Abstract
Background:
Severe hypoglycemic events (SHEs) in patients with diabetes are associated with substantial health care costs in the United States (US). Injectable glucagon (IG) is currently available for treatment of severe hypoglycemia but is associated with frequent handling errors. Nasal glucagon (NG) is a novel, easier-to-use treatment that is more often administered successfully. The economic impact of this usability advantage was explored in cost-offset and budget impact analyses for the US setting.
Methods:
A health economic model was developed to estimate mean costs per SHE for which treatment was attempted using NG or IG, which differed only in the probability of treatment success, based on a published usability study. The budget impact of NG was projected over 2 years for patients with type 1 diabetes (T1D) and type 2 diabetes treated with basal-bolus insulin (T2D-BB). Epidemiologic and cost data were sourced from the literature and/or fee schedules.
Results:
Mean costs were $992 lower if NG was used compared with IG per SHE for which a user attempted treatment. NG was estimated to reduce SHE-related spending by $1.1 million and $230 000 over 2 years in 10 000 patients each with T1D and T2D-BB, respectively. Reduced spending resulted from reduced professional emergency services utilization as successful treatment was more likely with NG.
Conclusions:
The usability advantage of NG over IG was projected to reduce SHE-related treatment costs in the US setting. NG has the potential to improve hypoglycemia emergency care and reduce SHE-related treatment costs.
Keywords: budget impact, cost-minimization, economic evaluation, nasal glucagon, severe hypoglycemia, United States
Severe hypoglycemic events (SHEs) are a major barrier to good glycemic control in people with diabetes.1 During an SHE, abnormally low blood glucose levels lead to cognitive impairment whereby external assistance is required for treatment.2,3 SHEs impose a substantial economic and quality of life burden. In the United States (US), SHEs are a frequent cause of emergency medical services (EMS) call-out and associated with considerable costs to health care payers.4-7 In addition, fear of hypoglycemia negatively affects quality of life due to concerns that SHE interrupts daily activities.8,9 In response, people with diabetes may increase calorie intake or reduce insulin doses to avoid hypoglycemia, thereby impairing glycemic control.9
Treatments that promote successful SHE resolution may contribute to avoiding EMS call-out and associated costs. Guidelines recommend glucagon as rescue therapy for hypoglycemia if a person cannot be treated using oral carbohydrates.3 Although efficacious, the currently available injectable glucagon (IG) formulations are challenging to use as the complex administration, including reconstitution, must be completed in a stressful emergency situation.10,11 In a recent usability study, 50% of caregivers of a person with diabetes failed to administer any glucagon when using IG during a simulated SHE.12 These results confirmed earlier findings of frequent handling errors and failed injections with IG.13 Overall, possession rates of glucagon are low, and glucagon is considered to be underused.13,14
Recently, nasal glucagon (NG; Eli Lilly and Company, Indianapolis, Indiana) has been undergoing development as a rescue treatment for SHE.15-18 Development of NG was informed by research dating back to the 1980s, in which the potential of intranasal glucagon to raise blood glucose levels was investigated in populations with and without diabetes.19-21 The novel NG is a single-use, portable, ready-to-use device containing 3 mg of dry glucagon powder. In a clinical trial of adults with type 1 diabetes (T1D), NG showed efficacy similar to intramuscular glucagon (1 mg).17 In real-world studies, NG was an effective treatment for SHEs in adult patients with T1D, and no additional emergency health services were required for patients to return to normal status.15 Importantly, a simulation study demonstrated much better usability for NG relative to IG.12 Administration of full glucagon doses and therefore successful treatment were more likely when using NG.
The aim of the present analysis was to evaluate the economic impact of NG’s usability advantage over IG in the US setting. Across the SHE treatment pathway, costs were compared between treatments to assess the per-event costs and budget impact of introducing NG to the US market.
Methods
Analytic Framework
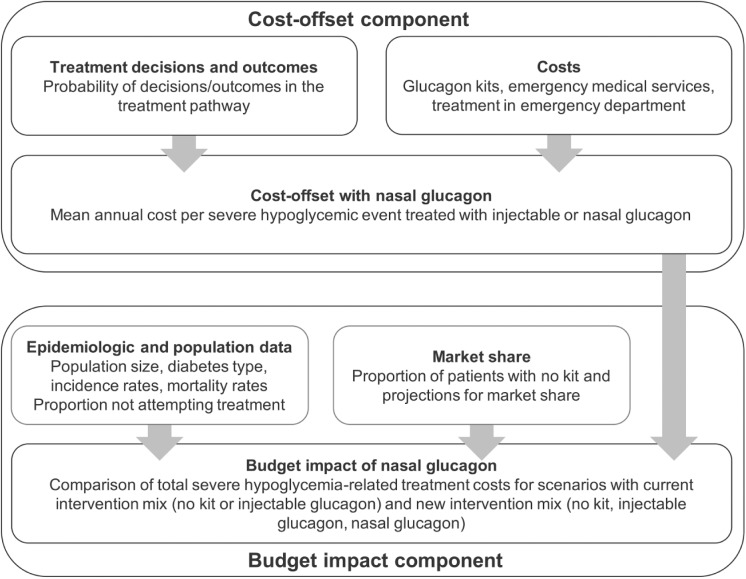
A de novo model was developed in Microsoft Excel to evaluate the economic impact of NG. The model combined a cost-offset component (a type of cost-minimization analysis [CMA]) and a budget impact component (Figure 1).
