Abstract
Aims
To assess whether hospitalization may assist in correcting errors in anticoagulant therapy among patients with atrial fibrillation (AF).
Methods
Our cohort included patients admitted to our institution with a history of AF between 2016 and 2018. We categorized patient's treatment upon admission and discharge as lacking (no treatment despite indication), inadequate (according to individual characteristics) or adequate. We assessed adequacy of treatment upon discharge and determined factors associated with correcting admission errors.
Results
Of 4427 patients admitted with a history of AF, the categorization to lacking, inadequate and adequate treatment was 1746 (39.4%), 1237 (27.9%) and 1444 (32.6%) patients, respectively. Of those with inadequate treatment, the most common types of errors were direct oral anticoagulant (DOAC) underdosing (n = 578; 46.7%), vitamin‐K antagonists when DOAC was indicated (n = 258; 20.9%), DOAC despite contraindication to DOAC (n = 166; 13.4%) and DOAC overdosing (n = 124; 10%). Upon discharge 688 (18.6%, out of n = 3694) corrections but also 316 (8.6%) new mistakes were found. On multivariate logistic regression, the factors associated with correction of an error on admission were hospitalization due to AF (odds ratio [OR] 2.94 [2.39–3.61]), hospitalization in the neurologic or geriatric wards (OR 2.79 [2.04–3.80]), female sex (OR 1.34 [1.10–1.63]) and a history of stroke (OR 1.47 [1.17–1.86]), while the presence of a contraindication to DOAC decreased the chance of correction (OR 0.10 [0.06–0.18]).
Conclusion
Hospitalization for any reason may contribute to correction of errors in AC treatment in patients with AF. Unfortunately, a significant portion of patients remains inadequately treated by both outpatient and inpatient providers.
Keywords: medication errors, stroke, anticoagulants, internal medicine, cardiovascular
What is already known about this subject
Correct use of anticoagulant treatment among patients with atrial fibrillation significantly reduces the risk of stroke.
Up to 40% of patients with atrial fibrillation do not receive anticoagulant therapy.
Direct oral anticoagulants (DOAC) are preferred over vitamin K antagonists; however, many patients on DOAC receive inadequate dosing.
What this study adds
Following nontreatment, DOAC underdosing and use of vitamin K antagonists instead of DOAC are the most common types of errors in anticoagulant treatment.
Hospitalization may contribute to identifying and fixing such errors, but many errors are still overlooked.
Patients with treatment errors hospitalized for atrial fibrillation or with a history of stroke are more likely to have such errors corrected.
1. INTRODUCTION
Ischaemic strokes are a major cause of morbidity and mortality in the modern world, with a lifetime risk of 14–18%.1 Approximately 15–25% of all ischaemic strokes are caused by embolization of a clot formed due to atrial fibrillation (AF).2, 3 The risk of emboli can be significantly reduced by the use of anticoagulant (AC) therapy, especially in patients at high risk according to risk stratification models such as the CHA2DS2‐VASc score.4
Despite an abundance of evidence5, 6, 7, 8 and multiple guidelines9, 10, 11 that support the use of AC therapy in patients with AF, approximately 40% of patients at risk are not prescribed such treatment.12 As it is estimated that up to 1/4 of adults in the USA and Europe will develop AF at some point during their lives,13 this represents a very large population of patients who are under‐treated.
In the past, the most common AC medications were vitamin K antagonists (VKA) such as https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6853. A low fraction of time in the therapeutic range limits the effectiveness of this treatment, and previous reports have found TTR to be as low as 50%.14 In recent years, however, the use of direct oral ACs (DOACs) have emerged. These drugs have been found to be superior to VKA in patients with AF, as they are associated with a lower risk of intracranial bleeding,15 have fewer drug interactions and revoke the need for periodical international normalized ratio monitoring.6, 7, 8, 15, 16 Accordingly, recent guidelines9, 10, 11 recommend the use of DOACs over the use of VKA for patients with AF at high risk of stroke, and DOACs are increasingly being used as AC of choice.17 It is imperative, however, to prescribe DOACs according to drug‐specific dosing guidelines, as these drugs differ in their pharmacokinetic and pharmacodynamic properties.18
Despite these clear recommendations, previous publications have suggested that in addition to those not treated with AC at all, a considerable amount of patients at high risk of stroke are still treated with VKA instead of DOAC,12 or are treated with a DOAC at an inappropriate dose.19 These errors are especially prevalent among elderly patients who are at high risk of stroke.20
Given the practice of reviewing patients' regular medications and providing them with an updated prescription upon discharge from hospitalization, we hypothesized that hospitalization due to any cause may assist in identifying patients not receiving adequate AC treatment on a regular basis and possibly facilitate correction of such errors.
2. METHODS
2.1. Study population
Initial screening was performed on the electronic health records (EHR) for patients with a past medical history of AF prior to hospitalization, aged 18 years or over, who were admitted for any reason to the neurological, cardiological, geriatric or 1 of the 9 internal medicine wards in the Tel Aviv Medical Center between 1 January 2016 and 31 July 2018. Exclusion criteria included pregnancy, treatment with parenteral AC agents or missing data regarding renal function or weight. In case of multiple visits by the same patient in the study period, only the first visit was included.
