the retina at the same time is to use both an anterior segment and retinal OCT system at the same time.20,21 In practice, this means two sets of scanning optics, two light sources, and two reference arms; the two systems must then merge at a single set of shared terminal optics to scan the patient (Fig. 3). The two light sources (or comparable ideas such as using two bands from a single wideband source) are necessary to ensure that one source of light goes to the anterior segment and the other goes to the retina. Ideally, there should be some form of filtering to prevent light from the anterior channel from crossing over to the posterior channel and vice versa which can cause complications in sending/receiving the light and producing the image.
FIGURE 3. Example of a two system combination, whole eye OCT system.21.
In this research system, there are effectively two separate OCT systems running simultaneously that share a final optic before the eye. In the system diagram on the left, the red path is a longer wavelength anterior segment system with optical path appropriate for focusing on the anterior segment. The green path is a shorter wavelength retinal system with optics designed to scan and focus on the retina. Representative images from this system are shown on the right (top is an eye undergoing accommodation with resultant smaller pupil and thicker lens while the bottom is the same eye dis-accommodated).
The benefits of a two system combination include flexibility in choosing the two light sources as in the system shown in Fig. 3. For water absorption reasons, relatively longer wavelengths are typically better for OCT scanning the anterior segment of the human eye while relatively shorter wavelengths are typically better for OCT scanning of the retina. In a two system combination, a > 1000 nm centered source could be used for the anterior segment while a < 1000 nm centered source could be used for the retina.21 The overall system designs for an anterior segment and retinal OCT system are also well established and need only to meet at a shared set of terminal optics.
The drawbacks of a two system combination include effectively doubled costs for having two OCT systems. Additionally, these two separate OCT systems need to be digitally synchronized to ensure simultaneous acquisition of scans and appropriate pairing of the anterior segment and retinal OCT images.
One System, One Single Long Imaging Depth
Another way to capture a whole eye OCT image is to take a single, long scan through the entire eye. However, as discussed earlier, contemporary commercial spectral domain OCT systems typically only have an axial imaging depth of 2 mm due to limitations inherent to the light sources used and the processing used to create the image. Advances in OCT light sources and associated engine design have removed some of these axial imaging constraints, though. These include swept source lasers and most notably, vertical cavity surface emitting lasers (VCSELs).22 Though VCSEL based OCT systems have currently only been described in research systems, they uniquely can have meter long coherence lengths. In practice, this means the 20+ mm length of the eye is well within the range of a VCSEL system. Images from research VCSEL systems have been used to measure ocular biometry such as those needed for cataract surgery.23,24
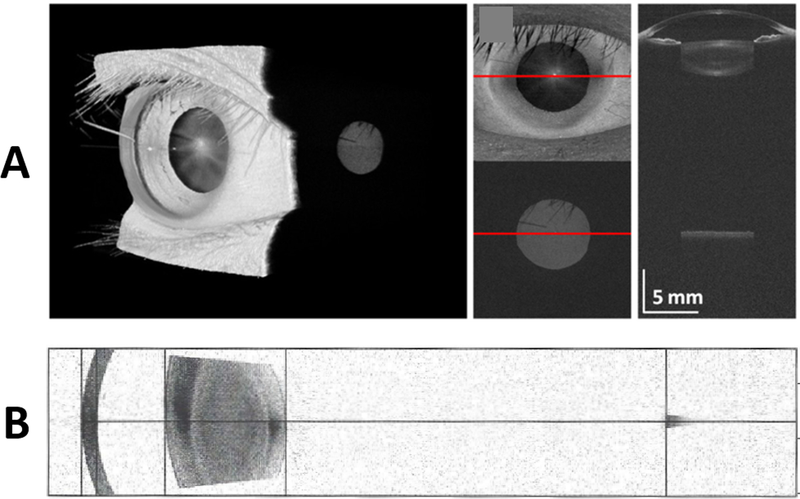
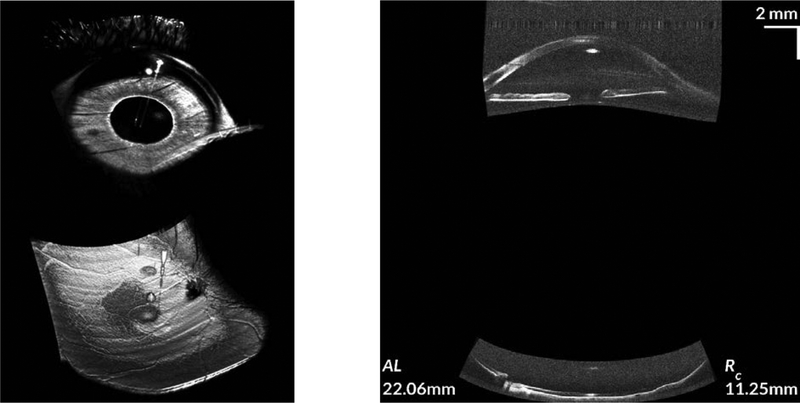
Similarly, though with less coherence length than VCSELs, there are swept source laser based OCT systems that have been commercially developed to measure ocular biometry. These include the clinically available Santec Movu and the Zeiss IOLMaster 700 OCT systems. These systems produce a full image of the anterior segment and then have sufficient imaging range to reach the retina (Fig. 4). As can been seen from Fig. 4, axial dimensions (corneal thickness, anterior chamber depth, axial length) can be directly measured from the single long depth image.
