Abstract
Objectives
Hunger training (HT) is an intervention designed to teach people to eat according to their hunger by connecting physical symptoms of appetite with glucose levels. HT is most effective for weight loss, and improving eating behaviours when adherence is high. However, adherence is a challenge that should be explored prior to wider dissemination. The aim of this study was to explore participants’ experience and self-reported adherence and behaviour change related to HT.
Design
A qualitative study, nested within a randomised controlled pilot study of two different methods of monitoring glucose during HT. Semistructured interviews were audio-recorded, transcribed verbatim and analysed thematically using a phenomenological approach.
Setting
Single-centre study with participants recruited from the local area.
Participants
40 participants began the pilot study and 38 participants (52.6% women) remained at 1 month and completed interviews.
Results
Most participants felt they were able to match their hunger to their glucose levels by the end of the intervention. The main adherence barriers were the social pressure to eat, lack of time and lack of flexibility in participants’ meal schedules. Common adherence enablers were having a set routine, social support and accountability. Participants described increased awareness of hungry versus non-hungry eating and better cognition of feelings of hunger and satiety as a result of the intervention, which in turn led to changes of food choice, portion size and adjusted meal timing and frequency.
Conclusions
Findings show that HT is acceptable from a patient perspective, and results can be used to inform the translation of HT programme to healthcare settings.
Trial registration number
ACTRN12618001257257.
Keywords: nutrition & dietetics, public health, qualitative research, obesity, translational research, adherence
Strengths and limitations of this study.
In-depth interviews allowed for detailed insight into participants’ experiences of hunger training, including adherence barriers and enablers as well as behaviour change.
Adherence to rigorous qualitative methods and analysis provided confidence in our findings, which are applicable to other lifestyle interventions.
While our sample was diverse in terms of sex, age, education and income, the New Zealand ‘university town’ setting, as well as the predominantly European ethnicity of participants may limit extrapolation to other countries and cultures.
As with all interviews, there was potential for response biases; however, we tried to limit this by introducing two independent researchers to conduct the interviews.
Introduction
Weight management is crucial to prevent chronic diseases; however, most weight loss diets prove unsustainable in the long term.1 2 A more viable approach may be to teach people to eat according to their appetite signals, which has been shown to benefit weight maintenance. However, they have been inconsistently effective for weight loss.3–7 This may be because overweight and obesity is linked with difficulty sensing and responding to physiological hunger and satiety cues, decreasing awareness of appetite.8–10
To overcome this barrier, an intervention known as hunger training (HT) uses glucose monitoring as an indicator of hunger to help people gain greater awareness of their appetite signals and eat accordingly.11 12 A limited body of research has found that HT produces clinically important weight loss, and reduces emotional and external eating13–15; however, more research into the efficacy of HT and the ability of participants to adhere to this novel method is needed.
The combination of the minimal human resources required for the delivery of HT and the potential of sustainable weight management makes it a promising intervention for primary healthcare. However, as with most health interventions, adherence is a challenge that must be investigated prior to wider dissemination.16 17 Previous work has shown that benefits of HT are greater for participants with higher adherence, and that only about one-third of participants sufficiently adhere to experience a clinically beneficial effect.15 Before HT can be widely implemented, the underlying mechanisms that contribute to the effectiveness of HT and the barriers and enablers to adherence must be determined. We recently undertook a randomised controlled pilot study of two different methods of monitoring glucose during HT, which included the theoretical approach of phenomenology to qualitatively examine personal participant experiences to arrive at a better understanding of how HT affected their behaviour as a whole.18 The aim of this manuscript was to qualitatively explore, from the participants’ perspective, their overall experiences with HT, their personal practice in adhering to HT and any resulting behaviour change they observed after experiencing HT, in order to inform translation of HT from research to practice, including whether any differences arose as a consequence of the different glucose monitoring methods.
Methods
Study design and participants
This study was approved by the New Zealand Southern Health and Disability Ethics Committee (18/STH/105) and was registered with the Australian New Zealand Clinical Trials Registry (ACTRN12618001257257). All participants provided written informed consent.
Forty adults were recruited from August to October 2018 from the local community through social media channels and local advertisement, and were included if they were 18 years of age or older, had a body mass index (BMI) of 30 kg/m2 or higher and were willing to measure their glucose by fingerprick blood sample and wear a continuous glucose monitor. Exclusion criteria were use of medication that affects weight; pregnancy or breastfeeding; allergy to surgical adhesive; skin changes or disease on the upper arm; or imaging appointments scheduled during the study.
Patient and public involvement
No patient or public were involved in the development of the research question, interpretation of the results or writing of this document. The results will be disseminated to participants via email.