Medications assessed were of the following Anatomical Therapeutic Chemical classification system (ATC) codes: B01AF01 (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6388), B01AF02 (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6390), B01AE07 (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=6380) and B01AA03 (warfarin). Notably, the drug B01AF03 (https://www.guidetopharmacology.org/GRAC/LigandDisplayForward?ligandId=7575) is not approved for use in Israel and thus was not included in the study. The adequacy of the regular treatment each patient received was evaluated according to accepted guidelines9: a calculated CHA2DS2‐VASc score of >1 in males or > 2 in females was considered an indication for AC treatment, and a score of 0 in males or 1 in females was considered a lack of indication for AC treatment. A platelet count <50 × 109/L was considered a contraindication to AC treatment. A diagnosis of active malignancy, dialysis status, rheumatic heart disease, severe mitral stenosis or an implanted mechanical heart valve was considered a contraindication to DOAC use. An estimated glomerular filtration rate (eGFR) of 30 mL/min or lower was considered a contraindication to the use of dabigatran and an eGFR of 15 mL/min or lower was considered a contraindication to the use of apixaban or rivaroxaban.
Dosing adequacy was evaluated for each patient according to individual characteristics such as age, weight, serum creatinine or eGFR, in adherence to the accepted indications for reduced dose for each DOAC9: an eGFR of <50 mL/min for rivaroxaban or dabigatran, an eGFR of <30 mL/min or the presence of 2 out of [A] weight <60 kg; [B] age >80 years; [C] serum creatinine >1.5 mg/dL for apixaban, and age >75 years for dabigatran. These criteria were then used to categorize each patient's treatment upon admission as lacking (i.e. no AC treatment despite indication and no contraindications), inadequate (i.e. AC without indication; AC of any type despite contraindication; DOAC despite contraindication to DOAC; VKA where DOAC was indicated; and DOAC at an inadequate dose) or adequate (no AC in the absence of indication or in the presence of a contraindication; VKA in the presence of a contraindication to DOAC; or DOAC at an adequate dose). A similar categorization was performed for the treatment prescribed at discharge. All of the above criteria are in general accordance with the individual agent's summary of product characteristics sheets. A detailed algorithm for this categorization is provided in the supplementary appendix. To exclude the possibility of inconsistent results over different time periods, the temporal trends in patient categorization and in the type of agent used were examined for each yearly quarter over the study period.
A second analysis was performed to categorize a subset of these patients into 4 groups according to the change in adequacy of treatment between admission and discharge: (i) continue lacking or inadequate treatment; (ii) continue adequate treatment; (iii) correct admission errors (switch from lacking or inadequate treatment to adequate treatment); (iv) create new error (switch from adequate treatment to lacking or inadequate treatment).
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions. Ethics approval and review according to the Declaration of Helsinki for this study was obtained from the institutional review board at the Tel Aviv Souraski Medical Center (Approval # TLVMC‐0549‐18).
2.2. Statistical analysis
Baseline characteristics were described for each of the groups. All continuous variables are displayed as means (standard deviation) for normally distributed variables or median (interquartile range) for variables with abnormal distribution. Categorical variables are displayed as numbers (%) of subjects within each group. For a subset of patients for whom data regarding prescribed medications at discharge were available, the treatment prescribed at discharge was described for each group and its adequacy assessed. To further investigate the association between patient variables and the correction of admission errors in AC treatment, logistic regression was performed: first, univariate regression was performed to assess the contribution of individual factors on correction of admission AC errors. Subsequently, multivariate logistic regression was performed, including in the model only the factors that displayed a significant contribution on univariate analysis. Patient variables used in the univariate analysis included age, sex, weight, height, individual components of the CHA2DS2‐VASc score, eGFR, serum platelet count, the change in eGFR and platelet count from admission to discharge, use of antiaggregants, the presence of a contraindication to AC in general or to DOAC in particular, AF as reason for admission, discharging ward, length of stay, and specific AC agent used. Patient characteristics between the groups were compared using a Student t test or analysis of variance (ANOVA) test for normally distributed continuous variables, the Kruskal–Wallis test for non‐normally distributed continuous variables, and a χ2 test or the Fisher exact test for categorical variables. Statistical analyses were performed using R software version 3.5.1 (R Core Team [2018]. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org.
2.3. Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY21.
3. RESULTS
Out of 42 629 visits to our institution that were screened, the final study population consisted of 4427 patients (Figure 1). Of these, 1746 (39.4%) patients were categorized as receiving lacking treatment; 1237 (27.9%) patients as receiving inadequate treatment; and 1444 (32.6%) patients as receiving adequate treatment. Baseline characteristics of the 3 groups are described in Table 1.
Figure 1.
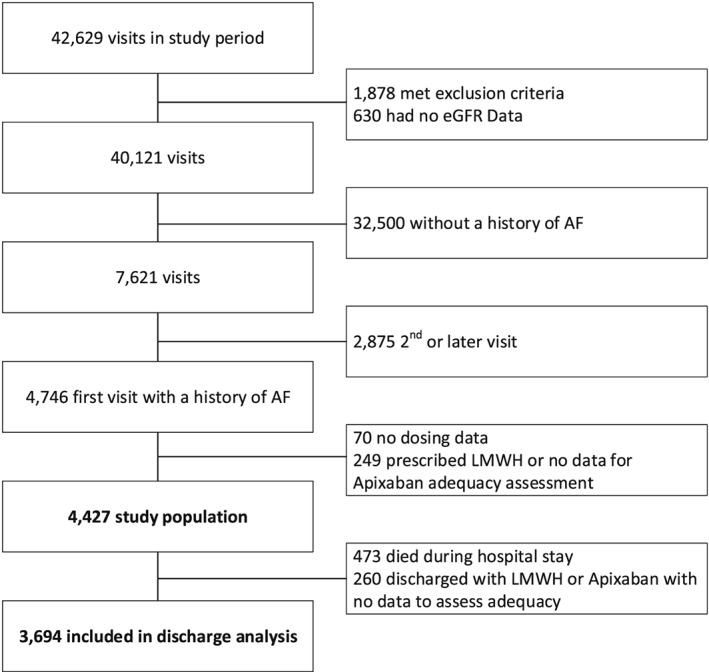
Study cohort derivation process. AF, atrial fibrillation; eGFR, estimated glomerular filtration rate; LMWH, low molecular weight heparin
Table 1.
