Abstract
Closed loop (CL) systems deliver insulin with a rapid onset and offset in action. Although favorable overall, the absence of a long-acting insulin increases the risk of diabetic ketoacidosis (DKA) which can occur with insulin delivery failure, acute illness, low carbohydrate diets, sodium glucose-linked transporter inhibitors, and high intensity exercise. A CL system relying entirely on interstitial glucose measurements may not provide an alert for DKA and many people with type 1 diabetes (T1D) do not carry a blood ketone meter and test-strips. Ketone sensing is theoretically feasible. A multianalyte platform incorporating a ketone sensor could provide an additional CL input without an increase in burden for the person with T1D, warning of impending DKA to allow remedial action to be taken. We outline the clinical case for inclusion of continuous ketone sensing as part of future CL systems.
Keywords: closed loop, ketones, sensor, diabetes
Type 1 diabetes (T1D), a state of absolute endogenous insulin deficiency, mandates exogenous insulin replacement therapy to preserve life. Failure to do so results in the life-threatening condition of diabetic ketoacidosis (DKA). The measurement of ketones is recognized as being part of mainstream care in the management of people with T1D. While ketone measurement is not required routinely on a daily basis, clinicians advise people with T1D to check their blood ketone levels in the face of rapidly increasing glucose levels or during times of illness. The detection of elevated ketone levels is critical in alerting the person and their health care professional to the presence of metabolic changes and potential danger of DKA, enabling early action to be taken to prevent or reverse the metabolic derangement, and potentially to avoid costly hospital visits and admissions. A recent US survey indicates that the incidence and cost of hospitalization for DKA in people with T1D is increasing,1 posing a significant burden to the person living with diabetes, their family and to the community.
There is an increasing body of evidence2 to support the contention that automated insulin delivery by closed loop (CL) systems can improve metabolic control in people with T1D by providing a flexible and responsive platform capable matching their changing insulin requirements. These systems deliver insulin analogues with a rapid onset and offset in action subcutaneously via an insulin pump. Insulin delivery is continuously adjusted by a control algorithm utilizing interstitial glucose measurements provided by an electrochemical sensor to maintain glucose levels within a healthy target range. This article discusses ketone sensing as a potential additional input to glucose, to improve CL safety and functionality.
Integration of Ketone Testing into CL Systems
Ketoacidosis remains a significant risk in those people living with T1D managed with insulin pumps with about 3% of those between 13 and 49 years experiencing >1 episode of DKA in the previous 3 months.3 Despite improvements in glycemic control ketosis remains a risk in people with T1D even on CL systems. This risk does not relate to the CL control of insulin delivery as such but is consequent upon the limitations of the method by which the hormone is delivered and the kinetics of the insulin preparations used. Attia et al demonstrated that ketosis occurred in those using insulin pump therapy 180 minutes postcessation of lispro insulin with 3-hydoxybutyrate (3HB) levels rising above 0.4 mmol/L and greater than 0.6 mmol/L at 240 minutes.4 Given that the same insulin delivery technologies are employed in CL systems as with conventional pump therapy, we expect that there will remain some risk for DKA despite the emergence of CL systems. Therefore, ketone testing will continue to be of relevance as automated control systems for insulin delivery evolve. In Bergenstal et al’s recent study5 evaluating the Medtronic 670G, the world’s first commercial hybrid CL insulin delivery system, there were no episodes of severe hypoglycemia or DKA. However, there were 17 device-related adverse events including severe hyperglycemia with blood ketones >0.6 mmol/L or accompanied by symptoms of nausea, vomiting, or abdominal pain. Eleven of these events were attributable to infusion set issues. The incidence of such events may be even greater outside of a clinical trial setting.
One envisages that there are a number of circumstances arising with CL glucose control where ketone testing would be important. These include:
1. Failure of insulin delivery: CL systems employ insulin formulations that have a rapid onset and offset in action to ensure responsiveness to the variable requirements associated with everyday living. Should delivery by the insulin infusion set fail, these same pharmacokinetic properties become maladaptive, increasing the risk of ketosis and DKA. While a failure of insulin delivery is also usually signaled by an increase in glucose levels, there are other causes of elevated glucose levels which are not associated with ketosis. For example, carbohydrate ingestion covered by an inadequate bolus, a prolonged sedentary period, or minor to moderate emotional stress may all cause elevations in glucose without requiring an insulin infusion set change. In these situations, the measurement of blood ketone levels may provide guidance as to whether insulin delivery is compromised or not.