Randomisation and procedures
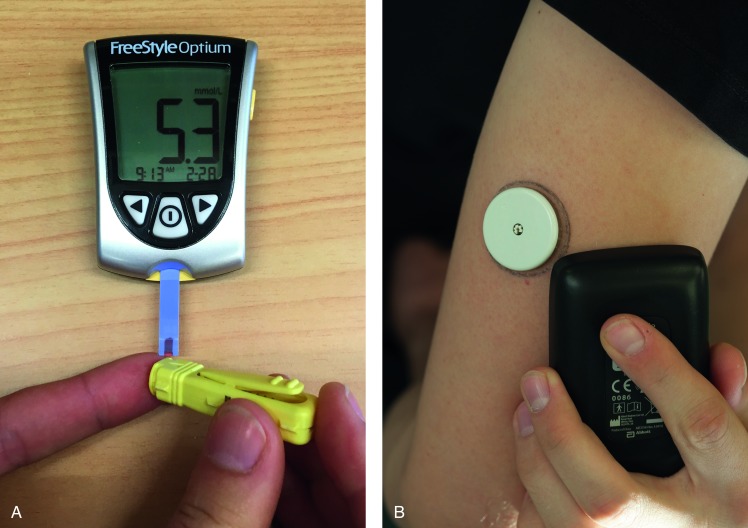
Participants were randomised to one of two groups using computerised block randomisation with random length blocks after stratification for sex. The ‘fingerpricking’ group measured their capillary glucose from a fingerprick sample by portable glucometer (Abbott Freestyle Optium Glucose Metre, Australia, figure 1A). The ‘scanning’ group used the Freestyle Libre Flash Glucose Monitoring system (Abbott Diabetes Care, Australia, figure 1B), which continuously measures interstitial glucose every 15 min. A thin water-resistant sensor was inserted just under the skin on the back of the arm, and remained there for 14 days, then replaced. When the participant wanted to test their glucose, they passed a reader over their arm to display current glucose levels. Both HT groups received the same guidance and support.
Figure 1.
Glucose measuring equipment, (a) the Freestyle Optium glucose Metre (Abbott Freestyle Optium glucose Metre, Australia), test strip and Lancet used by the “fingerpricking” group; (b) the Freestyle Libre flash glucose monitoring system (Abbott diabetes care, Australia), worn by the “scanner” group.
HT intervention
Participants were instructed to only eat or drink a caloric beverage if their glucose concentration was below their individualised cutoff, which was based on the average of fasting glucose from their first two mornings. If participants’ glucose was above their cutoff value, they were instructed to wait at least 20 min before retesting.
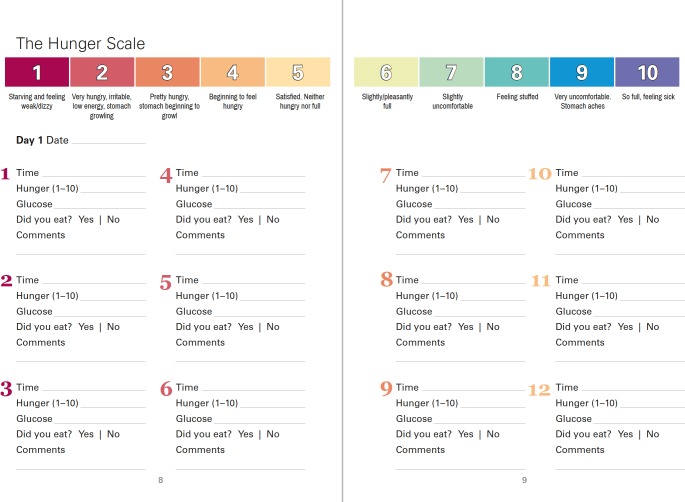
Alongside glucose monitoring, participants were asked to rate their hunger level (figure 2), and to note their glucose level and whether they ate, every time they wanted to consume food or caloric drink. Participants attended three HT appointments. At baseline, participants were introduced to HT and taught how to measure their glucose, based on their randomisation. At the day 14 visit, participants could ask questions and discuss challenges and successes, and were provided with a reading on intuitive eating using glucose monitoring.19 On the last visit (at 1 month), participants returned their equipment and participated in a semistructured interview with an independent interviewer (WEdB or ALW) not previously known to the participants.
Figure 2.
A page spread from the hunger training booklet.
Data collection
Researchers conducted in-depth interviews with each participant at the last visit. A semistructured interview guide (see online supplementary file) was developed to explore (1) participants’ experiences of HT; (2) perceived behaviour change due to HT; (3) self-reported adherence to the intervention; (4) future expectations; and (5) intervention feedback. All interviews were digitally recorded, and professionally transcribed verbatim. Transcripts were processed anonymously. After reviewing the transcribed interviews, it was clear that saturation had been reached and it was deemed unlikely that new topics would arise.20 21
bmjopen-2019-032248supp001.pdf (37.1KB, pdf)
Data analysis
The transcribed interviews were systematically scrutinised to guide coding development, key to employing grounded theory in analysing qualitative work.22 23 Codes were first piloted and refined using a subset of interviews; each interview was coded for themes by two researchers using NVivo.24 The thematic analyses took an inductive approach and included familiarisation with the interviews and transcripts, development of codes, coding of transcript and a convening meeting to dicuss coded content, to collate codes into themes and to reconsolidate any disagreements.23 25 Researchers conducting analyses (WEdB, ALW and MRJ) were blind to any participant classifications at the time of analyses.