Baseline characteristics of the different groups
| Overall | Group 1 (lacking treatment) | Group 2 (inadequate treatment) | Group 3 (adequate treatment) | Between‐group P‐value | |
|---|---|---|---|---|---|
| A. Admission | n = 4427 (100%) | n = 1746 (39.4%) | n = 1237 (27.9%) | n = 1444 (32.6%) | |
| Female sex, n (%) | 2197 (49.6) | 893 (51.1) | 619 (50.0) | 685 (47.4) | .107 |
| Age (y), mean (SD) | 79.54 (11.17) | 82.75 (9.19) | 80.09 (10.23) | 75.19 (12.61) | <.001 |
| Weight (kg), mean (SD) | 75.58 (17.65) | 72.89 (16.67) | 76.27 (17.40) | 78.02 (18.51) | <.001 |
| BMI (kg/m2), mean (SD) | 27.34 (5.77) | 26.61 (5.25) | 27.77 (6.27) | 27.81 (5.81) | <.001 |
| Length of stay (d), median (IQR) | 4 (2–8) | 5 (3–10) | 4 (2–8) | 3 (2–7) | <.001 |
| CI to all AC, n (%) | 44 (1.0) | 0 (0.0) | 19 (1.5) | 25 (1.7) | <.001 |
| CI to DOAC, n (%) | 589 (13.3) | 269 (15.4) | 173 (14.0) | 147 (10.2) | <.001 |
| CHADS2VASC score, mean (SD) | 3.41 (1.51) | 3.74 (1.23) | 3.47 (1.53) | 2.96 (1.67) | <.001 |
| CHADS2VASC score—categorial, n (%) | <.001 | ||||
| 0 | 144 (3.3) | 0 (0.0) | 46 (3.7) | 98 (6.8) | |
| 1 | 267 (6.0) | 0 (0.0) | 72 (5.8) | 195 (13.5) | |
| 2 | 731 (16.5) | 260 (14.9) | 166 (13.4) | 305 (21.1) | |
| 3 | 1279 (28.9) | 581 (33.3) | 376 (30.4) | 322 (22.3) | |
| 4 | 979 (22.1) | 455 (26.1) | 272 (22.0) | 252 (17.5) | |
| 5 | 648 (14.6) | 291 (16.7) | 188 (15.2) | 169 (11.7) | |
| 6 | 292 (6.6) | 122 (7.0) | 92 (7.4) | 78 (5.4) | |
| 7 | 71 (1.6) | 32 (1.8) | 19 (1.5) | 20 (1.4) | |
| 8 | 16 (0.4) | 5 (0.3) | 6 (0.5) | 5 (0.3) | |
| HASBLED score, mean (SD) | 1.89 (0.92) | 2.03 (0.82) | 1.94 (0.95) | 1.68 (0.98) | <.001 |
| CHF, n (%) | 780 (17.6) | 307 (17.6) | 237 (19.2) | 236 (16.3) | .162 |
| Hypertension, n (%) | 800 (18.1) | 350 (20.0) | 239 (19.3) | 211 (14.6) | <.001 |
| Stroke, n (%) | 1077 (24.3) | 493 (28.2) | 291 (23.5) | 293 (20.3) | <.001 |
| Diabetes, n (%) | 151 (3.4) | 69 (4.0) | 31 (2.5) | 51 (3.5) | .096 |
| Vascular disease, n (%) | 1827 (41.3) | 790 (45.2) | 530 (42.8) | 507 (35.1) | <.001 |
| Liver disease, n (%) | 59 (1.3) | 16 (0.9) | 16 (1.3) | 27 (1.9) | .064 |
| Treatment with antiplatelet agents, n (%) | 1108 (25.0) | 673 (38.5) | 201 (16.2) | 234 (16.2) | <.001 |
| Admission INR value (for patients on VKA), mean (SD) | 2.61 (1.35) | ‐ | 2.49 (1.26) | 2.97 (1.55) | .004 |
| Admission haemoglobin, (g/dL), mean (SD) | 12.34 (2.07) | 12.19 (2.06) | 12.29 (1.99) | 12.56 (2.13) | <.001 |
| Admission platelets (× 109/L), mean (SD) | 221.33 (92.69) | 229.07 (100.56) | 216.12 (87.23) | 216.48 (86.57) | <.001 |
| Admission eGFR (mL/min), mean (SD) | 52.02 (24.59) | 47.94 (23.63) | 51.52 (23.17) | 57.40 (25.87) | <.001 |
| Admission creatinine (mg/dL), mean (SD) | 1.33 (0.98) | 1.44 (1.19) | 1.28 (0.82) | 1.24 (0.78) | <.001 |
| Admission anticoagulation agent, n (%) | <.001 | ||||
| No anticoagulation | 2083 (47.1) | 1746 (100.0) | 0 (0.0) | 337 (23.3) | |
| >1 anticoagulant | 3 (0.1) | 0 (0.0) | 3 (0.2) | 0 (0.0) | |
| VKA | 377 (8.5) | 0 (0.0) | 282 (22.8) | 95 (6.6) | |
| Apixaban | 1136 (25.7) | 0 (0.0) | 594 (48.0) | 542 (37.5) | |
