Abstract
Intravenous flucloxacillin is one of the most frequently used high‐dose penicillin therapies in hospitalized patients, forming the cornerstone treatment of invasive Staphylococcus aureus infection. Being a nonreabsorbable anion, flucloxacillin has been suggested to cause hypokalaemia, although the frequency and magnitude of this unwanted effect is unknown. In a retrospective cohort, we investigated the incidence and extent of hypokalaemia after initiation of intravenous flucloxacillin or ceftriaxone therapy. In total, 77 patients receiving flucloxacillin (62% male, mean age 70.5 years) and 84 patients receiving ceftriaxone (46% male, mean age 70.8 years) were included. Hypokalaemia occurred significantly more often in patients receiving flucloxacillin than ceftriaxone (42% vs 14%, p < 10−4). Moreover, follow‐up potassium levels were significantly lower during flucloxacillin therapy. In general, women were more prone to develop hypokalaemia than men. In conclusion, intravenous flucloxacillin use is associated with a striking incidence of hypokalaemia. Therefore, standardized potassium measurements are necessary.
Keywords: flucloxacillin, hypokalaemia, nonreabsorbable anion, penicillins
What is already known about this subject
Intravenous flucloxacillin is one of the most frequently used high‐dose penicillin therapies.
Hypokalaemia has been described in penicillin treatment. So far, only 7 cases of hypokalaemia in flucloxacillin treatment have been described in the literature.
What this study adds
This study is the first to give a cross‐sectional estimate on the incidence of hypokalaemia in patients receiving intravenous flucloxacillin.
Patients receiving intravenous flucloxacillin are at considerable risk (over 40%) to develop hypokalaemia. Women are at higher risk to develop hypokalaemia than men.
In patients receiving intravenous flucloxacillin, frequent monitoring of potassium levels is warranted.
1. INTRODUCTION
Intravenous flucloxacillin is one of the most frequently used high‐dose penicillin therapies. Being a penicillinase‐resistant β‐lactam, flucloxacillin finds its main application in treatment of infections caused by penicillinase‐producing organisms. Currently, it is the cornerstone treatment of invasive Staphylococcus aureus infection in the Netherlands. Generally, the penicillins are considered safe, except for allergic manifestations and rare renal inflammatory effects. However, even in the absence of gross renal failure, the use of penicillins among other classes of antibiotics is associated with changes in fluid and electrolyte balance, in particular hypokalaemia.1 The first report of this phenomenon dates back half a century, when 6 patients were reported who were treated with massive sodium penicillin therapy of whom 3 developed a significant hypokalaemia. This decrease in serum potassium was not explained by other potassium‐reducing factors and resolved after cessation of therapy.2 Hypokalaemia is suggested to develop because penicillins act as nonreabsorbable anions, causing a transmembrane potential gradient in the cortical collecting duct that is negative on the luminal side, thus enhancing potassium secretion. This action as a nonreabsorbable anion is a class‐effect of penicillins. Indeed, more recently, several case reports and small cohort studies have warned us of hypokalaemia in a variety of penicillin classes3, 4, 5, 6, among which are flucloxacillin and related penicillinase‐resistant penicillins.7, 8 However, although the consequences of this potential side effect can be severe, the exact incidence and severity of hypokalaemia in flucloxacillin treatment is currently unknown. Hypokalaemia can cause muscle weakness, rhabdomyolysis, intestinal ileus, serious cardiac arrhythmias and death. It is considered a multifactorial condition, with several factors acknowledged to be of influence in the general in‐hospital populations, in particular kidney function and the use of kaliuretic diuretics.9, 10 This study aimed to investigate the incidence of hypokalaemia in patients who receive intravenous high‐dosed flucloxacillin therapy and identify patients that are particularly at risk of developing hypokalaemia during flucloxacillin treatment, guiding the clinician to patients in whom extra vigilance is needed.
2. METHODS
The study was conducted at the Canisius Wilhelmina Hospital, Nijmegen, the Netherlands, and approved by the local ethical committee. We retrospectively screened all adult patients admitted to our hospital from 2010 to 2015 who received intravenous flucloxacillin (≥6 g/day) or ceftriaxone (2 g/day). Only patients that were normokalaemic at the start of therapy (defined as at least 1 serum potassium level of 3.3–4.7 mmol/l, the lower and upper levels of normal in the Canisius Wilhelmina Hospital at the time, and no serum potassium levels below or above this range documented in the 24 hours before to 24 hours after start of antibiotic therapy). Moreover, a follow‐up potassium level had to be documented at 48–120 hours after initiation of antibiotic therapy. Patients were excluded if they had fewer than 48 consecutive hours of antibiotic treatment or received >1 antibiotic. Patients were also excluded if they were admitted to the intensive care unit, had kidney failure with a Modification of Diet in Renal Disease score (MDRD) <30 ml/min/1.73 m2 or received renal dialysis at start or during antibiotic treatment.
