Abstract
Introduction
Intermittent theta burst stimulation (iTBS) applied to primary motor cortex (M1) has been shown to modulate both the excitability and connectivity of the motor system. A recent proof-of-principle study, based on a small group of hospitalised patients with acute ischemic stroke, suggested that iTBS applied to the ipsilesional M1 combined with physical therapy early after stroke can amplify motor recovery with lasting after effects. A randomised controlled clinical trial using a double-blind design is warranted to justify the implementation of iTBS-assisted motor rehabilitation in neurorehabilitation from an acute ischaemic stroke.
Methods/design
We investigate the effects of daily iTBS on early motor rehabilitation after stroke in an investigator-initiated, longitudinal randomised controlled trial. Patients (n=150) with hemiparesis receive either iTBS (600 pulses) applied to the ipsilesional motor cortex (M1) or a control stimulation (ie, coil placement over the parieto-occipital vertex in parallel to the interhemispheric fissure and with a tilt of 45°). On 8 consecutive workdays, a 45 min arm-centred motor training follows the intervention . The relative grip strength, defined as the grip force ratios of the affected and unaffected hands, serves as the primary outcome parameter. Secondary outcome parameters are measures of arm function (Action Research Arm Test, Fugl-Meyer Motor Scale), stroke severity (National Institutes of Health Stroke Scale), stroke-induced disability (modified Rankin Scale, Barthel Index), duration of inpatient rehabilitation, quality of life (EuroQol 5D), motor evoked potentials and the resting motor threshold of the ipsilesional M1.
Ethics and dissemination
The study was approved by the Ethics Commission of the Medical Faculty, University of Cologne, Germany (reference number 15-343). Data will be disseminated through peer-reviewed publications and presentations at conferences. Study title: Theta-Burst Stimulation in Early Rehabilitation after Stroke (acronym: TheSiReS). Study registration at German Registry for Clinical Trials (DRKS00008963) and at ClinicalTrials.gov (NCT02910024).
Keywords: rehabilitation, hemiparesis, iTBS, TMS, motor recovery
Strengths and limitations of this study.
The present study is a randomised, controlled, double-blind, single-centre trial assessing the efficacy of intermittent theta burst stimulation (iTBS) in patients with acute cerebral ischaemia.
Interventions are applied before daily physiotherapy in the first few days after stroke since previous work suggests higher neural plasticity during the acute, compared with the chronic phase.
Patients receive iTBS during their hospitalisation warranting the adequate assessment of adverse events.
A limitation of the study is a potential selection bias, given the patients’ expected comorbidities, which may pose a risk for the application of repetitive transcranial magnetic stimulation or compromise the ability to provide informed consent.
Introduction
Stroke is a leading cause of acquired long-term disability in adults worldwide. From 1990 to 2010, the prevalence of stroke has reached numbers of 500–1000/100 000 people in North America and the European countries.1 Although recent developments in the acute treatment of a stroke such as thrombolysis or thrombectomy effectively reduce both morbidity and mortality,2 the majority of patients are still left with permanent motor deficits. More than 50% of stroke survivors keep a persisting impairment, affecting the patients’ activities of daily living.3 4
Functional recovery has been shown to arise, at least in part, from the reorganisation of functional brain networks, with intact neural structures compensating for the loss of specialised neural circuitry damaged by the lesion.5 6 Importantly, a focal stroke lesion also interferes with the neural processing in distant brain regions, thereby affecting the brain at a network level. In this context, neuroimaging studies have frequently reported altered brain activity in motor-related cortical areas of both hemispheres, even for lesions affecting primarily deep white matter.7–9 Longitudinal data revealed that in the first days after stroke, the activity of the primary motor cortex is typically decreased, particularly in patients with severe motor deficits, despite the structurally intact motor cortex.8 This pattern is typically followed by a bihemispheric increase of activity, which correlates with the amount of early motor recovery. However, the best predictors of functional motor recovery are high levels of activity in the ipsilesional motor cortex early after stroke as well as the activity pattern lateralised to the ipsilesional hemisphere.10 11 Thus, restoring neural activation, particularly in the lesioned hemisphere seems to be essential for functional recovery after stroke.