FIGURE 4. Images from long imaging depth OCT scanners.
A. The top row is from a research VCSEL based system with sufficient coherence length to image from the front to back of the eye.23 B. The bottom row is from a commercial swept-source OCT system (Zeiss IOLMaster 700). This more conventional swept-source system is designed to have a long imaging depth which allows it to image the cornea, phakic lens, and retina (small darker grey region on right of image in row B) in one single image.
Notice that in both these long imaging depth systems, the image of the retina (small grey disc in row A, small grey area on right in row B) is localized to the fovea and is laterally limited compared to a conventional retinal OCT image. The reason for this is due to the anterior segment scanning geometry (example in Fig. 1A) used by this these systems which limits imaging on the retina.
While these systems are mature enough to be commercially available, the primary disadvantage is the retinal field of view. Because of the anterior segment scanning geometry used by these systems, each A scan ends up imaging a single localized area on the retina. This retinal image is far smaller in lateral extent than that from a conventional retinal OCT system. Additionally, there are also sacrifices in focus given the long depth of the system (focused spot size is inversely related to the depth of focus). As a result, the retinal layer detail is also inferior to that of a conventional retinal OCT system.
One System, Two Imaging Depths Simultaneously
The most sophisticated and complex approach is a single light source system capable of providing appropriate focus and scanning geometry at both the anterior segment and retina simultaneously. The fundamental idea is that the single light source is divided in some fashion; to date, the division has been via polarization as first described by Dhalla, et al.25 In these systems, one polarization state travels through a dedicated anterior segment channel for that polarization state to image the anterior eye while the other polarization state simultaneously travels through a dedicated retinal channel to image the retina. A similar system with differences in the OCT engine (using two interferometers) was described in 2018.26 A contemporary version of the Dhalla system with wider fields of view on the retina (~55° compared to < 20°) while retaining a single interferometer has been recently described as well.27
These dual depth polarization encoded systems provide true simultaneous imaging of both the anterior segment and retina with, at minimum, standard fields of view and focus at each area (Fig. 5. Like the other systems described earlier, ocular biometry can be determined from the whole eye OCT image. Additionally, because both the anterior segment and retinal information was recorded simultaneously, those biometric measurements can then be used to perform spatial correction of the images for shape analysis (Fig. 5, right). The simultaneous acquisition allows investigation of global eye properties such as optical/visual axes of the eye26 (Fig. 6) and potentially kinetic events that affect both the anterior and posterior eye.
FIGURE 5. Simultaneous whole OCT of both the anterior segment and retina.
The image on the left is a volumetric rendering of the simultaneously acquired anterior segment and retina. From the volumetric rendering, the large fields of view on both the anterior segment and retina can be readily appreciated. The image on the right shows spatially corrected, simultaneously acquired cross-sections of the anterior segment and retina. Biometric information from the whole eye scan was used to perform the scan geometry and refraction corrections.28 (The left and right images are from two separate subjects. Additional detail on this whole eye OCT system can be found in McNabb, et al.27).
