Abstract
Background:
Optimal glucose control has been shown to be useful in critical care as well as in other settings. Glucose concentrations in patients admitted to critical care are characterized by marked variability and hypoglycemia due to inadequate sensing and treatment technologies.
Methods:
The insulin balanced infusion system (IBIS) is a closed-loop system that uses a system controller, two syringe pumps, and capillary glucose sensor intravenously infusing regular insulin and/or dextrose. The IBIS performance was evaluated in terms of glucose stability in response to various conditions in subjects with type 1 and insulin requiring type 2 diabetes mellitus (n = 15) with frequent intermittent capillary measurements, entered into the system and an adaptive algorithm adjusting the treatment modalities without other nursing intervention.
Results:
Target glucose concentrations (80-125 mg/dl) were achieved from hyperglycemic levels in 2.49 hours in the first study with mean and standard deviation of 105.2 mg/dl and 11.5 mg/dl, respectively.
Conclusion:
Preliminary studies using a prototype closed-loop glucose control system for critical care produced noticeable results. Improvements were initiated within the system and further studies performed.
Keywords: automated glucose monitoring, closed-loop glucose control, critical care glucose control, glucose stabilization trial
Among people with diabetes mellitus (DM) the most frequent cause of death is due to increased risk of cardiovascular disease. Furthermore, people with DM have higher preoperative mortality, worse outcome of surgery, longer stays at the hospitals, and postoperative strokes.1-5 Among other risk factors, excess cardiovascular morbidity and mortality is associated with hyperglycemia.6
It has been demonstrated that strict glycemic control has been useful both in intensive care units (ICU) and in other settings.7 Furthermore, glucose control in postoperative patients is found to be important in patients both in surgical and medical ICUs.1,3,8-12 Intravenous infusion of insulin has been demonstrated to yield better glucose control compared to subcutaneous insulin injection in situations such as critical care illness, cardiogenic shock or myocardial infarction, postoperative period after cardiac surgery, diabetic ketoacidosis and nonketotic hyperosmolar state.7,9,12-16
The landmark Van den Berghe study of 200117 initiated a debate on the benefits and practicability of tight glucose control in ICU populations. The study’s findings were compelling: a 34% reduction in mortality, and 30-50% decreases in morbidity across numerous indicators. Subsequent research has confirmed the morbidity findings, and noted declines in additional complications of hyperglycemia.17,18 In contrast, another prominent study highlighted the difficulty of achieving beneficial results consistently—showing increased mortality, strongly correlated with higher rates of hypoglycemia (74% of all individuals in the intensive treated group had hypoglycemia) and glucose variability.19 This dilemma of tight control and its limitations using present methods has produced significant dialogue and encouraged hesitancy regarding guidelines for intensive care application. There is a need for increased precision in adaptation to individual patient treatment needs. Metabolism in intensive care patients can be precarious and susceptible to rapid change in ways that defy easy classification.17 An evolving model of individual patient metabolism may offer resiliency in adapting to multifarious change from health, treatment, and environmental factors.20
In addition, this type of system must function in demanding situations without demanding workload or excessive risk of contamination seen from repeated access of indwelling catheters.7,21 Use of counterbalancing insulin and glucose infusion adjusted by a unified adaptive algorithm and predictive glucose modeling techniques designed specifically for high frequency sensing may be useful in addressing these points. Introduction of such a closed-loop treatment system, which leverages near continuous blood glucose sensing, could advance the state of diabetic control in ICUs. This research evaluates the insulin balanced infusion system (IBIS), a design based on the above mentioned principles.
The aim of this study is to evaluate the ability of this treatment system to normalize uncontrolled glucose levels and stabilize them in a defined target range (80-125 md/dl).
The protocol used in this study allows the performance of the treatment system to be evaluated with respect to correction of hyperglycemia, normoglycemic maintenance, and avoidance of hypoglycemia.