The results section includes the use of qualifiers that have been adapted from previous studies.26–28 When an issue was discussed by 1 to 9 participants, we referred to a ‘few’; for between 10 and 20 participants, we referred to ‘some’; for between 21 and 30 participants, we referred to ‘most’; for between 31 and 37, we referred to ‘almost all’; and for 38, we referred to ‘all’.
Results
The research team conducted a total of 38 semistructured interviews with HT participants; two participants dropped out of the study before their interview. Participants ranged in age from 20 to 78 years, had an HbA1c between 28 and 100 mmol/mol, and an individualised glucose cutoff between 4.0 and 14.0 mmol/L (table 1). Participants lost an average of 4 kg (SD 6.7 kg) at 6 months, with similar results between scanners and fingerprickers.
Table 1.
Baseline characteristics of participants
| Variable | All (n=38) |
| Randomised to scanning, n (%) | 19 (50.0) |
| Female, n (%) | 20 (52.6) |
| Age (years) | 45.0 (13.0) |
| HbA1C (mmol/mol) (median, IQR) | 37.0 (34–42) |
| Glucose cutoff (mmol/L) | 6.1 (1.9) |
| Diabetes status, n (%) | |
| Non-diabetic | 27 (71.1) |
| Prediabetic | 8 (21.1) |
| Type 2 diabetes | 3 (7.9) |
| Body mass index (kg/m2) | 38.3 (7.4) |
| Education, n (%) | |
| School only | 12 (31.6) |
| Postsecondary | 4 (10.5) |
| University | 22 (57.9) |
| Ethnicity, n (%)* | |
| New Zealand European | 36 (94.7) |
| Māori | 4 (10.5) |
| Samoan | 2 (5.3) |
| Other | 3 (7.9) |
| Partnered, n (%) | 25 (65.8) |
| Household income (New Zealand Dollar), n (%) | |
| <50 k | 14 (36.8) |
| 50–100 k | 11 (28.9) |
| 100–150 k | 12 (31.6) |
| >150 k | 1 (2.6) |
| Depression Anxiety Stress Scale (DASS-21) | |
| Stress | 12.2 (8.7) |
| Depression | 9.1 (9.3) |
| Anxiety | 7.7 (5.7) |
Values are mean (standard deviation) unless otherwise indicated.
*Multiple options are possible therefore responses surpass 100%.
Participants’ experiences with HT
Glucose measuring experience and self-reported adherence
Participants explained that is was useful to have an objective measure of their hunger; ‘It was helpful to see an actual concrete measurement of ‘Was I actually hungry? Or was I just imagining that kind of thing?’ […] it was actually something quite tangible.’ (#25, female, scanner). A few participants commented that HT increased their self-efficacy; ‘I was unprepared to feel empowered by taking that modicum of control. So that was really cool.’ (#63, male, scanner).
Almost all participants described situations where they were unable to adhere to the HT protocol, and ate without measuring their glucose levels or ate when their glucose was above their cutoff. Some reasons for not measuring were because they forgot equipment or forgot to measure; were too busy; or were in social situations that made measuring difficult. Reasons for eating above cutoff included social pressure to eat, feeling extremely hungry, a lack of flexibility to eat at different times, illness and eating out of habit.
Most participants discussed the social pressure to eat; ‘In a dinner situation where you’ve got to eat when everyone else is eating. You can’t not eat, can’t just say ‘sorry, I’m checking my glucose’.’ (#72, male, scanner). Participants also described the cultural importance of food in their families or social groups as reasons for not adhering.
There was a clear distinction in experiences and adherence barriers between participants randomised to fingerpricking compared with those who were randomised to scanning. Most of those who were ambivalent, and all three participants who were outspokenly negative about their glucose measuring experience, were randomised to fingerpricking. Almost all fingerprickers addressed the pain and inconvenience of testing, such as spilling blood, difficulty in obtaining sufficient blood, having to wash hands before pricking and disposing the lancets safely. A few explained that they were initially hindered by the pain but that they got used to it over time, whereas others thought that pain got worse. A few also explained the pain and inconvenience helped them be mindful; ‘That pain and inconvenience did help me, it set the routine because it’s like ok to eat I have to do this very inconvenient thing and cause myself a little bit of pain, do I really want to go ahead with that, is that chocolate biscuit really worth that and often the answer was no.’ (#9, female, fingerpricker). Those in the fingerpricking group were more likely to describe social situations in which they did not adhere since measuring their glucose made them feel self-conscious and a few expressed worries about being stigmatised.
Those who used the scanner were generally more positive about their glucose measuring experience. Almost all said scanning was quick, easy, discreet and convenient, which allowed for frequent checking.