Figure 1.
Cost-offset and budget impact model: schematic diagram.
In the cost-offset component, NG and IG were compared per SHE for which a user, either a caregiver (of a person with diabetes) or an acquaintance (willing to help a patient with SHE), attempted treatment with either glucagon, conditional on the SHE having already occurred. Mean per-SHE treatment costs over the entire treatment pathway (Figure 2) were compared for treatment attempts with NG and IG. This comparison provided an “instantaneous,” per-event perspective on how the usability advantage of NG affected SHE-related treatment costs. Cost-offset results depended on treatment outcome probabilities and costs but not on diabetes type (which did not affect treatment success probabilities), SHE incidence (as the SHE was assumed to have occurred already), mortality, or market share (which affected the population-level number of owners of a certain type of glucagon but not treatment success).
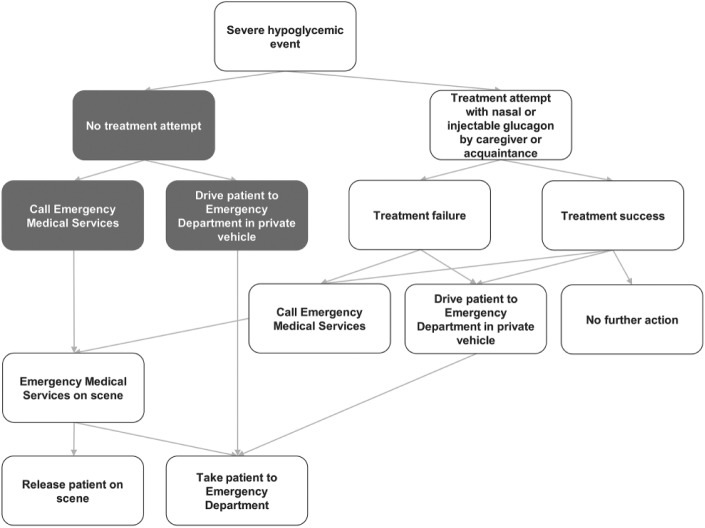
Figure 2.
Severe hypoglycemic event treatment pathway. Gray boxes were accounted for in the budget impact but not the cost-offset component (which considered costs per event with treatment attempted).
The budget impact component assessed the population-level impact of introducing NG to the market, by combining cost-offset results with population-specific SHE incidence and mortality data as well as market share projections. The analysis also accounted for costs incurred by individuals without glucagon and cases where treatment with glucagon was not attempted. Budget impact was assessed comparing a scenario with the current intervention mix (patients had no kit or an IG kit) with a scenario in which NG was available (so patients had no kit, an IG kit or NG). This analysis extended the per-event cost-offset perspective to patient populations, to assess the magnitude of overall changes in SHE-related spending.
Differences between treatments in the time to onset of action and administration time were not considered in the analysis as no evidence was available to suggest their impact on cost outcomes. In addition, all adverse events in clinical trials were of mild or moderate severity, which were considered unlikely to be associated with resource utilization and therefore not included in the analysis.18
SHE Treatment Pathway
The model was centered on a representation of the emergency treatment pathway for SHE (Figure 2). This pathway, which covers treatment decisions and outcomes, was developed based on reviews of the published literature, market research, treatment guidelines and emergency care pathways.3,22-25 Each decision and outcome was associated with a probability of occurrence and a cost. Mean treatment costs were calculated as expected costs per SHE over the entire pathway.
Inpatient stays were not considered in the treatment pathway. In inpatient stays following SHE, patients are usually admitted not solely for SHE but for other acute and/or chronic comorbidities.26 Glucagon was not expected to affect these comorbidity-related inpatient stays and their associated costs so they were excluded from the analysis.