Methods
All data were provided by Admetsys: Advanced Metabolic Systems™.
Study Sample
The study was approved by the United States Food and Drug Administration (FDA) under an Investigational Device Exemption (IDE) and performed under supervision of an independent Institutional Review Board (IRB). Inclusion criteria were the following: (1) male or female with no pregnancy; (2) age >21; (3) known people with DM type 1 or 2; (4) blood glucose 150-350 mg/dl at time of initiation; (5) nil per os (NPO); (6) stable blood pressure and cardiac rhythm; (7) adequate intravenous access. Exclusion criteria were (1) unwillingness to participate or dementia; (2) use of thiazolidinediones (TZD) or GLP-1 receptor agonists; (3) HbA1c >10%; (4) (a) serum creatinine >1.5 mg/dl, (b) serum potassium <3.5 mEq/l, (c) hematocrit <30% or >55%, (d) ALT/AST >3xnormal; (5) (a) anticoagulation therapy (other than aspirin), (b) corticosteroid use in previous 30 days, (c) supplemental oxygen pO2 <80 or >100 mmHg; (6) diabetic ketoacidosis in previous year; (7) history of seizures; (8) history of or symptomatic cardiac, peripheral, or cerebral vascular disease; (9) drug or alcohol dependency; (10) inclusion in a separate clinical trial. All subjects were given oral and written information and gave informed, written consent according to the Helsinki II declaration and to local ethical guidelines.
Study Treatment
The treatment protocol lasted 7.3-8.3 hours, which replicated the duration of a typical perioperative period including preoperative care, surgery, and recovery.
Study treatment was delivered intravenously, with access via 23 gauge peripheral catheter attached to a standard infusion set. Normal saline solution was infused at 30 ml/hr to insure vein patency. Separate IBIS insulin and glucose pumps were infused 50 ml syringes of regular human insulin at 1 unit/ml in normal saline and 50% dextrose (D50) in water, respectively. To prevent any irritation from D50 simultaneous infusion of normal saline at 30 ml/hr was infused in the common catheter for dilution as per FDA. Treatment channels were connected to the intravenous saline infusion using a 3-way connector proximate to the catheter, limiting space between each treatment channel and the point of infusion to less than 1 ml.
A commercial glucose strip meter (One Touch Ultra 2, LifeScan Inc, Milpitas, CA) was electronically connected to the system controller by USB cable. When receiving new measurements, the treatment system calculated the glucose and insulin dosage that were to be infused, and automatically altered the delivery rates of the pump. Other than a nurse applying blood to glucose test strips, no manual intervention was required. The LifeScan OneTouch Ultra 2 glucose strip was used in this study. The only input needed by the IBIS was the subjects weight.
To verify input data quality, confirmatory capillary glucose measurements were taken using a separate technology (HemoCue Glucose 201 Analyzer, Brea, CA) at minimum every two hours, and whenever glucose levels were below 80 mg/dl or over 250 mg/dl. Primary and confirmatory values were required to agree within 10% of one another or measurements were retaken.
Insulin Balanced Infusion System (IBIS)
The initial IBIS is a closed-loop treatment system comprised of three logical components: the system controller, two syringe pumps (AS 50, Baxter Healthcare, Round Lake, IL), and the glucose-sensing device (One Touch Ultra 2). The research presented uses a development prototype of the system in which the pumps are electronically slaved to the system controller, but physically separate. In more recent versions of the system, the pumps are physically integrated into the controller. The system is designed to interoperate with a variety of glucose sensor types, leveraging high-volume glucose data if available, or more moderate frequency data otherwise. The presented research employs the latter, sourced from an enzymatic strip based meter, electronically cabled to the controller. This is an interim sensor configuration, intended not for practical hospital use, but rather as a demonstration of concept using presently available, well-known sensing technique.
