Abstract
Background:
The efficacy/safety of device-supported versus routine titration with Gla-300 in type 2 diabetes (T2DM) was evaluated.
Method:
AUTOMATIX was a 16-week, randomized, open-label, parallel-group, multicenter, noninferiority trial in insulin-treated or insulin-naïve people with T2DM. The fasting self-monitored plasma glucose (FSMPG) target was 90-130 mg/dL (5.0-7.2 mmol/L). Primary endpoint: proportion of participants achieving target FSMPG at week 16 without severe hypoglycemia. Secondary endpoints included: proportion reaching FSMPG target without confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycemia; time to first achieve FSMPG target; mean FSMPG and HbA1c change (baseline to week 16). Safety endpoints included hypoglycemia and adverse events. Patient-reported outcomes (PROs) were also assessed.
Results:
Participants were randomized to device-supported (n = 75) or routine titration (n = 76); 17 participants in the device-supported group discontinued device use. Noninferiority was achieved for the primary endpoint (device-supported: 45.9%, routine: 36.8%; weighted difference: 9.04 [95% CI: −6.75, 24.83]), but not superiority (P = .262). The proportion reaching FSMPG target range without confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycemia was 34.3% vs 14.5%, respectively. The time at which 50% of the participants achieved the FSMPG target was less in the device-supported than routine titration arm (10 vs 13 weeks). Least squares mean HbA1c reduction, safety profiles, and PROs were similar in both arms. Mean “ease of use” score for the device, assessed by healthcare professionals and participants on a scale of 1-7, was ≥6.
Conclusions:
Device-supported self-titration had a good safety/efficacy profile, and was noninferior to routine titration and well accepted by diabetes specialists and patients.
Keywords: basal insulin, blood glucose meter, hypoglycemia, titration
Type 2 diabetes (T2DM) is usually treated initially with oral antihyperglycemic drugs (OADs), but as the disease progresses many individuals need insulin to maintain glycemic control.1 Despite recommendations that basal insulin (BI) therapy is initiated in those unable to achieve or maintain the recommended HbA1c target (<7.0 %, 53 mmol/mol) after 3 months at maximum tolerated doses of OADs,2 suboptimal glycemic control with OADs may continue for up to 7 years before insulin initiation.3 Even after BI is initiated, approximately 70% of individuals are unable to titrate the dose appropriately and fail to reach recommended glycemic targets;4 if targets are initially achieved, subsequently over 50% of these individuals fail to maintain long-term glycemic control.5 The failure to achieve or maintain glycemic control can be attributed to a significant delay in the initiation and dose optimization of BI, often termed as “clinical inertia,” and defined as the “failure of healthcare providers to initiate or intensify therapy when indicated.”6 Addressing issues that contribute to clinical inertia (eg, fear of undesirable side effects, lack of self-confidence in adhering to complex regimens, lack of trust in the efficacy of insulin, etc),7 and achieving optimal BI-dose titration is key to ensuring individuals achieve and maintain optimal-glucose control.3
Healthcare providers (HCPs) should strike a balance between the need for tight glycemic control early in T2DM, with its associated benefits of reduced risk of macrovascular and microvascular complications, myocardial infractions and death,8,9 with the risk of hypoglycemia.10 In the real-world setting BI dose is often titrated at the treating physician’s discretion during routine-clinic visits. While such visits provide support for individuals and allow HCPs to provide consistent advice and simple treatment algorithms, they may be infrequent (eg, at 3-month intervals or longer), and delay insulin intensification. Empowering and supporting people with T2DM to self-titrate their BI dose could enable more individuals to achieve optimal glycemic control with fewer delays.11 For example, better glycemic control with BI has been observed when dose titration was self-managed every 3 days rather than physician-led weekly dose titration.12 It has also been observed that when individuals with T2DM are involved in treatment decision-making, their understanding of diabetes care increases and positively impacts upon their self-management.13 Device-supported self-titration may empower individuals with T2DM by allowing them to make informed treatment decisions without having to rely as much on HCPs and may also improve understanding of dose optimization to better self-manage their condition.
MyStar DoseCoach® is an integrated titration device/blood glucose meter designed to assist people with T2DM to self-titrate insulin glargine by providing automated dosing suggestions. The AUTOMATIX study aimed to compare the efficacy and safety of a device-supported treat-to-target regimen versus diabetes knowledgeable investigator-recommended routine titration with Gla-300 in people with T2DM.
Methods
Study Design and Participants
AUTOMATIX (NCT02585674) was an open-label, randomized, controlled, parallel-group, multicenter, phase 3 study in people with T2DM conducted at 19 study centers (Supplementary Figure 1). The study was performed in accordance with the Good Clinical Practice and the Declaration of Helsinki. All participants provided written informed consent.