Analysis Settings
The analysis was conducted from the perspective of a US insurer similar to a Medicare Advantage Plan. The time horizon in the cost-offset component was instantaneous while a 2-year time horizon was used in the budget impact analysis, taking into account one glucagon lifecycle based on the 2-year shelf life of IG. Populations considered in the budget impact component were patients with insulin-treated diabetes, who were considered to be at increased hypoglycemia risk.3 Specifically, patients with T1D and type 2 diabetes treated with basal-bolus insulin (T2D-BB) were included. In the budget impact analysis, 10 000 patients were modeled for each population and scenario.
Input Data
Treatment Pathway Probabilities
Probabilities in the treatment pathway were derived from published sources wherever possible and equal for NG and IG, with the exception of treatment success (Table 1). While 94% of caregivers and 93% of acquaintances delivered a full glucagon dose if using NG, only 13% and 0%, respectively, did when using IG.12
Table 1.
Treatment Pathway Probabilities Used in Base Case Analyses.
| Item | Probability | Source |
|---|---|---|
| Attempting treatment with glucagon | 100% | Assumption |
| Caregivers among those attempting treatment | 90% | Assumption |
| Treatment success: caregivers (acquaintances) with NG | 94% (93%) | Yale et al12 |
| Treatment success: caregivers (acquaintances) with IG | 13% (0%) | Yale et al12 |
| Further action if treatment succeeded (failed) | 0% (100%) | Assumption |
| Further action if treatment failed: call-out EMS (drive patient to ED in private vehicle) | 50% (50%) | Assumption |
| Release on scene by EMS if prior caregiver/acquaintance treatment attempt failed | 43% | Kaufmann et al26 |
Costs
Costs were considered for decisions and outcomes in the treatment pathway. Costs associated with treatment were assumed to match fees published by the Centers for Medicare and Medicaid Services, supplemented from the literature where necessary (Table 2).27-29 Costs were expressed as 2017 US dollars ($).30 Costs projected by the model were distinguished into acquisition costs for glucagon and treatment costs associated with professional medical help.
Table 2.
Costs Used in Base Case Analyses.
| Cost item | Cost, $ | Source | Explanation/rationale |
|---|---|---|---|
| NG, IG acquisition | 280 | December 2017 IG list price | NG assumed to be priced at parity to IG |
| EMS, patient released on scene | 0 | Medicare Benefit Policy Manual28 | EMS costs incurred only in case of transport |
| EMS, transport to ED | 680 | Medicare Ambulance Fee Schedule Public Use File CY 201829 | Mean of urban and rural rates across contractors and localities for HCPCS A0427 (Advanced Life Support Emergency) |
| ED treatment | 1293 | Ward et al27 (inflated to 2017 values) | Cost per hypoglycemia-related ED visit |
SHE Incidence and Mortality
SHE incidence and background mortality were included in the budget impact component. For patients with T1D, an incidence rate of 366 SHEs per 1000 person-years (PY) was obtained from the conventional treatment arm of the Epidemiology of Diabetes Interventions and Complications (EDIC) cohort.2 For patients with T2D-BB, an incidence rate of 80 SHEs per 1000 PYs was assumed based on a systematic review.31 Background mortality was sourced from Diabetes Control and Complication Trial/EDIC data for patients with T1D (2.63 deaths per 1000 PYs) and a 5-year study of US patients with diabetes for patients with T2D-BB (32.3 deaths per 1000 PYs).32,33
Market Share
For the comparison of current and new intervention mix scenarios, glucagon ownership rates were projected. In both populations, a constant 40% of patients were assumed not to own any glucagon.34 Of patients owning glucagon, 80% were projected to own an IG kit in the first year after NG entered the market, compared with 70%, 60%, and 55% in years 2-4 and 50% from year 5 onward in both populations. For simplification, patients were assumed to own only one kit at any given time so glucagon acquisition costs were incurred once per 2-year glucagon lifecycle, unless glucagon was used and subsequently replaced. After either use or expiration, glucagon was assumed to be replaced with the same type of glucagon.
Uncertainty Analysis
A range of sensitivity analyses was conducted to investigate the impact of assumptions on and identify key drivers of results.
The influence of treatment success probabilities was explored using lower and upper 95% confidence interval bounds for proportions successfully administering at least partial glucagon doses. Lower (upper) bounds for caregivers were 24% (78%) when using IG and 81% (100%) when using NG. The corresponding values for acquaintances were 0% (42%) for IG and 79% (100%) for NG, respectively. In addition, a scenario was explored where EMS call-out was mandatory so all caregivers and acquaintances called out EMS regardless of treatment success with glucagon.