The core of the system is the proprietary intelligent controller developed by Admetsys (Admetsys, Boston, MA). It is a unique 3D proprietary adaptive AI algorithm, which has a feature to prevent race condition of glucose and insulin. The IBIS design accepts that each patient’s metabolism is unique and can change with changing condition. The controller therefore employs a patient-adaptive treatment model, which calculates active levels of biologics, sensitivities to treatment, and dosages over time using a predictive, feedback-driven technique to construct an evolving, real-time model of the patient’s insulin and glucose metabolism. The proprietary algorithm also suggests the frequency of glucose testing. In these studies, hyperglycemia triggered the device activation, and the IBIS learned infusion requirements over time.
In a series of trials the IBIS will be tested. The proprietary algorithm and treatment technique are the same throughout the trial protocols, but the system hardware components becomes more refined with each successive trial protocol.
Analysis of Results
The main measuring outcomes are divided into normalization and stability. Normalization being the reduction of blood glucose to the normoglycemic target range (80-125 mg/dl) and subsequently holding it for at least an hour, and stability being continued maintenance of glucose levels in the target range after normalization.
Descriptive statistics for blood glucose level and time are presented with central tendency described by mean and median, and dispersion described by standard deviation and range. The data were visualized in curves and tables.
Results
In all, 15 subject with diabetes were separately admitted to a research unit for trial participation. Subjects were heterogeneous in regards to degree of control, age, sex, and usual diabetic control regimens to illustrate general applicability of the system. A summary of the admitted and included subjects can be seen in Table 1. The subjects’ usual diabetic regimens were discontinued prior to admittance, leaving total dependency on the IBIS for glucose control.
Table 1.
Protocol Demographics.
| Stability protocol |
|
|---|---|
| Diabetes type | 9 type 2, 6 type 1 |
| Sex | 10 male, 5 female |
| Age | 27-75 years |
| Weight | 54-153 kg |
| A1c | 6.7-9.8% |
| Insulin | 10-190 units/day |
| Regimen | Oral agents Injected insulin Insulin pumps |
The stability protocol included a broad cross-section of subjects with diabetes to demonstrate general applicability of the system. A summary of the 15 admitted and 12 included individuals can be seen.
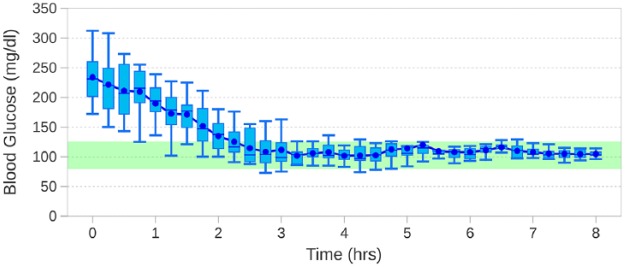
Results from all 15 subjects were collected and analyzed, as seen in Figure 1. Because of initial absence of proper glucose control, the subjects entered the study with hyperglycemic levels of 188-312 mg/dl, with a mean of 240 mg/dl.
Figure 1.
The tightness of control and target convergence with decreasing variability of the blood glucose of the subjects. The figure illustrates the progressive suppression of variability, simultaneous to correction of hyperglycemia.
Tightness of Control and Variability
The mean time to reach the normoglycemic target range of 80-125 mg/dl was 2.49 hours (SD 0.91), and the blood glucose levels stayed within the target range 97.04% of the time. Intervals between glucose sampling in the 15 subjects ranged between once every 10-16 minutes with a mean of 13.7 minutes. The mean blood glucose level was 105.2 mg/dl with no incidence of hypoglycemia (<70 mg/dl). It was observed that the initial glucose levels were brought to the target range with dampened variation in glucose. Furthermore, improvement in precision (tightening of control) and accuracy (convergence to target) was seen. The precision improved 5 fold from hours 3-8 compared to the first 2 hours. All of the subjects were stable in the target range by 4 hours into the study.
As seen in Figure 1, the subjects began the study with blood glucose levels of 188-312 mg/dl with a mean of 240 mg/dl. The first hour showed a large blood glucose range of 187 mg/dl (SD 42.5).