| Rivaroxaban | 647 (14.6) | 0 (0.0) | 287 (23.2) | 360 (24.9) | |
| Dabigatran | 181 (4.1) | 0 (0.0) | 71 (5.7) | 110 (7.6) | |
| Specific type of error on admission, n (%) | |||||
| >1 anticoagulant | 3 (0.1) | ‐ | 3 (0.2) | ‐ | ‐ |
| Receiving anticoagulation despite CI | 94 (2.1) | ‐ | 94 (7.6) | ‐ | ‐ |
| Receiving DOAC despite CI to DOAC | 166 (3.7) | ‐ | 166 (13.4) | ‐ | ‐ |
| Receiving VKA despite indication for DOAC | 258 (5.8) | ‐ | 258 (20.9) | ‐ | ‐ |
| DOAC underdosing | 578 (13.1) | ‐ | 578 (46.7) | ‐ | ‐ |
| DOAC overdosing | 124 (2.8) | ‐ | 124 (10.0) | ‐ | ‐ |
| Other DOAC dosing error | 14 (0.3) | ‐ | 14 (1.1) | ‐ | ‐ |
| B. Discharge | n = 3694 (100%) | n = 1362 (36.8%) | n = 1061 (28.7%) | n = 1271 (34.4%) | |
|---|---|---|---|---|---|
| Discharging ward, n (%) | <.001 | ||||
| Cardiology | 442 (12.0) | 85 (6.2) | 127 (12.0) | 230 (18.1) | |
| Geriatrics | 120 (3.2) | 68 (5.0) | 32 (3.0) | 20 (1.6) | |
| Internal medicine | 2914 (78.9) | 1109 (81.4) | 852 (80.3) | 953 (75.0) | |
| Neurology | 218 (5.9) | 100 (7.3) | 50 (4.7) | 68 (5.4) |
P values were calculated by ANOVA test for normally distributed continuous variables, Kruskal–Wallis test for non‐normally distributed continuous variables, and χ2 test for categorical variables. AC, anticoagulation; CHF, congestive heart failure; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; INR, international normalized ratio; SD, standard deviation; VKA, vitamin K antagonists
Compared to patients with lacking or inadequate treatment, the patients who received adequate treatment were younger, had better renal function, had less comorbidities, fewer contraindications to DOAC and consequently lower mean CHA2DS2VASc scores and shorter length of hospital stay.
Apixaban was the most commonly prescribed agent (n = 1136; 25.7% of patients), followed by rivaroxaban (n = 647; 14.6%), Warfarin (n = 377; 8.5%) and dabigatran (n = 181; 4.1%). Among the 1237 patients with inadequate treatment, the most common types of errors were DOAC underdosing (n = 578; 46.7%), VKA when DOAC was indicated (n = 258; 20.9%), DOAC despite contraindication to DOAC (n = 166; 13.4%) and DOAC overdosing (n = 124; 10.0%). The treatment upon discharge was evaluated for a subgroup of 3694 patients for whom data were available. The change in the adequacy of treatment between admission and discharge is displayed in Figure 2. The percentage of patients with adequate treatment rose from 34.4% on admission to 44.5% at the time of discharge from the hospital, representing a 29.3% improvement. Approximately 30% of patients with lacking or inadequate treatment on admission were discharged with adequate treatment; however, 1/4 of the patients who were admitted with adequate treatment did not receive adequate treatment at the time of discharge.
Figure 2.

Treatment adequacy on discharge (Y axis) according to treatment adequacy on admission (X axis)
Patients were then categorized to 4 groups according to the change in adequacy of treatment. Baseline characteristics and differences between the groups are displayed in Table 2.
Table 2.