For sensitivity analyses of budget impact, the proportion of users not attempting treatment was increased up to 75%. Glucagon acquisition costs were varied by ±20% and alternative cost estimates for emergency department (ED) visits were used.29,35 The impact of SHE incidence was explored by running the model with alternative rates from the literature.1,31,36 Budget impact was also evaluated over different time horizons (1-6 years) and for alternative market shares (±10% versus base case projections for IG).
Results
Base Case Results
Cost-offset analysis showed that using NG was associated with reduced per-event costs compared with using IG. Modeled mean costs per SHE for which treatment was attempted using glucagon were $1345 if an IG kit was used and $354 if NG was used, yielding a cost-offset of $992 with NG. As IG and NG acquisition costs were equal, the cost-offset resulted entirely from EMS transports and ED treatment avoided as professional medical help was less likely to be required given the usability advantage of NG (Table 3).
Table 3.
Cost-Offset for Nasal Versus Injectable Glucagon per Severe Hypoglycemic Event With Treatment Attempted Using Glucagon.
| Item | Mean cost with injectable glucagon, $ | Mean cost with nasal glucagon, $ | Cost-offset with nasal glucagon, $ |
|---|---|---|---|
| Total costs | 1345 | 354 | 992 |
| Cost breakdown | |||
| Glucagon acquisition costs | 280 | 280 | 0 |
| Costs for EMS treatment, patient released at scene | No cost included in model | ||
| Costs for EMS treatment with patient transported to ED | 170 | 12 | 159 |
| Costs for ED treatment | 895 | 62 | 833 |
At the population level, for 10 000 modeled patients with T1D, projected total SHE-related costs over 2 years were $10.84 million for the current intervention mix. The corresponding cost for the new intervention mix was projected at $9.76 million, leading to an estimated cost reduction of approximately $1.09 million over 2 years following the introduction of NG.
For 10 000 modeled patients with T2D-BB, the projected total SHE-related costs were $3.62 million and $3.39 million with the current and new intervention mix, respectively. Introduction of NG was estimated to reduce SHE-related treatment costs by approximately $230 000 over 2 years in this patient group.
In both populations and analyses, acquisition costs of IG and NG were equal so the entire reduction in SHE-related spending was due to reduced spending on EMS transports and ED treatment.
Sensitivity Analyses
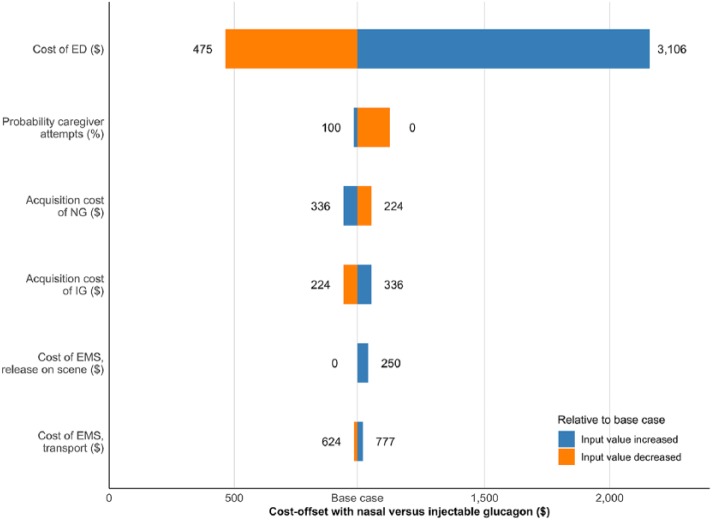
The cost-offset and reductions in total SHE-related spending associated with NG were confirmed in sensitivity analyses. In one-way sensitivity analyses, NG was consistently associated with cost-offset and reduced overall spending versus IG. ED treatment costs were identified as key drivers of results (Figure 3). With regard to glucagon costs, even when increasing NG costs by 20% (or, equivalently, decreasing IG kit costs by 20%), NG was still associated with cost-offset and reduced SHE-related treatment spending relative to IG. Relative to the base case, changes in input values and assumptions that reduced the costs or increased the use of professional medical help favored IG. In these cases, the usability advantage of NG translated into smaller cost reductions.
Figure 3.
Cost-offset results from sensitivity analyses. Horizontal bars indicate the range of cost-offset outcomes between minimum and maximum input values (indicated on either end of the bar).
In the budget impact analysis, SHE rates and time horizon were identified as key drivers of results (Figures S1 and S2). For the new intervention mix, higher SHE rates and longer time horizons were associated with larger reductions in spending for NG relative to IG.