Initial glucose levels were statistically dispersed across the trial population as a whole, and showed variability per subject. There was random behavior with a mean of standard deviations of 46.7 mg/dl, over the 1-3 hours of the trial.
The dispersion and variability was progressively suppressed during treatment and as the elevated blood glucose was reduced the fluctuations in blood glucose concentrations was decreased with a composite standard deviation of 11.3 mg/dl and mean of standard deviations of 9.6 mg/dl for hours 3-8, as seen in Figure 2.
Figure 2.
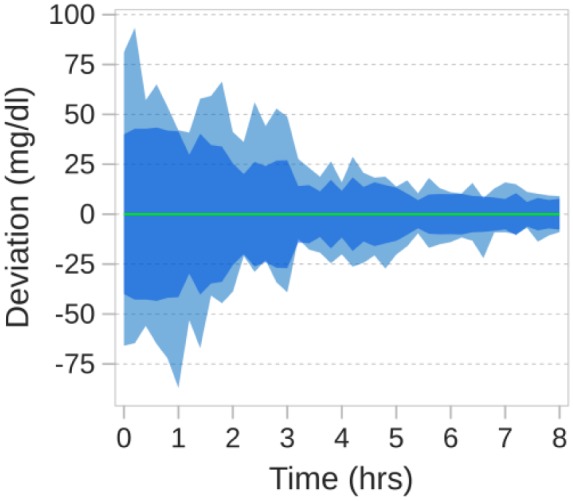
Observed range of glucose values, seen as the outer band, and standard deviation, seen as the darker inner band, plotted relative to the mean, shown as the zero reference line.
Target Range Stability
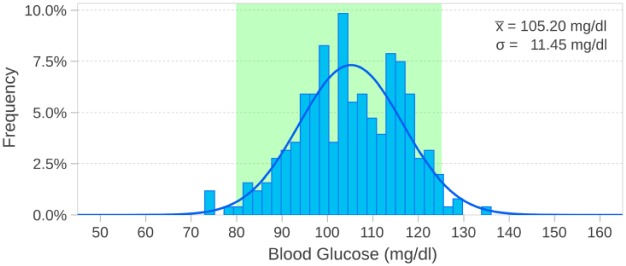
After blood glucose levels were brought to the target range, they were stable. Of measurements, 97.04% remained within the target range with the measured values distributed about a mean of 105.2 mg/dl with the two 95% thresholds in the target range, as seen in Figure 3.
Figure 3.
Target range stability once blood glucose levels had normalized. Values are approximately normally distributed with two standard deviations (95% threshold) within the target range (80-125 mg/dl).
Measurements in the target range predominates and no hypoglycemic events were noted. Furthermore, no acute hyperglycemia was noticed. Glucose control of the IBIS is seen in Table 2.
Table 2.
The Distribution of Measurements Across the Study.
| Description | Range (mg/dl) | Blood glucose |
Time/total (%) | |
|---|---|---|---|---|
| Mean | SD | |||
| Hypoglycemia, acute | <40 | — | — | 0.0 |
| Hypoglycemia | 40-69 | — | — | 0.0 |
| Acceptable low range | 70-79 | 75.0 | 2.2 | 1.3 |
| Target range | 80-125 | 105.2 | 10.5 | 97.0 |
| Hyperglycemia | 126-179 | 129.8 | 4.4 | 1.7 |
| Hyperglycemia, acute | 180+ | — | — | 0 |
Discussion
The results of the stabilization trial showed an overall arrangement of clear target convergence by the IBIS, with progressively tighter control. This was followed by target range stability, with pervasive lack of low blood glucose values and hypoglycemia (<70 mg/dl).
To suit the needs of critical care, a diabetic treatment system must address three mandates: minimization of glucose variability, elimination of hypoglycemia, and correction in a predictable manner. In the present study, the IBIS performance in these areas was investigated.