Characteristics of patients according to the change in adequacy of treatment between admission and discharge
| Group 1 (continue inadequate or lacking treatment) | Group 2 (continue adequate treatment) | Group 3 (correct admission error) | Group 4 (create new error on discharge) | Between‐group P‐value | |
|---|---|---|---|---|---|
| n = 1735 (46.9%) | n = 955 (25.8%) | n = 688 (18.6%) | n = 316 (8.5%) | ||
| Female sex, n (%) | 853 (49.2) | 467 (48.9) | 374 (54.4) | 133 (42.1) | .003 |
| Age (y), mean (SD) | 81.89 (9.80) | 74.35 (12.16) | 78.74 (9.46) | 75.30 (13.86) | <.001 |
| Length of stay (d), median (IQR) | 4 (2–7) | 3 (2–6.5) | 5 (3–10) | 3 (2–6) | <.001 |
| Discharging ward, n (%) | <.001 | ||||
| Cardiology | 151 (8.7) | 169 (17.7) | 61 (8.9) | 61 (19.3) | |
| Geriatrics | 52 (3.0) | 13 (1.4) | 48 (7.0) | 7 (2.2) | |
| Internal medicine | 1467 (84.6) | 714 (74.8) | 494 (71.8) | 239 (75.6) | |
| Neurology | 65 (3.7) | 59 (6.2) | 85 (12.4) | 9 (2.8) | |
| CHADSVASC, mean (SD) | 3.57 (1.39) | 2.90 (1.63) | 3.62 (1.37) | 2.95 (1.76) | <.001 |
| Treatment with antiplatelet agents, n (%) | 491 (28.3) | 151 (15.8) | 233 (33.9) | 54 (17.1) | <.001 |
| eGFR, mean (SD) | 60.46 (23.92) | 69.60 (24.76) | 66.89 (20.49) | 66.91 (25.57) | <.001 |
| Prescribed treatment at discharge, n (%) | <.001 | ||||
| No anticoagulation | 928 (53.5) | 170 (17.8) | 31 (4.5) | 139 (44.0) | |
| >1 anticoagulant | 1 (0.1) | 0 (0.0) | 0 (0.0) | 1 (0.3) | |
| VKA | 126 (7.3) | 39 (4.1) | 7 (1.0) | 5 (1.6) | |
| Apixaban | 402 (23.2) | 410 (42.9) | 416 (60.5) | 84 (26.6) | |
| Rivaroxaban | 221 (12.7) | 248 (26.0) | 194 (28.2) | 73 (23.1) | |
| Dabigatran | 57 (3.3) | 88 (9.2) | 40 (5.8) | 14 (4.4) | |
| Specific type of error on discharge, n (%) | <.001 | ||||
| Lacking treatment | 928 (53.5) | ‐ | ‐ | 139 (44.0) | |
| >1 anticoagulant | 1 (0.1) | ‐ | ‐ | 1 (0.3) | |
| Receiving anticoagulation despite CI | 69 (4.0) | ‐ | ‐ | 61 (19.3) | |
| Receiving DOAC despite CI to DOAC | 108 (6.2) | ‐ | ‐ | 5 (1.6) | |
| Receiving VKA despite indication for DOAC | 113 (6.5) | ‐ | ‐ | 2 (0.6) | |
| DOAC underdosing | 456 (26.3) | ‐ | ‐ | 98 (31.0) | |
| DOAC overdosing | 58 (3.3) | ‐ | ‐ | 10 (3.2) | |
| Other DOAC dosing error | 2 (0.1) | ‐ | ‐ | 0 (0.0) |
P values were calculated by ANOVA test for normally distributed continuous variables, Kruskal–Wallis test for non‐normally distributed continuous variables and χ2 test for categorical variables. AC, anticoagulation; CHF, congestive heart failure; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; VKA, vitamin K antagonists
The majority of new errors in treatment (inflicted on patients who were admitted with adequate treatment) were lacking treatment (n = 139, 44%) DOAC underdosing (n = 98, 31%) and AC despite the presence of a contraindication (n = 61, 19.3%). On in‐depth analysis of the patients in group 1 (who continued lacking or inadequate treatment) it was notable that the specific error on admission persisted to discharge in the majority of patients in each group, with the exception of DOAC overdosing which persisted in only 28 (27.2%) patients (Table S2). In multivariate logistic regression (Figure 3 and Table S1), the factors associated with correction of an admission AC error were AF as the reason for hospitalization (odds ratio [OR] 2.94 [2.39–3.61]), discharge from the neurological or geriatric wards (OR 2.79 [2.04–3.80]), female sex (OR 1.34 [1.10–1.63]) and a history of stroke (OR 1.47 [1.17–1.86]). Factors associated with a decreased chance of correcting an admission error were the presence of a contraindication to DOAC (OR 0.10 [0.06–0.18]) and, to a modest degree, age (OR 0.97 [0.96–0.98] for each increase of 1 year in age). Notably, the specific drug used was not found to influence the chance of correcting an AC error even on univariate analysis.
Figure 3.
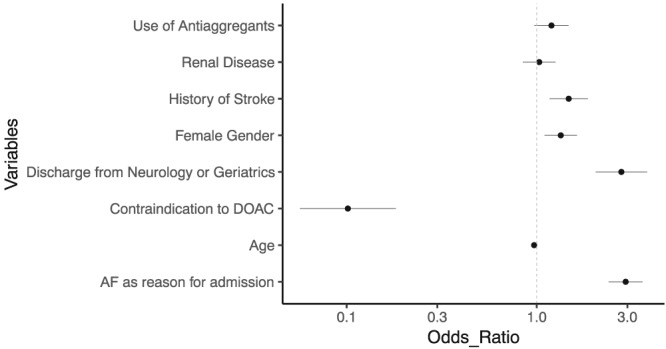
Forrest plot showing odds ratio for correction of an error present on admission for different patient variables, as found on multivariate logistic regression. The model included only variables that showed significant contribution on univariate analysis. See Table S1. AF, atrial fibrillation; DOAC, direct oral anticoagulants
The trends in prescribing patterns and in the frequency of errors over the study period are portrayed in Figure 4. Some variations that were observed in the beginning of the study period diminished during the last year of the study period.
Figure 4.