For the remaining themes, no clear differences were apparent between participants randomised to the different methods of gluicose measuring.
Booklet experience and self-reported adherence
A few participants explained the booklet helped them discover a pattern between their hunger and glucose and be more aware of food intake. A few explained how the hunger scale helped them understand hunger and fullness.
A few participants explained they occasionally forgot to complete the booklet, that it was impractical, and that it was ‘just another thing to carry around’. Hence, some participants explained that they only completed the booklet retrospectively. Several participants suggested developing a smartphone app to replace the booklet.
A few felt that the hunger scale should be personalised, or reflect feelings instead of numbers. Participants also talked about additional information that could be included in the booklet, including exercise and dietary recommendations, mindfulness and adherence techniques, and coping strategies for cravings.
Adherence enablers
Some participants stated that daily structure and normal routine helped them comply. Social support and accountability was another adherence enabler for some participants, specifically that provided by family and friends who helped them stick to the routine of checking their glucose and eating appropriately. A few mentioned their doctors expressed interest in the study and were supportive, providing another level of accountability.
Others indicated that adherence became easier; ‘I think it got easier as time’s gone on because as I was more mindful about eating breakfast and I guess doing preparations for lunch, knowing what’s coming and how to fit it in with the (glucose) levels.’ (#37, male, scanner).
Association between glucose and hunger
Most participants were able to determine a clear association between their glucose levels and hunger; ‘I noticed that […] when I was feeling really hungry, stomach growling, that my glucose was under four, which was like my threshold. I kind of almost got intuitive about it.’ (#38, female, scanner). Those who were able to detect an association described a learning curve, with the association became clearer over time; ‘Yeah, and I had the data in front of me that just said, well logically you're not hungry. You don't feel hungry with that grade of 1 to 10, your glucose says you're not hungry because you've still got obviously some sugars in your system providing energy, and I just thought ‘well alright then, I'll have a drink’. And then I just got in the habit of doing it and I found that really helpful.’ (#39, female, fingerpricker).
A few were unable to recognise an association and felt confused. While there was no clear delineation between groups regarding the association between glucose levels and hunger, confusion was slightly more common among fingerprickers. Common reasons for confusion were when glucose levels were high before breakfast, after physical activity, and when they felt very hungry. Some recognised that glucose readings were elevated when they were busy, stressed or unwell.
Awareness of hunger
The majority of participants reported that they became better at recognising hunger. This made HT different from other weight management strategies they had previously attempted; ‘I’ve tried listening to people, I’ve tried following routines, I’ve tried all that sort of thing and I occasionally lose (a few) kilos and then I go straight back to square one because I don’t know how to… read my body. I didn’t know what it felt like to be hungry. I didn’t know that the way I felt was actually what it feels like to be full. So, I was keeping myself constantly full.’ (#42, female, scanner). The HT scale opened their eyes to the fact there was a continuum of hunger sensations
Some struggled to identify with the provided descriptors of each of the hunger levels on the hunger scale. A few overcame this by personalising their scale and/or using half points. However, others referred to initially being unfamiliar with some cues but then experiencing them for the first time during the study; ‘I’ve been married 11 years and my wife heard my stomach rumble and she was like ‘I’ve never heard that before!’ (#64, male, fingerpricker). Only a few participants felt that HT did not improve their ability to recognise real hunger.
Awareness of non-hungry eating
Almost all participants reported they learnt to tell the difference between hungry and non-hungry eating; ‘It was interesting to start to find out whether there was an actual need for food from the blood glucose reading vs whether it was a mind thing. I was actually quite quick to adjust to it and it gave a good chance to understand the feelings of hunger that you get, whether it might be physical or psychological, or just the environment you're in.’ (#37, male, scanner). Some were surprised to discover that they regularly ate when they were not hungry; ‘I didn’t think that I would be susceptible, like at the start of the study they talked about eating when you’re bored, or eating when you’re emotional and I totally expected to be above all of that petty human (stuff) because I'm intelligent. It was really eye opening, especially in the first 2 weeks of the study, just how programmed or routine a lot of my eating is.’ (#63, male, scanner).
Once aware of their non-hungry eating, participants generally avoided it; ‘If I have anything now, even if blood glucose is fine, that’s going to affect my ability to have something, a meal later on when I’m really hungry and I then my blood glucose would be more than likely too high to allow me to eat when I wanted to later on’ (#9, female, fingerpricker).
The most popular reason for non-hungry eating was boredom; ‘I’d say I was a bored eater. Like I’d be at home, what do I want to do? Uh, I don’t really know, I’m just walking around the kitchen, just open up the pantry for no reason, and […], I’m here, so I’ll grab something.’ (#79, male, fingerpricker). A few turned to food when stressed or upset. Some believed that if they didn’t eat regularly, their body would go into ‘starvation mode’; ‘Well, often people tell you if you ate too little that you will put your body into starvation mode and then it will hold onto the fat.’ (#42, female, scanner).