Comparable effects have been found for changes in motor-cortical excitability as probed by transcranial magnetic stimulation (TMS).12 In parallel to the initial decrease of fMRI activity observed for ipsilesional M1,8 TMS studies have also found lower excitability of this region, which correlates with the severity and prognosis of motor deficits.13 14
To date, first-line rehabilitative strategies for improving motor deficits are based on functional training, that is, physical or occupational therapy early after stroke.15 16 Such behavioural interventions have been demonstrated to facilitate neural reorganisations.17 Accumulating evidence suggests that non-invasive brain stimulation techniques such as repetitive TMS (rTMS) may enhance neuroplasticity, thereby facilitating neural reorganisation and recovery from stroke deficits.18 19 Particularly the observation of decreased ipsilesional excitability early after stroke has led to the hypothesis that rTMS may be capable of increasing excitability and thus aiding functional recovery.20 This effect has been demonstrated for different rTMS protocols varying in stimulation frequency, pattern and the number of pulses.20 21 Of note, rTMS may not only aid neural reorganisation within the stimulated region, but it also modulates the activity of interconnected brain regions, for example, the dorsal premotor cortex or the supplementary motor area, as shown for both healthy subjects22 and patients with stroke .23 Thus, rTMS applied to M1 likely results in a system-wide change of neural activity in both hemispheres. At the behavioural level, proof-of-principle studies indicate that a single session of rTMS applied to ipsilesional M1 may transiently improve motor function of the paretic hand.24 25 Further, a critical factor for a therapeutic effect seems to be a combination of plasticity enhancing interventions with motor training, possibly leading to a better consolidation of (re-) learnt motor skills.26–28
While several rTMS studies in patients with stroke reported transient improvements in motor function, other studies failed to demonstrate lasting beneficial effects.29–32 A recently published large sample (n=167) trial (Navigated Inhibitory rTMS to Contralesional Hemisphere (NICHE) trial) revealed that application of inhibitory rTMS over contralesional M1 in patients with chronic stroke failed to demonstrate any beneficial effect of contralesional 1 Hz stimulation paired with arm-motor training in patients with chronic stroke,33 despite promising data from a relatively large number of pilot studies with small sample sizes (usually 10–20 patients). One likely reason may be the time window of intervention, which, in most studies, targeted the chronic phase after stroke. Substantial functional recovery alongside high levels of neural plasticity is observed in the acute and subacute phases after stroke.34
In contrast, the effectiveness of behavioural interventions gets more and more limited if more time elapses from the onset of the stroke. This negative effect may also be true for rTMS-mediated excitatory effects and their potential to support the recovery of function and neurorehabilitation. Hence, the amplification of neuroplasticity using rTMS may be most effective during the acute and early subacute phases after a stroke. While data on neuromodulatory effects within the first few days and weeks after stroke remain scarce, recent evidence from our group indicates the lasting beneficial effects of rTMS on motor recovery in a sample of patients with stroke in the first few days after the stroke.23 In this study, two groups of patients with early subacute stroke (each n=13, on average 7 days poststroke) received intermittent theta burst stimulation (iTBS; 600 pulses, 70% resting motor threshold (RMT)) for 5 days, either covering ipsilesional M1 or a control with the TMS coil tilted over the parieto-occipital vertex. Recovery of grip strength was stronger in the M1-stimulated group than in the control-stimulated group, with the beneficial effect persisting at least 3–6 months. As shown by fMRI before and after the rTMS intervention, patients in the verum rTMS group featured increased functional connectivity between the modulated stimulation site and a functionally related motor network, including the dorsal premotor cortex and the supplementary motor area, compared with patients in the control-stimulation group.23 Given that without rTMS intervention, patients during the first few days after the stroke featured a loss of activity and connectivity in the ipsilesional hemisphere,35–37 the finding of increased connectivity with the stimulated M1 suggests that the beneficial effects of rTMS may not only result from inducing local plasticity, but also from enhancing connectivity with a functionally related motor network. Taken together, these findings support the hypothesis that rTMS may be applied in addition to physiotherapy to induce plasticity in the ipsilesional M1 and thereby promote motor outcome. Of note, the small sample size of the follow-up groups and the heterogeneity of postinterventional treatments across patients preclude a reliable estimation of the clinical use of combined iTBS and physiotherapy in patients with (sub-)acute stroke to date. While studies with similar small sample sizes corroborate a positive effect of M1-modulation by non-invasive brain stimulation after stroke,30 large randomised controlled trials are widely lacking.
Aims and hypotheses
Accordingly, this study aims to investigate the efficacy of combining iTBS with ipsilesional M1 versus iTBS over a parieto-occipital control site, priming physiotherapy in the early rehabilitation of patients with stroke suffering from impaired hand–motor function. Thereby, the main goal of our study is to demonstrate the effectiveness of iTBS in supporting the recovery of motor function in a sufficiently powered sample, expecting stronger rehabilitation effects on relative grip strength (primary outcome parameter) in the M1-iTBS group compared with the control-stimulation treated group. Furthermore, by assessing the secondary outcome parameters (Action Research Arm Test (ARAT), Fugl-Meyer assessment (FM), we also test whether combining iTBS with physiotherapy during early rehabilitation may influence more complex motor functions of the impaired upper extremity. This study will be the first with a large sample of patients with early subacute stroke (n=150), systematically assessing clinical deficits, electrophysiological data, structural images, comorbidity, and medication before, during, and at least 3 months after the application of iTBS. We hypothesise that the combination of physical training with iTBS over ipsilesional M1 significantly enhances motor recovery after stroke compared with physical training combined with control stimulation.
Methods
Study design, recruitment and procedure
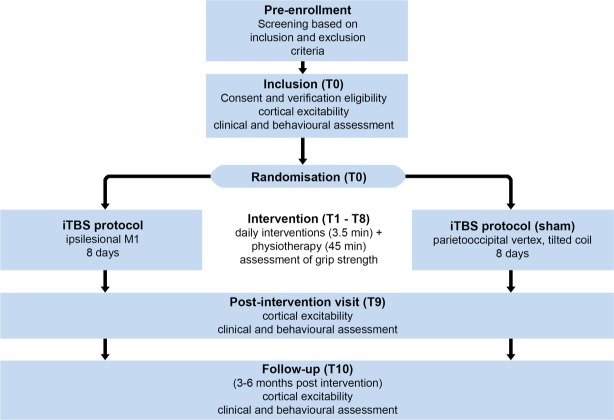
This prospective, randomised, controlled, double-blind, single-centre trial is conducted at the Department of Neurology University Hospital Cologne, Germany. Hospitalised patients with early subacute stroke (within the first 14 days poststroke), suffering from a hand–motor deficit due to ischaemic stroke, are screened for study participation by a stroke-specialised neurologist. Eligible patients are invited to participate in the study by the investigator, who has obtained the written informed consent. Several motor scores, as well as the general neurological status and electrophysiological measures of motor-cortical excitability, are assessed on the day of enrolment (T0) and 1 day after the last iTBS intervention (T9). A longitudinal follow-up after 3–6 months (T10) assesses the after effects that extend into the chronic poststroke phase. Of note, the first postintervention assessment at T9 takes place 1 day after stimulation, and hence does not reflect immediate stimulation after-effects. All patients undergo the same experimental procedure receiving iTBS interventions before physical therapy on days T1–T8 (figure 1), the latter conducted as a routine part of the early rehabilitation programme provided by the Department of Neurology, University Hospital Cologne. This programme (total duration of 300 min/day) includes daily physiotherapy, occupational therapy and speech therapy for at least 2 weeks. This timeframe determines the duration of the iTBS intervention phase, which aims at eight stimulations on consecutive workdays. Note that the intended stimulation period is longer than the five stimulations employed in our pilot study23 in order to increase the total stimulation dose. In case eight stimulations cannot be performed due to organisational reasons (eg, transfer of the patient to another rehab centre), a minimum of five stimulations is necessary to be included in the final analysis.23 A stimulation period longer than 8 days was not considered feasible without delaying further medical plans or subsequent treatment after transfer to a rehabilitation centre. Importantly, both groups receive the same amount of motor training, with cohorts solely differing in receiving M1-iTBS or control-iTBS before the physiotherapy session (see below). Details on trial characteristics based on the WHO trial registration dataset are provided in table 1.