Participants were aged ≥18 years, with T2DM for ≥1 year, either insulin-naïve or previously treated with BI, with HbA1c between 7.5-11.0% (58-97 mmol/mol inclusive) and fasting self-monitored plasma glucose (FSMPG) >130 mg/dL (7.2 mmol/L). Key exclusion criteria (Supplementary Materials) included diabetes other than T2DM, device-supported titration not being appropriate or use of device otherwise contraindicated (in the opinion of the investigator), and the use of mealtime insulin for more than 10 days in the last 3 months before screening.
Randomization and Treatment
All participants were given a titration device/blood glucose meter (MyStar DoseCoach, Agamatrix Inc, Salem, NH, USA), and self-administered Gla-300 subcutaneously once-daily. Participants were randomized 1:1 to either device-recommended titration (the titration feature was activated by investigator at randomization visit 3) or routine titration (titration feature of the device turned off) as recommended by the investigator, who were diabetes specialists, stratified by previous use of insulin (insulin naïve vs insulin treated). For insulin-naïve participants, the starting daily dose of Gla-300 was 0.2 U/kg body weight. Participants on previous BI therapy were switched to the same daily dose if they had been receiving once-daily Gla-100/neutral protamine Hagedorn (NPH) insulin/insulin detemir and to 80% of the previous daily dose if they had been receiving more than once-daily NPH insulin/insulin detemir.
Dosing recommendations (Supplementary Table 1) for participants randomized to the device-supported titration were provided by the device titration meter after a minimum of 3 consecutive days of FSMPG and insulin dose data, based on a FSMPG target range of 90-130 mg/dL (5.0-7.2 mmol/L). Participants who discontinued device-supported titration recommendations, for whatever reason, continued until the study end and followed the titration recommendations provided by the investigator from the time of discontinuation.
Outcomes
The primary efficacy endpoint was the percentage of participants reaching a FSMPG target of 90-130 mg/dL (5.0-7.2 mmol/L) following 16 weeks of treatment without severe hypoglycemia. Reaching the FSMPG target range required the mean of the last five FSMPG readings recorded in the previous 2 weeks to be within the target range before the end of the 16-week on-treatment period.
Secondary efficacy endpoints included percentages of participants reaching target FSMPG range (90-130 [5.0-7.2 mmol/L]) following 16 weeks of treatment without confirmed (≤70 mg/dL [3.9 mmol/L] or <54 mg/dL [3.0 mmol/L]) or severe hypoglycemia events, time to first reach FSMPG target, change in mean FSMPG, change in HbA1c and mean central laboratory measured fasting plasma glucose (FPG) from baseline to week 16, and percentage of participants with FPG in the target range of 90-130 mg/dL (5.0-7.2 mmol/L) without severe hypoglycemia at the week 16 time point.
Safety endpoints included hypoglycemia, categorized based upon American Diabetes Association (ADA) definitions (Supplementary Methods),2 adverse events (AEs), and meter- and pen-related events as reported by the participant or noted by the investigator. Hypoglycemia endpoints included the percentage of participants reporting ≥1 events.
Patient-reported outcomes (PROs) were assessed using PRO/questionnaires including the Diabetes Treatment Satisfaction Questionnaire (DTSQs),14 the Hypoglycemia Fear Survey (HFS-II),15 the Diabetes Distress Scale,16 the Glucose Monitoring Satisfaction Survey (GMS),17 with emotional well-being analyzed using the WHO-5 well-being index.18 Ease of use of the device was assessed in HCPs and participants during week 16 using questionnaires consisting of a series of questions to which responses were rated from 1 (extremely difficult) to 7 (extremely easy).
Statistical Analysis
A sample size of 148 participants (74 per titration arm) was estimated to demonstrate noninferiority of the device-supported arm with a 0.15 margin for the difference versus routine titration, 80% power and 2.5% one sided alpha; assuming the proportion reaching the FSMPG target range without severe hypoglycemia during the on-treatment period was 0.42 (routine-titration) and 0.50 (device-supported titration). All efficacy endpoints were analyzed or summarized for the 16-week on-treatment period using the modified intent-to-treat (mITT) population (Supplementary Materials), unless otherwise specified.
The primary endpoint was analyzed using a multiple imputation approach (Supplementary Materials), using effect estimators of titration regimen, weighted by the randomization stratum of previous use of insulin. A stepwise closed-testing approach was used to first assess noninferiority followed by superiority of device-supported versus routine titration. Noninferiority required the lower bound of the two-sided 95% CI for the difference in percentage of participants between titration arms to be greater than the predefined noninferiority margin of −15%. If the noninferiority was demonstrated, superiority required the lower bound of the two-sided 95% CI for the weighted difference in the percentage of participants between titration arms to be >0.