2. Sick days: Acute illness in people with T1D carries an increased risk of DKA because of an associated reduction in food intake and an increase in counterregulatory hormone release. Evidence suggests that sick day monitoring of blood ketone levels at home may reduce the incidence of hospitalization.6 The American Diabetes Association (ADA) guidelines to parents of children with T1D advises to “check blood or urine ketones as often as every 4 hours,”7 while the Australian Diabetes Educators Association (ADEA) advises that blood ketones should be checked every 2 hours for ketone levels between 0.6-1.0 mmol/L and every hour for ketone levels >1.0 mmol/L.8
3. Exercise: High-intensity exercise (HIIE) is associated with the acute release of counterregulatory hormones. Data from our studies challenging CL control with different forms of exercise indicate that following HIIE, a fall in glucose levels occurs which coincides with an increase in ketone levels (Figure 1).9 Because CL systems do not take into account ketone levels and only incorporate glucose levels in the algorithm, the postexercise fall in glucose levels may result in a reduction in insulin delivery following HIIE which may attenuate any further fall in glucose at the potential risk of a further increase in ketone levels. A more appropriate response under these circumstances would be for the CL system to signal the need for the individual to ingest carbohydrate. Ketone levels are not routinely measured following exercise, particularly if glucose levels are falling. A ketone sensor would provide an appropriate alert.
4. Reduced carbohydrate intake: In addition to sick days and acute illness mentioned above, there are other circumstances where low carbohydrate intake or fasting predispose to ketosis. These include religious requirements such as Ramadan, fasting for medical procedures, those aiming for weight reduction, or in those with coexisting eating disorders. More recently, data from an online survey indicate that very low carbohydrate diets have been reported to improve glucose control as reflected by HbA1c in people with T1D.10 While the authors Lennerz et al themselves advise caution, there has been an increased uptake of very low carbohydrate diets by people with T1D recently as a consequence of reports such as these. The 5:2 diet with at least two days a week of very low calorie intake is popular in the general population and with some people with T1D.11 Very low carbohydrate diets induce ketosis, and clinicians should advise people to monitor their ketone levels closely when fasting or if implementing a low carbohydrate diet.
5. Sodium glucose linked transporter inhibitors (SGLT inhibitors): SGLT inhibitors are a new class of oral hypoglycemic agents that inhibit the absorption of glucose in the proximal renal tubules (SGLT-2 inhibitors) or both in the renal tubules and the small intestine (SGLT-1/2 inhibitors). They have been approved by the FDA and the Australian regulatory authorities for use in people with type 2 diabetes (T2D). Studies evaluating these agents have been performed in people with T1D,12-14 demonstrating benefits in weight control and glucose control, and additional studies are currently underway. In fact, some people in Australia with T1D are using these agents off-label prior to regulatory approval.
Figure 1.