Figure 1.
Flow chart of study procedure. iTBS, Intermittent theta burst stimulation.
Table 1.
1Trial characteristics based on WHO trial registration dataset
| Data category | Trial information |
| Primary registry and trial identifying number | German Clinical Trials Register (DRKS) DRKS-ID: DRKS00008963 |
| Date of registration in primary registry | 16 February 2016 |
| Secondary identifying numbers | ClinicalTrials.gov (NCT02910024) |
| Source(s) of monetary or material support | The study is conducted as an investigator-initiated study supported by the Max-Delbrück Prize to GRF and by the University of Cologne Emerging Groups Initiative (CONNECT group; CG and GRF) implemented in the Institutional Strategy of the University of Cologne and the German Excellence Initiative. |
| Primary sponsor | University of Cologne, Albertus-Magnus-Platz 50 923 Cologne |
| Secondary sponsor | NA |
| Contact for public queries | Prof Gereon R. Fink (gereon.fink@uk-koeln.de) |
| Contact for scientific queries | Prof Gereon R. Fink (gereon.fink@uk-koeln.de) |
| Public title | Theta-Burst-Stimulation in early Rehabilitation of Stroke (TheSiReS) |
| Scientific title | Theta-Burst-Stimulation in early Rehabilitation of Stroke (TheSiReS) |
| Country of recruitment | Germany |
| Healthy conditions(s) or problems studied | Stroke with hemiparesis, including impaired hand–motor function |
| Interventions | Active comparator: repetitive transcranial magnetic stimulation (rTMS) applied to the primary motor cortex of the lesioned hemisphere using the intermittent theta burst stimulation protocol (application of 3 pulses with a frequency of 50 Hz, in a theta-rhythm of 5 Hz for 2 s, repeated every 10 s, duration of one session: about 3.5 min) before physical therapy for 8 days. Sham comparator: repetitive transcranial magnetic stimulation (rTMS) in control position (tilted coil over parieto-occipital vertex) before physical therapy for 8 days. |
| Key inclusion and exclusion criteria | Inclusion criteria: written consent, age: 40–90 years, ischaemic stroke. hemiparesis with impaired hand–motor function. Exclusion criteria: Subjects who are legally detained in an official institute (§20 MPG), participation in a clinical trial within the last 12 weeks, electronic implants or ferromagnetic implants located in the head, neck or thorax (eg, clips, intracranial shunt, artificial heart valve, pacemaker), medication pump (eg, insulin pump), metal splinters in eye or head, pregnancy/breastfeeding, severe neurodegenerative disease, severe neuroinflammatory disease, history of seizures/epilepsy, physical addiction to alcohol, medication or drugs (excluded: nicotine), insufficient compliance, present or past malignant tumour involving the central nervous system, severe psychiatric disease, clinically manifest bilateral hemiparesis or infarcts in the primary motor cortex or along the corticospinal tract in the hemisphere ipsilateral to the hemiparesis, pre-existing cerebral infarctions with hemiparesis or pre-existing cerebral infarctions in the primary motor cortex or along the corticospinal tract, excluding microvascular changes (eg, clinically asymptomatic lacunae <1 cm), known brain lesion (surgical, traumatic), evidence for enhanced cerebral pressure, severe cardiac dysfunction, life expectancy <12 months, National Institutes of Health Stroke Scale Score (NIHSS) >20, blood glucose imbalances resistant to treatment (<50 mg/dL or >300 mg/dL), elevated blood pressure resistant to treatment (>185/110 mm Hg), systemic thrombolysis using alteplase or thrombectomy within the last 24 hours before enrolment in study, medication with benzodiazepines, high-potency antipsychotics or tricyclic antidepressants before hospitalisation or long-term during hospitalisation. |
| Study type | Interventional Allocation: randomised intervention model Masking: double-blind (subject, caregiver, investigator, outcomes assessor). Assignment: parallel Primary purpose:treatment |
| Date of first enrolment | April 2016 |
| Target sample size | 150 |
| Recruitment status | Recruiting |
| Primary outcome(s) | Relative grip force (time frame: 3–6 months after enrolment) |
| Key secondary outcome | Relative grip force (time frame: after 8 days of intervention, and 3–6 months after enrolment). Action Research Arm Test (time frame: after 8 days of intervention, and 3–6 months after enrolment), Fugl-Meyer Motor Scale of the upper extremity (time frame: after 8 days of intervention, and 3–6 months after enrolment). NIHSS (time frame: after 8 days of intervention, and 3–6 months after enrolment). modified Rankin Scale (time frame: after 8 days of intervention, and 3–6 months after enrolment). Motor evoked potential induced by stimulation of the affected motor cortex as a measure of motorcortex excitability (time frame: after 8 days of intervention, and 3 –6 months after enrolment). Resting motor threshold as measured by stimulation of the affected motor cortex as a measure of motorcortex excitability (time frame: after 8 days of intervention, and 3–6 months after enrolment). EuroQol 5D questionnaire (time frame: after 8 days of intervention, and 3–6 months after enrolment). Barthel-Index at admission and discharge in external rehabilitation facility (time frame: 3–6 months after enrolment). Days of rehabilitation after intervention phase (time frame: 3–6 months after enrolment). |
Patient and public involvement
The study was designed based on the available literature related to optimising motor recovery in patients with stroke using iTBS, as described in the introduction. There was no public involvement in the study design.