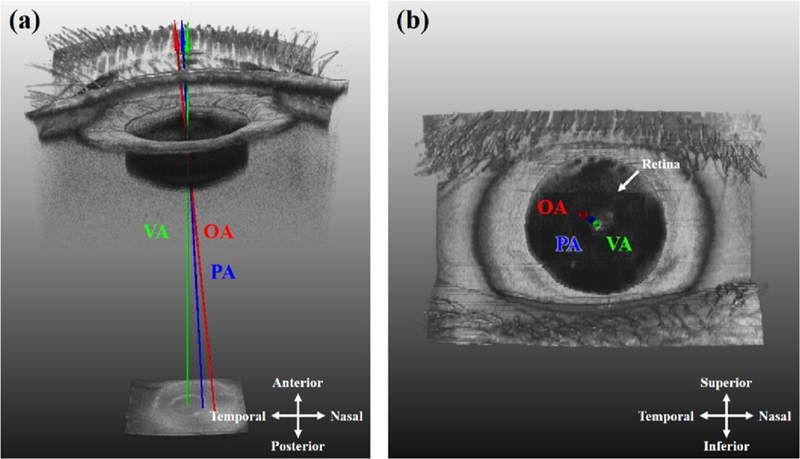
FIGURE 6. Using a whole eye OCT system to investigate axes of the eye.26.
Because a whole eye OCT system captures both the anterior segment (cornea apex, pupil) and the retina (fovea) simultaneously, the optical axis (OA), visual axis (VA), and pupillary axis (PA) can all be determined from the scan information. This enables investigation of relationships between these various axes.
Aside from system complexity, the major drawback of this type of system lies with the polarization division of light. While this division allows for the simultaneous dedicated anterior segment and retinal imaging, given an input of a finite amount of light, only ~50% of the light reaches each part of the eye which can affect image signal to noise ratio depending on the permissible limits. Newer limits (e.g. ANSI Z80.36–2016) and dedicated limit calculations for the anterior segment and retina help to provide adequate amounts of light at each area and overcome some of the signal to noise issues.
Conclusions
Unlike contemporary commercial OCT systems that are limited to imaging only the anterior segment or the retina (but not together at the same time), whole eye OCT systems have recently been described which are able to image both areas of the eye simultaneously. These systems allow for global ocular measurements such as the biometry required for cataract surgery and other applications such as spatial correction of OCT images to examine shape deformations from diseases such as pathologic myopia or elevated intracranial pressure. These whole eye OCT systems are also potentially the basis for comprehensive eye scanners that could be used for general eye screenings, remote or telemedicine applications, and in clinical research to record the state of the entire eye at a given point in time. Finally, removing the physical separation between the anterior and posterior eye changes our perspective from the eye as two separate, independent parts to again viewing and thinking of the eye as a whole, inter-related unit.
Acknowledgements
This work was supported by U.S. National Institutes of Health grant R01-EY024312
Footnotes
Conflicts of Interest:
ANK and RPM: none
JAI: Leica (P,R); Carl Zeiss Meditec (P,R)
References
- 1.Schuman JS, Puliafito CA, Fujimoto JG, Duker JS, editors. Optical Coherence Tomography of Ocular Diseases, 3rd edition. Thorafare, NJ: Slack Inc., 2013. [Google Scholar]
- 2.Huang D, Swanson EA, Lin CP, et al. Optical coherence tomography. Science. 1991. Nov 22;254(5035):1178–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Izatt JA, Hee MR, Swanson EA, et al. Micrometer-scale resolution imaging of the anterior eye in vivo with optical coherence tomography. Arch Ophthalmol. 1994. Dec;112(12):1584–9. [DOI] [PubMed] [Google Scholar]
- 4.Swanson EA, Izatt JA, Hee MR, et al. In vivo retinal imaging by optical coherence tomography. Opt Lett. 1993. Nov 1;18(21):1864–6. [DOI] [PubMed] [Google Scholar]
- 5.Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol. 1995. Mar;113(3):325–32. [DOI] [PubMed] [Google Scholar]
- 6.Panozzo G, Mercanti A. Optical coherence tomography findings in myopic traction maculopathy. Arch Ophthalmol. 2004. Oct;122(10):1455–60. [DOI] [PubMed] [Google Scholar]
- 7.You QS, Peng XY, Xu L, et al. Myopic maculopathy imaged by optical coherence tomography: the beijing eye study. Ophthalmology. 2014. Jan;121(1):220–224. [DOI] [PubMed] [Google Scholar]
- 8.Ohno-Matsui K Pathologic Myopia. Asia Pac J Ophthalmol (Phila). 2016. Nov/Dec;5(6):415–423. [DOI] [PubMed] [Google Scholar]
- 9.Ng DS, Cheung CY, Luk FO, et al. Advances of optical coherence tomography in myopia and pathologic myopia. Eye (Lond). 2016. Jul;30(7):901–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radhakrishnan S, Goldsmith J, Huang D, et al. Comparison of optical coherence tomography and ultrasound biomicroscopy for detection of narrow anterior chamber angles. Arch Ophthalmol. 2005. Aug;123(8):1053–9. [DOI] [PubMed] [Google Scholar]