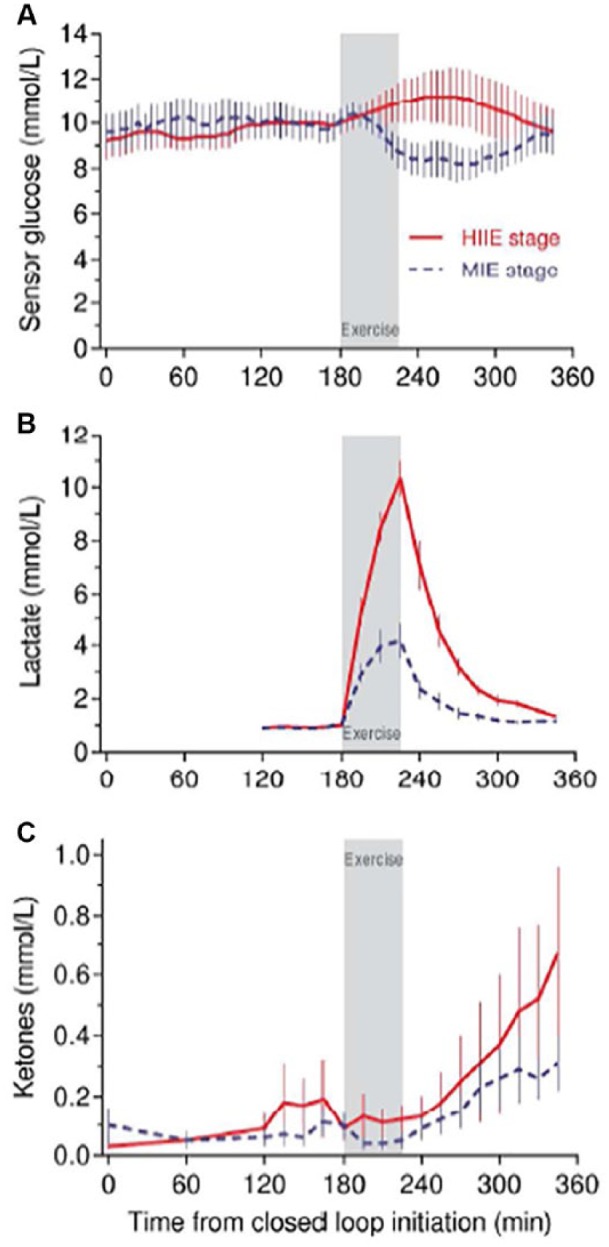
Biochemical profiles during CL with high-intensity exercise (HIIE) and moderate-intensity exercise (MIE) in participants with type 1 diabetes.9
However, SGLT inhibitor use is associated with an increase in risk of DKA. A recent Australian audit15 found that 38% of patients presenting with DKA secondary to SGLT inhibitor use had a diagnosis of T1D despite the fact that SGLT inhibitors have not been approved for use in this group. A feature of DKA associated with SGLT inhibitors is euglycemia, that is, glucose levels that remain largely within normal range. Therefore, a CL system relying entirely on interstitial glucose measurements may not provide warning of the impending metabolic derangement. The mechanisms underpinning the increased risk of DKA with SGLT inhibitors remain to be fully determined. Combining the results of the three inTandem studies12-14 a total of 1748 T1D participants received 24 weeks sotagliflozin versus 1228 in the placebo group. Within the intervention group, those on insulin pump therapy experienced more episodes of DKA relative to MDI participants (23/723 vs 15/1025), equating to an annual risk of 6.4% and 2.9%, respectively. In contrast, within the placebo group, 2/506 in the pump group and 2/722 on multiple daily insulin injections experienced DKA.
Therefore, while there may be benefits with weight control, reduced glucose variability, diminution of hyperglycemia and importantly cardiovascular protection (should the T2D data translate to the T1D population), balanced against the aforementioned is the significant increase in risk of DKA. It should also be noted that there are those who argue that the cardiovascular protection is in part mediated by ketosis.16 It is conceivable that these oral agents may be used as an adjunct to CL systems for glucose control. However, for the risk-benefit ratio to be acceptable in people with T1D, we will need to have robust mechanisms for monitoring ketones and strategies in place to mitigate the increased risk of DKA, the cornerstone of which will be patient and clinician education regarding risk factors, ketone detection and management.
- 6. Other groups at risk for DKA: In addition to those described above, other groups at risk for DKA who may benefit from a continuous ketone monitor include:
- i. Pregnant women with T1D: CL glucose control has been studied in pregnant women with T1D.17-20 Some early studies have shown promise and we await the outcomes of larger, longer-duration studies. Pregnancy itself is a ketogenic state particularly in women suffering with hyperemesis gravidarum, or vomiting secondary to urinary tract infections to which they are susceptible and during labor.
- ii. People with a history of recurrent DKA: Factors such as chronic medical illness or psychological stress may be important, and in these circumstances automated insulin delivery by CL systems may reduce the opportunities for missed doses. In addition, the ability to monitor ketone levels and input this information into the CL algorithm may further diminish metabolic instability and mitigate DKA rates. However, we recognize that a common factor contributing to recurrent DKA is nonadherence to insulin therapy or missed insulin doses and it would be expected that those intentionally omitting insulin doses to cause hyperglycemia and ketosis for weight loss, would deliberately circumvent any safeguards including those provided by a ketone sensor.