iTBS protocol
As a predominantly facilitatory rTMS protocol, iTBS has been rendered safe and effective, increasing cortical excitability in healthy subjects38 and in patients with acute stroke .39 One session of iTBS consists of 3 pulses delivered at a frequency of 50 Hz every 200 ms during 2 s (10 bursts), which are repeated every 10 s for a total duration of 3.5 min (600 pulses).38 For patients assigned to the study arm receiving an effective intervention, the protocol is applied over the ipsilesional M1, whereas patients in the control group receive iTBS over the parieto-occipital vertex, corresponding to the POz location of the 10–20 electroencephalography (EEG) system. Importantly, to prevent effective stimulation of cortical tissue in the control condition, the handle of the coil is placed parallel to the interhemispheric fissure pointing to the front. Besides, the coil is tilted upwards at about 45°, touching the skull not with the centre but with the rim to increase the coil-brain distance. This procedure induces similar acoustic and tactile effects as M1 stimulation without leading to a change of motor behaviour, motor-cortical excitability or neural activity as measured with fMRI.22 23 40–42 Compared with other facilitatory rTMS protocols, the short duration of the intervention (3.5 min) enables a good integration of iTBS in training schedules even when patients are severely affected. The second advantage of iTBS is its relatively low stimulation intensity, reducing the risk of adverse reactions, particularly seizures.43 The stimulation intensity of iTBS is individually adapted in each patient according to the excitability of the ipsilesional motor cortex. The original iTBS protocol, as published by Huang et al 38 set the stimulation intensity to 80% of the active motor threshold (AMT). However, assessment of the AMT requires subjects to perform constant contractions of the hand muscles which is often impossible for stroke patients with severe hand–motor weakness. The present study, therefore, set stimulation intensities to 70% of the RMT, which is independent of the patients’ motor abilities. Of note, using 70% RMT instead of 80% AMT has been repeatedly demonstrated to induce comparable after effects on cortical excitability,22 40 44 allowing effective application of iTBS in stroke.23 As shown in our proof-of-principle study,23 stimulation thresholds may exceed the maximum stimulator output (MSO) in case of a severe disruption of the corticospinal tract leading to no recordable motor-evoked potentials (MEPs). Here, the stimulation intensity is set to 50% MSO, which represents the upper limit for 50 Hz stimulation using a standard Magstim SuperRapid2 stimulator and which has been proven to be safe.
Inclusion and exclusion criteria
Inclusion and exclusion criteria are defined in line with previous iTBS studies in stroke22 23 and the guidelines for the use of rTMS in clinical practice and research.43 45 46
Inclusion criteria are
Written informed consent.
Age 40–90 years.
Ischaemic stroke.
Hemiparesis with impaired unilateral hand motor function.
Exclusion criteria are
Subjects legally detained in an official institute.
Participation in a clinical trial within the last 12 weeks.
Electronic or ferromagnetic implants located in the head, neck or thorax (eg, clips, intracranial shunt, artificial heart valve, pacemaker, medication pump).
Metal splinters in eye or head.
Pregnancy/breastfeeding.
Severe neurodegenerative disease (eg, Parkinson’s disease, Alzheimer’s disease).
Severe neuroinflammatory disease (eg, multiple sclerosis).
History of seizures/epilepsy.
Physical addiction to alcohol, medication or drugs (excluded: nicotine).
Insufficient compliance.
Present or past malignant tumour involving the central nervous system.
Severe psychiatric disease (eg, schizophrenia).
Bilateral hemiparesis or infarcts to the primary motor cortex or the corticospinal tract in the hemisphere ipsilateral to the hemiparesis.
Pre-existing cerebral infarctions with hemiparesis or pre-existing cerebral infarctions affecting the primary motor cortex or the corticospinal tract, excluding minor small vessel disease changes (eg, clinically asymptomatic lacunae <1 cm).
Known brain lesion (surgical, traumatic).
Evidence of enhanced cerebral pressure.
Severe cardiac dysfunction.
Life expectancy <12 months.
National Institutes of Health Stroke Scale (NIHSS) score at enrolment >20.
Blood glucose imbalances resistant to treatment (<50 mg/dL or >300 mg/dL).
Elevated blood pressure resistant to treatment (RR >185/110 mm Hg).
Systemic thrombolysis using r-tPA or thrombectomy within the last 24 hours before enrolment in the study.
Medication with benzodiazepines, antipsychotics or tricyclic antidepressants before hospitalisation or long-term during hospitalisation.