In two-way sensitivity analyses, cost-offsets and reduced expenditure were also observed for NG relative to IG (Table S1). When assuming higher success probabilities for IG and lower probabilities for NG relative to the base case, cost-offset and budget impact were reduced but still indicated reduced spending associated with NG. NG was also associated with cost-offset and reduced spending if EMS call-out was mandatory although reductions were smaller than in the base case. In this scenario, patients who had returned to normal by the time EMS arrived due to successful prior treatment with glucagon were assumed to be more likely released on the scene than patients in whom glucagon treatment had failed. When lower IG acquisition costs were combined with higher NG acquisition costs, the new intervention mix increased glucagon costs but still reduced professional medical treatment costs, leading to a cost-offset with NG relative to IG and a decrease in overall SHE-related expenditure relative to the current intervention mix.
Discussion
This study provided evidence that, in the United States, the usability advantage of NG can reduce the costs of SHEs with treatment attempted by non–medically trained users using glucagon. In addition, reduced overall SHE-related spending was observed for populations with T1D and T2D-BB. Reduced spending resulted from less professional medical help required, namely avoidance of EMS transports and ED treatment.
Results from this analysis suggested that NG’s usability advantage translated to lower SHE-related costs in patients with insulin-treated diabetes. For SHE prevention, novel approaches have been explored in recent years.37,38 Treatment for SHEs, however, has seen little progress despite the challenges associated with IG.13,34,39 In the 1980s and 1990s, initial research was conducted on nasally administered glucagon formulations but none was brought to market.21 NG was the first innovative glucagon formulation to undergo a clinical development program, which showed NG to have clinical efficacy similar to IG.18
The de novo model was designed to be flexible and accommodate a range of different assumptions. For example, while a closed cohort of 10 000 patients owning at most one glucagon kit at any one time was used for clarity of presentation, this could easily be modified and explored in future analyses. However, the present analysis has potential shortcomings that should be acknowledged. First, due to a lack of studies investigating decision-making during SHE, published data were not available for all treatment decision and outcomes in the treatment pathway so simplifying assumptions had to be made. Extensive sensitivity analyses were performed, which consistently showed reduced per-event costs and overall SHE-related spending with NG. No further action following successful treatment with glucagon was assumed in budget impact base case analyses. This assumption was considered to reflect real-world practice as most SHEs take place outside the health care system, indicating that they are resolved without professional medical intervention.40 While data on SHE occurring outside the health care system are still sparse, results from real-world studies of NG showed that no professional medical help was required for treating SHE.15 In addition, sensitivity analyses showed that the impact of this assumption on budget impact results was comparatively small.
Second, the study on which treatment success probabilities were based was small (n = 31).12 The study sample was based on sample size calculations to achieve ≥95% power, assuming a within-subject mean difference of ≥40 seconds between glucagon formulations in the time required to deliver a dose (due to higher than expected drop-out in the acquaintance arm, statistical power for acquaintance analysis was 94%).13 Overall, the study was well aligned with previous results regarding the poor usability of IG so bias, if any, was probably small.13 In addition, while the study was a simulation and may not have captured the full emotional distress associated with SHE, it seems plausible that distress would more negatively affect administration of IG than of NG, given the complexity of administration. The difference between the two treatments obtained in the simulation might therefore underestimate the real-world usability advantage of NG.
Third, the cost perspective was not straightforward to define for the US setting. Medicare Part B covers nonhospital medical services but not drugs, while the opposite holds for Part D.41 Medicare Advantage Plans cover both but are provided by a wide range of commercial payers, for which data on cost and fee structures are not generally available. To obtain a coherent set of costs for the present analysis, fees were sourced from published Medicare Part B schedules.29 This choice was likely conservative from the perspective of commercial payers, which, for example, may also cover EMS transport with release at the scene. Results are therefore likely to provide a lower bound for the reduction in spending with NG relative to IG for these payers.
The present study used a cost-offset and budget impact analysis design, which was considered appropriate to model the economic impact of the usability advantage of NG. The costs and benefits considered were short-term, relating to the SHE and subsequent emergency treatment. No changes in long-term benefits or costs following improved hypoglycemia emergency treatment were assumed. Together with the “economic hypothesis of weak dominance”—that NG is as safe and effective as IG but less costly—the short-term focus justified the use of CMA.17,42 CMA is also appropriate if based on a noninferiority trial where the difference in cost was so large that plausible efficacy differences would not change conclusions.42 This criterion was met as the underlying noninferiority trial showed pharmacodynamic responses to IG and NG to be very similar while differences in administration success probabilities were so large that any changes were unlikely to affect conclusions (as shown in sensitivity analyses).12,17
Current uptake and use of IG kits are poor given the complexity of IG administration, indicating that novel hypoglycemia treatments are urgently required.11,13,14,39,43 Compared with the currently available IG, NG is associated with a usability advantage that translates into benefits to patients, due to a higher probability of treatment success, and to payers, due to reduced costs associated with professional medical help for SHE. Relative to IG, NG was considered to represent a more efficient use of health care payers’ resources. In addition, NG may not only provide clinical and economic benefits but also reduce fear of hypoglycemia and improve better quality of life due to increased confidence in treatment and avoidance of ED visits while reducing costs by avoiding use of professional medical help.8,44
Conclusions
As a novel treatment option for SHE, NG is easier to administer than IG and therefore has the potential to improve the probability of successful treatment for SHEs relative to IG. This usability advantage of NG was estimated to translate into reduced per-event costs and reduced overall SHE-related spending as SHEs are more likely to be resolved without professional medical help.