In terms of physical symptoms, some realised that they had confused hunger and thirst. Others realised they ate when tired, in order to give them energy. For a few, non-hungry cravings reduced once they stopped responding to them.
A few mentioned they ate when they were not hungry because of their environment; ‘I walked down past the café this morning, I went, ‘Ah, food’. It was really good to go to my brain, ‘No, you're not hungry, this is just your body pretending’.’ (#62, female, scanner). A lot mentioned they used to eat out of habit or routine, related to time of day, ‘Eat when I’m hungry, rather than eat because it's 12 o’clock.’ (#22, female, fingerpricker), or activity, ‘Have chips and dip and watch the rugby’ (#69, male, scanner).
Participants developed strategies when faced with triggers. The most common coping mechanism to avoid non-hungry eating was to drink water or another sugar-free beverage. Some diverted themselves with chores, a walk, or other activities. Participants dealt with social eating by planning ahead; ‘There’s another gathering this Saturday, so if I’m going to go and don’t feel like I’m really hungry to eat, I can take the food and do a takeaway and say, ‘oh I’ll take this and I’ll have it later’.’ (#76, male, scanner). One participant used strategies she used when quitting smoking; ‘I’d implement the breathing like you would have (when) you were having a cigarette. I would talk to someone or talk to myself if no one was around, ‘you know what this is you know that this is a craving, you just have to ride through it. ’ ’ (#42, female, scanner).
Awareness of fullness
A couple of participants randomised to the scanner connected their physical sensations with their glucose; ‘I could scan myself within half an hour of a heavy meal and be able to go see ‘you’re full, look what that’s done’ and show myself that actually this is what your body needs vs what it wants.’ (#42, female, scanner. Participants also used the hunger scale to identify their satiety; however, they expressed they were less confident about recognising fullness.
Some participants became aware of feeling uncomfortable from overeating, especially after their evening meal. A couple of participants noticed their sleep improved after reducing overeating.
Behaviour change
The main self-reported behaviour changes were changing their portions, food choices, and timing and frequency of meals (table 2).
Table 2.
Behaviour changes due to hunger training
| Theme description | Representative quotes |
| Portion size reduction | |
| Most participants reduced the amount of food they ate at a given meal | “This could show me in a physical way that you're not actually starving yourself eating this small amount. I learnt really quickly that actually if I get a six-inch subway sandwich, I'm just as full and satisfied for just as long a time period as I am if I have (the amount) that I'd normally get.”- #42, female, scanner |
| Some specifically reduced their portions of unhealthy foods, or foods that spiked their glucose | “Instead of buying a decent size cake of chocolate I bought the little bars and that was because I had in my mind the spike that would then come and associated the spike with what then is happening in your body.” - #25, female, scanner “I'd actually stop and think before I ate. So in the past I would have my cup of tea and just automatically reach for the biscuit tin. And maybe have two or three, instead of stopping at one. It's like ‘I only need that one, I'm fine now’. So definitely changed, my behaviours there. Even just being aware of when I was eating, of having that sensation of fullness, instead of just carrying on eating.” - #57, female, fingerpricker |
| Others reduced their intake in order to be able to eat when desired | “I've noticed that if I'm having less at lunch(…)then I'm able to eat my dinner at dinner time vs having a huge lunch and then my blood sugar is still so high that I couldn't have dinner.” – #38, female, scanner. |
| A few participants noticed their evening meal influenced morning glucose levels, often modifying their evening meal to eat breakfast at a convenient time | “We had a friend round and I had a dessert. I had to wait 20 min or longer in the morning. But I don't have 20 min in the morning. So, I was like, ‘okay, let's not do that’.” - #79, female, fingerpricker. |
| Food choice | |
| Participants from both groups found particular foods delayed their next subsequent meals due to being over their glucose cut-offs, and those wearing the scanner reported seeing a spike in glucose levels after consuming certain foods (or “spikey” foods). People also noticed which foods kept them satisfied for longer, which they viewed as positive. | “It made me acutely aware of what foods lasted me longer before the start growl (stomach growl) level was achieved.” - #50, male, scanner. |
| Reduced intake of “sugary foods” and “sweet stuff”, bread, chips, biscuits, chocolate, cakes, takeaways and fast food, sugar-sweetened beverages (SSBs), and alcohol. | “It was more around the drinking because I take a lot of convincing that things (are) right loaded with sugar and if I can't see it, I'm probably not going to believe a word you say. So, it was good to see it(…)After (I drank) I pricked my finger and saw it did shoot up, I would think a bit and have a look at what I was drinking and what was in it.” – #40, female, fingerpricker. |
| Increased intake of vegetables, salads, homemade meals, nuts, eggs, water, and coffee. | “Especially on a day off if I go up into the hills. I tend to bring things like muesli bars and just keep snacking all day, whereas now I'm not doing that. I'm waiting ' til I'm hungry and have a proper sit down. I'm drinking a lot more water, as well. ” - #20, male, fingerpricker |
| Increased planning of meals | “Actually I probably have thought about planning my day out meal wise a little bit more ‘cause I’d just grab whatever and just eat till I was full or you know, it used to be I’d come home from work and grab a snack and have dinner some time after that and yeah, I don’t snack anymore.” - #9, female, fingerpricker |
| Timing and frequency of meals | |
| Most reduced their number of eating occasions by eliminating snacking. The majority of this group changed their habit of grazing to eating a fixed number of meals a day, because they realised that they were not hungry, did not want to delay their next meal, and/or to avoid fingerpricking. Most chose to have a sugar-free beverage instead of food; a few combined their snack food with their main meal. | I would amalgamate (a snack) into a meal. So, this last weekend we were away so you know, we'd have a handful of chips, one or two crackers, some bits and pieces(…), and then you go, okay well I've had it. Now the old me, prior to this (study), would be having it a bit later on when the blood sugar would still be high and two, I'd probably be having the entire pack – #64, male, fingerpricker. |
| For most, monitoring their glucose confirmed their normal morning food pattern. However, some had glucose levels that were too high to eat breakfast at their usual time. For these participants, elevated morning glucose was frustrating. Some ate later, and others ignored their glucose and ate anyway. | “Because based on my monitoring, I'm pretty good, and I don't need to have breakfast, which was a relief, because I'm not a fan of breakfast to begin with.” - #62, female, scanner |
Themes listed in order of frequency.