Outcome measures
The primary endpoint of this study is relative grip strength defined as of the maximum grip strength of the affected (paretic) hand compared with that of the unaffected hand, assessed 3–6 months after the intervention, that is, in the chronic phase poststroke. While motor recovery after stroke may be assessed with several measures, we selected grip strength based on the following rationale: first, grip strength represents a fundamental feature of hand motor function, and is typically reduced in patients suffering from stroke-induced hemiparesis. In turn, recovery of grip strength usually precedes the recovery of other motor domains such as dexterity or movement speed.47 Second, the assessment of grip strength can be conducted efficiently at the bedsite, even in severely affected patients.
Furthermore, improvements in grip strength predominantly reflect the restitution of neurological function as grip strength is less dependent on alternative strategies such as compensatory movements. Besides, grip strength is mediated by contralateral M1 activity.48 Therefore, given that in the present study iTBS is applied to enhance M1 activity, grip strength seems to be a sensitive readout to monitor improvements of M1. Finally, as the present study design is based on a pilot study that also used grip force as the primary outcome parameter,23 we aimed at reproducing the beneficial effects of iTBS on the recovery of grip force. Besides, we further assess the impact of iTBS on the motor recovery in other parameters frequently used to study motor performance after stroke. These secondary endpoints comprise different measures of gross and fine upper limb function assessed by the ARAT49 and the FM50 of the upper extremity, stroke severity measured by NIHSS, general disability (modified Rankin Scale)51 and quality of life (EuroQol 5D including the visual analogue scale). Moreover, in order to obtain electrophysiological measures of corticospinal integrity, MEP and the RMT of the ipsilesional M1 are included as secondary endpoints. Finally, to account for the differences in rehabilitation treatments between completion of the intervention (T9) and the follow-up assessment (T10), we document the performance in activities of daily living assessed by the Barthel scale as well as the duration of stay in external rehabilitation facilities.
In sum, these tests provide a detailed assessment, monitoring the clinical and electrophysiological condition of patients before and after iTBS (table 2).
Table 2.
: Overview of data collection and study timings
| Study period | Pre-enrolment | T0 | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 |
| Visits | ||||||||||||
| Screening (in-/exclusion criteria) | X | |||||||||||
| Written informed consent | X | |||||||||||
| Randomisation | X | |||||||||||
| Medical history | X | X | ||||||||||
| Neuroimaging (MRI/CT) | X | |||||||||||
| TMS-intervention (M1 iTBS/control iTBS) | X | X | X | X | X | X | X | X | ||||
| Physiotherapy | X | X | X | X | X | X | X | X | ||||
| Assessment of adverse events | X | X | X | X | X | X | X | X | X | X | X | |
| Relative grip strength | X | X | X | X | X | X | X | X | X | X | X | X |
| Documentation of medication | X | X | X | X | X | X | X | X | X | X | X | |
| Neurological examination | X | X | X | X | ||||||||
| Electrophysiological examination (RMT, MEPs) | X | X | X | |||||||||
| Upper limb motor function (ARAT, FM) | X | X | X | |||||||||
| Stroke severity (NIHSS) | X | X | X | |||||||||
| Disability (mRS) | X | X | X | |||||||||
| Quality of life (EQ-5D) | X | X | X | |||||||||
| Assessment of external rehabilitation time | X |
ARAT, Action Research Arm Test; EQ-5D, EuroQol 5D including the visual analogue scale; FM, Fugl-Meyer Motor Scale of the upper extremity;iTBS, intermittent theta burst stimulation; MEPs, Motor evoked potentials; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale;RMT, resting motor threshold.
Randomisation and stratification
After obtaining informed consent, randomisation is performed using the 24/7 online randomisation tool ALEA (FormsVision BV, Abcoude, NL). Patients are allocated 1:1 into the intervention groups, receiving ‘verum’ or ‘control’ iTBS. In order to balance groups for potential confounding factors, randomisation is stratified based on patients’ age (≤68, >68 years), motor impairment (relative grip strength <10%, 10%–70%,>70%) and stimulation intensity (≤50%,>50% maximal stimulator output), as these factors are known to impact motor recovery poststroke.52 53
Statistical analysis
After data collection, confirmatory and descriptive analyses will be conducted. In our proof-of-principle study, we obtained data from a smaller sample21 which revealed, 3– 6 months after the intervention, an increase in grip strength of 38.1%±28.7% in patients treated with iTBS versus 26.2%±11.7% in the control-stimulation group. Thus, the observed strength of the effect amounted to 0.54. Using an unpaired, two-sided t-test with a type I error of 5% and a power of 80%, a sample of 110 patients is required (calculated using the software G*Power V.3.1.7). Assuming a dropout rate of 25% based on the cohort of Volz et al (2016), an estimated sample of 150 recruited patients is needed.
Variables are analysed descriptively using mean, SD, quantiles (0, 25, 50, 75, 100), or count and frequency, respectively. The final statistical analysis is carried out with an intention to treat) collective including all patients who received at least one intervention (verum or control) with a subsequent grip strength testing, to assess the safety and efficacy of iTBS. Moreover, a supportive analysis is performed based on the ‘per protocol’ collective which includes all patients who underwent at least five23 interventions (verum or control) and provided grip force measures at baseline and at 3–6-month follow-up.
The primary endpoint, that is, the change in grip strength after 3 months (T10), is analysed using a linear mixed model with repeated measurements, in which the factors group (verum, control), time, group x time and strata at baseline (age, motor impairment, stimulation intensity) will be entered. Moreover, the model will account for the number of data points obtained during the intervention phase (T1–T9). The primary hypothesis is addressed using a customised test (contrast) to compare the change from baseline (T0) to 3–6 months (T10) between the two treatment groups. Mean difference, the corresponding 95% CI, and the p-value (two-sided) will be presented.