Supplemental Material
Supplemental material, POEHLMANN_Supplementary_Figures_S1_and_S2 for Nasal Glucagon Versus Injectable Glucagon for Severe Hypoglycemia: A Cost-Offset and Budget Impact Analysis by Johannes Pöhlmann, Beth D. Mitchell, Sanjay Bajpai, Beatrice Osumili and William J. Valentine in Journal of Diabetes Science and Technology
Supplemental Material
Supplemental material, POEHLMANN_Table_S1 for Nasal Glucagon Versus Injectable Glucagon for Severe Hypoglycemia: A Cost-Offset and Budget Impact Analysis by Johannes Pöhlmann, Beth D. Mitchell, Sanjay Bajpai, Beatrice Osumili and William J. Valentine in Journal of Diabetes Science and Technology
Footnotes
Abbreviations: CMA, cost-minimization analysis; ED, emergency department; EDIC, Epidemiology of Diabetes Interventions and Complications cohort; EMS, emergency medical services; HCPCS, Healthcare Common Procedure Coding System; IG, injectable glucagon; NG, nasal glucagon; PY, person-year; SHE, severe hypoglycemic event; T1D, type 1 diabetes; T2D-BB, type 2 diabetes, treated with basal-bolus insulin; US, United States.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JP: full-time employee of Ossian, which received consulting fees from Eli Lilly for model development and preparation of this article. BDM: full-time employee and stock owner of Eli Lilly. SB: full-time employee and stock owner of Eli Lilly. BO: full-time employee of Eli Lilly. WJV: full-time employee of Ossian, which received consulting fees from Eli Lilly for model development and preparation of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The development of the model described in this article and the preparation of the article itself were funded by Eli Lilly and Company, which manufactures glucagon and nasal glucagon, in the form of consultancy fees to Ossian Health Economics and Communications.
Data Availability: Data used to develop and populate the model are all publicly available and referenced in the article. The model itself is proprietary and will not be made publicly available. Results of the study are presented in full in the article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Johannes Pöhlmann  https://orcid.org/0000-0002-8065-755X
https://orcid.org/0000-0002-8065-755X
References
- 1. International Hypoglycaemia Study Group. Minimizing hypoglycemia in diabetes. Diabetes Care. 2015;38:1583-1591. [DOI] [PubMed] [Google Scholar]
- 2. Gubitosi-Klug RA, Braffett BH, White NH. et al. Risk of severe hypoglycemia in type 1 diabetes over 30 years of follow-up in the DCCT/EDIC study. Diabetes Care. 2017;40:1010-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. American Diabetes Association. Glycemic targets: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(suppl 1):S55-S64. [DOI] [PubMed] [Google Scholar]
- 4. Foos V, Varol N, Curtis BH. et al. Economic impact of severe and non-severe hypoglycemia in patients with type 1 and type 2 diabetes in the United States. J Med Econ. 2015;18:420-432. [DOI] [PubMed] [Google Scholar]
- 5. Basu S, Berkowitz SA, Seligman H. The monthly cycle of hypoglycemia: an observational claims-based study of emergency room visits, hospital admissions, and costs in a commercially insured population. Med Care. 2017;55:639-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parsaik AK, Carter RE, Pattan V. et al. Population-based study of severe hypoglycemia requiring emergency medical service assistance reveals unique findings. J Diabetes Sci Technol. 2012;6:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moffet HH, Warton EM, Siegel L, Sporer K, Lipska KJ, Karter AJ. Hypoglycemia patients and transport by EMS in Alameda County, 2013-15. Prehosp Emerg Care. 2017;21:767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King J, Overland J, Fisher M, White K. Severe hypoglycemia and the role of the significant other: expert, sentry, and protector. Diabetes Educ. 2015;41:698-705. [DOI] [PubMed] [Google Scholar]
- 9. Fidler C, Elmelund Christensen T, Gillard S. Hypoglycemia: an overview of fear of hypoglycemia, quality-of-life, and impact on costs. J Med Econ. 2011;14:646-655. [DOI] [PubMed] [Google Scholar]
- 10. Pontiroli AE. Intranasal glucagon: a promising approach for treatment of severe hypoglycemia. J Diabetes Sci Technol. 2015;9:38-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pontiroli AE, Ceriani V. Intranasal glucagon for hypoglycaemia in diabetic patients. An old dream is becoming reality? Diabetes Obes Metab. 2018;20:1812-1816. [DOI] [PubMed] [Google Scholar]