A similar multiple imputation approach was used to assess the secondary efficacy endpoints related to percentage of participants reaching FSMPG target range without a hypoglycemic event. Change in mean FSMPG from baseline to the end of the 16-week on-treatment period was analyzed using a mixed model for repeated measures (MMRM) approach (Supplementary Materials). Time to first FSMPG target range of 90-130 mg/dL (5.0-7.2 mmol/L) was defined by the first 2-week period in which the mean FSMPG of the last 5 values was within target (Supplementary Materials). Change in HbA1c from baseline to week 16 was analyzed using an analysis of covariance (ANCOVA) model (Supplementary Materials). Change in mean FPG from baseline to week 16 was analyzed using a MMRM approach, while the prespecified FPG target was analyzed using a Cochran Mantel Haenszel (CMH) method (Supplementary Materials). Safety analyses were descriptive and based on the safety population (Supplementary Materials).
The change in score from baseline to week 16 for each PRO/questionnaire was analyzed in the mITT population using ANCOVA. The percentage of PROs responders based on the minimum clinically important difference (MCID) was analyzed using a CMH method (Supplementary Materials).
Results
Study Population
In total, 151 participants with T2DM were enrolled from 19 centers (device-supported titration, n = 75; routine titration, n = 76) (Supplementary Figure 2). All participants were exposed to Gla-300 and included in the safety and mITT populations. Five participants (6.7%) in the device-supported titration arm did not complete the study period and permanently discontinued Gla-300 treatment (Supplementary Figure 2). Overall, 17/75 (23%) of the participants randomized to the device-supported arm discontinued use of device, 9 (12%) due to misunderstanding the device titration function and 8 (11%) due to other reasons including withdrawal of consent (Supplementary Figure 2). Baseline characteristics (Table 1) were generally well balanced across the two arms with a slightly higher proportion of females in the device-supported titration versus routine titration arm (36.0% vs 26.3%). All enrolled participants were Caucasians.
Table 1.
Baseline Demographics and Patient Characteristics (Randomized Population).
| Device-supported titration n = 75 |
Routine titration n = 76 |
All N = 151 |
|
|---|---|---|---|
| Age, years, mean (SD) | 61.2 (9.5) | 62.9 (9.4) | 62.1 (9.5) |
| Age group, years, n (%) | |||
| <65 | 43 (57.3) | 42 (55.3) | 85 (56.3) |
| 65-75 | 29 (38.7) | 24 (31.6) | 53 (35.1) |
| ≥75 | 3 (4.0) | 10 (13.2) | 13 (8.6) |
| Sex, n (%) | |||
| Male | 48 (64.0) | 56 (73.7) | 104 (68.9) |
| Female | 27 (36.0) | 20 (26.3) | 47 (31.1) |
| Race, Caucasian, n (%) | 75 (100) | 76 (100) | 151 (100) |
| Body Weight, kg, mean (SD) | 96.9 (24.0) | 100.0 (23.8) | 98.5 (23.8) |
| BMI, kg/m2, mean (SD) | 33.2 (6.9) | 33.3 (7.0) | 33.2 (6.9) |
| BMI categories, kg/m2, n (%) | |||
| <25 | 5 (6.7) | 5 (6.6) | 10 (6.6) |
| 25-30 | 24 (32.0) | 20 (26.3) | 44 (29.1) |
| 30-40 | 35 (46.7) | 37 (48.7) | 72 (47.7) |
| ≥40 | 11 (14.7) | 14 (18.4) | 25 (16.6) |
| Estimated GFR, L/min/1.73m2, mean (SD) | 82.02 (27.60) | 84.04 (24.02) | 83.04 (25.80) |
| Estimated GFR categories, n (%) | |||
| ≥90 | 22 (29.3) | 33 (43.4) | 55 (36.4) |
| 60-90 | 40 (53.3) | 30 (39.5) | 70 (46.4) |
| 30-60 | 13 (17.3) | 13 (17.1) | 26 (17.2) |
| Randomization stratuma (previous insulin use) | |||
| Insulin-naïve | 30 (40.0) | 30 (39.5) | 60 (39.7) |
| Insulin-pretreated | 45 (60.0) | 46 (60.5) | 91 (60.3) |
Due to stratification errors, 4 insulin pretreated participants were randomized as insulin-naïve and 1 insulin-naïve participant was randomized as insulin pretreated.