All secondary variables will be analysed similarly or using unpaired t-tests or Mann-Whitney U tests. Serious adverse events (SAE) are listed. Subgroup analyses will be performed for randomisation stratification variables and length of rehabilitation therapy. The current version of SPSS statistics (IBM Corp) will be used for the statistical analyses.
Blinding
The study is carried out using a double-blinded design, in which neither the patients nor the testing physicians nor statisticians are aware of the intervention arm (verum or control). As applying iTBS over different stimulation sites (depending on the patients’ intervention arm) implies that physicians performing the intervention cannot be blinded, the intervention team needs to be separated into blinded physicians performing patient recruitment and examinations, and unblinded physicians exclusively applying iTBS. Thereby, we ensure that both patients and investigators are blinded during the assessment of outcome parameters throug the entire study procedure. In case of an emergency unblinding, investigators at the department of neurology have access to sealed envelopes labelled with the patients’ randomisation numbers. To maintain the quality of the trial, a patient’s allocation should only be unblinded in exceptional circumstances when knowledge of the actual treatment is essential for the management of the patient.
Safety
The exclusion criteria of the present trial follow the latest safety recommendations for rTMS,43 46 thereby reducing the risk of adverse events (AEs) or reactions to iTBS to a minimum. AEs or SAEs are assessed throughout observation period of the study, including all scheduled visits T0–T10. All events are reported to the federal authorities (Federal Institute for Drugs and Medical Devices, BfArM). In our pilot study,23 no SAE occurred, especially no focal or generalised seizures.
Documentation and quality assurance
All data assessed during the trial are documented promptly after data acquisition and entered into the electronic case report form (eCRF) by the responsible investigators. Regular monitor inspections ensure high quality of documentation and correct implementation of the study protocol. The Clinical Trials Centre Cologne (CTCC Cologne) is responsible for the monitoring. Besides the initiation visit at the beginning and the closeout visit at the end of the study, monitoring visits are performed, on average, after every tenth patient included. Thus, at least 15 visits are scheduled. Monitoring visits include a review of source data documented in the eCRF, written consent, inclusion and exclusion criteria.
Data collection and management
CTCC Cologne performs the data management. The commercial online software TrialMasterTM (OmniComm.com) is used as a data management system, ensuring data safety with a firewall and backup system, including multiple data storage sites. The database was developed and validated by CTCC Cologne.
All data collectors are stroke-specialised neurologists who have been trained in good clinical practice . After the investigators enter the data into the eCRF, CTCC Cologne reviews the data for completeness and plausibility. The data manager and investigators resolve discrepancies and implausible entries.
Only researchers involved in the data collection, management and data analysis will have access to the final dataset. However, the principal investigator allows direct access to all source data and documents at monitoring and inspection from federal authorities (Federal Institute for Drugs and Medical Devices, BfArM).
Ethics and dissemination
The study was approved by the Ethics Commission of the Medical Faculty/University of Cologne (reference number: 15-343). The amendments leading to the current version (V.3, 15 November 2018) were made to increase the number of patients eligible for the study. Before entering the study, all participants are informed that their participation is entirely voluntary, and that their withdrawal of consent is possible at any time without further consequences. All requirements regarding the well-being, insurance, rights, and privacy of participants are fulfilled. The study findings will be reported at conferences and in peer-reviewed journals.
Trial status
At the time of submission, recruitment has not been completed.
Supplementary Material
Footnotes
Contributors: CG, LH, LJV and GRF developed the study design and wrote the statistical analysis plan in collaboration with DK and SH. LH, CR, CG and GRF will perform the clinical evaluation. CT will conduct rTMS interventions. LH and CG wrote the first draft of the manuscript. CT, LJV and GRF revised it for technical content. All authors read and approved the final manuscript.
Funding: This trial is supported by the Max-Delbrück Prize to GRF and by the University of Cologne Emerging Groups Initiative (CONNECT group; CG and GRF) implemented into the Institutional Strategy of the University of Cologne and the German Excellence Initiative. University of Cologne, Albertus-Magnus-Platz 50923 Cologne.
Competing interests: None declared.
Patient consent for publication: Written informed consent is obligatory before study participation. The study is registered at the German Clinical Trials Register (DRKS, www.drks.de, DRKS-ID: DRKS00008963) and the ClinicalTrials.gov database (Identifier: NCT02910024).
Ethics approval: The Ethics Commission of the Medical Faculty of the University of Cologne (reference number 15 - 343) approved the study and its amendments: first approval 2 November 2015 (original version). Amendment (V.2.22) approved and implemented 20 December 2016. Specification of exclusion criteria. Amendment (V.3) approved and implemented 15 November 2018. Change of inclusion and exclusion criteria.
Provenance and peer-review: Not commissioned; externally peer-reviewed.