Future expectations
Almost all participants expressed they were motivated and hopeful about continuing with their recent behaviour changes; ‘It seems an easy way to do it, 'cause it's not a diet. It's just eating sensibly and just waiting 'til your body's ready to eat. Rather than just eating for the sake of eating.’ - #20, male, fingerpricker. A few explained they would have liked to measure their glucose for longer to gain confidence about their hunger levels and some (mostly those who scanned) expressed concern about being without their equipment. This feeling of concern generally revolved around the fact that they would be without immediate feedback. On the contrary, other participants were happy and confident to leave their equipment behind. A few participants reflected that following HT without equipment would be the next step.
Discussion
Our use of pheonomenology to explore the experience of participants using HT provided rich descriptions that aided our understanding of the daily lived experiences of those monitoring their glucose on a regular basis. Most participants had a positive experience of HT, and were able to match their hunger to their glucose levels by the end of the study, which is consistent with other findings29 and our previous results.14 While the majority found an association between hunger and glucose, some experienced confusion, which is likely related to the homeostatic control of glucose.12
The main adherence barriers of social pressure to eat, lack of time, and lack of flexibility in meal schedule, and the main enablers of routine, social support and accountability, are consistent with those of a systematic review of determinants of adherence to lifestyle interventions in adults with obesity.16 Participants realised that they were previously unaware of feelings of appetite, supporting the theory that some with overweight/obesity have blunted sensations of hunger and satiety.8–10 Participants primarily changed their behaviour by becoming aware of hungry vs non-hungry eating, recognising feelings of hunger and satiety, reducing their number of meals, and exploring the effect of different types of foods on their glucose. This is in line with a review of mindful and intuitive eating interventions wherein participants became more aware of and reduced non-hungry eating.30 Although historically HT enhances recognition of hunger rather than satiety,29 our results indicate our participants felt they learnt to recognise feelings of fullness. Whether this translated to long-term behaviour change is unknown.
Participants randomised to use the scanners generally described fewer negative experiences and less adherence barriers, and were more inclined to try different foods to see the effects on their glucose, due to the ease of scanning. However, those randomised to fingerpricking may have become more mindful of their hunger, since they carefully considered their hunger before submitting to the effort and discomfort of fingerpricking. Fingerprickers were more confident returning their glucose measuring equipment, perhaps due to their established awareness of hunger. As suggested by our participants, a mobile app instead of a paper booklet, and including nutrition and exercise recommendations, and strategies to cope with emotional and social eating may increase adherence, and this agrees with current recommendations.31 Social support and involving family and friends may improve adherence and benefits, as demonstrated elsewhere.32–34
Our analysis was robust; the researchers were blinded for participant characteristics, all transcripts were double-coded, and the results were analysed and interpreted by three researchers. As with all interviews there is potential for response bias, with a chance of study participants providing socially desirable answers to appease researchers.35 We tried to reduce this by introducing an independent researcher for the interviews. Our study also has some limitations, principally related to the limited diversity obtained in our sample. Our participants were highly educated, perhaps in part a consequence of recruitment occurring in a university town, were predominantly European, and a smaller proportion of participants were diabetic than was anticipated.36 Anecdotal feedback indicated that diabetic patients were unwilling to be involved in research that might require additional blood glucose testing than was already required by their condition. Given the limited nature of our sample it is possible that different findings would be evident in a more diverse range of participants, particularly had we been able to recruit a significant number of diabetics with varying levels of glycaemic control. It is possible that participants reported on HT experiences that were intertwined with their own past struggles with other weight loss programme. Thus it is impossible to know if participants’ self-reported experiences here are a result solely of the HT protocol, or exposure to weight management strategies in general. This would be a relevant topic for further research.