Efficacy Outcomes
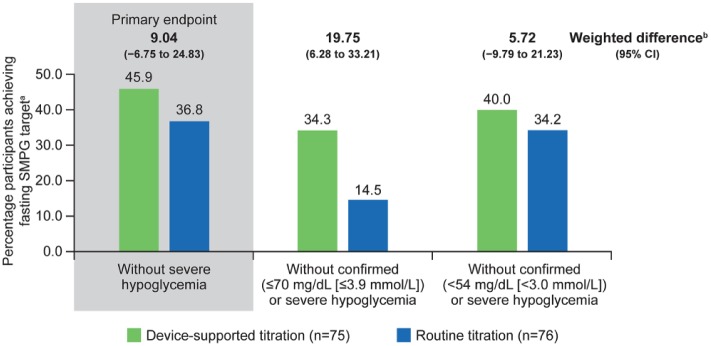
The percentage of participants who achieved the primary endpoint, FSMPG in the target range of 90-130 mg/dL after 16-weeks of treatment without severe hypoglycemia, was 45.9% in the device-supported titration arm compared with 36.8% in the routine-titration arm (weighted difference: 9.04 [95% CI: −6.75 to 24.83]; Figure 1). Noninferiority of the device-supported versus the routine-titration arm was demonstrated as the lower bound of the 95% CI for the weighted difference in percentage of patients between arms (9.04; 95% CI [−6.748 to 24.829]) was greater than the predefined noninferiority margin of −15%. Superiority of device-supported versus routine-titration was not statistically shown (P = .262).
Figure 1.
Estimated percentage of participants achieving target FSMPG without hypoglycemia during the 16-week on-treatment period (modified intent-to-treat population). aEstimated proportion of participants was obtained using a multiple imputation method to address missing values in the mITT population. bEstimated weighted difference of proportions obtained by combining the difference in percentage, weighted by the randomization stratum of previous use of insulin (insulin naïve, insulin pretreated), between titration groups of all different imputed data sets.
The percentage of participants who reached the FSMPG target range without confirmed (≤70 mg/dL [≤3.9 mmol/L]) or severe hypoglycemia was higher in the device-supported than the routine titration group (34.3% vs 14.5%; [weighted difference: 19.75 (95% CI: 6.28 to 33.21)]; Figure 1). A comparable proportion of participants in the device-supported and routine-titration arm (40.0% vs 34.2%) reached the FSMPG target range without confirmed (<54 mg/dL [<3.0 mmol/L]) or severe hypoglycemia.
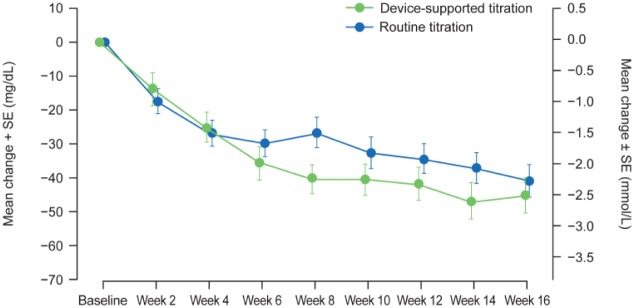
The Kaplan-Meier cumulative incidence curves of participants reaching the FSMPG target of 90-130 mg/dL showed an overall shorter time to reach the FSMPG target in the device-supported titration arm than the routine arm, but this trend was driven by participants who did not reach the target within the first 8 weeks (P = .171). The time at which 50% of the participants achieved the FSMPG target was less in the device-supported than in the routine-titration arm (10 weeks [95% CI: 8-10] vs 13-weeks [95% CI: 6-16], respectively). Mean FSMPG (mean of the last 5 readings recorded over the last 2 weeks) reduced from baseline to the week 16 time-point in both titration arms (least squares [LS] mean change −41.7 mg/dL vs −43.3 mg/dL; Table 2 and Figure 2).
Table 2.
Secondary Efficacy Outcomes During the 16 Week On-treatment Period (Modified Intent-to-Treat Population).
| Device-supported titration n = 75 |
Routine titration n = 76 |
LS mean difference (SE) vs routine titration, 95% CI | |
|---|---|---|---|
| Change in mean FSMPG from baseline to week 16 time-point, LS mean (SE) mg/dL | −41.70 (3.32) | −43.26 (3.18) | 1.56 (4.60), −7.55 to 10.66 |
| Change in HbA1c from baseline to week 16, LS mean (SE) % | −1.12 (0.09) | −1.07 (0.08) | −0.05 (0.12), −0.29 to 0.19 |
| Change in FPG from baseline to week 16, LS mean (SE) mg/dL | −44.05 (4.26) | −49.46 (4.08) | 5.40 (5.91), −6.28 to 17.09 |
| Device-supported titration n = 75 |
Routine titration n = 76 |
RR (95% CI) vs routine titrationa | |
| Laboratory measured FPG at target (90-130 mg/dL [5.0-7.2 mmol/L]) at week 16 without severe hypoglycemia, n (%) | 22 (29.3) | 33 (43.4) | 0.67 (0.438 to 1.039) |
Based on RR stratified by randomization stratum of previous use of insulin (insulin naïve, insulin pretreated), using a CMH (Cochran Mantel Haenszel) methodology.
Figure 2.