References
- 1. Feigin VL, Forouzanfar MH, Krishnamurthi R, et al. Global and regional burden of stroke during 1990–2010: findings from the global burden of disease study 2010. The Lancet 2014;383:245–55. 10.1016/S0140-6736(13)61953-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–95. 10.1056/NEJMoa1415061 [DOI] [PubMed] [Google Scholar]
- 3. Gresham GE, Fitzpatrick TE, Wolf PA, et al. Residual disability in survivors of stroke — the Framingham study. N Engl J Med 1975;293:954–6. 10.1056/NEJM197511062931903 [DOI] [PubMed] [Google Scholar]
- 4. Carod-Artal J, Egido JA, González JL, et al. Quality of life among stroke survivors evaluated 1 year after stroke: experience of a stroke unit. Stroke 2000;31:2995–3000. [DOI] [PubMed] [Google Scholar]
- 5. Cramer SC. Repairing the human brain after stroke: I. mechanisms of spontaneous recovery. Ann Neurol 2008;63:272–87. 10.1002/ana.21393 [DOI] [PubMed] [Google Scholar]
- 6. Nudo RJ. Recovery after brain injury: mechanisms and principles. Front Hum Neurosci 2013;7:887 10.3389/fnhum.2013.00887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chollet F, Dipiero V, Wise RJS, et al. The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol 1991;29:63–71. 10.1002/ana.410290112 [DOI] [PubMed] [Google Scholar]
- 8. Ward NS, et al. Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 2003;126:2476–96. 10.1093/brain/awg245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Grefkes C, Nowak DA, Eickhoff SB, et al. Cortical connectivity after subcortical stroke assessed with functional magnetic resonance imaging. Ann Neurol 2008;63:236–46. 10.1002/ana.21228 [DOI] [PubMed] [Google Scholar]
- 10. Rehme AK, Volz LJ, Feis D-L, et al. Individual prediction of chronic motor outcome in the acute post-stroke stage: behavioral parameters versus functional imaging. Hum Brain Mapp 2015;36:4553–65. 10.1002/hbm.22936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rehme AK, Eickhoff SB, Rottschy C, et al. Activation likelihood estimation meta-analysis of motor-related neural activity after stroke. Neuroimage 2012;59:2771–82. 10.1016/j.neuroimage.2011.10.023 [DOI] [PubMed] [Google Scholar]
- 12. Volz LJ, Hamada M, Rothwell JC, et al. What makes the muscle twitch: motor system connectivity and TMS-Induced activity. Cerebral Cortex (Published Online First: 7 March 2014). [DOI] [PubMed] [Google Scholar]
- 13. Pennisi G, et al. Transcranial magnetic stimulation after pure motor stroke. Clin Neurophysiol 2002;113:1536–43. 10.1016/S1388-2457(02)00255-9 [DOI] [PubMed] [Google Scholar]
- 14. Stinear CM, Barber PA, Petoe M, et al. The PreP algorithm predicts potential for upper limb recovery after stroke. Brain 2012;135:2527–35. 10.1093/brain/aws146 [DOI] [PubMed] [Google Scholar]
- 15. Maulden SA, Gassaway J, Horn SD, et al. Timing of initiation of rehabilitation after stroke. Arch Phys Med Rehabil 2005;86:34–40. 10.1016/j.apmr.2005.08.119 [DOI] [PubMed] [Google Scholar]
- 16. Pollock A, Farmer SE, Brady MC, et al. Interventions for improving upper limb function after stroke. Cochrane Database Syst Rev 2014;23 10.1002/14651858.CD010820.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pin-Barre C, plasticity JLN. Physical exercise as a diagnostic, rehabilitation, and preventive tool: influence on neuroplasticity and motor recovery after stroke. Hindawicom 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grefkes C, Fink GR. Disruption of motor network connectivity post-stroke and its noninvasive neuromodulation. Curr Opin Neurol 2012;25:670–5. 10.1097/WCO.0b013e3283598473 [DOI] [PubMed] [Google Scholar]
- 19. Grefkes C, Fink GR. Noninvasive brain stimulation after stroke: it is time for large randomized controlled trials! Curr Opin Neurol 2016;29:714–20. 10.1097/WCO.0000000000000395 [DOI] [PubMed] [Google Scholar]
- 20. Hummel FC, Cohen LG. Non-Invasive brain stimulation: a new strategy to improve neurorehabilitation after stroke? Lancet Neurol 2006;5:708–12. 10.1016/S1474-4422(06)70525-7 [DOI] [PubMed] [Google Scholar]
- 21. Ziemann U. Improving disability in stroke with rTMS. Lancet Neurol 2005;4:454–5. 10.1016/S1474-4422(05)70126-5 [DOI] [PubMed] [Google Scholar]
- 22. Nettekoven C, Volz LJ, Kutscha M, et al. Dose-Dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci 2014;34:6849–59. 10.1523/JNEUROSCI.4993-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Volz LJ, Rehme AK, Michely J, et al. Shaping early reorganization of neural networks promotes motor function after stroke. Cerebral Cortex 2016;26:2882–94. 10.1093/cercor/bhw034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ameli M, Grefkes C, Kemper F, et al. Differential effects of high-frequency repetitive transcranial magnetic stimulation over ipsilesional primary motor cortex in cortical and subcortical middle cerebral artery stroke. Ann Neurol 2009;66:298–309. 10.1002/ana.21725 [DOI] [PubMed] [Google Scholar]
- 25. Khedr EM, Ahmed MA, Fathy N, et al. Therapeutic trial of repetitive transcranial magnetic stimulation after acute ischemic stroke. Neurology 2005;65:466–8. 10.1212/01.wnl.0000173067.84247.36 [DOI] [PubMed] [Google Scholar]
- 26. Ackerley SJ, Stinear CM, Barber PA, et al. Combining theta burst stimulation with training after subcortical stroke. Stroke 2010;41:1568–72. 10.1161/STROKEAHA.110.583278 [DOI] [PubMed] [Google Scholar]
- 27. Talelli P, Wallace A, Dileone M, et al. Theta burst stimulation in the rehabilitation of the upper limb: a semirandomized, placebo-controlled trial in chronic stroke patients. Neurorehabil Neural Repair 2012;26:976–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reis J, Robertson EM, Krakauer JW, et al. Consensus: can transcranial direct current stimulation and transcranial magnetic stimulation enhance motor learning and memory formation? Brain Stimul 2008;1:363–9. 10.1016/j.brs.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 29. Volz LJ, Grefkes C. Basic principles of rTMS in motor recovery after stroke. Therapeutic rTMS in Neurology 2016:23–37. [Google Scholar]