Stabilization and Target Range
The results of the stabilization trial demonstrates the glycemic control capabilities of the IBIS. It is noteworthy that the high blood glucose levels were brought to the target range while the glucose oscillations simultaneously were dampened. A study by Cox et al22 investigating the prediction of hypoglycemia, showed that fluctuation of the blood glucose and specific patterns of fluctuation are often followed by severe hypoglycemia.22 A reason could be that large fluctuations can lead to overtreatment and thereby leading to hypoglycemia. Thus, precision seen as tightening of control and accuracy seen as convergence to target (Figures 1-2), by the IBIS may lessen the risk of against hypoglycemia.
In general, the target range (80-125 mg/dl) was reached in 2.5 hours. The target range was 80-125 mg/dl in this study. This is in concordance with Furnary et al,16 who concluded that intravenous insulin compared to conventional diabetes management after coronary artery bypass grafting reduced death from cardiovascular events and removed death from infection when the mean blood glucose levels was held at 150 mg/dl. Other studies have suggested target ranges between 80 and 139 and would be optimal if achievable, suggesting that this target range of 80-125 mg/dl may be appropriate.7,23-26
Comparison With Other Devices
Control with the IBIS resulted mean time to target of 2.5 hours and 97.0% target range stability and absence of hypoglycemia.
Davidson and colleagues investigated a computer-directed intravenous insulin software called Glucommander and found it to be safe and simple. The mean time to reach target range (being 80-120 mg/dl for the study) for was 7.5 hours and the target range stability was shown to be 58.0%.7 Furthermore, 16.5% of the subjects had incidence of hypoglycemia (<60 mg/dl for the study).7 Compared with the results of the present study the Glucommander showed less stability and precision, underlining the effectiveness the IBIS. However, the IBIS was tested on a relatively small population in contrast to the large amount of data that was available with the Glucommander that may have a statistical impact on the results. Another reason for improved control with the IBIS may be the use of the counterbalancing glucose infusion.
Another intravenous insulin system is the GlucoStabilizer™, a commercial software program to improve insulin infusion and glucose control, which was tested by Juneja and colleagues.24 The GlucoStabilizer demonstrated a mean time to reach target range of 6.9 hours and a target range (being 80-110 mg/dl for the study) stability of 61.0% with 18.0% of subjects showing incidence of hypoglycemia (<70 mg/dl for the study).24 A possible reason for the better performance of the IBIS in this comparison, besides the technical advantages, may be the restricted target range used.24 The 15 mg/dl greater target range in the IBIS study, may have allowed for a faster time to target and improved statistical stability. Furthermore, the subjects using the GlucoStabilizer were intensive care patients with unstable conditions.
The Van den Berghe et al23 landmark study investigating the effects of intensive insulin therapy in medical ICU, found that the therapy using point-of-care glucometer and insulin pump resulted in mean time to target range (80-120 mg/dl for this study) of 6.2 hours and with a target range stability of 48.0%.23 Furthermore, 18.7% of subjects had occurring hypoglycemia (<40 for the present study). Yet again the IBIS seems to contrast with notable programs and metrics. Though there are study differences and population difference, which could explain the better performance of the IBIS, the significant results seems to be persistent in the trial and seems to be replicable in each subject. Furthermore, the Van den Berghe et al23 study results have been shown to be difficult to replicate. The IBIS demonstrates a possible way of gaining stabilization without reaching hypoglycemic levels.27
As seen in Figure 3 and Table 2, the IBIS allowed marginal hyperglycemia 1.7% of the time. However, the overall measurements were predominantly in restricted target range. Dispersion of glucose values around the mean glucose levels appeared to be less compared to other studies,7,23-26 demonstrating the utility of the IBIS.
There may be factors, which influence the comparison at this stage, such as stable patient population and restricted food and medications. While the principles of the IBIS seems to have been established in this study, further trials with automated sensing and a larger diverse patient group are needed to verify these results.