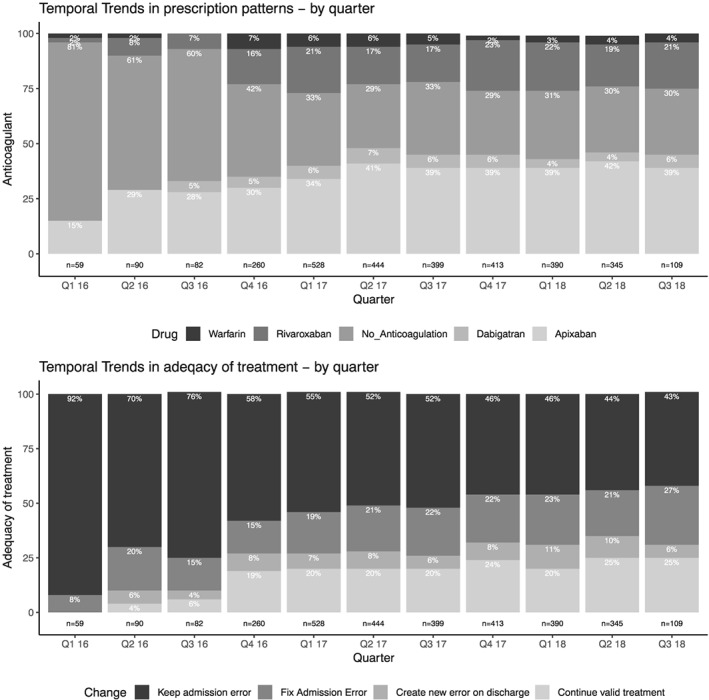
Temporal trends in anticoagulant agent prescription patterns (top panel) and in adequacy of treatment (bottom panel) over the course of the study period
4. DISCUSSION
This study had 2 objectives: (i) to assess the frequency and types of errors in AC treatment administered on an outpatient basis, as documented upon admission for any cause; and (ii) to retrospectively evaluate the changes in AC therapy during hospitalization.
Cross‐sectional evaluation of oral AC treatment reveals that the overall adherence rate was approximately 60% and is similar to that reported from a large American database by Marzec et al.12 Our study adds details with regard to the specific types of errors prevalent among patients with AF, demonstrating that lacking treatment is by far the most common type of error (39.4% of patients) followed by DOAC underdosing (13.1%) and use of VKA when DOAC use was indicated (5.8%). In our cohort, only 1/3 of patients were receiving adequate treatment on admission. In addition, among those with lacking treatment there was a disproportionally higher frequency of use of antiplatelet agents (38.5% vs 16.2% among the other 2 groups). This possibly indicates that antiplatelet agents were used as an alternative to AC agents, although this practice has long been known to be ineffective22 and has recently been abandoned.9 Unfortunately, this trend is still observed, especially among older patients.20 The patients that received adequate treatment in our study were younger and had fewer comorbidities. Taken together, these data support the trend suggested in several other studies, that providers often choose to avoid treatment23 or under‐treat24, 25, 26 with AC, especially in old or frail patients, possibly because of a perceived higher risk of bleeding than of embolic stroke. This, despite numerous studies that have shown no benefit and possible harm in lacking treatment or unwarranted reduced‐dose DOAC.19, 27, 28
In our study, there was a 29.3% increase in adequate treatment between admission to discharge from hospitalization (from 34.4% to 44.5%), demonstrating that hospitalization due to any cause may serve as an effective intervention point for correction of errors in AC treatment, and plausibly other medication types as well. Disappointingly, our data also show that there is a substantial number of patients that are either admitted and discharged with an error in AC treatment (46.9% of patients) or are discharged with a de novo error in AC therapy (8.5% of patients). In the former, most cases are due to uncorrected errors, which may reflect unawareness of practitioners to AC prescription and dosing guidelines. Some types of errors, such as treatment despite a contraindication (to AC in general or DOAC in particular) had especially low rates of correction, while others such as DOAC over and underdosing, were more commonly fixed. These findings shed a light on possible caveats in practitioners' proficiency in AC treatment guidelines. Regarding the latter group, the majority of new errors were lacking treatment, followed by AC despite, contraindication and DOAC underdosing.
While at least some of these errors reflect a true error in judgement by practitioners, there are alternative explanations. For example, it is plausible that some of the patients lacking treatment on discharge reflect cases that were discharged with a prescription only for new medications and a continue all other medications as usual statement in the discharge letter (free‐text fields in the EHR were not scanned in this study). As for the other types of errors, those could potentially be caused by keeping the same medication regimen in the face of changing patient characteristics, which are also indicative of inattention to AC treatment (especially when this was not the cause for admission). Interestingly, in univariate logistic regression, the change in GFR and platelet count from admission to discharge did not seem to affect the chance of creating a new error on discharge (data not shown). Not surprisingly, on multivariate logistic regression, only admission due to AF, a history of stroke, or admission in the neurological or geriatric wards (which may reflect stroke as the reason for admission) were associated with an increased chance of correcting an AC error present on admission. This probably represents a higher level of attention to medications directly related to the cause of admission.
Our findings are generally in agreement with those recently published by Antoniazzi et al.29 on a smaller (yet multicentre‐derived) Italian population, albeit with a higher baseline rate of errors in our cohort. This illustrates that, even on a global perspective, providers still lack knowledge of and awareness to AC guidelines and highlight the importance of continued education of medical personnel on its use.