- iii. People who drink excessive alcohol, which causes a marked increase in lipolysis and fatty acid delivery to the liver, leading to ketogenesis and subsequent ketosis.
- iv. People with T1D who live remotely or are socially isolated as they are at greater risk of the consequences of unrecognized DKA.
Ketone Sensing Versus Blood Ketone Measurements
Health professionals advise people diagnosed with T1D to check their blood ketone levels in the face of rapidly increasing glucose levels, or if they are experiencing nausea and vomiting or while fasting.21 However, clinical experience indicates that often this does not occur in a timely manner as ketone testing strips have not been carried with the person or these strips have passed their expiry date. In our recently published survey of 205 adults with T1D from two tertiary referral diabetes clinics, one third of people did not have in-date ketone testing strips.22 In general, the capability to measure ketones requires the person to carry with them an additional device, with testing strips with a finite shelf-life as a precaution in the event of circumstances which occur infrequently and unexpectedly. In addition, finger-prick blood testing is painful and requires a conscious decision and actions on the part of the person. The development of combined meters able to measure blood glucose and ketone levels in one test is already underway. In the UK, the KEYA® meter (Inside Biometrics International Ltd, Dingwall, Scotland, UK) has recently been approved and combines a blood glucose and ketone test into one. Its ability to automatically check for ketones and notify users via an on-screen alert of abnormal levels enables a similar concept to that which we propose though does require the individual to initiate the blood test.
We suggest that a CL system utilizing a single insertion multianalyte glucose sensor probe and incorporating ketone sensing capability could provide greater functionality and safety in comparison with blood ketone testing by providing key information on ketone levels without increasing the burden or requiring a conscious action on the user’s part. Importantly, ketone sensing should be integrated into the system’s glucose sensing platform to minimize any additional costs of the system.
The Technical Feasibility of Ketone Sensing
Commercially available point of care (POC) devices measure blood ketone concentrations using the 3-hydroxybutyrate dehydrogenase (3HBDH) enzyme.23 These devices detect the concentration of 3HB, which is a ketone body highly associated with the onset of DKA. 3HBDH belongs to the family of oxidoreductase enzymes which requires the availability of a cofactor—nicotinamide adenine dinucleotide (NAD+)—to catalyze the oxidation of 3HB to acetoacetate and NADH. NADH is in turn oxidized at the electrode surface to generate a current proportional to the concentration of 3HB.
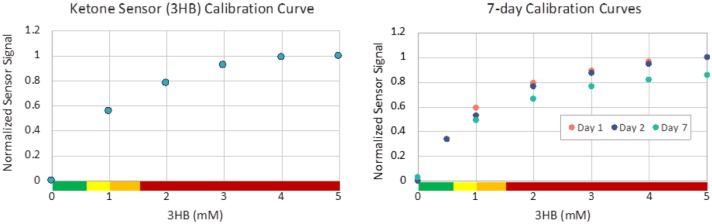
Unlike POC devices, an in vivo ketone sensor operated continuously will need to ensure that the enzyme remains stable and sensitive across the relevant operating conditions and physiological ranges. This can be a challenge because of the low concentration of NAD+ in the human body and the potential for electrode poisoning which reduces the efficiency of NADH oxidation.24 While many POC devices use redox mediators to support the enzyme reaction, these additives produce more challenges in in vivo sensing. A new sensor design is proposed for continuous ketone sensing that operates a high surface area electrode to prevent electrode fouling. A geometrically deployed hydrogel membrane provides a pathway for NAD+ diffusion into an immobilized enzyme hydrogel containing 3HBDH, while also serving to reduce the effect of other interferents at the electrode surface. Proof of concept in vitro studies at physiological 3HB and NAD+ levels and extremely high concentration of NAD+ and 3HB designed to test continuous ketone sensor operational stability have demonstrated the response of the sensor to increasing ketone concentration even as the ratio of 3HB to NAD+ approaches 100. The preliminary results (see Figure 2) indicate that the enzyme kinetics support sensor development specific to critical sensor attributes such as linearity and stability.