- 30. Hsu W-Y, Cheng C-H, Liao K-K, et al. Effects of repetitive transcranial magnetic stimulation on motor functions in patients with stroke. Stroke 2012;43:1849–57. 10.1161/STROKEAHA.111.649756 [DOI] [PubMed] [Google Scholar]
- 31. Adeyemo BO, Simis M, Macea DD, et al. Systematic review of parameters of stimulation, clinical trial design characteristics, and motor outcomes in non-invasive brain stimulation in stroke. Front Psychiatry 2012;3 10.3389/fpsyt.2012.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hao Z, Wang D, Zeng Y, et al. Repetitive transcranial magnetic stimulation for improving function after stroke. Cochrane Database Syst Rev 2013;78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Harvey RL, Edwards D, Dunning K, et al. Randomized sham-controlled trial of Navigated repetitive transcranial magnetic stimulation for motor recovery in stroke. Stroke 2018;49:2138–46. 10.1161/STROKEAHA.117.020607 [DOI] [PubMed] [Google Scholar]
- 34. Buma F, Kwakkel G, Ramsey N. Understanding upper limb recovery after stroke. Restor Neurol Neurosci 2013;31:707–22. 10.3233/RNN-130332 [DOI] [PubMed] [Google Scholar]
- 35. Rehme AK, Volz LJ, Feis D-L, et al. Identifying neuroimaging markers of motor disability in acute stroke by machine learning techniques. Cerebral Cortex 2015;25:3046–56. 10.1093/cercor/bhu100 [DOI] [PubMed] [Google Scholar]
- 36. Rehme AK, Fink GR, von Cramon DY, et al. The role of the contralesional motor cortex for motor recovery in the early days after stroke assessed with longitudinal fMRI. Cerebral Cortex 2011;21:756–68. 10.1093/cercor/bhq140 [DOI] [PubMed] [Google Scholar]
- 37. Rehme AK, Eickhoff SB, Wang LE, et al. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. Neuroimage 2011;55:1147–58. 10.1016/j.neuroimage.2011.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang Y-Z, Edwards MJ, Rounis E, et al. Theta burst stimulation of the human motor cortex. Neuron 2005;45:201–6. 10.1016/j.neuron.2004.12.033 [DOI] [PubMed] [Google Scholar]
- 39. Di Lazzaro V, Profice P, Pilato F, et al. Motor cortex plasticity predicts recovery in acute stroke. Cerebral Cortex 2010;20:1523–8. 10.1093/cercor/bhp216 [DOI] [PubMed] [Google Scholar]
- 40. Cárdenas-Morales L, Volz LJ, Michely J, et al. Network connectivity and individual responses to brain stimulation in the human motor system. Cerebral Cortex 2014;24:1697–707. 10.1093/cercor/bht023 [DOI] [PubMed] [Google Scholar]
- 41. Nettekoven C, Volz LJ, Leimbach M, et al. Inter-Individual variability in cortical excitability and motor network connectivity following multiple blocks of rTMS. Neuroimage 2015;118:209–18. 10.1016/j.neuroimage.2015.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Diekhoff-Krebs S, Pool E-M, Sarfeld A-S, et al. Interindividual differences in motor network connectivity and behavioral response to iTBS in stroke patients. Neuroimage 2017;15:559–71. 10.1016/j.nicl.2017.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rossi S, Hallett M, Rossini PM, et al. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009;120:2008–39. 10.1016/j.clinph.2009.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gentner R, Wankerl K, Reinsberger C, et al. Depression of human corticospinal excitability induced by magnetic theta-burst stimulation: evidence of rapid polarity-reversing metaplasticity. Cerebral Cortex 2008;18:2046–53. 10.1093/cercor/bhm239 [DOI] [PubMed] [Google Scholar]
- 45. Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International workshop on the safety of repetitive transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol 1998;38:1–16. [DOI] [PubMed] [Google Scholar]
- 46. Rossini PM, Burke D, Chen R, et al. Non-Invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: basic principles and procedures for routine clinical and research application. An updated report from an I.F.C.N. Committee. Clin Neurophysiol 2015;126:1071–107. 10.1016/j.clinph.2015.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heller A, Wade DT, Wood VA, et al. Arm function after stroke: measurement and recovery over the first three months. J Neurol Neurosurg Psychiatry 1987;50:714–9. 10.1136/jnnp.50.6.714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ward NS, Newton JM, Swayne OBC, et al. The relationship between brain activity and peak grip force is modulated by corticospinal system integrity after subcortical stroke. Eur J Neurosci 2007;25:1865–73. 10.1111/j.1460-9568.2007.05434.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lyle RC. A performance test for assessment of upper limb function in physical rehabilitation treatment and research. Int J Rehabil Res 1981;4:483–92. 10.1097/00004356-198112000-00001 [DOI] [PubMed] [Google Scholar]
- 50. Fugl-Meyer AR, Jääskö L, Leyman I, et al. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. [PubMed] [Google Scholar]
- 51. Rankin J. Cerebral vascular accidents in patients over the age of 60: I. General considerations. Scott Med J 1957;2:127–36. 10.1177/003693305700200401 [DOI] [PubMed] [Google Scholar]
- 52. Bembenek JP, Kurczych K, Karli Nski M, et al. The prognostic value of motor-evoked potentials in motor recovery and functional outcome after stroke − a systematic review of the literature. Funct Neurol 2012;27:79–84. [PMC free article] [PubMed] [Google Scholar]
- 53. Kwakkel G, Kollen BJ. Predicting activities after stroke: what is clinically relevant? Int J Stroke 2013;8:25–32. 10.1111/j.1747-4949.2012.00967.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.