4.1. Strengths and limitations
The main strength of our study was the in‐depth, patient‐level determination of optimal therapy and dosing, which allowed us to obtain a more accurate estimation of the true rate of errors in AC treatment as well as the specific types of errors. In addition, we focused on the change in AC treatment over the course of the hospitalization, a field that has only recently come under investigation.29 However, our study had several limitations: first, it was a single‐centre study, which limits the applicability of our results to other environments; however, the relatively large cohort, the robustness of the results over time and the similarity to other published studies affirm our confidence in our findings. Second, laboratory results (which determined adequacy of dosing) were based on samples drawn on admission to the hospital and thus may have inaccurately represented the renal function used when the dose was prescribed and contributed to overestimation of the true error rates. Third, as we only included hospitalized patients, the study was subject to selection bias, which may have overestimated the true prevalence of AC errors in the outpatient setting. It does not, however, affect the validity of our results obtained regarding the change in AC during hospitalization. Similar research on AC treatment in an outpatient setting will assist in overcoming this selection bias. Fourth, the study was retrospective and EHR‐based, which makes it susceptible to potential confounders or errors in documentation and prevents determination of causality. While the inadequacy of oral AC treatment presented in our report has already been reported widely, our methodology and findings represent a novel perspective on the issue, specifically with regard to the role of hospital clinicians in the effort to improve medication appropriateness. Further studies, possibly prospective, are warranted to reaffirm our results and determine which interventions would improve adherence to AC treatment guidelines and reduce AC error rates.
5. CONCLUSIONS
Hospitalization for any reason may contribute to correction of errors in AC treatment in patients with AF; however, a significant portion of patients remains inadequately treated by both outpatient and inpatient providers. A predischarge review of current patient characteristics and medications is needed to ensure adequacy of care. More effort should be put into raising physician competence and awareness to AC dosing guidelines, and further studies are required to establish what interventions would best aid in achieving this goal.
COMPETING INTERESTS
M.I. declares personal fees from Bayer outside the scope of this work. All other authors report no conflict of interests. Personal declaration forms are attached.
CONTRIBUTORS
Contributors Y.A., S.S.T., D.Z., S.B. and O.R. were responsible for the concept and design of the study. Y.A. performed data collection. Y.A., S.S.T. and O.R. performed statistical analysis. Y.A. and S.S.T. wrote the manuscript. All authors revised the manuscript and approved its final version.
Supporting information
TABLE S1
Odds ratios and 95% confidence intervals for univariate and multivariate analyses for the chance to correct an admission error in anticoagulant treatment (pertains to Figure 3 of the manuscript)
TABLE S2 Specific group on discharge according to specific group on admission
FIGURE S1 Population categorization algorithm used in the study
ACKNOWLEDGEMENTS
This study was supported by an independent research grant from Pfizer Pharmaceuticals. The authors wish to thank Hila Zadka, Naama Warman‐Alaluf and Eli Raykhshtat of the Tel Aviv Medical Center computing division for their assistance in obtaining the data for this research, as well as Adi Silberman of the Tel Aviv Medical Center and Avital Angel‐Korman of the Boston Medical Center for their contributions to this manuscript.
Angel Y, Zeltser D, Berliner S, et al. Hospitalization as an opportunity to correct errors in anticoagulant treatment in patients with atrial fibrillation. Br J Clin Pharmacol. 2019;85:2838–2847. 10.1111/bcp.14116
The authors confirm that the PI for this paper is Ori Rogowski and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Seshadri S, Beiser A, Kelly‐Hayes M, et al. The lifetime risk of stroke: estimates from the Framingham study. Stroke. 2006;37(2):345‐350. 10.1161/01.STR.0000199613.38911.b2 [DOI] [PubMed] [Google Scholar]
- 2. Xian Y, O'Brien EC, Liang L, et al. Association of preceding antithrombotic treatment with acute ischemic stroke severity and in‐hospital outcomes among patients with atrial fibrillation. JAMA ‐ J am Med Assoc. 2017;317(10):1057‐1067. 10.1001/jama.2017.1371 [DOI] [PubMed] [Google Scholar]
- 3. Murtagh B, Smalling RW. Cardioembolic stroke. Curr Atheroscler Reports. 2006;8(4):310‐316. 10.1007/s11883-006-0009-9 [DOI] [PubMed] [Google Scholar]
- 4. Lip GYH, Nieuwlaat R, Pisters R, Lane DA, Crijns HJGM. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: The euro heart survey on atrial fibrillation. Chest. 2010;137(2):263‐272. 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 5. Hart RG, Pearce LA, Koudstaal PJ. Transient ischemic attacks in patients with atrial fibrillation. Implications for secondary prevention: The European atrial fibrillation trial and stroke prevention in atrial fibrillation III trial. Stroke. 2004;35(4):948‐951. 10.1161/01.STR.0000120741.34866.1D [DOI] [PubMed] [Google Scholar]
- 6. Granger CB, Alexander JH, McMurray JJV, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365(11):981‐992. 10.1056/NEJMoa1107039 [DOI] [PubMed] [Google Scholar]
- 7. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in Nonvalvular atrial fibrillation. N Engl J Med. 2011;365(10):883‐891. 10.1056/NEJMoa1009638 [DOI] [PubMed] [Google Scholar]