Mean change in FSMPG over the 16-week on treatment period (modified intent-to-treat population).
The LS mean reduction in HbA1c from baseline to week 16 was similar in the device-supported (−1.12%) and the routine-titration (−1.07%) arms (Table 2).
For laboratory measured FPG, both titration groups showed reductions from baseline to week 16, although the reductions were slightly lower in the device-supported than the routine-titration arm (−44.05 mg/dL vs −49.46 mg/dL). The percentage of participants with laboratory measured FPG in the target range of 90-130 mg/dL (5.0-7.2 mmol/L) without severe hypoglycemia at week 16 was also higher in the routine-titration arm (29.3% vs 43.4%; Table 2), which may be due to a higher mean (standard deviation [SD]) FPG value at baseline in the device-supported arm (192.30 [39.83] mg/dL vs 186.78 [47.15] mg/dL).
Basal Insulin Dose
During the study, BI dose rose steadily in both treatment arms and the change in average BI dose from baseline to week 16 was 0.213 (SD: 0.185) U/kg and 0.157 (SD: 0.153) U/kg in the device-supported and routine-titration arms, respectively.
Safety
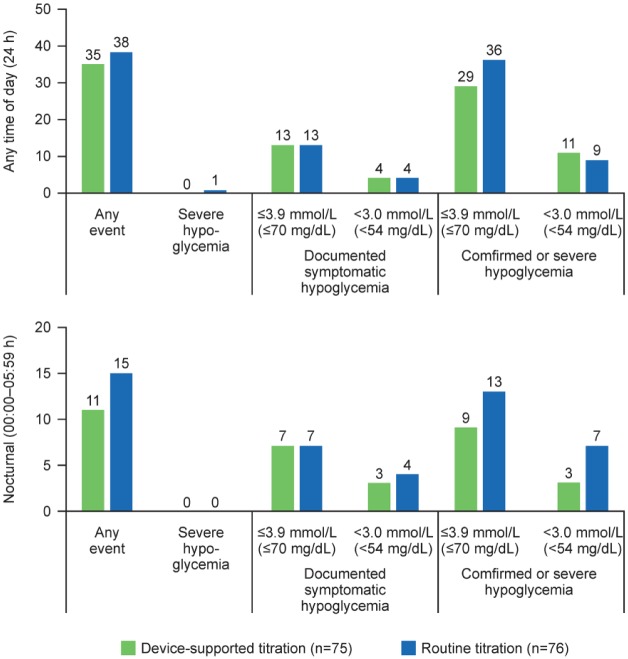
The percentage of participants with at least one hypoglycemic event in any category including nocturnal (00:00-05:59 h) hypoglycemia during the on-treatment period was generally comparable between both the titration arms (Figure 3). A slightly higher proportion of participants in the routine-titration arm reported at least one asymptomatic hypoglycemic event (27.6%), and at least one confirmed (≤70 mg/dL [3.9 mmol/L]) or severe hypoglycemic event (35.5%) during the on-treatment period compared with that in the device-supported arm (20.0% and 29.3%, respectively). One case of severe hypoglycemia was reported in the routine-titration arm.
Figure 3.
Incidence (%) of participants experiencing ≥1 hypoglycemic event during the on-treatment period (safety population).
The safety profile was comparable between the titration arms (Supplementary Table 2). For participants in the device-supported arm, the proportion of treatment-emergent adverse events (TEAEs) during on-treatment period and device-support period were similar. The proportion of TEAEs was slightly higher in the device-supported versus routine titration arm (45.3% vs 38.2%). However, the proportion of serious TEAEs was slightly lower in the device-supported versus routine-titration arm (2.7% vs 3.9%). No TEAE resulted in treatment discontinuation or death.
The percentage of participants with at least one meter-related event (MRE) was higher in the device-supported arm compared with the routine-titration arm (70.7% vs 9.2%) and mainly related to the functionality of the device (not activated in the routine-titration arm as per protocol) (Supplementary Table 2). The percentage of participants with at least one pen-related event(s) was low and comparable between the device-supported and routine-titration arms (4.0% vs 3.9%).
Patient-Reported Outcomes
Overall, there were no major differences in PROs between the device-supported and routine-titration arms (Supplementary Table 3). For DTSQ total treatment satisfaction score, the LS mean change from baseline to week 16 was 4.46 using routine-titration versus 2.90 using device-supported titration (Supplementary Table 3). The LS mean change in HFS-II scores were similar for both titration arms at week 16 (Supplementary Table 3). There was little change in Diabetes Distress Scale scores from baseline to week 16 for both titration arms with no clinically relevant differences between arms in the proportion of participants reaching the MCID (Supplementary Table 3). Improvements in GMS from baseline to week 16 was seen in both titration arms (Supplementary Table 3). LS mean change in WHO-5 well-being index scores from baseline to week 16 were −0.03 and 6.20 for device-supported group and routine group, respectively (Supplementary Table 3).