The primary endpoint of the study was the incidence of hypokalaemia during antibiotic therapy. Hypokalaemia was defined as ≥1 serum potassium level of <3.3 mmol/l measured at 48–120 hours after initiation of antibiotic therapy. The secondary endpoint was the difference in serum potassium level between the start of treatment and the follow‐up potassium documented. In the occasional situation where the potassium level was measured more than once in the follow‐up period, the lowest serum potassium level was used to calculate the total decline in serum potassium level for this patient during antibiotic treatment. In addition, information on the indication for antibiotic treatment, kidney function, use of diuretics and in‐hospital mortality (an indicator of disease severity) was documented.
Data are displayed as mean ± standard deviation for linear, normally distributed data and median (interquartile range) for non‐normally distributed data. Categorical data are represented by percentages (%). Statistical analysis of differences in baseline characteristics was performed using an independent samples t‐test or Mann–Whitney U test, and a χ2 test for categorical data. Within‐person potassium levels before and after treatment were tested with a paired t‐test. Differences in incidence of hypokalaemia between flucloxacillin‐ and ceftriaxone‐treated patients were tested using a stepwise logistic regression with forward selection to correct for potential confounders, with 3 variables in the final regression model: antibiotic treatment, potassium level at baseline and sex (tested independent variables: antibiotic treatment, age, sex, potassium levels at baseline, mortality rate, use of kaliuretic and potassium‐sparing diuretics, and kidney function). Differences in lowest potassium levels during treatment or decrease in potassium levels between the 2 investigated antibiotic treatments were tested using an ANCOVA model with age, sex, baseline potassium levels and the use of kaliuretic diuretics as covariates. In this analysis, the categorical covariates sex and use of kaliuretic drugs were recoded into dummy variables.
3. RESULTS
In total, 77 patients receiving flucloxacillin (62% male, mean age 70.5 years, range 32–96 years) and 84 patients receiving ceftriaxone (46% male, mean age 70.8 years, range 28–96 years) were included; both groups had similar potassium levels at baseline (mean 3.9 mmol/l, range 3.3–4.7 mmol/l). Men had slightly higher potassium levels than women at baseline (4.0 mmol/l vs 3.9 mmol/l, P < .05). There were more men in the flucloxacillin group than in the ceftriaxone group (62% and 46%, P <.05). As expected, the indication for antibiotic treatment differed between those receiving ceftriaxone and flucloxacillin. In those receiving ceftriaxone, the top 3 diagnoses at admission were complicated urinary tract infection or urosepsis (32%), pneumonia (21%), and sepsis of unknown origin (19%). In those receiving flucloxacillin therapy, this was Staphylococcus aureus bacteraemia (35%), cellulitis or wound infections (23%), or spondylodiscitis, arthritis or osteomyelitis (9%). Mild to moderate kidney failure (MDRD 30–60 ml/min/1.73 m2) occurred more often in patients who received ceftriaxone than flucloxacillin therapy (49% and 27%, P <.01). Kaliuretic diuretics (loop and thiazide diuretics), alone or in combination with potassium‐sparing diuretics, were used at least on 1 occasion by 26 patients receiving flucloxacillin, and 17 patients receiving ceftriaxone (34% vs 20%, P = .05). Potassium sparing diuretics were used by 5 patients receiving flucloxacillin, and by 1 patient receiving ceftriaxone, always in combination with kaliuretic diuretics. Finally, 28 patients died in‐hospital or were dismissed from hospital to receive palliative care at home and died within 4 weeks (18 patients receiving flucloxacillin, and 10 receiving ceftriaxone, P = .04). These patients were significantly older than nondeceased patients (mean age 78 years, range 43–96 years, compared to 69 years, range 24–96 years, respectively). Of these deceased patients, 36% had developed hypokalaemia during antibiotic treatment, compared to 26% in the nondeceased patients (P = .31). Cohort characteristics are displayed in Table 1.
Table 1.