Our interviews allowed insight into how participants undergoing HT felt it influenced their eating behaviour, and suggestions for how to better support participants in establishing healthy eating routines, both of which can be used to inform future HT programme and other healthy eating interventions in both primary care and public health settings.
Supplementary Material
Acknowledgments
We would like to thank all participants involved in the hunger training pilot study (ACTRN12618001257257). Thank you to Associate Professor Ben Wheeler and Dr Sara Boucher for sharing their expertise with the Freestyle Libre Flash Glucose Monitoring system.
Footnotes
Twitter: @wiwa21, @michellejospe
Contributors: Conceptualization, WEB., ALW., RT and MRJ; Methodology, WEB., ALW. and MRJ; Formal Analysis, WEB., ALW. and MRJ; Resources, MRJ; Data Curation, WEB., ALW. and MRJ; Writing-Original Draft Preparation, WEB and MRJ; Writing-Review & Editing, WEB., ALW., RT. and MRJ; Visualization, MRJ; Project Administration, WEB. and MRJ; Funding Acquisition, RT. and MRJ.
Funding: This research was supported by a University of Otago Research Grant.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request.
References
- 1. Franz MJ, VanWormer JJ, Crain AL, et al. Weight-Loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc 2007;107:1755–67. 10.1016/j.jada.2007.07.017 [DOI] [PubMed] [Google Scholar]
- 2. Johnston BC, Kanters S, Bandayrel K, et al. Comparison of weight loss among named diet programs in overweight and obese adults: a meta-analysis. JAMA 2014;312:923–33. 10.1001/jama.2014.10397 [DOI] [PubMed] [Google Scholar]
- 3. Borkoles E, Carroll S, Clough P, et al. Effect of a non-dieting lifestyle randomised control trial on psychological well-being and weight management in morbidly obese pre-menopausal women. Maturitas 2016;83:51–8. 10.1016/j.maturitas.2015.09.010 [DOI] [PubMed] [Google Scholar]
- 4. Leblanc V, Provencher V, Bégin C, et al. Impact of a Health-At-Every-Size intervention on changes in dietary intakes and eating patterns in premenopausal overweight women: results of a randomized trial. Clin Nutr 2012;31:481–8. 10.1016/j.clnu.2011.12.013 [DOI] [PubMed] [Google Scholar]
- 5. Provencher V, Bégin C, Tremblay A, et al. Health-At-Every-Size and eating behaviors: 1-year follow-up results of a size acceptance intervention. J Am Diet Assoc 2009;109:1854–61. 10.1016/j.jada.2009.08.017 [DOI] [PubMed] [Google Scholar]
- 6. Van Dyke N, Drinkwater EJ. Relationships between intuitive eating and health indicators: literature review. Public Health Nutr 2014;17:1757–66. 10.1017/S1368980013002139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bacon L, Keim NL, Van Loan MD, et al. Evaluating a ‘non-diet’ wellness intervention for improvement of metabolic fitness, psychological well-being and eating and activity behaviors. Int J Obes 2002;26:854–65. 10.1038/sj.ijo.0802012 [DOI] [PubMed] [Google Scholar]
- 8. Barkeling B, King NA, Näslund E, et al. Characterization of obese individuals who claim to detect no relationship between their eating pattern and sensations of hunger or fullness. Int J Obes 2007;31:435–9. 10.1038/sj.ijo.0803449 [DOI] [PubMed] [Google Scholar]
- 9. Blundell JE, Stubbs RJ, Golding C, et al. Resistance and susceptibility to weight gain: individual variability in response to a high-fat diet. Physiol Behav 2005;86:614–22. 10.1016/j.physbeh.2005.08.052 [DOI] [PubMed] [Google Scholar]
- 10. Drapeau V, Blundell J, Gallant AR, et al. Behavioural and metabolic characterisation of the low satiety phenotype. Appetite 2013;70:67–72. 10.1016/j.appet.2013.05.022 [DOI] [PubMed] [Google Scholar]
- 11. Flint A, Gregersen NT, Gluud LL, et al. Associations between postprandial insulin and blood glucose responses, appetite sensations and energy intake in normal weight and overweight individuals: a meta-analysis of test meal studies. Br J Nutr 2007;98:17–25. 10.1017/S000711450768297X [DOI] [PubMed] [Google Scholar]
- 12. Lemmens SG, Martens EA, Kester AD, et al. Changes in gut hormone and glucose concentrations in relation to hunger and fullness. Am J Clin Nutr 2011;94:717–25. 10.3945/ajcn.110.008631 [DOI] [PubMed] [Google Scholar]