Participants were asked to rate how easy or difficult it was to use the device on a scale of 1-7 with 1 = very difficult and 7 = very easy. The mean scores for how easy it was “to decide what insulin dose to take,” “to do the dose calculations correctly,” and “to adjust their insulin dose” were 6.11, 6.07, and 6.24 indicating that individuals found the device easy to use.
Discussion
AUTOMATIX indicates that device-supported titration with Gla-300 was statistically noninferior to diabetes knowledgeable, investigator-led routine titration in achieving the FSMPG target of 90-130 mg/dL (5.0-7.2 mmol/L) without experiencing severe hypoglycemia. Most people with T2DM and HCPs found the device easy to use. As discussed below, however, the higher number of MRE, and the finding that 23% of individuals in the device-supported arm discontinued using the device indicates that further improvements to the training/support provided to users, or changes to improve device ease of use, may be required.
Previously it has been suggested that based on their knowledge, expertise or interest in the use of technology, some individuals may need additional guidance and support as they may not like technical language while others may need more extensive details.19 Participants had a mean age >60 years, with 44% being aged 65 years or over, a group at higher risk of cognitive impairment compared with younger-age groups,2 which may impact on their ability to correctly understand instructions and use the device. In total, 17 participants (23%, mean age: 60.6 years; range: 42-74 years) discontinued device-supported titration, of whom 9 (12%) discontinued due to a misunderstanding of the device function. This limited number of participants does not allow an assessment of whether older age and cognitive ability may have been a contributing factor. However, as approximately 70% of individuals in the device-supported group experienced MREs, which included user error in operating the device (eg, not tagging the FSMPG reading or the incorrect inputting of the insulin dose used), misunderstanding device instructions, and device malfunctions, it appears that increasing participant age is unlikely to underpin these errors. There were also some minor differences in patient-reported treatment satisfaction and well-being that favored routine titration that did not reflect any disparities in HCP contact between the groups. Overall, these findings suggest that discontinuations could be a potential challenge in managing compliance with device use in a minority of individuals, and that this device may not be suitable for all individuals with T2DM who wish to use device-supported titration. These outcomes highlight the need for careful selection of patients in whom use of the device is most appropriate and the need to provide appropriate training in its use. To facilitate this, based on AUTOMATIX, the device user interface has subsequently been optimized and the training materials reworked, for example, to simplify tagging of the FSMPG reading (Supplementary Table 4).
Technological advances to aid insulin titration have demonstrated improved outcomes and safety in both type 1 diabetes (T1DM) and T2DM in several studies.20-25 The INNOVATE study examined titration with insulin glargine 100 U/mL (Gla-100) using the long-acting insulin glargine titration web tool (LTHome) in T2DM,26 using the same rules and engine-based algorithm for titration as that for MyStar DoseCoach. Of note, a similar percentage of participants reached FPG targets without experiencing hypoglycemia with LTHome as with device-supported titration observed in AUTOMATIX (47% vs 45.9%, respectively). There were similar HbA1c reductions in the LTHome versus the enhanced usual therapy (EUT) arm, but with the EUT arm receiving more HCP resources. While cost-effectiveness data were not collected in AUTOMATIX, the potential for device-supported titration to cut healthcare costs by enabling people with T2DM to achieve glycemic targets while reducing the involvement of physicians and other ancillary healthcare services is of interest.
In AUTOMATIX, outcomes reported for the routine-titration arm were probably better than that observed in the real world. Participants in AUTOMATIX were instructed by investigators (all diabetes specialists with extensive experience working with T2DM) on the recommended method for titration of Gla-300 at each scheduled visit, whereas in real life, routine BI titration in many people with T2DM is not as well managed and follow-up visits are generally less frequent than the clinical-trial setting. Therefore, the numerical difference seen in AUTOMATIX might be predictive of more clinically meaningful differences in real-life clinical practice, and it is possible that differences in outcomes between device-titration and routine-titration could be better demonstrated using a real-world study. While the performance of device-supported titration is less likely to be affected by infrequent clinic visits in clinical practice, outcomes in the routine-titration group may be poorer.
The limitations of AUTOMATIX are those inherently associated with the use of devices and device-titration. The open-label trial design and the use of block randomization instead of cluster randomization could have been a potential source of bias. Dosing recommendations were not standardized for the routine-titration arm, and were at the discretion of each investigator, which may have contributed to dosing variability and influenced target achievement; although, this may also be considered a strength of the study as it ensures that the control is more representative of real-life practice despite the caveats mentioned above. Several variables may have influenced target achievement in the routine-titration arm that were not able to be controlled (eg, participant behavior, potential investigator bias, and participant education on how to titrate). Lastly, the short trial duration may have been insufficient to allow observations on long-term challenges in adherence to titration device, and longer term, real-world studies would be of interest.