Cohort characteristics
| Ceftriaxone (n = 84) | Flucloxacillin (n = 77) | ||
|---|---|---|---|
| Sex (% male) | 46% | 62% | P < .05 |
| Age, mean ± SD (y) | 70.8 ± 14.2 | 70.5 ± 14.9 | Ns |
| Baseline K+ level, mean ± SD (mmol/L) | 3.9 ± 0.38 | 3.9 ± 0.35 | Ns |
| Diuretic treatment | |||
| • kaliuretic diuretic (%) | 20% | 34% | P = .05 |
| • potassium‐sparing diuretic (%) (combined with kaliuretic diuretic) | 1% | 6% | Ns |
| MDRD >60 ml/min/1.73 m2 (%) | 51% | 73% | P < .01 |
| Mortality (%) | 12% | 23% | P < .05 |
MDRD, Modification of Diet in Renal Disease score.
After 48–120 hours of antibiotic treatment, 44 cases of hypokalaemia occurred. Of these, 32 cases occurred in the patients treated with flucloxacillin. Overall, hypokalaemia occurred in 42% of patients treated with flucloxacillin, compared to 14% of patients treated with ceftriaxone (Figure 1B; uncorrected P < 10−3, regression coefficient β = −1.52). Of all women receiving i.v. antibiotics, 38% developed hypokalaemia compared to 18% of all men. In those receiving kaliuretic diuretics, the incidence of hypokalaemia was slightly higher to those not receiving these drugs, although this did not reach statistical significance (36% and 23%, respectively, P = .09). In a forward‐entry stepwise logistic regression model, we found that next to antibiotic treatment (P < 10−4), sex (P < .001) and potassium levels at baseline (P = .03) were associated with the occurrence of hypokalaemia; however, incorporating these variables only strengthened the regression coefficient between antibiotic treatment and hypokalaemia (β = −2,06).
Figure 1.
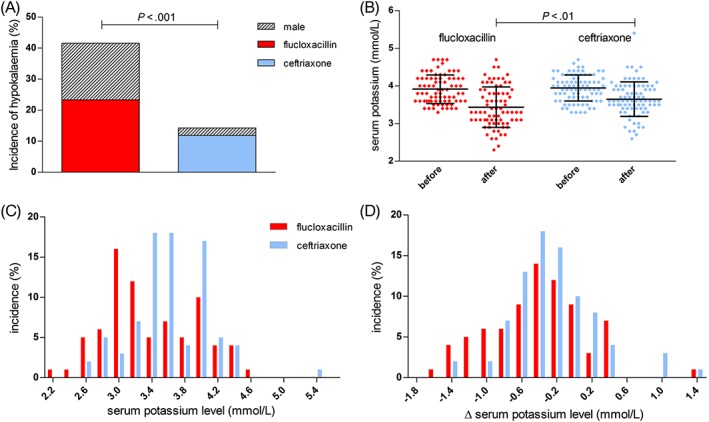
Intravenous flucloxacillin treatment is associated with a high incidence of hypokalaemia. A, Hypokalaemia was more frequent in patients on flucloxacillin than ceftriaxone treatment. Most patients developing hypokalaemia were female. B, after treatment, serum potassium levels were lower in patients on flucloxacillin treatment than ceftriaxone treatment (mean, SD). C, Histogram showing the distribution of potassium levels during treatment and D, the change in potassium level during treatment. ∆ delta, defined as change from baseline to follow‐up; K+, potassium; ns, nonsignificant
In 76% of patients, potassium levels were lower after treatment than at baseline (P < 10−7 for ceftriaxone, P < 10−10 for flucloxacillin). The decrease in potassium levels was larger in patients receiving flucloxacillin, with a mean decrease during antibiotic treatment of 0.5 ± 0.55 mmol/l compared to 0.3 ± 0.47 mmol/), reflected in a lower mean lowest level of potassium during treatment of 3.4 ± 0.54 mmol/l compared 3.6 ± 0.46 mmol/l in patients receiving ceftriaxone (both P < .01). The distribution of potassium levels and change in potassium level from baseline are shown in Figure 1B–D. Importantly, moderate to severe hypokalaemia (defined as a serum potassium level of ≤3.0 mmol/l) also occurred more frequently during flucloxacillin than ceftriaxone use: 23% of patients receiving flucloxacillin treatment developed moderate to severe hypokalaemia, compared to 10% of patients receiving ceftriaxone (P = .03). Compared to men, women showed a larger decrease in potassium level during treatment (0.5 ± 0.54 mmol/l vs 0.3 ± 0.43 mmol/l), and a lower follow‐up potassium level (3.4 ± 0.53 mmol/l vs 3.7 ± 0.43 mmol/l, P = .01).