Figure 2.
Continuous ketone sensor tested in phosphate buffer solution. Left: Ketone sensor calibration at physiologically relevant levels of NAD+ (0.04 mM). Right: Sensor operated continuously in high concentration (5 mM) 3HBU shows limited sensitivity.
The sensor platform simplifies integration of glucose and ketone sensing on a single sensor through the use of a shared membrane structure. This membrane structure is compatible with oxidase enzymes that are used in many continuous glucose monitoring (CGM) systems, as well as dehydrogenase enzymes that enable continuous ketone sensing. Spacing of the sensing electrodes achieves isolation between the different sensing elements. By minimizing the increase in cost and size when integrating a ketone sensing element and delivering calibration-free performance of both glucose and ketone sensors, there is an opportunity to improve CL safety without increased user burden. While there are several challenges related to the successful design of an in vivo continuous ketone monitor, innovative sensor design such as described can overcome these challenges.
Accuracy of a Proposed Ketone Sensor
Acceptable performance parameters for a continuous ketone sensor would not need to be as stringent as for glucose sensing. There is no equivalent of the narrow physiological range, rapid fluctuations of significant amplitude, and risk associated with hypoglycemia, which determine glucose sensor performance requirements. Rather, a ketone sensor would need to recognize trends in an upward direction above a predetermined series of thresholds signifying increments in urgency. The early recognition of ketosis would facilitate preemptive intervention (eg, infusion set change with unexplained increasing glucose levels or carbohydrate administration with fasting states).
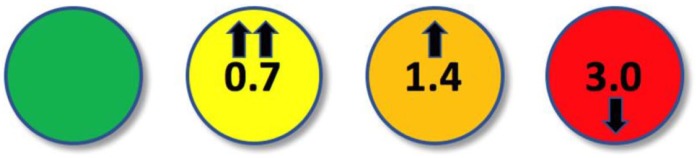
While a continuous display of glucose levels is conventional for CL systems, we propose a different approach for ketone sensing in recognition of the above and so as to reduce the burden of excess information that does not require action (Figure 3). A displayed green light indicates that the ketone sensor is activated and functioning. Below a ketone level of 0.6 mmol/L, no measured level will be provided. At ketone levels between 0.6-1.0 mmol/L, the device will alarm, the light will change to yellow and the ketone level will be displayed with a trend arrow indicating direction and rate of change. Should ketone levels continue to increase and measure between 1.0-1.5 mmol/L, the device will alarm once more, the light will become orange and the measured ketone level and trend arrows will be displayed. If levels continue to rise above 1.5 mmol/L the device will alarm, the display will become red with ketone levels and trend arrows. A message advising the person to contact their diabetes team will also be displayed.
Figure 3.
Proposed display for ketone sensor measurements: <0.6 mmol/L = green light and no reading; 0.6-1.0 mmol/L = yellow light with ketone level and trend arrows displayed; 1.0-1.5 mmol/L = orange light with ketone level and trend arrows displayed; >1.5 mmol/L = red light with ketone level and trend arrows displayed.
As with early generation glucose sensing we would advise people with elevated ketone levels on their ketone sensor to confirm this with a blood ketone meter. Conversely, if a person with T1D feels unwell or experiences nausea, vomiting, or abdominal pain, they should be advised to check their blood ketone levels even if the display was green.
Utility of Information Provided by Ketone Sensing
There are ketosis management guidelines in Australia by the ADEA8 and internationally by the ADA.25 For example, if a person with T1D on an insulin pump has moderately elevated ketone levels (1.0-1.5 mmol/L) and feels otherwise well, we advise that they replace the insulin delivery set immediately and administer rapid acting insulin via injection to correct elevated glucose and ketone levels. Increase in fluid intake is also encouraged and both blood glucose and ketone levels should be rechecked in 1 hour. If there is no improvement or should symptoms develop they are advised to report to the emergency department. Nevertheless, given that ketone sensing and CL systems represent new technologies, we would recommend that existing guidelines are reviewed in recognition of this.