Conclusions
Device-supported titration with Gla-300 demonstrated a good safety profile and was noninferior to routine titration (led by diabetes specialists), with a trend toward shorter times being needed to reach FSMPG target. While further work to support people with T2DM in terms of making the device easier to use and providing suitable training materials is required, this study provides additional support for device-supported insulin titration. By helping individuals to make timely and sensible dosing choices, devices such as MyStar DoseCoach and other innovative technologies may help to address the clinical inertia in optimizing insulin dosing.
Supplemental Material
Supplemental material, DAVIES_AUTOMATIX_Supplementary_Material for A Randomized Controlled, Treat-to-Target Study Evaluating the Efficacy and Safety of Insulin Glargine 300 U/mL (Gla-300) Administered Using Either Device-Supported or Routine Titration in People With Type 2 Diabetes by Melanie Davies, Steve Bain, Guillaume Charpentier, Frank Flacke, Harmonie Goyeau, Michael Woloschak, Christoph Hasslacher, Giacomo Vespasiani and Steven Edelman in Journal of Diabetes Science and Technology
Acknowledgments
Editorial and writing assistance was provided by Sharon Eastwood (DPhil) of Fishawack Communications and was funded by Sanofi.
Footnotes
Abbreviations: ADA, American Diabetes Association; AE, adverse event; ANCOVA, analysis of covariance; BI, basal insulin; BMI, body mass index; CI, confidence interval; CMH, Cochran Mantel Haenszel; DTSQs, Diabetes Treatment Satisfaction Questionnaire status version; EUT, enhanced usual therapy; FPG, fasting plasma glucose; FSMPG, fasting self-monitored plasma glucose; GFR, glomerular filtration rate; GMS, Glucose Monitoring Satisfaction Survey; HCP, health care provider; HFS-II, Hypoglycemia Fear Survey; LS, least squares; MCID, minimum clinically important difference; mITT, modified intent-to-treat; MMRM, mixed model for repeated measures; MRE, meter-related event; NPH, neutral protamine Hagedorn; OAD, oral antihyperglycemic drug; PRO, patient-reported outcome; RR, relative risk; SD, standard deviation; SE, standard error; T1DM, type 1 diabetes; T2DM, type 2 diabetes; TEAE, treatment-emergent adverse event.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MD—Advisory board: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Novo Nordisk, Sanofi, Servier; Consultant: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi; Speaker’s bureau: AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, Takeda, Tanabe; Research support: Boehringer Ingelheim, Janssen, Novo Nordisk. SB—Advisory panel: Sanofi, Novo Nordisk, Eli Lilly, Boehringer Ingelheim, Merck, Johnson & Johnson; Research support: Novo Nordisk, Merck. GC—Advisory panel: Eli Lilly; Board member: Sanofi, Eli Lilly, Novo Nordisk, Boehringer Ingelheim; Consultant: AstraZeneca. FF—Employee: Sanofi; Stock/shareholder: Sanofi. HG—Employee: Sanofi; Stock/shareholder: Sanofi. MW—Employee: Sanofi; Stock/shareholder: Sanofi. CH—No conflicts of interest to declare. GV—Advisory panel: Sanofi; Consultant: Meteda. SE—Board member: Senseonics; Speaker’s bureau and advisory panel: AstraZeneca, Dexcom, Eli Lilly, MannKind, Merck, Novo Nordisk, Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The AUTOMATIX study was sponsored by Sanofi.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Nelson SE, Palumbo PJ. Addition of insulin to oral therapy in patients with type 2 diabetes. Am J Med Sci. 2006;331:257-263. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association. Standards of medical care in diabetes—2017. Diabetes Care. 2017;39:S1-S135. [Google Scholar]
- 3. Khunti K, Wolden ML, Thorsted BL, Andersen M, Davies MJ. Clinical inertia in people with type 2 diabetes: a retrospective cohort study of more than 80,000 people. Diabetes Care. 2013;36:3411-3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stark CS, Fradkin JE, Saydah SH, Rust KF, Cowie CC. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988-2010. Diabetes Care. 2013;36:2271-2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu N, Aagren M, Boulanger L, Friedman M, Wilkey K. Assessing achievement and maintenance of glycemic control by patients initiating basal insulin. Curr Med Res Opin. 2012;28:1647-1656. [DOI] [PubMed] [Google Scholar]
- 6. Khunti S, Davies MJ, Khunti K. Clinical inertia in the management of type 2 diabetes mellitus: a focused literature review. Br J Diabetes. 2015;15:65-69. [Google Scholar]
- 7. Khunti K, Millar-Jones D. Clinical inertia to insulin initiation and intensification in the UK: a focused literature review. Prim Care Diabetes. 2017;11:3-12. [DOI] [PubMed] [Google Scholar]