Conclusion
In conclusion, the IBIS has demonstrated an appropriate level of glucose control without hypoglycemia under a wide range of conditions and individual treatment requirements. More research is needed to illuminate the full potential of the IBIS for critical care glucose management, and to investigate the ability of the IBIS to correct hyperglycemia in a disciplined manner. As glucose sensing technology progresses, treatment systems must progress as well to realize maximum possible benefit, and advance the standard of care in this area of clear need.
Footnotes
Abbreviations: DM, diabetes mellitus; D50, 50% dextrose; FDA, US Food and Drug Administration; IBIS, insulin balanced infusion system; ICU, intensive care unit; IDE, Investigational Device Exemption; IRB, Institutional Review Board; NPO, nil per os; SD, standard deviation; TZD, thiazolidinediones.
Authors’ Note: Any underlying research material can be accessed by emailing the corresponding author. ClinicalTrials.gov Identifier: NCT01291719.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: TV is a full-time employee of Admetsys.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was funded by Admetsys: Advanced Metabolic Systems™.
ORCID iD: Nasseh Hashemi  https://orcid.org/0000-0001-9775-2919
https://orcid.org/0000-0001-9775-2919
References
- 1. Furnary AP, Zerr KJ, Grunkemeier GL. et al. Continuous intravenous insulin infusion reduces the incidence of deep sternal wound infection in diabetic patients after cardiac surgical procedures. Ann Thorac Surg. 1999;67:352-360; discussion 60-62. [DOI] [PubMed] [Google Scholar]
- 2. Lauruschkat AH, Arnrich B, Albert AA. et al. Prevalence and risks of undiagnosed diabetes mellitus in patients undergoing coronary artery bypass grafting. Circulation. 2005;112:2397-2402. [DOI] [PubMed] [Google Scholar]
- 3. Schmeltz LR, DeSantis AJ, Thiyagarajan V. et al. Reduction of surgical mortality and morbidity in diabetic patients undergoing cardiac surgery with a combined intravenous and subcutaneous insulin glucose management strategy. Diabetes Care. 2007;30:823-828. [DOI] [PubMed] [Google Scholar]
- 4. Thourani VH, Weintraub WS, Stein B. et al. Influence of diabetes mellitus on early and late outcome after coronary artery bypass grafting. Ann Thorac Surg. 1999;67:1045-1052. [DOI] [PubMed] [Google Scholar]
- 5. Weintraub WS, Wenger NK, Jones EL, Craver JM, Guyton RA. Changing clinical characteristics of coronary surgery patients. Differences between men and women. Circulation. 1993;88:II79-II86. [PubMed] [Google Scholar]
- 6. Laakso M, Kuusisto J. Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat Rev Endocrinol. 2014;10:293-302. [DOI] [PubMed] [Google Scholar]
- 7. Davidson PC, Steed RD, Bode BW. Glucommander: a computer-directed intravenous insulin system shown to be safe, simple, and effective in 120,618 h of operation. Diabetes Care. 2005;28:2418-2423. [DOI] [PubMed] [Google Scholar]
- 8. DeJournett L. Essential elements of the native glucoregulatory system, which, if appreciated, may help improve the function of glucose controllers in the intensive care unit setting. J Diabetes Sci Technol. 2010;4:190-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Van den Berghe G, Wouters P, Weekers F. et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. [DOI] [PubMed] [Google Scholar]
- 10. Krinsley JS. Glycemic control, diabetic status, and mortality in a heterogeneous population of critically ill patients before and during the era of intensive glycemic management: six and one-half years experience at a university-affiliated community hospital. Semin Thorac Cardiovasc Surg. 2007;18:317-325. [DOI] [PubMed] [Google Scholar]