While we recognize that a continuous ketone monitor integrated into a CL system may not necessarily be suitable for all, our ultimate goal is to provide a multianalyte sensing platform, at the same cost and with the same physical footprint as a current generation glucose sensor. The aim is to enhance the safety of CL systems without an increase in either the financial, intellectual, or physical burden to the user. Therefore, we envisage that the majority of the T1D cohort willing to use a current generation CL system would benefit from a system configured to detect elevated ketone levels
Conclusion
The early recognition of ketosis could facilitate timely intervention addressing the underlying problem and may prevent metabolic instability and possibly hospitalization. We envisage a future CL system incorporating a single subcutaneous insertion with multiple sensing modalities including ketones with glucose remaining as the primary analyte. The input of ketone levels could enhance CL performance and safety, enabling earlier recognition of insulin line failure and potentially reducing unnecessary insulin delivery set replacements as well as inform more appropriate responses to metabolic changes with stress and exercise. In addition, while this discussion has specifically addressed CL functionality, benefits may extend beyond this group as ketone measurements are relevant to all people with T1D regardless of the mode of insulin delivery.
We propose that continuous ketone sensing be considered as part of future CL systems. The analogy would be one of an airbag in a car. One may rarely require it, but it could save your life.
Footnotes
Abbreviations: ADA, American Diabetes Association; ADEA, Australian Diabetes Educators Association; CGM, continuous glucose monitoring; CL, closed loop; DKA, diabetic ketoacidosis; HIIE, high-intensity exercise; MIE, moderate-intensity exercise; POC, point of care; SGLT, sodium glucose linked transporter; T1D, type 1 diabetes; T2D, type 2 diabetes; 3HB, 3-hydoxybutyrate; 3HBDH, 3-hydroxybutyrate dehydrogenase.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: MHL has received research support and honoraria from Medtronic. BP has received research support and honoraria from Medtronic, Novo-Nordisk, and Sanofi. BK has no conflicts of interest. RS is the founder of PercuSense. AJJ, and DNO have received research support and is on advisory boards for Medtronic, Novo-Nordisk, and Sanofi. DNO has received honoraria from Medtronic, AMSL, Novo-Nordisk, and Sanofi. SAM has received support for research from Medtronic.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Sybil A. McAuley  https://orcid.org/0000-0001-7035-489X
https://orcid.org/0000-0001-7035-489X
References
- 1. Desai D, Mehta D, Mathias P, Menon G, Schubart UK. Health care utilization and burden of diabetic ketoacidosis in the U.S. Over the past decade: a nationwide analysis. Diabetes Care. 2018;41:1631-1638. doi: 10.2337/dc17-1379 [DOI] [PubMed] [Google Scholar]
- 2. Weisman A, Bai JW, Cardinez M, Kramer CK, Perkins BA. Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol. 2017;5:501-512. [DOI] [PubMed] [Google Scholar]
- 3. Miller KM, Foster NC, Beck RW. et al. Current state of type 1 diabetes treatment in the U.S.: updated data from the T1D exchange clinic registry. Diabetes Care. 2015;38:971-978. [DOI] [PubMed] [Google Scholar]
- 4. Attia N, Jones TW, Holcombe J, Tamborlane WV. Comparison of human regular and lispro insulins after interruption of continuous subcutaneous insulin infusion and in the treatment of acutely decompensated IDDM. Diabetes Care. 1998;21:817-821. [DOI] [PubMed] [Google Scholar]
- 5. Bergenstal RM, Garg S, Weinzimer SA. et al. Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA. 2016;316:1407-1408. [DOI] [PubMed] [Google Scholar]
- 6. Laffel LMB, Wentzell K, Loughlin C, Tovar A, Moltz K, Brink S. Sick day management using blood 3-hydroxybutyrate (3-OHB) compared with urine ketone monitoring reduces hospital visits in young people with T1DM: a randomized clinical trial. Diabet Med. 2006;23:278-284. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association. Sick Days; Available at: http://www.diabetes.org/living-with-diabetes/parents-and-kids/everyday-life/sick-days.html [Google Scholar]
- 8. Australian Diabetes Educators Association. Clinical Guiding Principles for Sick Day Management of Adults with Type 1 and Type 2 Diabetes. Technical document. Canberra: Australian Diabetes Educators Association; 2016. [Google Scholar]
- 9. Jayawardene DC, McAuley SA, Horsburgh et al. Closed-loop insulin delivery for adults with type 1 diabetes undertaking high-intensity interval exercise versus moderate-intensity exercise: a randomized, crossover study. Diabetes Technol Ther. 2017;19:340-348. [DOI] [PubMed] [Google Scholar]
- 10. Lennerz BS, Barton A, Bernstein RK. et al. Management of type 1 diabetes with a very low-carbohydrate diet. Pediatrics. 2018;141:e20173349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Diabetes.co.uk: The Global Diabetes Community. 5:2 Fasting Diet. 2018. Available at: https://www.diabetes.co.uk/diet/5-2-intermittent-fast-diet.html.