- 11.Cheung CY, Liu S, Weinreb RN, et al. Dynamic analysis of iris configuration with anterior segment optical coherence tomography. Invest Ophthalmol Vis Sci. 2010. Aug;51(8):4040–6. [DOI] [PubMed] [Google Scholar]
- 12.Narayanaswamy A, Sakata LM, He MG, et al. Diagnostic performance of anterior chamber angle measurements for detecting eyes with narrow angles: an anterior segment OCT study. Arch Ophthalmol. 2010. Oct;128(10):1321–7. [DOI] [PubMed] [Google Scholar]
- 13.Porporato N, Baskaran M, Aung T. Role of anterior segment optical coherence tomography in angle-closure disease: a review. Clin Exp Ophthalmol. 2018. Mar;46(2):147–157. [DOI] [PubMed] [Google Scholar]
- 14.Kuo AN, Verkicharla PK, McNabb RP, et al. Posterior Eye Shape Measurement With Retinal OCT Compared to MRI. Invest Ophthalmol Vis Sci. 2016. Jul 1;57(9):OCT196–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggeri M, Uhlhorn SR, De Freitas C, Ho A, Manns F, Parel JM. Imaging and full-length biometry of the eye during accommodation using spectral domain OCT with an optical switch. Biomed Opt Express. 2012. Jul 1;3(7):1506–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nankivil D, Waterman G, LaRocca F, et al. Handheld, rapidly switchable, anterior/posterior segment swept source optical coherence tomography probe. Biomed Opt Express. 2015. Oct 21;6(11):4516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grulkowski I, Mazanera S, Cwiklinski L, et al. Swept source optical coherence tomography and tunable lens technology for comprehensive imaging and biometry of the whole eye. Optica. 2018. Jan;5(1):52–9. [Google Scholar]
- 18.Jeong HW, Lee SW, Kim BM. Spectral-domain OCT with dual illumination and interlaced detection for simultaneous anterior segment and retina imaging. Opt Express. 2012. Aug 13;20(17):19148–59. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Kim PU, Hyeon MG, Choi Y, Kim J, Kim BM. High-resolution, dual-depth spectral-domain optical coherence tomography with interlaced detection for whole-eye imaging. Appl Opt. 2016. Sep 10;55(26):7212–7. [DOI] [PubMed] [Google Scholar]
- 20.Dai C, Zhou C, Fan S, et al. Optical coherence tomography for whole eye segment imaging. Opt Express. 2012. Mar 12;20(6):6109–15. [DOI] [PubMed] [Google Scholar]
- 21.Fan S, Li L, Li Q, et al. Dual band dual focus optical coherence tomography for imaging the whole eye segment. Biomed Opt Express. 2015. Jun 12;6(7):2481–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jayaraman V, Cole GD, Robertson M, et al. High-sweep-rate 1310 nm MEMS-VCSEL with 150 nm continuous tuning range. Electron Lett. 2012. Jul 5;48(14):867–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grulkowski I, Liu JJ, Potsaid B, et al. Retinal, anterior segment and full eye imaging using ultrahigh speed swept source OCT with vertical-cavity surface emitting lasers. Biomed Opt Express. 2012. Nov 1;3(11):2733–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grulkowski I, Liu JJ, Zhang JY, et al. Reproducibility of a long-range swept-source optical coherence tomography ocular biometry system and comparison with clinical biometers. Ophthalmology. 2013. Nov;120(11):2184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhalla AH, Nankivil D, Bustamante T, et al. Simultaneous swept source optical coherence tomography of the anterior segment and retina using coherence revival. Opt Lett. 2012. Jun 1;37(11):1883–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim HJ, Kim M, Hyeon MG, et al. Full ocular biometry through dual-depth whole-eye optical coherence tomography. Biomed Opt Express. 2018. Jan 2;9(2):360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McNabb RP, Polans J, Keller B, et al. Wide-field whole eye OCT system with demonstration of quantitative retinal curvature estimation. Biomed Opt Express. 2018. Dec 21;10(1):338–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuo AN, McNabb RP, Chiu SJ, et al. Correction of ocular shape in retinal optical coherence tomography and effect on current clinical measures. Am J Ophthalmol. 2013. Aug;156(2):304–11. [DOI] [PMC free article] [PubMed] [Google Scholar]