- 11. Lazar HL, Chipkin SR, Fitzgerald CA, Bao Y, Cabral H, Apstein CS. Tight glycemic control in diabetic coronary artery bypass graft patients improves perioperative outcomes and decreases recurrent ischemic events. Circulation. 2004;109:1497-1502. [DOI] [PubMed] [Google Scholar]
- 12. Malmberg K. Prospective randomised study of intensive insulin treatment on long term survival after acute myocardial infarction in patients with diabetes mellitus. DIGAMI (Diabetes Mellitus, Insulin Glucose Infusion in Acute Myocardial Infarction) Study Group. BMJ. 1997;314:1512-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kitabchi AE, Umpierrez GE, Murphy MB. et al. Management of hyperglycemic crises in patients with diabetes. Diabetes Care. 2001;24:131-153. [DOI] [PubMed] [Google Scholar]
- 14. Lazar HL, Chipkin S, Philippides G, Bao Y, Apstein C. Glucose-insulin-potassium solutions improve outcomes in diabetics who have coronary artery operations. Ann Thorac Surg. 2000;70:145-150. [DOI] [PubMed] [Google Scholar]
- 15. Trence DL, Kelly JL, Hirsch IB. The rationale and management of hyperglycemia for in-patients with cardiovascular disease: time for change. J Clin Endocrinol Metab. 2003;88:2430-2437. [DOI] [PubMed] [Google Scholar]
- 16. Furnary AP, Gao G, Grunkemeier GL. et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1007-1021. [DOI] [PubMed] [Google Scholar]
- 17. van den Berghe G, Wouters P, Weekers F. et al. Intensive insulin therapy in critically ill patients. N Engl J Med. 2001;345:1359-1367. [DOI] [PubMed] [Google Scholar]
- 18. Lanspa MJ, Hirshberg EL, Phillips GD, Holmen J, Stoddard G, Orme J. Moderate glucose control is associated with increased mortality compared with tight glucose control in critically ill patients without diabetes. Chest. 2013;143:1226-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Finfer S, Liu B, Chittock DR. et al. Hypoglycemia and risk of death in critically ill patients. N Engl J Med. 2012;367:1108-1118. [DOI] [PubMed] [Google Scholar]
- 20. Feng J, Hajizadeh I, Yu X. et al. Multi-level supervision and modification of artificial pancreas control system. Comput Chem Eng. 2018;112:57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Weiss R, Lazar I. The need for continuous blood glucose monitoring in the intensive care unit. J Diabetes Sci Technol. 2007;1:412-414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cox DJ, Gonder-Frederick L, Ritterband L, Clarke W, Kovatchev BP. Prediction of severe hypoglycemia. Diabetes Care. 2007;30:1370-1373. [DOI] [PubMed] [Google Scholar]
- 23. Van den Berghe G, Wilmer A, Hermans G. et al. Intensive insulin therapy in the medical ICU. N Engl J Med. 2006;354:449-461. [DOI] [PubMed] [Google Scholar]
- 24. Juneja R, Roudebush C, Kumar N. et al. Utilization of a computerized intravenous insulin infusion program to control blood glucose in the intensive care unit. Diabetes Technol Ther. 2007;9:232-240. [DOI] [PubMed] [Google Scholar]
- 25. Braithwaite SS, Edkins R, Macgregor KL. et al. Performance of a dose-defining insulin infusion protocol among trauma service intensive care unit admissions. Diabetes Technol Ther. 2006;8:476-488. [DOI] [PubMed] [Google Scholar]
- 26. Goldberg PA, Roussel MG, Inzucchi SE. Clinical results of an updated insulin infusion protocol in critically ill patients. Diabetes Spectr. 2005;18:188-191. [Google Scholar]
- 27. Meijering S, Corstjens AM, Tulleken JE, Meertens JH, Zijlstra JG, Ligtenberg JJ. Towards a feasible algorithm for tight glycaemic control in critically ill patients: a systematic review of the literature. Crit Care. 2006;10:R19. [DOI] [PMC free article] [PubMed] [Google Scholar]