- 12. Buse JB, Garg SK, Rosenstock J. et al. Sotagliflozin in Combination With Optimized Insulin Therapy in Adults With Type 1 Diabetes: The North American inTandem1 Study. Diabetes Care. 2018;41:1970-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Danne T, Cariou B, Banks P. et al. Fifty-two-week efficacy and safety of sotagliflozin, a dual SGLT1 and SGLT2 inhibitor, as adjunct therapy to insulin in adults with type 1 diabetes (The European in Tandem2 Study). Diabetes. 2018;67(suppl 1):1122-P. [Google Scholar]
- 14. Garg SK, Henry RR, Banks P. et al. Effects of sotagliflozin added to insulin in patients with type 1 diabetes. N Engl J Med. 2017;377:2337-2348. [DOI] [PubMed] [Google Scholar]
- 15. Meyer EJ, Gabb G, Jesudason D. SGLT2 inhibitor–associated euglycemic diabetic ketoacidosis: a South Australian clinical case series and Australian spontaneous adverse event notifications. Diabetes Care. 2018;41:e47-e49. [DOI] [PubMed] [Google Scholar]
- 16. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39:1115-1122. [DOI] [PubMed] [Google Scholar]
- 17. Murphy HR, Elleri D, Allen JM. et al. Closed-loop insulin delivery during pregnancy complicated by type 1 diabetes. Diabetes Care. 2011;34:406-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy HR, Kumareswaran K, Elleri D. et al. Safety and efficacy of 24-h closed-loop insulin delivery in well-controlled pregnant women with type 1 diabetes: a randomized crossover case series. Diabetes Care. 2011;34:2527-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stewart ZA, Wilinska ME, Hartnell S. et al. Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med. 2016;375:644-654. [DOI] [PubMed] [Google Scholar]
- 20. Stewart ZA, Wilinska ME, Hartnell S. et al. Day-and-night closed-loop insulin delivery in a broad population of pregnant women with type 1 diabetes: a randomized controlled crossover trial. Diabetes Care. 2018;41:1391-1399. [DOI] [PubMed] [Google Scholar]
- 21. American Diabetes Association. Standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S1-S159.29222369 [Google Scholar]
- 22. Larsson CR, Januszewski AS, McGrath RT. et al. Suboptimal behaviour and knowledge regarding overnight glycaemia in adults with type 1 diabetes is common. Intern Med J. 2018;48:1080-1086. doi: 10.1111/imj.13798. [DOI] [PubMed] [Google Scholar]
- 23. Sheikh-Ali M, Karon BS, Basu A. et al. Can serum β-hydroxybutyrate be used to diagnose diabetic ketoacidosis? Diabetes Care. 2008;31:643-647. Available at: http://care.diabetesjournals.org/content/31/4/643. [DOI] [PubMed] [Google Scholar]
- 24. Cardosi M, Liu Z. Amperometric glucose sensors for whole blood measurement based on dehydrogenase enzymes. In: Canuto RA. ed. Dehydrogenases. Rijeka: Intech Open Access; 2012. [Google Scholar]
- 25. American Diabetes Association. Checking For Ketones; Available at: http://www.diabetes.org/living-with-diabetes/treatment-and-care/blood-glucose-control/checking-for-ketones.html [Google Scholar]