- 8. Stratton IM, Adler AI, Neil HA. et al. Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study. BMJ. 2000;321:405-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359:1577-1589. [DOI] [PubMed] [Google Scholar]
- 10. Bailon RM, Cook CB, Hovan MJ. et al. Temporal and geographic patterns of hypoglycemia among hospitalized patients with diabetes mellitus. J Diabetes Sci Technol. 2009;3:261-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Khunti K, Davies MJ, Kalra S. Self-titration of insulin in the management of people with type 2 diabetes: a practical solution to improve management in primary care. Diabetes Obes Metab. 2013;15:690-700. [DOI] [PubMed] [Google Scholar]
- 12. Davies M, Storms F, Shutler S, Bianchi-Biscay M, Gomis R, Group AS. Improvement of glycemic control in subjects with poorly controlled type 2 diabetes: comparison of two treatment algorithms using insulin glargine. Diabetes Care. 2005;28:1282-1288. [DOI] [PubMed] [Google Scholar]
- 13. Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. J Gen Intern Med. 2002;17:243-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bradley C, Plowright R, Stewart J, Valentine J, Witthaus E. The Diabetes Treatment Satisfaction Questionnaire change version (DTSQc) evaluated in insulin glargine trials shows greater responsiveness to improvements than the original DTSQ. Health Qual Life Outcomes. 2007;5:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Graue M, Iversen MM, Wentzel-Larsen T, Rokne B, Haugstvedt A. Assessing fear of hypoglycemia among adults with type 1 diabetes—psychometric properties of the Norwegian version of the Hypoglycemia Fear Survey II questionnaire. Norsk Epidemiologi. 2013;23:75-81. [Google Scholar]
- 16. Polonsky WH, Fisher L, Earles J. et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626-631. [DOI] [PubMed] [Google Scholar]
- 17. Polonsky WH, Fisher L, Hessler D, Edelman SV. Development of a new measure for assessing glucose monitoring device-related treatment satisfaction and quality of life. Diabetes Technol Ther. 2015;17:657-663. [DOI] [PubMed] [Google Scholar]
- 18. Hajos TR, Pouwer F, Skovlund SE. et al. Psychometric and screening properties of the WHO-5 well-being index in adult outpatients with type 1 or type 2 diabetes mellitus. Diabet Med. 2013;30:e63-e69. [DOI] [PubMed] [Google Scholar]
- 19. Turner J, Larsen M, Tarassenko L, Neil A, Farmer A. Implementation of telehealth support for patients with type 2 diabetes using insulin treatment: an exploratory study. Inform Prim Care. 2009;17:47-53. [DOI] [PubMed] [Google Scholar]
- 20. Russell SJ, Hillard MA, Balliro C. et al. Day and night glycaemic control with a bionic pancreas versus conventional insulin pump therapy in preadolescent children with type 1 diabetes: a randomised crossover trial. Lancet Diabetes Endocrinol. 2016;4:233-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Charpentier G, Benhamou PY, Dardari D. et al. The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care. 2011;34:533-539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franc S, Borot S, Ronsin O. et al. Telemedicine and type 1 diabetes: is technology per se sufficient to improve glycaemic control? Diabetes Metab. 2014;40:61-66. [DOI] [PubMed] [Google Scholar]
- 23. Daoudi A, Joubert M, Franc S. et al. A Smartphone for adjustment of basal insulin dose and for coaching: benefits in terms of glycaemic control for type 2 diabetes patients. Diabetologia. 2013;56:S426. [Google Scholar]
- 24. Hsu WC, Lau KH, Huang R. et al. Utilization of a cloud-based diabetes management program for insulin initiation and titration enables collaborative decision making between healthcare providers and patients. Diabetes Technol Ther. 2016;18:59-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergenstal RM, Bashan E, McShane M, Johnson M, Hodish I. Can a tool that automates insulin titration be a key to diabetes management? Diabetes Technol Ther. 2012;14:675-682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bajaj HS, Venn K, Ye C, Aronson R. Randomized trial of long-acting insulin glargine titration web tool (LTHome) versus enhanced usual therapy of glargine titration (INNOVATE Trial). Diabetes Technol Ther. 2016;18:610-615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DAVIES_AUTOMATIX_Supplementary_Material for A Randomized Controlled, Treat-to-Target Study Evaluating the Efficacy and Safety of Insulin Glargine 300 U/mL (Gla-300) Administered Using Either Device-Supported or Routine Titration in People With Type 2 Diabetes by Melanie Davies, Steve Bain, Guillaume Charpentier, Frank Flacke, Harmonie Goyeau, Michael Woloschak, Christoph Hasslacher, Giacomo Vespasiani and Steven Edelman in Journal of Diabetes Science and Technology