4. DISCUSSION
Although hypokalaemia is described as a class‐effect of the penicillins, the awareness of this effect among clinicians is low, and the extent of this electrolyte imbalance had previously not been investigated in detail. We studied the incidence and magnitude of hypokalaemia in high‐dosed intravenous flucloxacillin treatment, one of the most commonly used high‐dose intravenous penicillin therapies in the Netherlands. In this retrospective cohort, treatment with high‐dosed intravenous flucloxacillin was associated with a 3 times higher risk of hypokalaemia than treatment with ceftriaxone. Hypokalaemia occurred in 42% of patients treated with flucloxacillin dosed ≥6 g/day, compared to 14% in ceftriaxone‐treated patients. This latter incidence is comparable to the general in‐hospital occurrence of hypokalaemia according to previous studies.11 Moreover, moderate to severe hypokalaemia—which can cause a spectrum of symptoms ranging from muscle weakness to cardiac arrhythmias and death—developed strikingly more during flucloxacillin than ceftriaxone therapy, occurring in over 23% compared to 10% of patients. In all cases, hypokalaemia occurred within 5 days after start of antibiotic therapy. This exemplifies the need of frequent potassium level monitoring after start of intravenous antibiotic treatment in general, but in particular of high‐dosed flucloxacillin.
Drug‐induced hypokalaemia is common in the in‐hospital setting. It is estimated that hypokalaemia occurs in 20% of all hospitalized patients, with 40% of cases attributed to drug use.11 In general, medication use can cause hypokalaemia via 2 mechanisms: either through renal or gastrointestinal losses, or through transcellular shifts. Both in the in‐hospital setting and in the general population, drug‐induced hypokalaemia is most frequently associated with the use of loop‐and thiazide diuretics, causing excessive renal potassium excretion.10, 11, 12 In the current cohort we found an incidence of hypokalaemia in those using kaliuretic diuretics of 36%, which further underlines the strikingly high incidence of hypokalaemia of 42% in those on flucloxacillin treatment. Being a multifactorial condition, we also investigated patient factors that could modify the risk of hypokalaemia. Kidney function did not predict the occurrence of hypokalaemia. However, we only included patients with mildly to moderately impaired kidney function, and only 12 patients had an MDRD <40 ml/min/1.73 m2. In contrast, female sex significantly predicted the risk of hypokalaemia and, in our cohort, 64% of all patients suffering from hypokalaemia after start of the antibiotic treatment was female. In several recent studies on dyskalaemia, hypokalaemia was also found to be more common in women than in men.10
There are several potential limitations of this study. First, its retrospective nature makes that group differences in baseline characteristics could potentially influence our outcomes. More men were included in the flucloxacillin than ceftriaxone group, but since we found male sex to be protective for hypokalaemia, this does not explain our results. Kaliuretic diuretics were used slightly more often in patients who received intravenous flucloxacillin, but correcting for kaliuretic drug use did not change our results. Also, the mortality rate was higher in patients receiving flucloxacillin, which could indicate that these patients had more severe disease. However, correction for this factor did not alter our results, nor did these patients develop hypokalaemia more often than nondeceased patients. Second, we lack information on the exact severity of illness of the study subjects, as well as information on food intake and possible gastrointestinal potassium loss. Third, the retrospective nature of this study does not allow us to prove causality. However, from a mechanistic perspective it is plausible that hypokalaemia occurs more frequently in flucloxacillin than ceftriaxone therapy. Both β‐lactam antibiotics, they are composed of a salt (most often sodium) and an attached nonreabsorbable anion that increases kaliuresis.1 The amount of nonreabsorbable anion can be estimated by calculating the amount of molars of sodium present in the daily dose of antibiotic therapy; the same amount of anion is administered. Since flucloxacillin dosed ≥6 g/day contains at least twice the amount of nonreabsorbable anion present in ceftriaxone, it has the potential to cause more potassium secretion in the distal tubule. Another mechanism that has been suggested to contribute to the hypokalaemia observed in high‐dosed penicillin administration is that of solute diuresis. Because of the high amount of sodium administered with intravenous fluids that accompany intravenous penicillin treatment, the flow rate in the cortical collecting duct is increased, as proportionally is the excretion of osmoles (including potassium).13 Indeed, in a case series of patients that developed hypokalaemia during flucloxacillin therapy, the presence of a high urine chloride concentration and polyuria argued for a combined pathophysiology of nonreabsorbable anion effects as well as osmotic diuresis as causal factors in the extensive kaliuresis.8 However, since the amount of sodium in high‐dose flucloxacillin treatment does not differ much from ceftriaxone treatment when compared to daily sodium administration in the in‐hospital setting, this is not likely to explain the differences found between ceftriaxone‐ and flucloxacillin‐treated patients. For example, approximately 300 mg sodium is administered with flucloxacillin 6 g/day compared to approximately 160 mg in ceftriaxone 2 g/day, both neglectable compared to the amount of sodium administered with standard intravenous fluid therapy (2 l/day NaCL 0.9%, or 18 g sodium chloride per day). Future studies are necessary to establish the exact mechanism by which the strong potassium lowering effect of flucloxacillin is caused.