- 12. Yale JF, Dulude H, Egeth M. et al. Faster use and fewer failures with needle-free nasal glucagon versus injectable glucagon in severe hypoglycemia rescue: a simulation study. Diabetes Technol Ther. 2017;19:423-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Harris G, Diment A, Sulway M, Wilkinson M. Glucagon administration: undervalued and undertaught. Pract Diab Int. 2001;18:22-25. [Google Scholar]
- 14. Kedia N. Treatment of severe diabetic hypoglycemia with glucagon: an underutilized therapeutic approach. Diabetes Metab Syndr Obes. 2011;4:337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Seaquist ER, Dulude H, Zhang XM. et al. Prospective study evaluating the use of nasal glucagon for the treatment of moderate to severe hypoglycaemia in adults with type 1 diabetes in a real-world setting. Diabetes Obes Metab. 2018;20:1316-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sherr JL, Ruedy KJ, Foster NC. et al. Glucagon nasal powder: a promising alternative to intramuscular glucagon in youth with type 1 diabetes. Diabetes Care. 2016;39:555-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rickels MR, Ruedy KJ, Foster NC. et al. Intranasal glucagon for treatment of insulin-induced hypoglycemia in adults with type 1 diabetes: a randomized crossover noninferiority study. Diabetes Care. 2016;39:264-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Deeb LC, Dulude H, Guzman CB. et al. A phase 3 multicenter, open-label, prospective study designed to evaluate the effectiveness and ease of use of nasal glucagon in the treatment of moderate and severe hypoglycemia in children and adolescents with type 1 diabetes in the home or school setting. Pediatr Diabetes. 2018;19:1007-1013. [DOI] [PubMed] [Google Scholar]
- 19. Pontiroli AE, Alberetto M, Pozza G. Intranasal glucagon raises blood glucose concentrations in healthy volunteers. Br Med J (Clin Res Ed). 1983;287:462-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pontiroli AE, Calderara A, Pajetta E, Alberetto M, Pozza G. Intranasal glucagon as remedy for hypoglycemia. Studies in healthy subjects and type I diabetic patients. Diabetes Care. 1989;12:604-608. [DOI] [PubMed] [Google Scholar]
- 21. Boido A, Ceriani V, Pontiroli AE. Glucagon for hypoglycemic episodes in insulin-treated diabetic patients: a systematic review and meta-analysis with a comparison of glucagon with dextrose and of different glucagon formulations. Acta Diabetol. 2015;52:405-412. [DOI] [PubMed] [Google Scholar]
- 22. Sibley T, Jacobsen R, Salomone J. Successful administration of intranasal glucagon in the out-of-hospital environment. Prehosp Emerg Care. 2013;17:98-102. [DOI] [PubMed] [Google Scholar]
- 23. National Association of State EMS Officials. National model EMS guidelines: version 2.0; September 2017. Available at: https://www.ems.gov/pdf/advancing-ems-systems/Provider-Resources/National-Model-EMS-Clinical-Guidelines-September-2017.pdf. Accessed October 9, 2018.
- 24. Rostykus P, Kennel J, Adair K. et al. Variability in the treatment of prehospital hypoglycemia: a structured review of EMS protocols in the United States. Prehosp Emerg Care. 2016;20:524-530. [DOI] [PubMed] [Google Scholar]
- 25. Villani M, de Courten B, Zoungas S. Emergency treatment of hypoglycaemia: a guideline and evidence review. Diabet Med. 2017;34:1205-1211. [DOI] [PubMed] [Google Scholar]
- 26. Kaufmann M, Nelson DR, Kaushik P, Mann NC, Mitchell BD. Hypoglycemia emergencies: factors associated with prehospital care, transportation status, emergency department disposition, and cost? [published online ahead of print November 2, 2018]. Prehosp Emerg Care. doi: 10.1080/10903127.2018.1528322. [DOI] [PubMed] [Google Scholar]
- 27. Ward A, Alvarez P, Vo L, Martin S. Direct medical costs of complications of diabetes in the United States: estimates for event-year and annual state costs (USD 2012). J Med Econ. 2014;17:176-183. [DOI] [PubMed] [Google Scholar]
- 28. Centers for Medicare & Medicaid Services. Medicare benefit policy manual: chapter 10—ambulance services. Rev. 243. March 2018. Available at: https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c10.pdf. Accessed October 9, 2018.