- 13. Ciampolini M, Lovell-Smith D, Sifone M. Sustained self-regulation of energy intake. loss of weight in overweight subjects. maintenance of weight in normal-weight subjects. Nutr Metab 2010;7 10.1186/1743-7075-7-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jospe MR, Brown RC, Roy M, et al. Adherence to hunger training using blood glucose monitoring: a feasibility study. Nutr Metab 2015;12 10.1186/s12986-015-0017-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jospe M, Taylor R, Athens J, et al. Adherence to hunger training over 6 months and the effect on weight and eating behaviour: secondary analysis of a randomised controlled trial. Nutrients 2017;9:1260 10.3390/nu9111260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burgess E, Hassmén P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes 2017;7:123–35. 10.1111/cob.12183 [DOI] [PubMed] [Google Scholar]
- 17. Leung AWY, Chan RSM, Sea MMM, et al. An overview of factors associated with adherence to lifestyle modification programs for weight management in adults. Int J Environ Res Public Health 2017;14:922 10.3390/ijerph14080922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Creswell JW, Plano Clark VL. Choosing a mixed method design In: Designing and conducting mixed method research. 2nd ed, 2011: 58–88. [Google Scholar]
- 19. Bacon L, Matz J. Intuitive eating: enjoy your food, respect your body. Diabetes Self Manag 2010;27:44–51. [PubMed] [Google Scholar]
- 20. Mason M. Sample size and saturation in PHD studies using qualitative interviews. Forum Qual Soc Res 2010;11 10.17169/fqs-11.3.1428 [DOI] [Google Scholar]
- 21. Saunders B, Sim J, Kingstone T, et al. Saturation in qualitative research: exploring its conceptualization and operationalization. Qual Quant 2018;52:1893–907. 10.1007/s11135-017-0574-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sandelowski M. Whatever happened to qualitative description? Res Nurs Health 2000;23:334–40. <334::AID-NUR9>3.0.CO;2-G [DOI] [PubMed] [Google Scholar]
- 23. Strauss A, Corbin J. Basics of qualitative research: techniques and procedures for developing grounded theory. 2nd ed Thousand Oaks, CA, US: Sage Publications, Inc, 1998. [Google Scholar]
- 24. NVivo qualitative data analysis software [program] 12 version 2018.
- 25. Thomas DR. A general inductive approach for analyzing qualitative evaluation data. Am J Eval 2006;27:237–46. 10.1177/1098214005283748 [DOI] [Google Scholar]
- 26. Sandelowski M. Real qualitative researchers do not count: the use of numbers in qualitative research. Res Nurs Health 2001;24:230–40. 10.1002/nur.1025 [DOI] [PubMed] [Google Scholar]
- 27. Van Cauwenberg J, Van Holle V, Simons D, et al. Environmental factors influencing older adults' walking for transportation: a study using walk-along interviews. Int J Behav Nutr Phys Act 2012;9 10.1186/1479-5868-9-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ward AL, Freeman C, McGee R. The influence of transport on well-being among teenagers: a photovoice project in New Zealand. J Transp Health 2015;2:414–22. 10.1016/j.jth.2015.06.004 [DOI] [Google Scholar]
- 29. Ciampolini M, Bianchi R. Training to estimate blood glucose and to form associations with initial hunger. Nutr Metab 2006;3 10.1186/1743-7075-3-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Warren JM, Smith N, Ashwell M. A structured literature review on the role of mindfulness, mindful eating and intuitive eating in changing eating behaviours: effectiveness and associated potential mechanisms. Nutr Res Rev 2017;30:272–83. 10.1017/S0954422417000154 [DOI] [PubMed] [Google Scholar]
- 31. The diabetes prevention program (DPP) Research Group The diabetes prevention program (DPP). Diabetes Care 2002;25 10.2337/diacare.25.12.2165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lemstra M, Bird Y, Nwankwo C, et al. Weight loss intervention adherence and factors promoting adherence: a meta-analysis. Patient Prefer Adherence 2016;10:1547–59. 10.2147/PPA.S103649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Verheijden MW, Bakx JC, van Weel C, et al. Role of social support in lifestyle-focused weight management interventions. Eur J Clin Nutr 2005;59:S179–86. 10.1038/sj.ejcn.1602194 [DOI] [PubMed] [Google Scholar]
- 34. Elfhag K, Rössner S. Who succeeds in maintaining weight loss? A conceptual review of factors associated with weight loss maintenance and weight regain. Obes Rev 2005;6:67–85. 10.1111/j.1467-789X.2005.00170.x [DOI] [PubMed] [Google Scholar]
- 35. Stec JA. Bias : Lavrakas PJ, Encyclopedia of survey research methods. Thousand Oaks, California: Sage Publications, Inc, 2011: 57–60. [Google Scholar]
- 36. Leung MYM, Carlsson NP, Colditz GA, et al. The burden of obesity on diabetes in the United States: medical expenditure panel survey, 2008 to 2012. Value Health 2017;20:77–84. 10.1016/j.jval.2016.08.735 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2019-032248supp001.pdf (37.1KB, pdf)