In conclusion, in patients treated with high‐dosed intravenous flucloxacillin, hypokalaemia occurs with a strikingly higher incidence than in those treated with ceftriaxone. However, even in the latter, moderate to severe hypokalaemia is observed in 1 in 10 patients. Therefore, frequent and standardized monitoring of potassium levels is warranted after start of intravenous antibiotic treatment in general, but in particular of intravenous flucloxacillin therapy.
COMPETING INTERESTS
There are no competing interests to declare.
CONTRIBUTORS
Conceptualization, C.D.C.C.H., C.K., A.T.S.M.D., T.S., B.A.V., H.W.H.A.F.; methodology, C.D.C.C.H., C.K., A.T.S.M.D., H.W.H.A.F., B.A.V.; formal analysis, C.D.C.C.H., M.L.D. investigation, C.D.C.C.H., M.L.D.; writing—original draft, C.D.C.C.H.; writing—review and editing, C.D.C.C.H., M.L.D., C.K.; Supervision, C.K., A.T.S.M.D.
van der Heijden CDCC, Duizer ML, Fleuren HWHA, et al. Intravenous flucloxacillin treatment is associated with a high incidence of hypokalaemia. Br J Clin Pharmacol. 2019;85:2886–2890. 10.1111/bcp.13969
The authors confirm that the PI for this paper is Cornelis Kramers, MD, PhD, and that he had direct clinical responsibility for patients.
REFERENCES
- 1. Zietse R, Zoutendijk R, Hoorn EJ. Fluid, electrolyte and acid‐base disorders associated with antibiotic therapy. Nat Rev Nephrol. 2009;5(4):193–202. [DOI] [PubMed] [Google Scholar]
- 2. Brunner FP, Frick PG. Hypokalaemia, metabolic alkalosis, and hypernatraemia due to "massive" sodium penicillin therapy. Br Med J. 1968;4(5630):550–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoffbrand BI, Stewart JD. Carbenicillin and hypokalaemia. Br Med J. 1970;4(5737):746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zaki SA, Lad V. Piperacillin‐tazobactam‐induced hypokalemia and metabolic alkalosis. Indian J Pharmacol. 2011;43(5):609–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nanji AA, Lindsay J. Ticarcillin associated hypokalemia. Clin Biochem. 1982;15(2):118–119. [DOI] [PubMed] [Google Scholar]
- 6. Kibbler CC, Prentice HG, Sage RJ, et al. A comparison of double beta‐lactam combinations with netilmicin/ureidopenicillin regimens in the empirical therapy of febrile neutropenic patients. J Antimicrob Chemother. 1989;23(5):759–771. [DOI] [PubMed] [Google Scholar]
- 7. Johnson DW, Kay TD, Hawley CM. Severe hypokalaemia secondary to dicloxacillin. Intern Med J. 2002;32(7):357–358. [DOI] [PubMed] [Google Scholar]
- 8. Hoorn EJ, Zietse R. Severe hypokalaemia caused by flucloxacillin. J Antimicrob Chemother. 2008;61(6):1396–1398. [DOI] [PubMed] [Google Scholar]
- 9. Paltiel O, Salakhov E, Ronen I, Berg D, Israeli A. Management of severe hypokalemia in hospitalized patients: a study of quality of care based on computerized databases. Arch Intern Med. 2001;161(8):1089–1095. [DOI] [PubMed] [Google Scholar]
- 10. Nilsson E, Gasparini A, Ärnlöv J, et al. Incidence and determinants of hyperkalemia and hypokalemia in a large healthcare system. Int J Cardiol. 2017;245:277–284. [DOI] [PubMed] [Google Scholar]
- 11. Paice BJ, Paterson KR, Onyanga‐Omara F, Donnelly T, Gray JM, Lawson DH. Record linkage study of hypokalaemia in hospitalized patients. Postgrad Med J. 1986;62(725):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013;126(3):256–263. [DOI] [PubMed] [Google Scholar]
- 13. Halperin ML, Kamel KS. Potassium. Lancet. 1998;352(9122):135–140. [DOI] [PubMed] [Google Scholar]