- 29. Centers for Medicare & Medicaid Services. Ambulance Fee Schedule (AFS) public use files: CY 2018. February 2018. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AmbulanceFeeSchedule/afspuf.html. Accessed October 9, 2018.
- 30. U.S. Bureau of Labor Statistics. Consumer price index for all urban consumers: medical care (CPIMEDSL). July 2018. Available at: https://fred.stlouisfed.org/series/CPIMEDSL. Accessed October 9, 2018.
- 31. Czech M, Rdzanek E, Pawęska J, Adamowicz-Sidor O, Niewada M, Jakubczyk M. Drug-related risk of severe hypoglycaemia in observational studies: a systematic review and meta-analysis. BMC Endocr Disord. 2015;15:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Diabetes Control and Complications Trial (DCCT)/Epidemiology of Diabetes Interventions and Complications (EDIC) Study Research Group. Mortality in type 1 diabetes in the DCCT/EDIC versus the general population. Diabetes Care. 2016;39:1378-1383.27411699 [Google Scholar]
- 33. McCoy RG, Van Houten HK, Ziegenfuss JY, Shah ND, Wermers RA, Smith SA. Increased mortality of patients with diabetes reporting severe hypoglycemia. Diabetes Care. 2012;35:1897-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yanai O, Pilpel D, Harman I, Elitzur-Leiberman E, Phillip M. IDDM patients’ opinion on the use of Glucagon Emergency Kit in severe episodes of hypoglycaemia. Pract Diabetes Int. 1997;14:40-42. [Google Scholar]
- 35. Dalal MR, Kazemi M, Ye F, Xie L. Hypoglycemia after initiation of basal insulin in patients with type 2 diabetes in the United States: implications for treatment discontinuation and healthcare costs and utilization. Adv Ther. 2017;34:2083-2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Elliott L, Fidler C, Ditchfield A, Stissing T. Hypoglycemia event rates: a comparison between real-world data and randomized controlled trial populations in insulin-treated diabetes. Diabetes Ther. 2016;7:45-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yeoh E, Choudhary P. Technologies to reduce hypoglycemia. J Diabetes Sci Technol. 2015;9:911-916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Elliott J, Jacques RM, Kruger J. et al. Substantial reductions in the number of diabetic ketoacidosis and severe hypoglycaemia episodes requiring emergency treatment lead to reduced costs after structured education in adults with type 1 diabetes. Diabet Med. 2014;31:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilson LM, Castle JR. Stable liquid glucagon: beyond emergency hypoglycemia rescue. J Diabetes Sci Technol. 2018;12:847-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Karter AJ, Moffet HH, Liu JY, Lipska KJ. Surveillance of hypoglycemia: limitations of emergency department and hospital utilization data. JAMA Intern Med. 2018;178:987-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Centers for Medicare & Medicaid Services. What Medicare covers. October 2018. Available at: https://www.medicare.gov/what-medicare-covers/index.html. Accessed October 9, 2018. [PubMed]
- 42. Dakin H, Wordsworth S. Cost-minimisation analysis versus cost-effectiveness analysis, revisited. Health Econ. 2013;22:22-34. [DOI] [PubMed] [Google Scholar]
- 43. Murata T, Okazaki K, Yanagisawa K. et al. Glucagon underutilized among type 1 diabetes mellitus patients in Japan. Diabetes Technol Ther. 2013;15:748-750. [DOI] [PubMed] [Google Scholar]
- 44. Anderbro T, Amsberg S, Adamson U. et al. Fear of hypoglycaemia in adults with type 1 diabetes. Diabet Med. 2010;27:1151-1158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, POEHLMANN_Supplementary_Figures_S1_and_S2 for Nasal Glucagon Versus Injectable Glucagon for Severe Hypoglycemia: A Cost-Offset and Budget Impact Analysis by Johannes Pöhlmann, Beth D. Mitchell, Sanjay Bajpai, Beatrice Osumili and William J. Valentine in Journal of Diabetes Science and Technology
Supplemental material, POEHLMANN_Table_S1 for Nasal Glucagon Versus Injectable Glucagon for Severe Hypoglycemia: A Cost-Offset and Budget Impact Analysis by Johannes Pöhlmann, Beth D. Mitchell, Sanjay Bajpai, Beatrice Osumili and William J. Valentine in Journal of Diabetes Science and Technology