- 8. Connolly SJ, Ezekowitz MD, Yusuf S, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361(12):1139‐1151. 10.1056/NEJMoa0905561 [DOI] [PubMed] [Google Scholar]
- 9. Kirchhof P, Benussi S, Zamorano JL, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893‐2962. 10.1093/eurheartj/ehw210 [DOI] [PubMed] [Google Scholar]
- 10. January CT, Wann LS, Calkins H, et al 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation. 2019. 10.1161/CIR.0000000000000665 [DOI]
- 11. Steffel J, Verhamme P, Potpara TS, et al. The 2018 European heart rhythm association practical guide on the use of non‐vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39(16):1330‐1393. 10.1093/eurheartj/ehy136 [DOI] [PubMed] [Google Scholar]
- 12. Marzec LN, Wang J, Shah ND, et al. Influence of direct Oral anticoagulants on rates of Oral anticoagulation for atrial fibrillation. J am Coll Cardiol. 2017;69(20):2475‐2484. 10.1016/j.jacc.2017.03.540 [DOI] [PubMed] [Google Scholar]
- 13. Heeringa J, van der Kuip DAM, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur Heart J. 2006;27(8):949‐953. 10.1093/eurheartj/ehi825 [DOI] [PubMed] [Google Scholar]
- 14. Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M. Prospective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with incident non‐valvular atrial fibrillation. J am Heart Assoc. 2015;4(1):e001486 10.1161/JAHA.114.001486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Raccah BH, Perlman A, Danenberg HD, Pollak A, Muszkat M, Matok I. Major bleeding and hemorrhagic stroke with direct Oral anticoagulants in patients with renal failure. Chest. 2016;149(6):1516‐1524. 10.1016/j.chest.2015.12.029 [DOI] [PubMed] [Google Scholar]
- 16. Lip GYH, Keshishian A, Li X, et al. Effectiveness and safety of Oral anticoagulants among Nonvalvular atrial fibrillation patients. Stroke. 2018;49(12):2933‐2944. 10.1161/STROKEAHA.118.020232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huisman MV, Rothman KJ, Paquette M, et al. The changing landscape for stroke prevention in AF. J am Coll Cardiol. 2017;69(7):777‐785. 10.1016/j.jacc.2016.11.061 [DOI] [PubMed] [Google Scholar]
- 18. Ieko M, Naitoh S, Yoshida M, Takahashi N. Profiles of direct oral anticoagulants and clinical usage‐dosage and dose regimen differences. J Intensive Care. 2016;4:19 10.1186/s40560-016-0144-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non–vitamin K antagonist Oral anticoagulant dosing in patients with atrial fibrillation and renal dysfunction. J am Coll Cardiol. 2017;69(23):2779‐2790. 10.1016/j.jacc.2017.03.600 [DOI] [PubMed] [Google Scholar]
- 20. Franchi C, Antoniazzi S, Proietti M, Nobili A, Mannucci PM, on behalf of the SIM‐AF Collaborators . Appropriateness of oral anticoagulant therapy prescription and its associated factors in hospitalized older people with atrial fibrillation. Br J Clin Pharmacol. 2018;84(9):2010‐2019. 10.1111/bcp.13631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Harding SD, Sharman JL, Faccenda E, et al. The IUPHAR/BPS Guide to PHARMACOLOGY in 2018: updates and expansion to encompass the new guide to IMMUNOPHARMACOLOGY. Nucl Acids Res. 2018;46:D1091‐D1106. http://www.ncbi.nlm.nih.gov/pubmed/17577005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hart RG, Pearce LA, Aguilar MI. Meta‐analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. 2007;146(12):857‐867. http://www.ncbi.nlm.nih.gov/pubmed/17577005 [DOI] [PubMed] [Google Scholar]
- 23. Hess PL, Kim S, Fonarow GC, et al. Absence of Oral anticoagulation and subsequent outcomes among outpatients with atrial fibrillation. Am J Med. 2017;130(4):449‐456. 10.1016/j.amjmed.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 24. Nguyen E, White CM, Patel MR, et al. Doses of apixaban and rivaroxaban prescribed in real‐world United States cardiology practices compared to registration trials. Curr Med Res Opin. 2016;32(7):1277‐1279. 10.1185/03007995.2016.1170672 [DOI] [PubMed] [Google Scholar]
- 25. Leef GC, Perino AC, Askari M, et al. Appropriateness of direct Oral anticoagulant dosing in patients with atrial fibrillation: insights from the veterans health administration. J Pharm Pract. 2019;Epub ahead of print. 10.1177/0897190019828270 [DOI] [PubMed] [Google Scholar]
- 26. Perlman A, Horwitz E, Hirsh‐Raccah B, et al. Clinical pharmacist led hospital‐wide direct oral anticoagulant stewardship program. Isr J Health Policy Res. 2019;8:19 10.1186/s13584-019-0285-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arbel R, Sergienko R, Hammerman A, et al. Effectiveness and safety of off‐labeldose‐reduced direct Oral anticoagulants in atrial fibrillation. Am J Med. 2019;Epub ahead of print. 10.1016/j.amjmed.2019.01.025 [DOI] [PubMed] [Google Scholar]
- 28. Steinberg BA, Shrader P, Pieper K, et al. Frequency and outcomes of reduced dose non‐vitamin K antagonist anticoagulants: results from ORBIT‐AF II (The outcomes registry for better informed treatment of atrial fibrillation II). J am Heart Assoc. 2018;7(4). 10.1161/JAHA.117.007633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Antoniazzi S, Ardoino I, Proietti M, et al. Appropriateness of prescription of oral anticoagulant therapy in acutely hospitalized older people with atrial fibrillation. Secondary analysis of the SIM‐AF cluster randomized clinical trial. Br J Clin Pharmacol. 2019;85(9):2134‐2142. 10.1111/bcp.14029 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
TABLE S1
Odds ratios and 95% confidence intervals for univariate and multivariate analyses for the chance to correct an admission error in anticoagulant treatment (pertains to Figure 3 of the manuscript)
TABLE S2 Specific group on discharge according to specific group on admission
FIGURE S1 Population categorization algorithm used in the study


